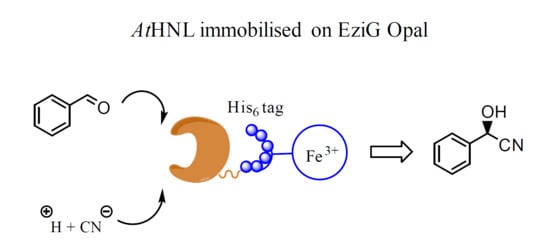
Immobilization of Arabidopsis thaliana Hydroxynitrile Lyase (AtHNL) on EziG Opal
Abstract
1. Introduction
2. Results and Discussion
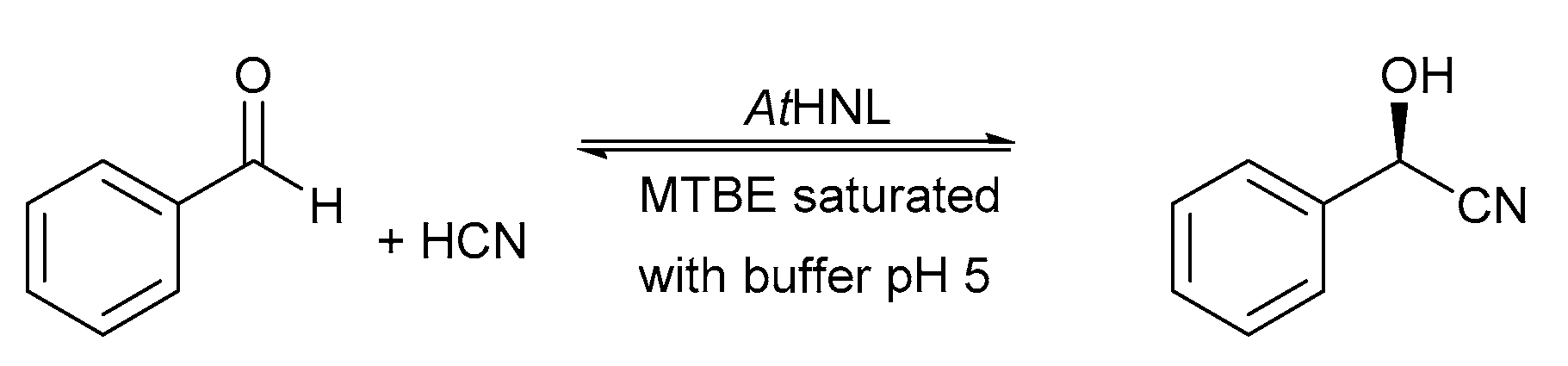
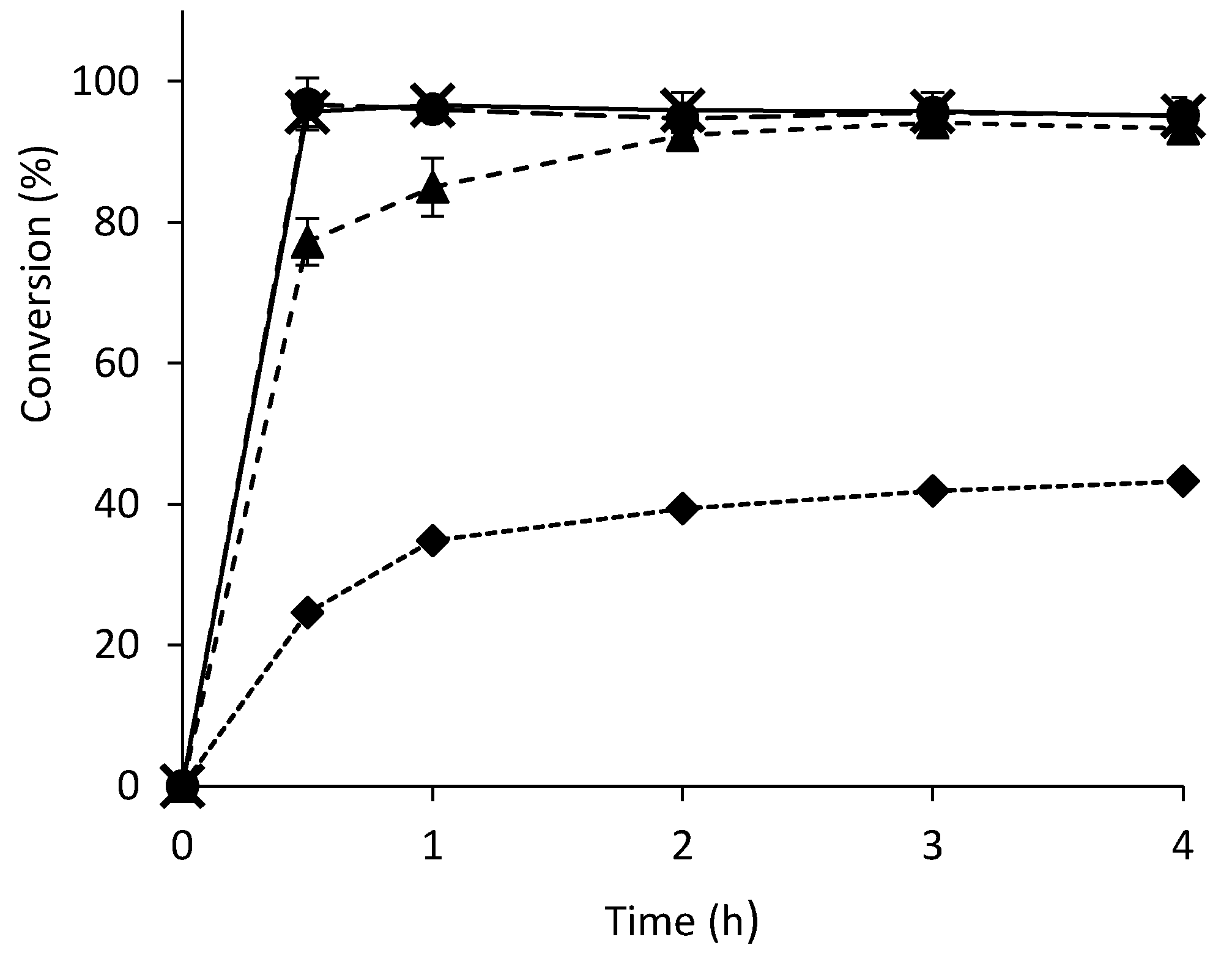
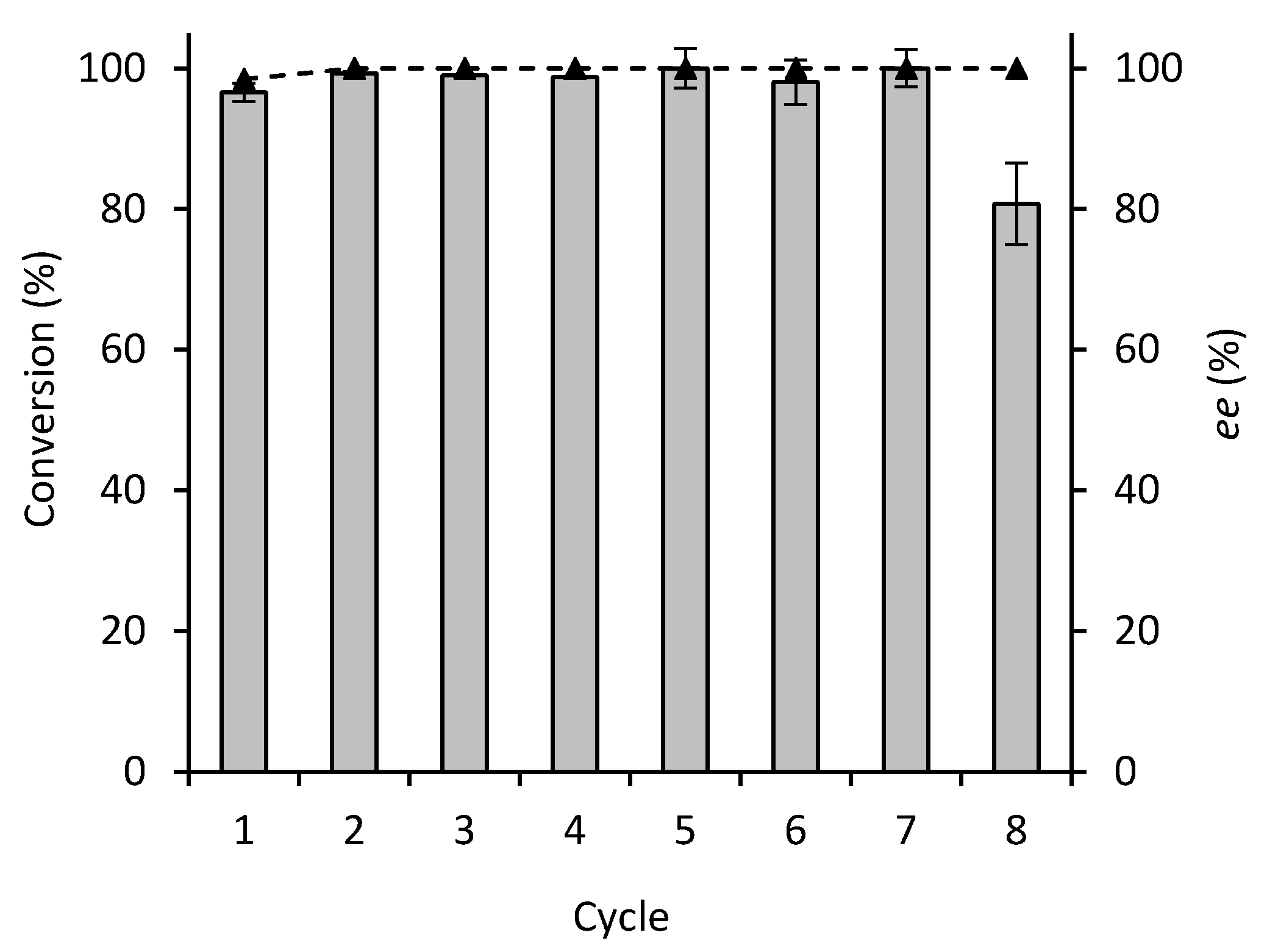
2.1. Batch Reactions
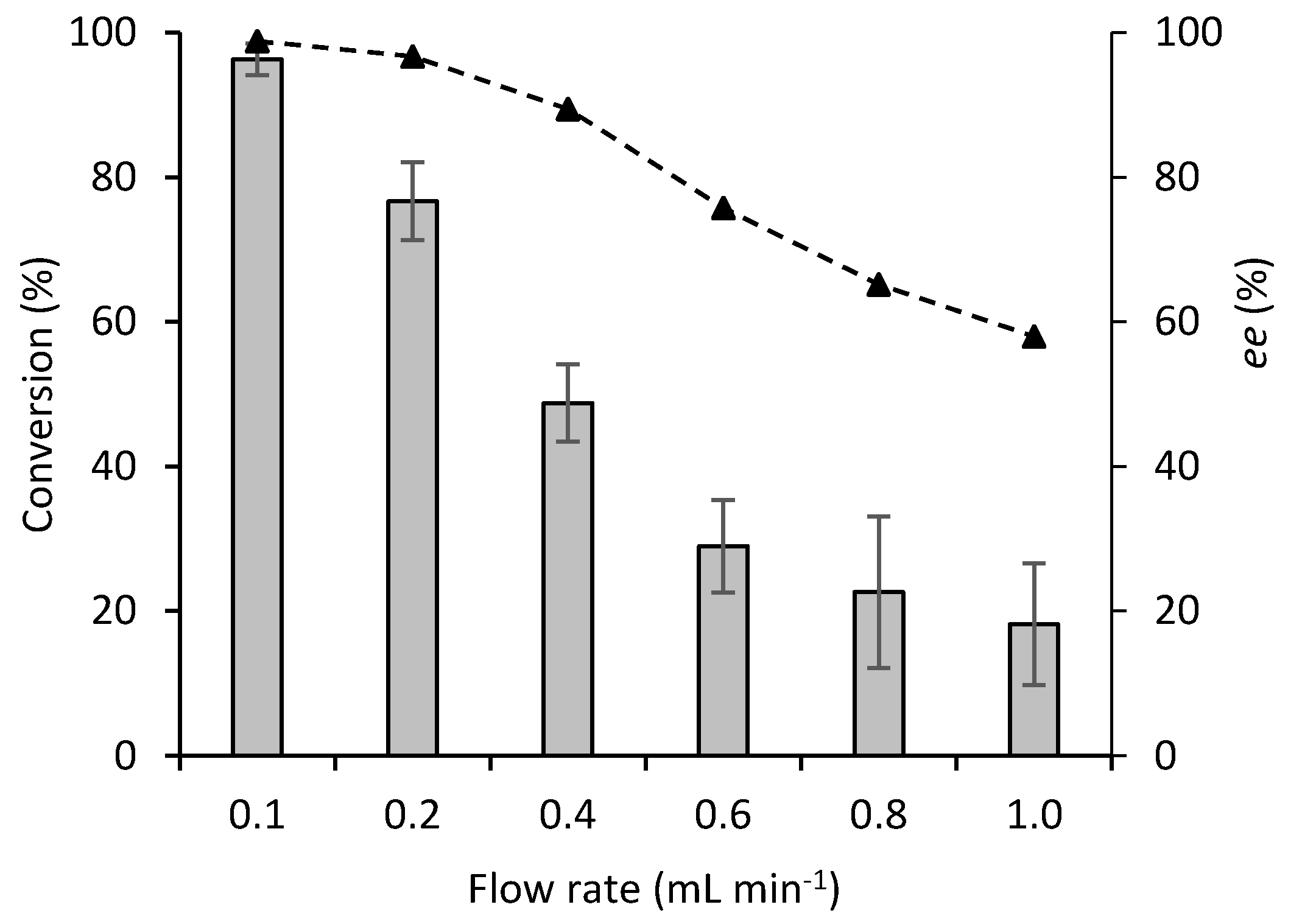
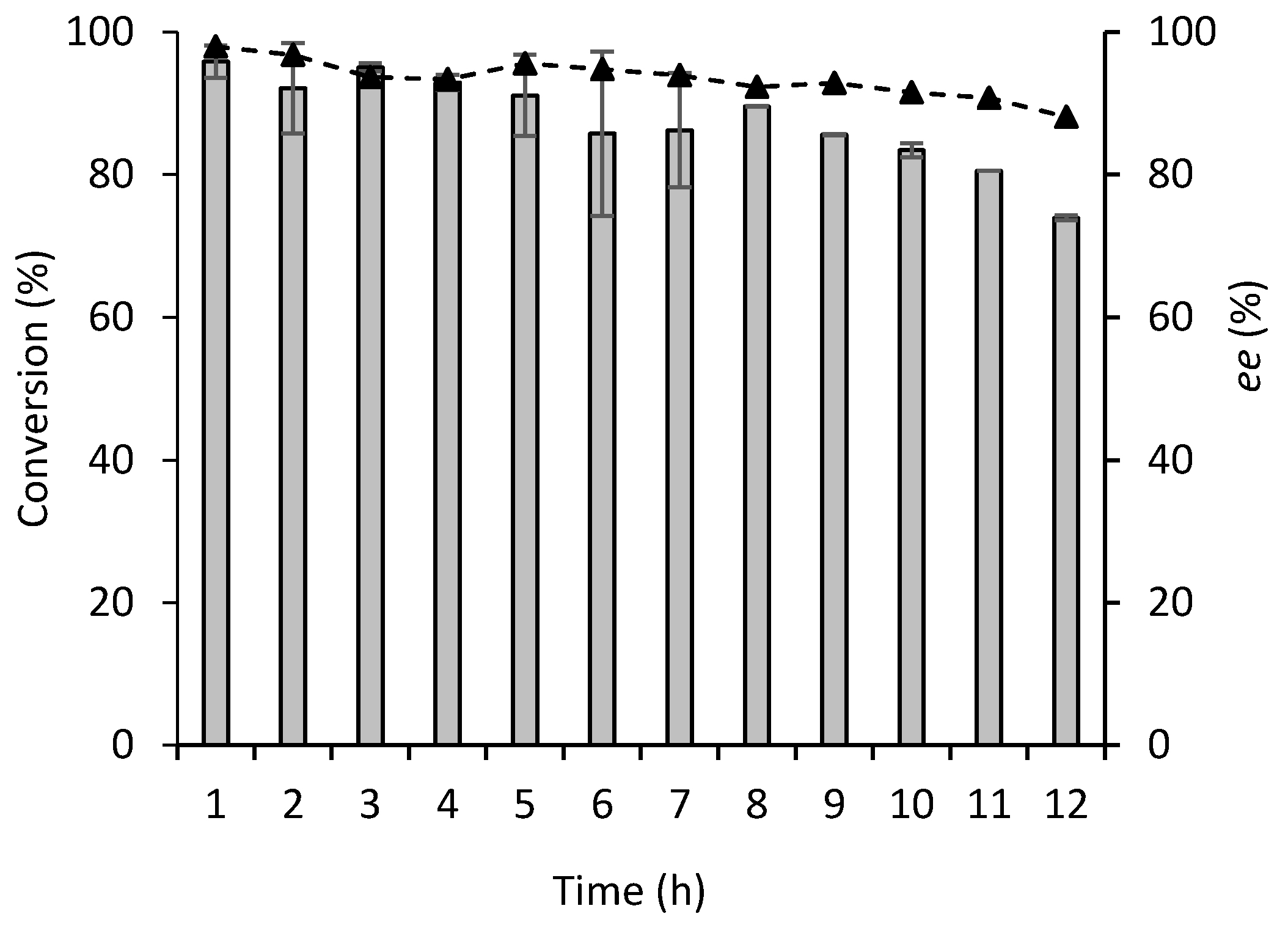
2.2. Continuous Flow Reactions
2.3. Comparison between Batch and Continuous Flow Systems
3. Materials and Methods
3.1. Chemicals
3.2. Heterologous Expression of Arabidopsis Thaliana HNL (AtHNL)
3.3. Enzyme Purification
3.4. Enzymatic Activity Aassay
3.5. Synthesis of Hydrogen Cyanide (HCN) Solution in MTBE
3.6. Immobilization of AtHNL on EziG Opal by Adsorption
3.7. Immobilization of AtHNL on EziG Opal by Incubation
3.8. Synthesis of (R)-Mandelonitrile in Batch
3.9. Enzyme Recyclability in Batch
3.10. Synthesis of (R)-Mandelonitrile in Continuous Flow
3.11. Enzyme Stability in Continuous Flow
3.12. Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dadashipour, M.; Asano, Y. Hydroxynitrile lyases: Insights into biochemistry, discovery, and engineering. ACS Catal. 2011, 1, 1121–1149. [Google Scholar] [CrossRef]
- Steiner, K.; Glieder, A.; Gruber-Khadjawi, M. Cyanohydrin formation/Henry reaction. In Science of Synthesis: Biocatalysis in Organic Synthesis; Georg Thieme Verlag: Stuttgart, Germany, 2015; Volume 2, pp. 1–30. [Google Scholar]
- Lanfranchi, E.; Steiner, K.; Glieder, A.; Hajnal, I.; Sheldon, R.A.; van Pelt, S.; Winkler, M. Mini-Review: Recent developments in hydroxynitrile lyases for industrial biotechnology. Recent Pat. Biotechnol. 2013, 7, 197–206. [Google Scholar] [CrossRef]
- Bracco, P.; Busch, H.; von Langermann, J.; Hanefeld, U. Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases—A review. Org. Biomol. Chem. 2016, 14, 6375–6389. [Google Scholar] [CrossRef]
- Faber, K. Biotransformations in Organic Chemistry, 7th ed.; Springer Nature: Basel, Switzerland, 2018; pp. 224–233. [Google Scholar] [CrossRef]
- Hanefeld, U. Immobilisation of hydroxynitrile lyases. Chem. Soc. Rev. 2013, 42, 6308–6321. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Q.; Shao, L.; Jia, Y.; Zhang, X. Microfluidic immobilized enzyme reactors for continuous biocatalysis. React. Chem. Eng. 2020, 5, 9–32. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Block, H.; Maertens, B.; Spriestersbach, A.; Brinker, N.; Kubicek, J.; Fabis, R.; Labahn, J.; Schäfer, F. Immobilized-Metal Affinity Chromatography (IMAC) in Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2009; pp. 439–473. [Google Scholar] [CrossRef]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity—An overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Andexer, J.N.; Staunig, N.; Eggert, T.; Kratky, C.; Pohl, M.; Gruber, K. Hydroxynitrile lyases with α/β-hydrolase fold: Two enzymes with almost identical 3D structures but opposite enantioselectivities and different reaction mechanisms. ChemBioChem 2012, 13, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, K.E.; Kadow, M.; Wikmark, Y.; Humble, M.S.; Rothstein, M.L.; Rothstein, D.M.; Bäckvall, J.-E. A general protein purification and immobilization method on controlled porosity glass: Biocatalytic applications. Chem. Commun. 2014, 50, 9134–9137. [Google Scholar] [CrossRef] [PubMed]
- Okrob, D.; Paravidino, M.; Orru, R.V.A.; Wiechert, W.; Hanefeld, U.; Pohl, M. Hydroxynitrile lyase from Arabidopsis thaliana: Identification of reaction parameters for enantiopure cyanohydrin synthesis by pure and immobilized catalyst. Adv. Synth. Catal. 2011, 353, 2399–2408. [Google Scholar] [CrossRef]
- Bracco, P.; Torrelo, G.; Noordam, S.; de Jong, G.; Hanefeld, U. Immobilization of Prunus amygdalus hydroxynitrile lyase on Celite. Catalysts 2018, 8, 287. [Google Scholar] [CrossRef]
- van der Helm, M.P.; Bracco, P.; Busch, H.; Szymańska, K.; Jarzębski, A.; Hanefeld, U. Hydroxynitrile lyases covalently immobilized in continuous flow microreactors. Catal. Sci. Technol. 2019, 9, 1189–1200. [Google Scholar] [CrossRef]
- Torrelo, G.; van Midden, N.; Stloukal, R.; Hanefeld, U. Immobilized hydroxynitrile lyase: A comparative study of recyclability. ChemCatChem 2014, 6, 1096–1102. [Google Scholar] [CrossRef]
- Brahma, A.; Musio, B.; Ismayilova, U.; Nikbin, N.; Kamptmann, S.; Siegert, P.; Jeromin, G.E.; Ley, S.V.; Pohl, M. An orthogonal biocatalytic approach for the safe generation and use of HCN in a multistep continuous preparation of chiral O-Acetylcyanohydrins. Synlett 2015, 27, 262–266. [Google Scholar] [CrossRef]
- Böhmer, W.; Knaus, T.; Volkov, A.; Slot, T.K.; Shiju, N.R.; Cassimjee, K.E.; Mutti, F.G. Highly efficient production of chiral amines in batch and continuous flow by immobilized ω-transaminases on controlled porosity glass metal-ion affinity carrier. J. Biotechnol. 2019, 291, 52–60. [Google Scholar] [CrossRef]
- Tentori, F.; Bavaro, T.; Brenna, E.; Colombo, D.; Monti, D.; Semproli, R.; Ubiali, D. Immobilisation of old yellow enzymes via covalent or coordination bonds. Catalysts 2020, 10, 260. [Google Scholar] [CrossRef]
- Aßmann, M.; Mügge, C.; Gaßmeyer, S.K.; Enoki, J.; Hilterhaus, L.; Kourist, R.; Liese, A.; Kara, S. Improvement of the process stability of Arylmalonate decarboxylase by immobilization for biocatalytic profen synthesis. Front. Microbiol. 2017, 8, 448. [Google Scholar] [CrossRef]
- Yu, T.; Ding, Z.; Nie, W.; Jiao, J.; Zhang, H.; Zhang, Q.; Xue, C.; Duan, D.; Yamada, Y.M.A.; Li, P. Recent advances in continuous-flow enantioselective catalysis. Chem. Eur. J. 2020, 26, 5729–5747. [Google Scholar] [CrossRef]
- Yoo, W.J.; Ishitani, H.; Saito, Y.; Laroche, B.; Kobayashi, S. Reworking organic synthesis for the modern age: Synthetic strategies based on continuous-flow addition and condensation reactions with heterogeneous catalysts. J. Org. Chem. 2020, 85, 5132–5145. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.P.; Peñafiel, I.; Cosgrove, S.C.; Turner, N.J. Biocatalysis using immobilized enzymes in continuous flow for the synthesis of fine chemicals. Org. Process Res. Dev. 2019, 23, 9–18. [Google Scholar] [CrossRef]
- Akwi, F.M.; Watts, P. Continuous flow chemistry: Where are we now? Recent applications, challenges and limitations. Chem. Commun. 2018, 54, 13894–13928. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef]
- Coloma, J.; Guiavarc’h, Y.; Hagedoorn, P.L.; Hanefeld, U. Probing batch and continuous flow reactions in organic solvents: Granulicella tundricola hydroxynitrile lyase (GtHNL). Catal. Sci. Technol. 2020, 10, 3613–3621. [Google Scholar] [CrossRef]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Guterl, J.K.; Andexer, J.N.; Sehl, T.; von Langermann, J.; Frindi-Wosch, I.; Rosenkranz, T.; Fitter, J.; Gruber, K.; Kragl, U.; Eggert, T.; et al. Uneven twins: Comparison of two enantiocomplementary hydroxynitrile lyases with α/β-hydrolase fold. J. Biotechnol. 2009, 166–173. [Google Scholar] [CrossRef]
- Paravidino, M.; Sorgedrager, M.J.; Orru, R.V.; Hanefeld, U. Activity and enantioselectivity of the hydroxynitrile lyase MeHNL in dry organic solvents. Chem. Eur. J. 2010, 16, 7596–7604. [Google Scholar] [CrossRef]
- Effenberger, F.; Eichhorn, J.; Roos, J. Enzyme catalyzed addition of hydrocyanic acid to substituted pivalaldehydes—A novel synthesis of (R)-pantolactone. Tetrahedron Asymmetry 1995, 6, 271–282. [Google Scholar] [CrossRef]
- Costes, D.; Wehtje, E.; Adlercreutz, P. Hydroxynitrile lyase-catalyzed synthesis of cyanohydrins in organic solvents: Parameters influencing activity and enantiospecificity. Enzym. Microb. Technol. 1999, 25, 384–391. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis explained: From pharmaceutical to bulk chemical production. React. Chem. Eng. 2019, 4, 1878–1894. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow bioreactors as complementary tools for biocatalytic process intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef]
- Affinity Chromatography: Principles and Methods. Available online: www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/General_Information/1/ge-affinity-chromatography.pdf (accessed on 14 October 2019).
- PD-10 Desalting Columns. Available online: http://wwwuser.gwdg.de/~jgrossh/protocols/protein-purification/PD10.pdf (accessed on 14 October 2019).
- Van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. Enzymatic hydrocyanation of a sterically hindered aldehyde. Optimization of a chemoenzymatic procedure for (R)-2-Chloromandelic Acid. Org. Process. Res. Dev. 2003, 7, 828–831. [Google Scholar] [CrossRef]
- User Guide: Pierce BCA Protein Assay Kit. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A%2F%2Fassets.thermofisher.com%2FTFS-Assets%2FLSG%2Fmanuals%2FMAN0011430_Pierce_BCA_Protein_Asy_UG.pdf&title=VXNlciBHdWlkZTogUGllcmNlIEJDQSBQcm90ZWluIEFzc2F5IEtpdA (accessed on 15 October 2019).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coloma, J.; Lugtenburg, T.; Afendi, M.; Lazzarotto, M.; Bracco, P.; Hagedoorn, P.-L.; Gardossi, L.; Hanefeld, U. Immobilization of Arabidopsis thaliana Hydroxynitrile Lyase (AtHNL) on EziG Opal. Catalysts 2020, 10, 899. https://doi.org/10.3390/catal10080899
Coloma J, Lugtenburg T, Afendi M, Lazzarotto M, Bracco P, Hagedoorn P-L, Gardossi L, Hanefeld U. Immobilization of Arabidopsis thaliana Hydroxynitrile Lyase (AtHNL) on EziG Opal. Catalysts. 2020; 10(8):899. https://doi.org/10.3390/catal10080899
Chicago/Turabian StyleColoma, José, Tim Lugtenburg, Muhammad Afendi, Mattia Lazzarotto, Paula Bracco, Peter-Leon Hagedoorn, Lucia Gardossi, and Ulf Hanefeld. 2020. "Immobilization of Arabidopsis thaliana Hydroxynitrile Lyase (AtHNL) on EziG Opal" Catalysts 10, no. 8: 899. https://doi.org/10.3390/catal10080899
APA StyleColoma, J., Lugtenburg, T., Afendi, M., Lazzarotto, M., Bracco, P., Hagedoorn, P.-L., Gardossi, L., & Hanefeld, U. (2020). Immobilization of Arabidopsis thaliana Hydroxynitrile Lyase (AtHNL) on EziG Opal. Catalysts, 10(8), 899. https://doi.org/10.3390/catal10080899