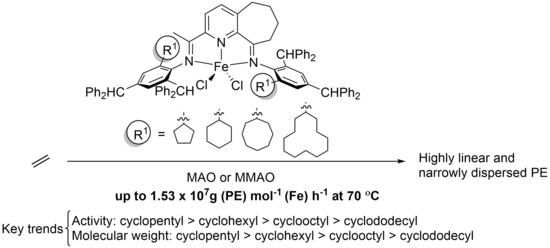
Adjusting Ortho-Cycloalkyl Ring Size in a Cycloheptyl-Fused N,N,N-Iron Catalyst as Means to Control Catalytic Activity and Polyethylene Properties
Abstract
1. Introduction
2. Results
2.1. Materials and Methods
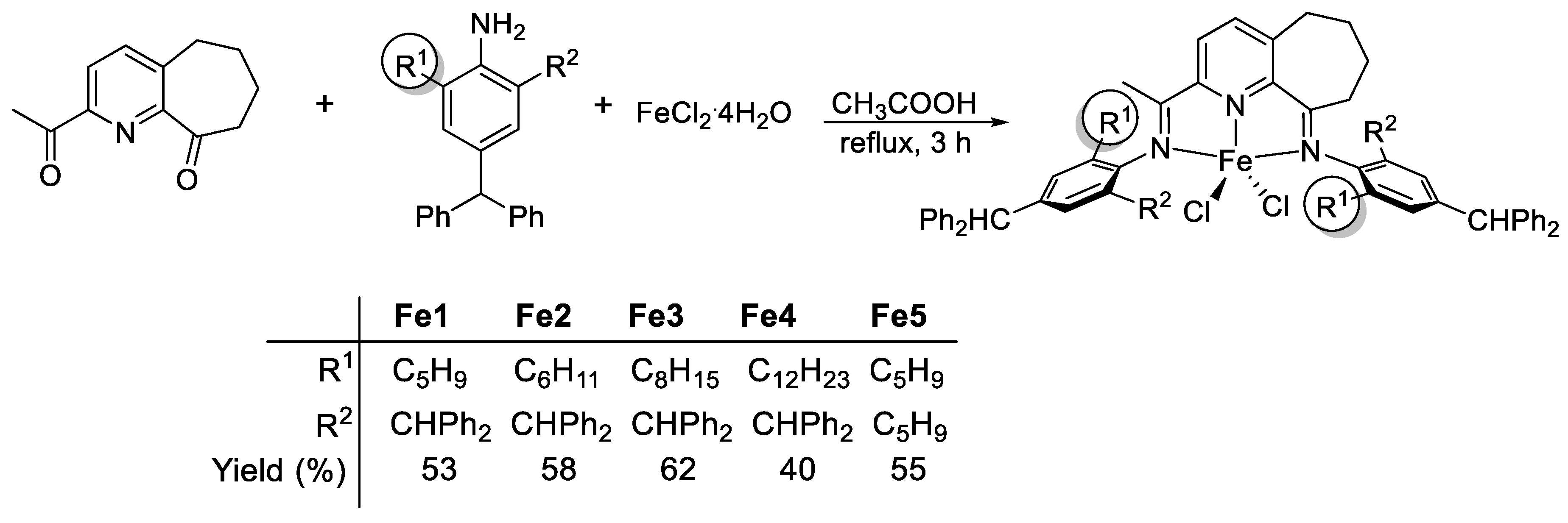
2.2. [2-{(Ar)N=CMe}-9-{N(Ar)}C10H10N]FeCl2 (Fe1–Fe5)
2.3. General Procedure for Ethylene Polymerization
2.4. X-ray Diffraction Studies
3. Results and Discussion
3.1. Synthesis and Characterization of Fe1–Fe5
3.2. Ethylene Polymerization
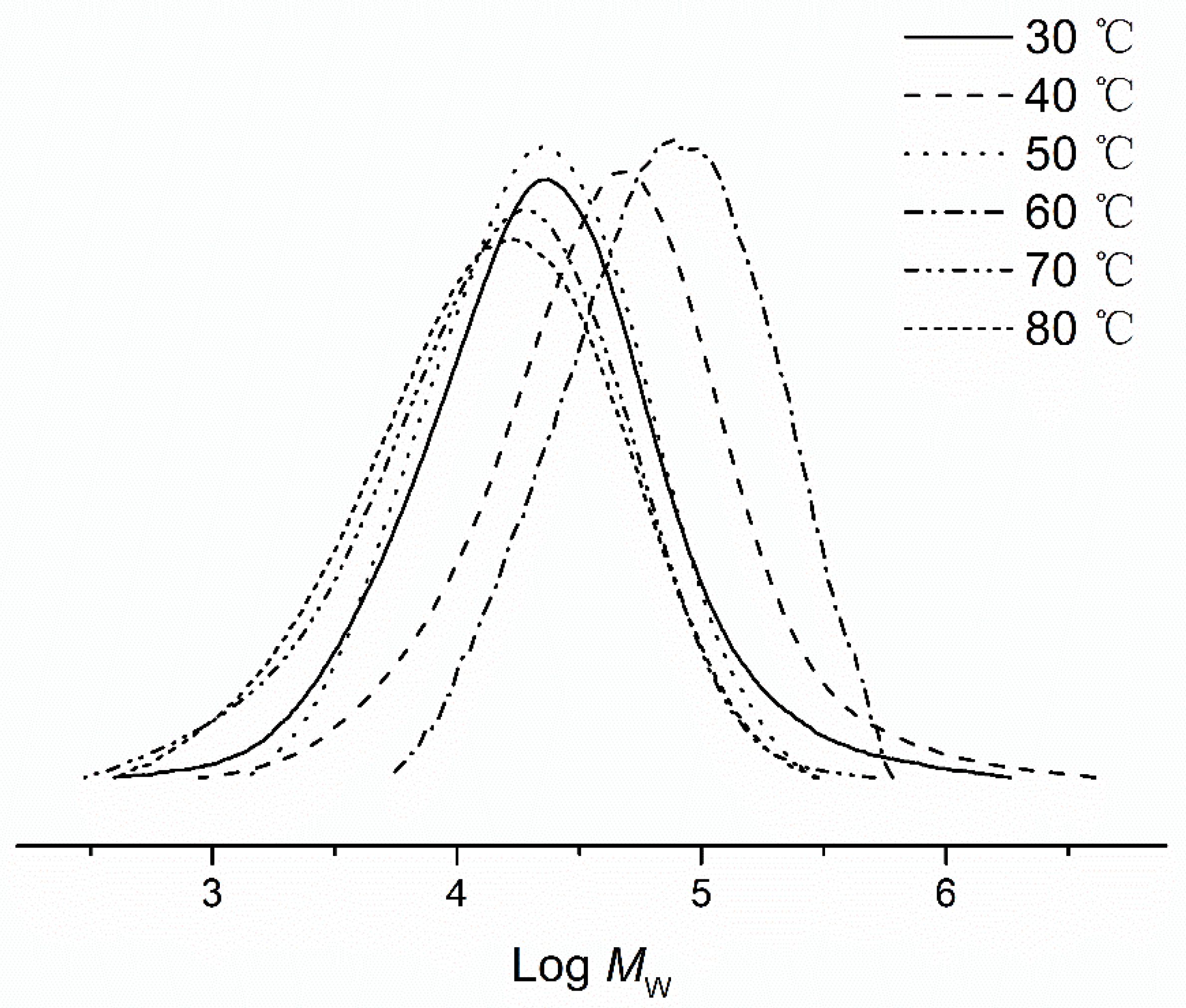
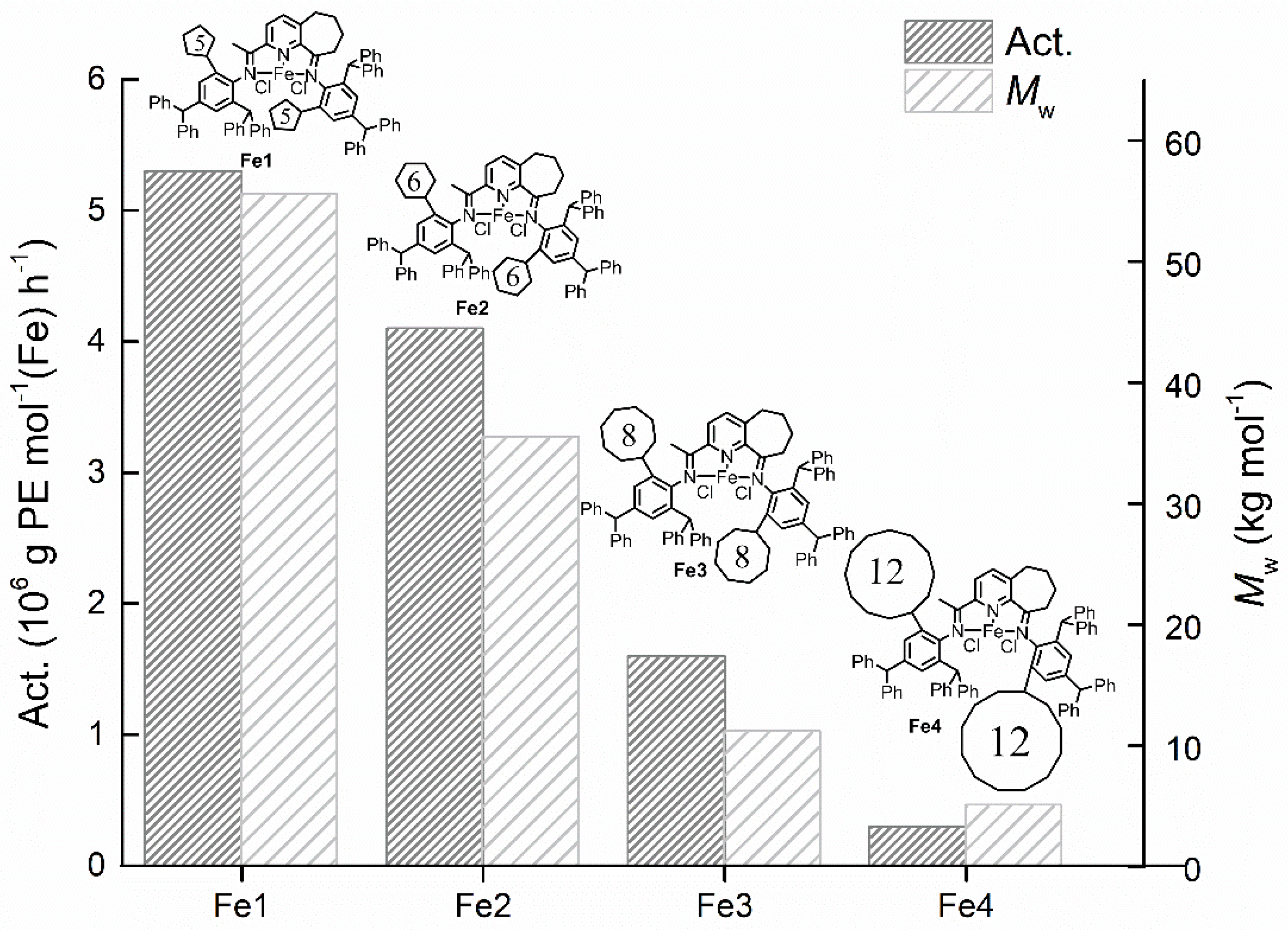
3.2.1. Catalytic Evaluation Using Fe1–Fe5/MMAO
3.2.2. Catalytic Evaluation Using Fe1–Fe5/MAO
3.3. Microstructural Properties of the Polyethylene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natta, G.; Pino, P.; Corradini, P.; Danusso, F.; Mantica, E.; Mazzanti, G.; Moraglio, G. Crystalline high polymers of α-olefins. J. Am. Chem. Soc. 1955, 77, 1708–1710. [Google Scholar] [CrossRef]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Flisak, Z.; Sun, W.-H. Progression of Diiminopyridines: From Single Application to Catalytic Versatility. ACS Catal. 2015, 5, 4713–4724. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Burcher, B.; Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Iron-Catalyzed Oligomerization and Polymerization Reactions. Top. Organomet. Chem. 2015, 50, 217–258. [Google Scholar]
- Britovsek, G.J.P.; Mastroianni, S.; Solan, G.A.; Baugh, S.P.D.; Redshaw, C.; Gibson, V.C.; White, A.J.P.; Williams, D.J.; Elsegood, M.R.J. Oligomerisation of Ethylene by Bis(imino)pyridyliron and -cobalt Complexes. Chem. Eur. J. 2000, 6, 2221–2231. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Hoarau, O.D.; Spitzmesser, S.K.; White, A.J.P.; Williams, D.J. Iron and Cobalt Ethylene Polymerization Catalysts: Variations on the Central Donor. Inorg. Chem. 2003, 42, 3454–3465. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, N.; Xiang, J.; Solan, G.A.; Suo, H.; Ma, Y.; Liang, T.; Sun, W.-H. Bis-cycloheptyl-fused bis(imino)pyridine-cobalt catalysts for PE wax formation: Positive effects of fluoride substitution on catalytic performance and thermal stability. Dalton Trans. 2020, 49, 9425–9437. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M. Highly Active Iron and cobalt Catalysts for the Polymerization of Olefins. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 7, 849–850. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Strömberg, S.; et al. Iron and cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Small, B.L. Discovery and Development of Pyridine-bis(imine) and Related Catalysts for Olefin Polymerization and Oligomerization. Acc. Chem. Res. 2015, 48, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Small, B.L.; Brookhart, M. Iron-Based Catalysts with Exceptionally High Activities and Selectivities for Oligomerization of Ethylene to Linear r-Olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Yuan, S.; Fan, Z.; Zhang, Q.; Flisak, Z.; Ma, Y.; Sun, W.-H. Enhancing performance of α-diiminonickel precatalyst for ethylene polymerization by substitution with the 2,4-bis(4,4′-dimethoxybenzhydryl)-6-methylphenyl group. Appl. Organomet. Chem. 2020, 34, e5638. [Google Scholar] [CrossRef]
- Bariashir, C.; Huang, C.; Solan, G.A.; Sun, W.-H. Recent advances in homogeneous chromium catalyst design for ethylene tri-, tetra-, oligo and polymerization. Coord. Chem. Rev. 2019, 385, 208–229. [Google Scholar] [CrossRef]
- Gansukh, B.; Zhang, Q.; Flisak, Z.; Liang, T.; Ma, Y.; Sun, W.-H. The chloro-substituent enhances performance of 2,4-bis(imino)pyridylchromium catalysts yielding highly linear polyethylene. Appl. Organomet. Chem. 2020, 34, e5471. [Google Scholar] [CrossRef]
- Phillips, A.; Suo, H.; Silva, M.; Pombeiro, A.; Sun, W.-H. Recent developments in vanadium-catalyzed olefin coordination polymerization. Coord. Chem. Rev. 2020, 416, e213332. [Google Scholar] [CrossRef]
- Du, S.; Wang, X.; Zhang, W.; Flisak, Z.; Sun, Y.; Sun, W.-H. A practical ethylene polymerization for vinyl-polyethylenes: Synthesis, characterization and catalytic behavior of α,α′-bisimino-2,3:5,6-bis(pentamethylene)pyridyliron chlorides. Polym. Chem. 2016, 7, 4188–4197. [Google Scholar] [CrossRef]
- Bariashir, C.; Wang, Z.; Ma, Y.; Vignesh, A.; Hao, X.; Sun, W.-H. Finely Tuned α,α′-Bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridine-Based Practical Iron Precatalysts for Targeting Highly Linear and Narrow Dispersive Polyethylene Waxes with Vinyl Ends. Organometallics 2019, 38, 4455–4470. [Google Scholar] [CrossRef]
- Huang, F.; Xing, Q.; Liang, T.; Flisak, Z.; Ye, B.; Hu, X.; Yang, W.; Sun, W.-H. 2-(1-Aryliminoethyl)-9-arylimino-5,6,7,8-tetrahydrocycloheptapyridyl iron(II) dichloride: Synthesis, characterization, and the highly active and tunable active species in ethylene polymerization. Dalton Trans. 2014, 43, 16818–16829. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, W.; Oleynik, I.I.; Solan, G.A.; Oleynik, I.V.; Liang, T.; Sun, W.-H. Probing the effect of ortho-cycloalkyl ring size on activity and thermostability in cycloheptyl-fused N,N,N-iron ethylene polymerization catalysts. Dalton Trans. 2020, 49, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Li, Z.; Oleynik, I.V.; Wang, Z.; Oleynik, I.I.; Ma, Y.; Liu, Q.; Sun, W.-H. Achieving strictly linear polyethylenes by the NNN-Fe precatalysts finely tuned with different sizes of ortho-cycloalkyl substituents. Appl. Organomet. Chem. 2020, e5937. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Ma, Y.; Liu, Q.; Liang, T.; Sun, W.-H. Fusing carbocycles of inequivalent ring size to a bis(imino)pyridine-iron ethylene polymerization catalyst; distinctive effects on activity, PE molecular weight and dispersity. Research 2019, 2019, e9426063. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Huang, H.; Mo, W.; Fan, X.; Shen, Z.; Sun, N.; Hu, B.; Hu, X. Tetrahedron: Asymmetry. Sci. Direct 2009, 20, 1425–1432. [Google Scholar]
- Oleinik, I.I.; Oleinik, I.V.; Abdrakhmanov, I.B.; Ivanchev, S.S.; Tolstikov, G.A. Design of Arylimine Postmetallocene Catalytic Systems for Olefin Polymerization: I. Synthesis of Substituted 2-Cycloalkyl and 2,6-Dicycloalkylanilines. Russ. J. Gen. Chem. 2004, 74, 1423–1427. [Google Scholar] [CrossRef]
- Chartoire, A.; Claver, C.; Corpet, M.; Krinsky, J.; Mayen, J.; Nelson, D.; Nolan, S.P.; Penafiel, I.; Woodward, R.; Meadows, R.E. Recycle NHC Catalyst for the Development of a Generalized Approach to Continuous Buchwald-Hartwing Reaction and Workup. Org. Process Res. Dev. 2016, 20, 551–557. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Q.; Oleinik, I.I.; Suo, H.; Solan, G.A.; Oleinik, I.V.; Ma, Y.; Liang, T.; Sun, W.-H. High molecular weight polyethylenes of narrow dispersity promoted using bis(arylimino)cyclohepta[b]pyridine-cobalt catalysts ortho-substituted with benzhydryl & cycloalkyl groups. Dalton Trans. 2020, 49, 4774–4784. [Google Scholar]
- Sheldrick, G.M. SHELXT: Integrated space-group and crystalstructure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Guo, J.J.; Wang, Z.; Zhang, W.; Oleynik, I.I.; Vignesh, A.; Oleynik, I.V.; Hu, X.; Sun, Y.; Sun, W.-H. Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand. Molecules 2019, 24, 1176. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; Han, M.; Solan, G.A.; Pi, Y.; Sun, Y.; Sun, W.-H. Exceptionally high molecular weight linear polyethylene by using N,N,N′-Co catalysts appended with a N′-2,6-bis{di(4-fluorophenyl)methyl}-4-nitrophenyl group. Appl. Organomet. Chem. 2019, 33, e5157. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Suo, H.; Solan, G.A.; Liang, T.; Sun, W.-H. Co-catalyst effects on the thermal stability/activity of N,N,N-Co ethylene polymerization Catalysts Bearing Fluoro-Substituted N-2,6-dibenzhydrylphenyl groups. Appl. Organomet. Chem. 2019, 33, e5134. [Google Scholar] [CrossRef]
- Zada, M.; Guo, L.; Ma, Y.; Zhang, W.; Flisak, Z.; Sun, Y.; Sun, W.-H. Activity and Thermal Stability of Cobalt(II)-Based Olefin Polymerization Catalysts Adorned with Sterically Hindered Dibenzocycloheptyl Groups. Molecules 2019, 24, 2007. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Controlling the molecular weights of polyethylene waxes using the highly active precatalysts of 2-(1-aryliminoethyl)-9-arylimino-5,6,7,8-tetrahydrocycloheptapyridylCobalt chlorides: Synthesis, characterization, and catalytic behavior. Dalton Trans. 2016, 45, 657–666. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Solan, G.A.; Liang, T.; Sun, W.-H. Methylene-bridged bimetallic bis(imino)pyridinecobaltous chlorides as precatalysts for vinyl-terminated polyethylene waxes. Dalton Trans. 2018, 47, 6124–6133. [Google Scholar] [CrossRef]
- Suo, H.; Oleynik, I.I.; Bariashir, C.; Oleynik, I.V.; Wang, Z.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Strictly linear polyethylene using Fe-catalysts chelated by fused bis(arylimino)pyridines: Probing ortho-cycloalkyl ring-size effects on molecular weight. Polymer 2018, 149, 45–54. [Google Scholar] [CrossRef]
- Appukuttan, V.K.; Liu, Y.; Son, B.C.; Ha, C.-S.; Suh, H.; Kim, I.I. Iron and cobalt complexes of 2,3,7,8-tetrahydroacridine-4,-5(1H,6H)-diimine sterically modulated by substituted aryl rings for the selective oligomerization to polymerization of ethylene. Organometallics 2011, 30, 2285–2294. [Google Scholar] [CrossRef]
- Xiao, T.; Hao, P.; Kehr, G.; Hao, X.; Erker, G.; Sun, W.-H. Dichlorocobalt(II) Complexes Ligated by Bidentate 8-(Benzoimidazol-2-yl)quinolines: Synthesis, Characterization, and Catalytic Behavior toward Ethylene. Organometallics 2011, 30, 4847–4853. [Google Scholar] [CrossRef]
- Hansen, E.; Blom, R.; Bade, O. NMR characterization of polyethylene with emphasis on internal consistency of peak intensities and estimation of uncertainties in derived branch distribution numbers. Polymer 1997, 38, 4295–4304. [Google Scholar] [CrossRef]
- Galland, G.; Quijada, R.; Rolas, R.; Bazan, G.; Komon, Z. NMR Study of Branched Polyethylenes Obtained with Combined Fe and Zr Catalysts. Macromolecules 2002, 35, 339–345. [Google Scholar] [CrossRef]
- Semikolenova, N.; Sun, W.-H.; Soshnikov, I.; Matsko, M.; Kolesova, O.; Zakharov, V.; Bryliakov, K. Origin of “Multisite-like” Ethylene Polymerization Behavior of the Single-Site Nonsymmetrical Bis(imino)pyridine Iron(II) Complex in the Presence of Modified Methylaluminoxane. ACS Catal. 2017, 7, 2868–2877. [Google Scholar] [CrossRef]





| - | Fe3 | Fe5 |
|---|---|---|
| Crystal color | blue | gray |
| Empirical formula | C92H91Cl2FeN3 | 2 C70H75Cl2FeN3·3CH2Cl2 |
| Formula weight | 1363.41 | 2424.93 |
| T (K) | 172(2) | 173(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Tetragonal | Orthorhombic |
| Space group | I41/a | P212121 |
| a/Å | 32.4190(2) | 18.8805(6) |
| b/Å | 32.4190(2) | 19.1069(5) |
| c/Å | 31.8280(3) | 35.1775(10) |
| α/° | 90 | 90 |
| β/° | 90 | 90 |
| γ/° | 90 | 90 |
| Volume/Å3 | 33,451.0(5) | 12,690.2(6) |
| Z | 16 | 4 |
| ρcalcg/cm3 | 1.083 | 1.269 |
| μ/mm−1 | 2.359 | 0.492 |
| F(000) | 11,552.0 | 5112 |
| Crystal size/mm3 | 0.15 × 0.1 × 0.05 | 0.184 × 0.181 × 0.062 |
| Θ range (°) | 6.7 to 151.132 | 3.032 to 50 |
| Limiting indices | −34 ≤ h ≤ 36 | −22 ≤ h ≤ 22 |
| −35 ≤ k ≤ 40 | −22 ≤ k ≤ 22 | |
| −39 ≤ l ≤ 37 | −41 ≤ l ≤ 39 | |
| No. of rflns collected | 65,052 | 76,355 |
| No. unique rflns [R(int)] | 16,088(0.0368) | 21,892(0.1219) |
| Completeness to θ (%) | 96.2 | 99.8 |
| Goodness of fit on F2 | 1.024 | 1.024 |
| Final R indices [I > 2σ(I)] | R1 = 0.0531 | R1 = 0.0953 |
| wR2 = 0.1370 | wR2 = 0.2468 | |
| R indices (all data) | R1 = 0.0623 | R1 = 0.1449 |
| wR2 = 0.1425 | wR2 = 0.2910 | |
| Largest diff peak and hole (e Å−3) | 0.99/−0.35 | 0.92/−0.56 |
| - | Fe3 | Fe5 |
|---|---|---|
| - | Bond lengths (Å) | - |
| Fe(1)-N(1) | 2.2256(18) | 2.287(8) |
| Fe(1)-N(2) | 2.0826(17) | 2.132(8) |
| Fe(1)-N(3) | 2.1972(17) | 2.292(8) |
| Fe(1)-Cl(1) | 2.3250(6) | 2.316(4) |
| Fe(1)-Cl(2) | 2.2456(6) | 2.282(4) |
| - | Bond Angles (deg) | - |
| N(1)-Fe(1)-N(2) | 73.48(6) | 73.5(3) |
| N(1)-Fe(1)-N(3) | 141.04(6) | 144.8(3) |
| N(2)-Fe(1)-N(3) | 73.23(6) | 72.1(3) |
| N(1)-Fe(1)-Cl(2) | 97.70(5) | 102.0(2) |
| N(2)-Fe(1)-Cl(2) | 152.55(6) | 135.5(3) |
| N(3)-Fe(1)-Cl(2) | 102.58(5) | 97.2(2) |
| N(1)-Fe(1)-Cl(1) | 104.23(5) | 97.0(2) |
| N(2)-Fe(1)-Cl(1) | 92.60(5) | 113.0(2) |
| N(3)-Fe(1)-Cl(1) | 96.92(5) | 103.1(3) |
| Cl(2)-Fe(1)-Cl(1) | 114.84(2) | 111.51(13) |
| Entry | Precat. | Al:Fe | T/°C | t/min | Activity b | Mwc | Mw/Mnc | Tmd/°C |
|---|---|---|---|---|---|---|---|---|
| 1 | Fe2 | 2000 | 50 | 30 | 4.2 | 7.0 | 2.7 | 127.4 |
| 2 | Fe2 | 2000 | 60 | 30 | 7.9 | 9.4 | 3.0 | 128.6 |
| 3 | Fe2 | 2000 | 70 | 30 | 11.2 | 12.2 | 3.1 | 130.3 |
| 4 | Fe2 | 2000 | 80 | 30 | 8.8 | 7.2 | 2.3 | 127.5 |
| 5 | Fe2 | 2000 | 90 | 30 | 7.5 | 9.3 | 2.3 | 126.4 |
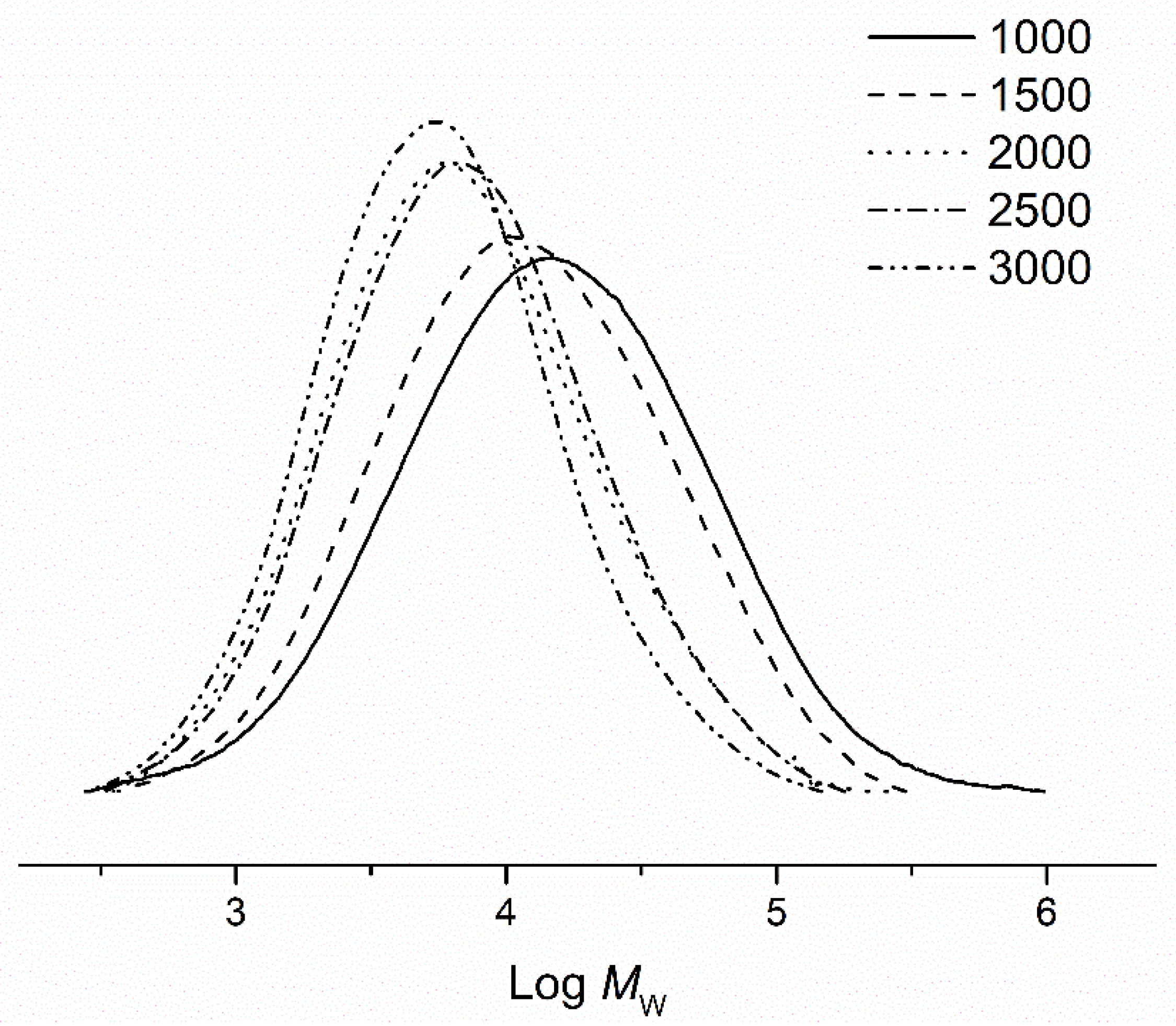
| 6 | Fe2 | 1000 | 70 | 30 | 7.4 | 31.1 | 4.5 | 132.6 |
| 7 | Fe2 | 1500 | 70 | 30 | 9.4 | 21.8 | 3.7 | 131.9 |
| 8 | Fe2 | 2500 | 70 | 30 | 9.0 | 12.5 | 3.0 | 130.1 |
| 9 | Fe2 | 3000 | 70 | 30 | 7.8 | 9.4 | 2.8 | 128.4 |
| 10 | Fe2 | 2000 | 70 | 5 | 23.8 | 10.9 | 2.6 | 129.3 |
| 11 | Fe2 | 2000 | 70 | 15 | 15.3 | 14.5 | 3.3 | 129.9 |
| 12 | Fe2 | 2000 | 70 | 45 | 9.9 | 41.9 | 4.7 | 132.2 |
| 13 | Fe2 | 2000 | 70 | 60 | 9.0 | 64.8 | 4.0 | 132.5 |
| 14 e | Fe2 | 2000 | 70 | 30 | 6.4 | 9.2 | 2.8 | 127.9 |
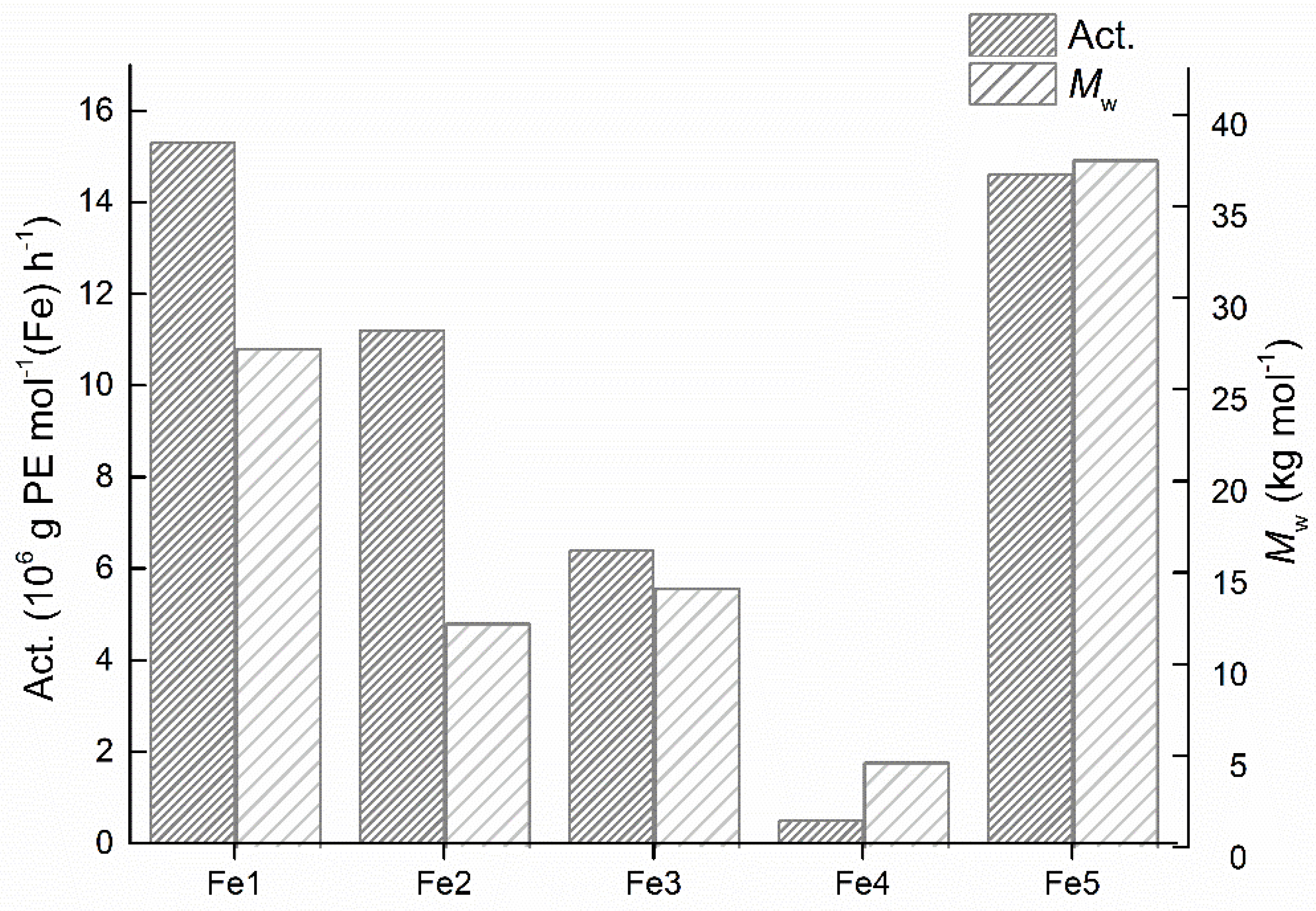
| 15 | Fe1 | 2000 | 70 | 30 | 15.3 | 27.2 | 4.2 | 130.4 |
| 16 | Fe3 | 2000 | 70 | 30 | 6.4 | 14.1 | 2.9 | 129.7 |
| 17 | Fe4 | 2000 | 70 | 30 | 0.5 | 4.6 | 2.8 | 125.7 |
| 18 | Fe5 | 2000 | 70 | 30 | 14.6 | 37.5 | 4.0 | 128.7 |
| Entry | Precat | Al:Fe | T/°C | t/min | Activity b | Mwc | Mw/Mnc | Tmd/°C |
|---|---|---|---|---|---|---|---|---|
| 1 | Fe2 | 2000 | 30 | 30 | 2.7 | 45.8 | 2.7 | 135.0 |
| 2 | Fe2 | 2000 | 40 | 30 | 2.9 | 90.6 | 3.0 | 132.6 |
| 3 | Fe2 | 2000 | 50 | 30 | 3.1 | 32.0 | 3.1 | 134.6 |
| 4 | Fe2 | 2000 | 60 | 30 | 3.3 | 103.5 | 2.3 | 131.9 |
| 5 | Fe2 | 2000 | 70 | 30 | 1.2 | 25.9 | 2.3 | 133.8 |
| 6 | Fe2 | 2000 | 80 | 30 | 1.0 | 24.7 | 4.5 | 131.1 |
| 7 | Fe2 | 1000 | 60 | 30 | 1.0 | 186.8 | 3.7 | 135.1 |
| 8 | Fe2 | 1500 | 60 | 30 | 2.9 | 41.6 | 3.0 | 132.2 |
| 9 | Fe2 | 2500 | 60 | 30 | 4.1 | 35.5 | 2.8 | 131.7 |
| 10 | Fe2 | 3000 | 60 | 30 | 2.7 | 13.6 | 2.6 | 130.3 |
| 11 | Fe2 | 2500 | 60 | 5 | 7.2 | 88.9 | 3.3 | 133.1 |
| 12 | Fe2 | 2500 | 60 | 15 | 5.6 | 52.8 | 4.7 | 132.3 |
| 13 | Fe2 | 2500 | 60 | 45 | 2.9 | 44.5 | 4.0 | 132.5 |
| 14 | Fe2 | 2500 | 60 | 60 | 2.4 | 71.2 | 2.8 | 134.2 |
| 15 e | Fe2 | 2500 | 60 | 30 | 1.2 | 28.8 | 3.2 | 133.0 |
| 16 | Fe1 | 2500 | 60 | 30 | 5.3 | 55.6 | 2.9 | 131.8 |
| 17 | Fe3 | 2500 | 60 | 30 | 1.6 | 11.2 | 2.8 | 131.3 |
| 18 | Fe4 | 2500 | 60 | 30 | 0.3 | 5.1 | 4.0 | 129.6 |
| 19 | Fe5 | 2500 | 60 | 30 | 6.0 | 25.6 | 2.7 | 131.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Zhang, Q.; Oleynik, I.I.; Suo, H.; Oleynik, I.V.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Adjusting Ortho-Cycloalkyl Ring Size in a Cycloheptyl-Fused N,N,N-Iron Catalyst as Means to Control Catalytic Activity and Polyethylene Properties. Catalysts 2020, 10, 1002. https://doi.org/10.3390/catal10091002
Han M, Zhang Q, Oleynik II, Suo H, Oleynik IV, Solan GA, Ma Y, Liang T, Sun W-H. Adjusting Ortho-Cycloalkyl Ring Size in a Cycloheptyl-Fused N,N,N-Iron Catalyst as Means to Control Catalytic Activity and Polyethylene Properties. Catalysts. 2020; 10(9):1002. https://doi.org/10.3390/catal10091002
Chicago/Turabian StyleHan, Mingyang, Qiuyue Zhang, Ivan I. Oleynik, Hongyi Suo, Irina V. Oleynik, Gregory A. Solan, Yanping Ma, Tongling Liang, and Wen-Hua Sun. 2020. "Adjusting Ortho-Cycloalkyl Ring Size in a Cycloheptyl-Fused N,N,N-Iron Catalyst as Means to Control Catalytic Activity and Polyethylene Properties" Catalysts 10, no. 9: 1002. https://doi.org/10.3390/catal10091002
APA StyleHan, M., Zhang, Q., Oleynik, I. I., Suo, H., Oleynik, I. V., Solan, G. A., Ma, Y., Liang, T., & Sun, W.-H. (2020). Adjusting Ortho-Cycloalkyl Ring Size in a Cycloheptyl-Fused N,N,N-Iron Catalyst as Means to Control Catalytic Activity and Polyethylene Properties. Catalysts, 10(9), 1002. https://doi.org/10.3390/catal10091002