Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects
Abstract
1. Introduction
2. Produced Water Characteristics, Treatment and Reuse
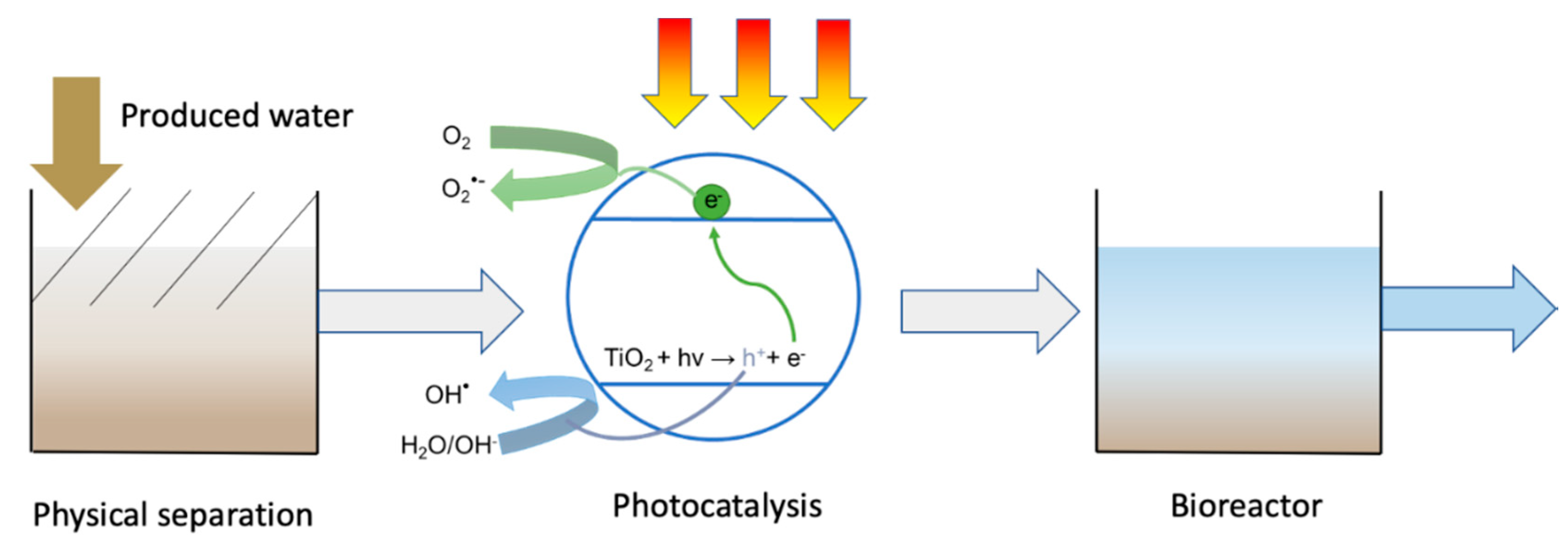
3. Photocatalytic Treatment of Produced Water
3.1. Decomposition and Mineralization
3.2. Toxicity
4. Factors Affecting Photocatalytic Performance
4.1. Ionic Species in Produced Water
4.2. Organics in Produced Water
5. Future Research Perspectives
5.1. Biodegradability Improved with Photocatalysis
5.2. Toxicity of Catalysts
5.3. Photoinduced Intermediates
5.4. Photo-Detoxication of Heavy Metals
5.5. Catalysts
6. System Integration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fakhru’L-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas Produced Water Treatment Technologies; All Consulting, LLC: Tulsa, OK, USA, 2005. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low Carbon Technol. 2012, 9, 157–177. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E. Viability of nanfiltration and ultra-low pressure reverse osmosis membranes for multi-beneficial use of methane produced water. Sep. Purif. Technol. 2006, 52, 67–76. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D. Beneficial use of co-produced water through membrane treatment: technical-economic assessment. Desalination 2008, 225, 139–155. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Hancock, N.T.; Hutchings, N.R.; Appleton, E.W.; Beaudry, E.G.; Xu, P.; Cath, T.Y. Forward osmosis treatment of drilling mud and fracturing wastewater from oil and gas operations. Desalination 2013, 312, 60–66. [Google Scholar] [CrossRef]
- Stoll, Z.A.; Forrestal, C.; Ren, Z.J.; Xu, P. Shale gas produced water treatment using innovative microbial capacitive desalination cell. J. Hazard. Mater. 2015, 283, 847–855. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous recovery of ammonium, potassium and magnesium from produced water by struvite precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Geza, M.; Ma, G.; Kim, H.; Cath, T.Y.; Xu, P. iDST: An integrated decision support tool for treatment and beneficial use of non-traditional water supplies–Part I. Methodology. J. Water Process. Eng. 2018, 25, 236–246. [Google Scholar] [CrossRef]
- Dores, R.; Hussain, A.; Katebah, M.; Adham, S.S. Using Advanced Water Treatment Technologies to Treat Produced Water from the Petroleum Industry. In Proceedings of the SPE International Production and Operations Conference & Exhibition, Doha, Qatar, 14–16 May 2012. [Google Scholar]
- Hussain, A.; Minier-Matar, J.; Janson, A.; Gharfeh, S.; Adham, S. Advanced technologies for produced water treatment and reuse. In Proceedings of the International Petroleum Technology Conference, European Association of Geoscientists & Engineers, Doha, Qatar, 19–22 January 2014; No. 1. pp. 1–11. [Google Scholar]
- Munirasu, S.; Abu Haija, M.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process. Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process. Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Boschee, P. Handling Produced Water from Hydraulic Fracturing. Oil Gas Facil. 2012, 1, 22–26. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Butkovskyi, A.; Bruning, H.; Kools, S.A.; Rijnaarts, H.; Van Wezel, A.P. Organic Pollutants in Shale Gas Flowback and Produced Waters: Identification, Potential Ecological Impact, and Implications for Treatment Strategies. Environ. Sci. Technol. 2017, 51, 4740–4754. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.; Fakhru’L-Razi, A.; Chuah, T.; Radiah, A.D.; Madaeni, S.; Zurina, Z. Biological treatment of produced water in a sequencing batch reactor by a consortium of isolated halophilic microorganisms. Environ. Technol. 2010, 31, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Saba, B. Potential Treatment Options for Hydraulic Fracturing Return Fluids: A Review. ChemBioEng Rev. 2014, 1, 273–279. [Google Scholar] [CrossRef]
- Abousnina, R.M.; Nghiem, L.D.; Bundschuh, J. Comparison between oily and coal seam gas produced water with respect to quantity, characteristics and treatment technologies: A review. Desalin. Water Treat. 2014, 54, 1793–1808. [Google Scholar] [CrossRef]
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Xu, P.; Stoll, Z.; Ma, G.; Geza, M.; Cath, T.Y.; Drewes, J. Technical Assessment of Produced Water Treatment Technologies. An Integrated Framework for Treatment and Management of Produced Water; Final Report to Department of Energy Project 11122-53; New Mexico State University-Colorado School of Mines: Las Cruces, NM, USA; Golden, CO, USA, 2016. [Google Scholar]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Ely, J.W.; Fraim, M.; Horn, A.D.; Jakhete, S.D. Game changing technology for treating and recycling frac water. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Jiang, W.; Mkaouar, A.R.; Xu, P. Comparison study on photocatalytic oxidation of pharmaceuticals by TiO2-Fe and TiO2-reduced graphene oxide nanocomposites immobilized on optical fibers. J. Hazard. Mater. 2017, 333, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, H.; Luo, H.; Xu, P. Enhanced photocatalysis using side-glowing optical fibers coated with Fe-doped TiO2 nanocomposite thin films. J. Photochem. Photobiol. A: Chem. 2015, 307, 88–98. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef]
- Litter, M.I. Mechanisms of removal of heavy metals and arsenic from water by TiO2-heterogeneous photocatalysis. Pure Appl. Chem. 2015, 87, 557–567. [Google Scholar] [CrossRef]
- Veil, J. U.S. Produced Water Volumes and Management Practices in 2012; Ground Water Protection Council: Oklahoma City, OK, USA, 2015; Available online: http://www.veilenvironmental.com/publications/pw/final_report_CO_note.pdf (accessed on 1 August 2020).
- U.S. EIA. Annual Energy Outlook 2019: With Projections to 2050; US Energy Information Administration Office of Energy Analysis, U.S. Department of Energy: Washington, DC, USA, 2019. Available online: https://www.eia.gov/outlooks/aeo/pdf/aeo2019.pdf (accessed on 1 August 2020).
- U.S. EIA. Drilling Productivity Report; US Energy Information Administration Independent Statistics & Analysis: Washington, DC, USA, 2020. Available online: https://www.eia.gov/petroleum/drilling/ (accessed on 1 August 2020).
- Produced Water Report: Regulations, Current Practices, and Research Needs. 2019. Available online: http://www.gwpc.org/sites/default/files/files/Produced%20Water%20Full%20Report%20-%20Digital%20Use.pdf (accessed on 1 August 2020).
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.A.; Nicot, J.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can we beneficially reuse produced water from oil and gas extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef]
- Chaudhary, B.K.; Sabie, R.; Engle, M.A.; Xu, P.; Willman, S.; Carroll, K.C. Spatial variability of produced-water quality and alternative-source water analysis applied to the Permian Basin, USA. Hydrogeol. J. 2019, 27, 2889–2905. [Google Scholar] [CrossRef]
- Study of Oil and Gas Extraction Wastewater Management under the Clean Water Act. 2019. Available online: https://www.epa.gov/sites/production/files/2019-05/documents/oil-and-gas-study_draft_05-2019.pdf (accessed on 1 August 2020).
- Ma, G.; Geza, M.; Cath, T.Y.; Drewes, J.E.; Xu, P. iDST: An integrated decision support tool for treatment and beneficial use of non-traditional water supplies—Part II. Marcellus and Barnett Shale case studies. J. Water Process. Eng. 2018, 25, 258–268. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Nasr, M.; Bechelany, M.; Miele, P.; Wang, H.; Xu, P.; Lu, L. Enhanced visible light photocatalysis by TiO2–BN enabled electrospinning of nanofibers for pharmaceutical degradation and wastewater treatment. Photochem. Photobiol. Sci. 2019, 18, 2921–2930. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Bechelany, M.; Nasr, M.; Jarvis, J.; Schaub, T.; Sapkota, R.R.; Miele, P.; Wang, H.; Xu, P. Adsorption and photocatalytic oxidation of ibuprofen using nanocomposites of TiO2 nanofibers combined with BN nanosheets: Degradation products and mechanisms. Chemosphere 2019, 220, 921–929. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Luo, H.; Xu, P. Photocatalytic Treatment of Desalination Concentrate Using Optical Fibers Coated With Nanostructured Thin Films: Impact of Water Chemistry and Seasonal Climate Variations. Photochem. Photobiol. 2016, 92, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhu, X.; Butler, E.C. Comparison of four advanced oxidation processes for the removal of naphthenic acids from model oil sands process water. J. Hazard. Mater. 2011, 190, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Ibañez, P.F.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Kiss, J.; Kukovecz, Á.; Kónya, Z. Beyond Nanoparticles: The Role of Sub-nanosized Metal Species in Heterogeneous Catalysis. Catal. Lett. 2019, 149, 1441–1454. [Google Scholar] [CrossRef]
- László, B.; Baán, K.; Varga, E.; Oszkó, A.; Erdőhelyi, A.; Konya, Z.; Kiss, J. Photo-induced reactions in the CO 2 -methane system on titanate nanotubes modified with Au and Rh nanoparticles. Appl. Catal. B: Environ. 2016, 199, 473–484. [Google Scholar] [CrossRef]
- Hong, S.; Ratpukdi, T.; Sivaguru, J.; Khan, E. Photolysis of glutaraldehyde in brine: A showcase study for removal of a common biocide in oil and gas produced water. J. Hazard. Mater. 2018, 353, 254–260. [Google Scholar] [CrossRef]
- Andreozzi, M.; Álvarez, M.; Contreras, S.; Medina, F.; Clarizia, L.; Vitiello, G.; Llorca, J.; Marotta, R. Treatment of saline produced water through photocatalysis using rGO-TiO2 nanocomposites. Catal. Today 2018, 315, 194–204. [Google Scholar] [CrossRef]
- Al-Sabahi, J.; Bora, T.; Claereboudt, M.; Al-Abri, M.; Dutta, J. Visible light photocatalytic degradation of HPAM polymer in oil produced water using supported zinc oxide nanorods. Chem. Eng. J. 2018, 351, 56–64. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Jing, L.; Zhang, H.; Lee, K. Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbons in Offshore Produced Water: Effects of Water Matrix. J. Environ. Eng. 2016, 142, 04016054. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.; Kebria, D.Y.; Qaderi, F. Investigation of photocatalytic degradation of BTEX in produced water using γ-Fe2O3 nanoparticle. J. Therm. Anal. Calorim. 2018, 135, 1617–1627. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Y.; Zhang, X.; Dong, Y. Construction of the elements based on lifted multiwavelet and its applications. Integr. Ferroelectr. 2016, 172, 132–141. [Google Scholar] [CrossRef]
- Hasegawa, M.C.; Daniel, J.F.D.S.; Takashima, K.; Batista, G.A.; Da Silva, S.M.C.P. COD removal and toxicity decrease from tannery wastewater by zinc oxide-assisted photocatalysis: A case study. Environ. Technol. 2014, 35, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Çifçi, D.I.; Meric, S.; Optimization of Suspended photocatalytic treatment of two biologically treated textile effluents using TiO2 and ZnO catalysts. Glob. NEST J. 2015, 17, 653–663. Available online: https://journal.gnest.org/sites/default/files/Submissions/gnest_01715/gnest_01715_published.pdf (accessed on 1 August 2020).
- Tsoumachidou, S.; Velegraki, T.; Antoniadis, A.; Poulios, I. Greywater as a sustainable water source: A photocatalytic treatment technology under artificial and solar illumination. J. Environ. Manag. 2017, 195, 232–241. [Google Scholar] [CrossRef]
- Saverini, M.; Catanzaro, I.; Sciandrello, G.; Avellone, G.; Indelicato, S.; Marci, G.; Palmisano, L. Genotoxicity of citrus wastewater in prokaryotic and eukaryotic cells and efficiency of heterogeneous photocatalysis by TiO2. J. Photochem. Photobiol. B Biol. 2012, 108, 8–15. [Google Scholar] [CrossRef]
- Calleja, A.; Baldasano, J.M.; Mulet, A. Toxicity analysis of leachates from hazardous wastes via microtox anddaphnia magna. Environ. Toxicol. Water Qual. 1986, 1, 73–83. [Google Scholar] [CrossRef]
- Corrêa, A.X.; Tiepo, E.N.; Somensi, C.A.; Sperb, R.M.; Radetski, C.M. Use of ozone-photocatalytic oxidation (O 3/UV/TiO 2) and biological remediation for treatment of produced water from petroleum refineries. J. Environ. Eng. 2010, 136, 40–45. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.; Saint, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Sirtori, C.; Agüera, A.; Gernjak, W.; Malato, S. Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways. Water Res. 2010, 44, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; McDonagh, A.; Tijing, L.D.; Shon, H.K. Fouling and inactivation of titanium dioxide-based photocatalytic systems. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1880–1915. [Google Scholar] [CrossRef]
- Rincón, A. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2Implications in solar water disinfection. Appl. Catal. B Environ. 2004, 51, 283–302. [Google Scholar] [CrossRef]
- Keen, O.; McKay, G.; Mezyk, S.; Linden, K.G.; Rosario-Ortiz, F.L. Identifying the factors that influence the reactivity of effluent organic matter with hydroxyl radicals. Water Res. 2014, 50, 408–419. [Google Scholar] [CrossRef]
- Nogueira, A.A.; Bassin, J.P.; Cerqueira, A.C.; Dezotti, M. Integration of biofiltration and advanced oxidation processes for tertiary treatment of an oil refinery wastewater aiming at water reuse. Environ. Sci. Pollut. Res. 2016, 23, 9730–9741. [Google Scholar] [CrossRef]
- Liu, P.; Ren, Y.; Ma, W.; Ma, J.; Du, Y. Degradation of shale gas produced water by magnetic porous MFe2O4 (M = Cu, Ni, Co and Zn) heterogeneous catalyzed ozone. Chem. Eng. J. 2018, 345, 98–106. [Google Scholar] [CrossRef]
- Friehs, E.; AlSalka, Y.; Jonczyk, R.; Lavrentieva, A.; Jochums, A.; Walter, J.-G.; Stahl, F.; Scheper, T.; Bahnemann, D.W. Toxicity, phototoxicity and biocidal activity of nanoparticles employed in photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2016, 29, 1–28. [Google Scholar] [CrossRef]
- Lee, W.-M.; An, Y.-J. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 2013, 91, 536–544. [Google Scholar] [CrossRef]
- Miller, R.; Bennett, S.; Keller, A.A.; Pease, S.; Lenihan, H.S. TiO2 Nanoparticles Are Phototoxic to Marine Phytoplankton. PLoS ONE 2012, 7, e30321. [Google Scholar] [CrossRef]
- Dalai, S.; Pakrashi, S.; Kumar, R.S.S.; Chandrasekaran, N.; Mukherjee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 2012, 1, 116. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.-F.; Gray, K.A. Cytotoxicity of commercial nano-TiO2 to Escherichia coli assessed by high-throughput screening: Effects of environmental factors. Water Res. 2013, 47, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—the role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Barberet, P.; Delville, M.-H.; Moretto, P.; Seznec, H. Titanium dioxide nanoparticles induced intracellular calcium homeostasis modification in primary human keratinocytes. Towards anin vitroexplanation of titanium dioxide nanoparticles toxicity. Nanotoxicology 2010, 5, 125–139. [Google Scholar] [CrossRef]
- Yin, J.-J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes--generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, C.; Alloy, M.; Hamilton, J.; Verbeck, G.; Newton, K.; Klaine, S.; Roberts, A.P. Photo-induced toxicity of titanium dioxide nanoparticles to Daphnia magna under natural sunlight. Chemosphere 2015, 120, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Kim, C.-M.; Seo, J.; Park, Y.; Kwon, D.; Lee, S.-H.; Yoon, T.H.; Choi, K. Non-monotonic concentration–response relationship of TiO2 nanoparticles in freshwater cladocerans under environmentally relevant UV-A light. Ecotoxicol. Environ. Saf. 2014, 101, 240–247. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Louis, K.M.; Yang, S.P.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology 2011, 6, 670–679. [Google Scholar] [CrossRef]
- Faria, M.; Navas, J.M.; Soares, A.; Barata, C. Oxidative stress effects of titanium dioxide nanoparticle aggregates in zebrafish embryos. Sci. Total Environ. 2014, 470, 379–389. [Google Scholar] [CrossRef]
- Dasari, T.P.; Pathakoti, K.; Hwang, H.-M. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J. Environ. Sci. 2013, 25, 882–888. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Yoon, T.H.; Yoon, C.; Choi, K. Phototoxicity of CdSe/ZnSe quantum dots with surface coatings of 3-mercaptopropionic acid or tri-n-octylphosphine oxide/gum arabic in Daphnia magna under environmentally relevant UV-B light. Aquat. Toxicol. 2010, 97, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.-C.; Blinova, I.; Ivask, A.; Kasemets, K. Biotests and Biosensors for Ecotoxicology of Metal Oxide Nanoparticles: A Minireview. Sensors 2008, 8, 5153–5170. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Pinelli, E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. J. Hazard. Mater. 2015, 283, 764–777. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2015, 20, 1–11. [Google Scholar] [PubMed]
- Pinedo, A.; Lopez, M.; Leyva, E.; Zermeño, B.; Serrano, B.; Moctezuma, E. Photocatalytic Decomposition of Metoprolol and Its Intermediate Organic Reaction Products: Kinetics and Degradation Pathway. Int. J. Chem. React. Eng. 2016, 14, 809–820. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Lambropoulou, D.A.; Michailidou, D.; Kouras, A.; Poulios, I. Decomposition and detoxification of the insecticide thiacloprid by TiO 2-mediated photocatalysis: kinetics, intermediate products and transformation pathways. J. Chem. Technol. Biotechnol. 2019, 94, 2475–2486. [Google Scholar] [CrossRef]
- Trinh, D.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A. Investigation of Intermediate Compounds of Phenol in Photocatalysis Process. Int. J. Chem. Eng. Appl. 2016, 7, 273–276. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, D.; Monllor-Satoca, D.; Kim, K.; Lee, W.; Choi, W. Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr (VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates. J. Hazard. Mater. 2019, 372, 121–128. [Google Scholar] [CrossRef]
- Santos, I.; Hildenbrand, Z.L.; Schug, K.A. A Review of Analytical Methods for Characterizing the Potential Environmental Impacts of Unconventional Oil and Gas Development. Anal. Chem. 2018, 91, 689–703. [Google Scholar] [CrossRef]
- Oetjen, K.; Giddings, C.G.; McLaughlin, M.; Nell, M.; Blotevogel, J.; Helbling, D.E.; Mueller, D.; Higgins, C.P. Emerging analytical methods for the characterization and quantification of organic contaminants in flowback and produced water. Trends Environ. Anal. Chem. 2017, 15, 12–23. [Google Scholar] [CrossRef]
- Luek, J.L.; Gonsior, M. Organic compounds in hydraulic fracturing fluids and wastewaters: A review. Water Res. 2017, 123, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Akyon, B.; McLaughlin, M.; Hernandez, F.; Blotevogel, J.; Bibby, K. Characterization and biological removal of organic compounds from hydraulic fracturing produced water. Environ. Sci. Process. Impacts 2019, 21, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Sitterley, K.A.; Linden, K.G.; Ferrer, I.; Thurman, E.M. Identification of Proprietary Amino Ethoxylates in Hydraulic Fracturing Wastewater Using Liquid Chromatography/Time-of-Flight Mass Spectrometry with Solid-Phase Extraction. Anal. Chem. 2018, 90, 10927–10934. [Google Scholar] [CrossRef]
- Pichtel, J. Oil and Gas Production Wastewater: Soil Contamination and Pollution Prevention. Appl. Environ. Soil Sci. 2016, 2016, 1–24. [Google Scholar] [CrossRef]
- Ray, J.P.; Engelhardt, F.R. Produced Water: Technological/Environmental Issues and Solutions; Springer Science & Business Media: Berlin, Germany, 2012; Volume 46. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Integrated Risk Information System (IRIS) on Baygon; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999. [Google Scholar]
- Litter, M.I.; Quici, N. New Advances in Heterogeneous Photocatalysis for Treatment of Toxic Metals and Arsenic. Nanomater. Environ. Prot. 2014, 143–167. [Google Scholar] [CrossRef]
- Litter, M.I. Treatment of Chromium, Mercury, Lead, Uranium, and Arsenic in Water by Heterogeneous Photocatalysis. Chem. Eng. Renew. Convers. 2009, 36, 37–67. [Google Scholar] [CrossRef]
- Litter, M.I.; Morgada, M.E.; Bundschuh, J. Possible treatments for arsenic removal in Latin American waters for human consumption. Environ. Pollut. 2010, 158, 1105–1118. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Ciminelli, V.S.; Morgada, M.E.; Cornejo, L.; Hoyos, S.E.G.; Hoinkis, J.; Alarcón-Herrera, M.T.; Armienta-Hernández, M.A.; Bhattacharya, P. Emerging mitigation needs and sustainable options for solving the arsenic problems of rural and isolated urban areas in Latin America—A critical analysis. Water Res. 2010, 44, 5828–5845. [Google Scholar] [CrossRef]
- Fostier, A.H.; Pereira, M.D.S.S.; Rath, S.; Guimarães, J.R. Arsenic removal from water employing heterogeneous photocatalysis with TiO2 immobilized in PET bottles. Chemosphere 2008, 72, 319–324. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.H.; Ramadan, A.R. TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Kukovecz, A.; Kordas, K.; Kiss, J.; Konya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J. Phys. Chem. Solids 2002, 63, 1909–1920. [Google Scholar] [CrossRef]
- Yu, H.; Irie, H.; Hashimoto, K. Conduction Band Energy Level Control of Titanium Dioxide: Toward an Efficient Visible-Light-Sensitive Photocatalyst. J. Am. Chem. Soc. 2010, 132, 6898–6899. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Ferrero, E.; Malfeito, J.J.; Medina, F.; Contreras, S. Integrated processes for produced water polishing: Enhanced flotation/sedimentation combined with advanced oxidation processes. Chemosphere 2017, 168, 309–317. [Google Scholar] [CrossRef]
- Mazzeo, D.E.C.; Levy, C.E.; Angelis, D.D.F.D.; Marin-Morales, M.A. BTEX biodegradation by bacteria from effluents of petroleum refinery. Sci. Total Environ. 2010, 408, 4334–4340. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Pérez, J.S. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.-H.; Govindwar, S.P. Sequential photocatalysis and biological treatment for the enhanced degradation of the persistent azo dye methyl red. J. Hazard. Mater. 2019, 371, 115–122. [Google Scholar] [CrossRef]
- Parrino, F.; Corsino, S.F.; Bellardita, M.; Loddo, V.; Palmisano, L.; Torregrossa, M.; Viviani, G. Sequential biological and photocatalysis based treatments for shipboard slop purification: A pilot plant investigation. Process. Saf. Environ. Prot. 2019, 125, 288–296. [Google Scholar] [CrossRef]
- Hamdi, H.; Namane, A.; Hank, D.; Hellal, A. Coupling of photocatalysis and biological treatment for phenol degradation: application of factorial design methodology. J. Mater. 2017, 8, 3953–3961. [Google Scholar]

| Parameters | Range | Parameters | Range |
|---|---|---|---|
| pH | 4.3–8.9 | Ca (mg/L) | 18–132,687 |
| TDS (g/L) | 1.0–470.3 | Mg (mg/L) | 4–18,145 |
| TSS (mg/L) | 2–21,820 | Na (mg/L) | 316–134,652 |
| DOC (mg/L) | 3.4–5960 | K (mg/L) | 8.6–14,649 |
| Alkalinity (CaCO3, mg/L) | 6.1–2000 | SO4 (mg/L) | 0.5–7851 |
| Total Ra (pCi/L) | 0.2–18,045 | Cl (mg/L) | 1405–310,561 |
| HEM (mg/L) | 0.6–2000 | HCO3 (mg/L) | 1.9–7355 |
| MBAS (mg/L) | 0.01–54 | Ba (mg/L) | 0–22,400 |
| Catalyst | System Setup | Test Solution | Characterization | Primary Results | Ref. |
|---|---|---|---|---|---|
| Photolysis/without catalyst | pH 5–9 UV 25 mL reactor | Synthetic PW Glutaraldehyde (0.1 mM) 0–300 g/L NaCl | GC–FID TOC HR–MS |
| [47] |
| P25 TiO2 | UVA pH 3 0.1–0.5 g/L of P25 | Synthetic PW: toluene (10 mg/L), xylene (10 mg/L), naphthalene (3 mg/L), phenol (10 mg/L), acetic (150 mg/L), malonic acids (10 mg/L), seawater matrix (56 mS/cm), COD 262 mg/L, TOC 92 mg/L | TOC GC HPLC |
| [19] |
| rGO-TiO2 | slurry system 0.5 L UVA | Synthetic PW: acetic acid (150 mg/L), phenol (10 mg/L), toluene (10 mg/L), (o, m, p)-xylenes (10 mg/L) and naphthalene (3 mg/L) | TOC GC |
| [48] |
| ZnO | ZnO nanorod coated glass substrate | Synthesized PW: 25–150 mg/L petroleum hydrocarbons and partially hydrolyzed polyacrylamide (HPAM) | viscosity HPLC |
| [49] |
| TiO2 | 1.3 L UV pH 8–12 P25 3 g/L | Synthesized oil sands process waters: 100 mg/L naphthenic acids, 110 mg/L dissolved silicate, 91 mg/L colloidal SiO2, 3920 mg/L NaCl | TOC |
| [42] |
| TiO2 | 500 mL UVC TiO2 0.1 g/L | PW: alkalinity 2.16 mg/L, PAHs 0.06 mg/L, Na 16.4 g/L, K 240 mg/L, Mg 417 mg/L, Ca 1.1 g/L, S 730 mg/L, pH 6.8, Turbidity 17.6 NTU, COD 1247 mg/L Synthesized solutions to simulate PW | GC–MS |
| [50] |
| Maghemite (γ-Fe2O3) | pH 3–7 0–0.25 g/L catalyst 0–100 W UV 0–225 W visible light | synthetic PW: 600 mg/L BTEX | COD |
| [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts 2020, 10, 924. https://doi.org/10.3390/catal10080924
Lin L, Jiang W, Chen L, Xu P, Wang H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts. 2020; 10(8):924. https://doi.org/10.3390/catal10080924
Chicago/Turabian StyleLin, Lu, Wenbin Jiang, Lin Chen, Pei Xu, and Huiyao Wang. 2020. "Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects" Catalysts 10, no. 8: 924. https://doi.org/10.3390/catal10080924
APA StyleLin, L., Jiang, W., Chen, L., Xu, P., & Wang, H. (2020). Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts, 10(8), 924. https://doi.org/10.3390/catal10080924








