Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Fresh Catalysts
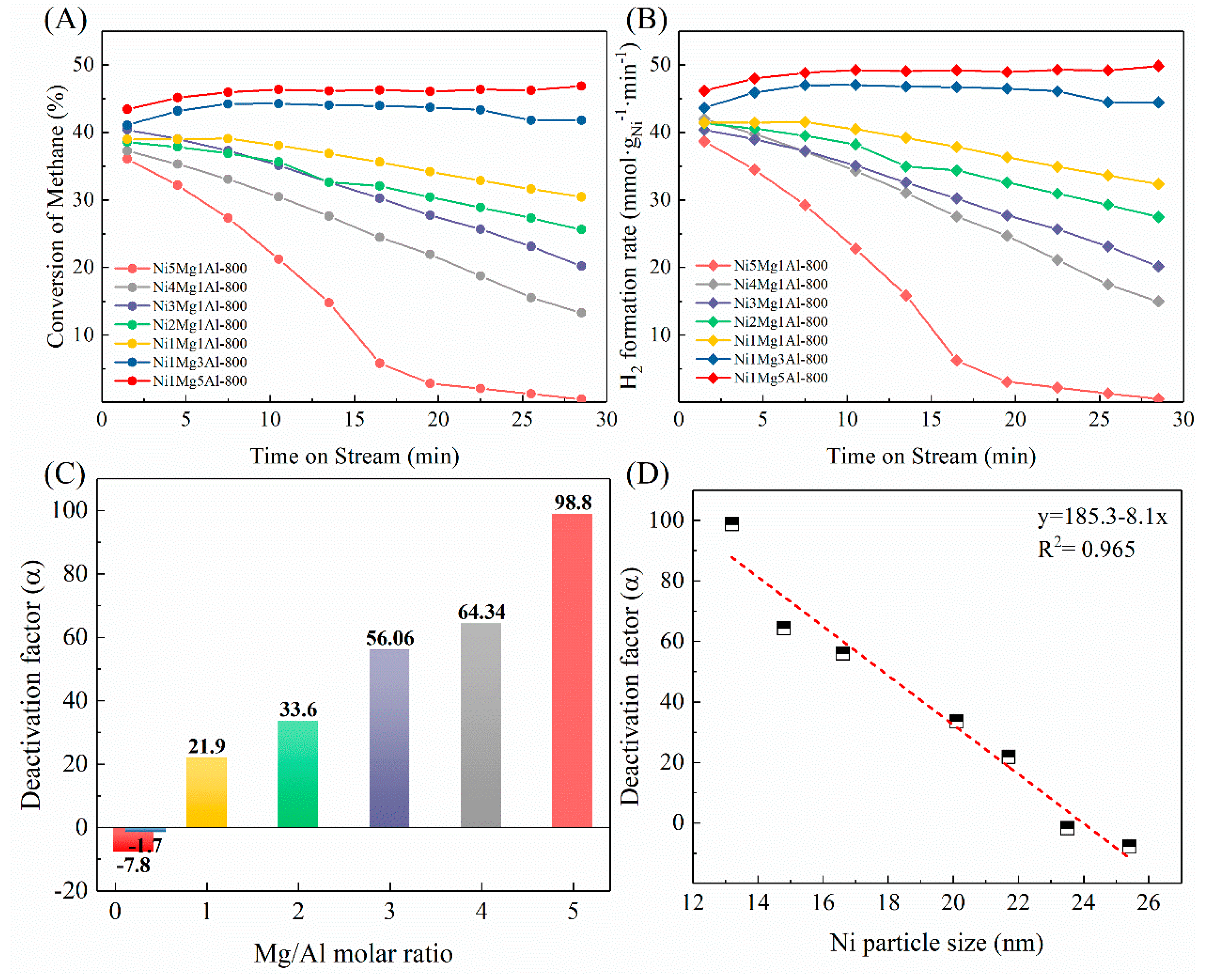
2.2. The Catalytic Activity of NixMgyAl-800 Catalysts on CDM Reaction
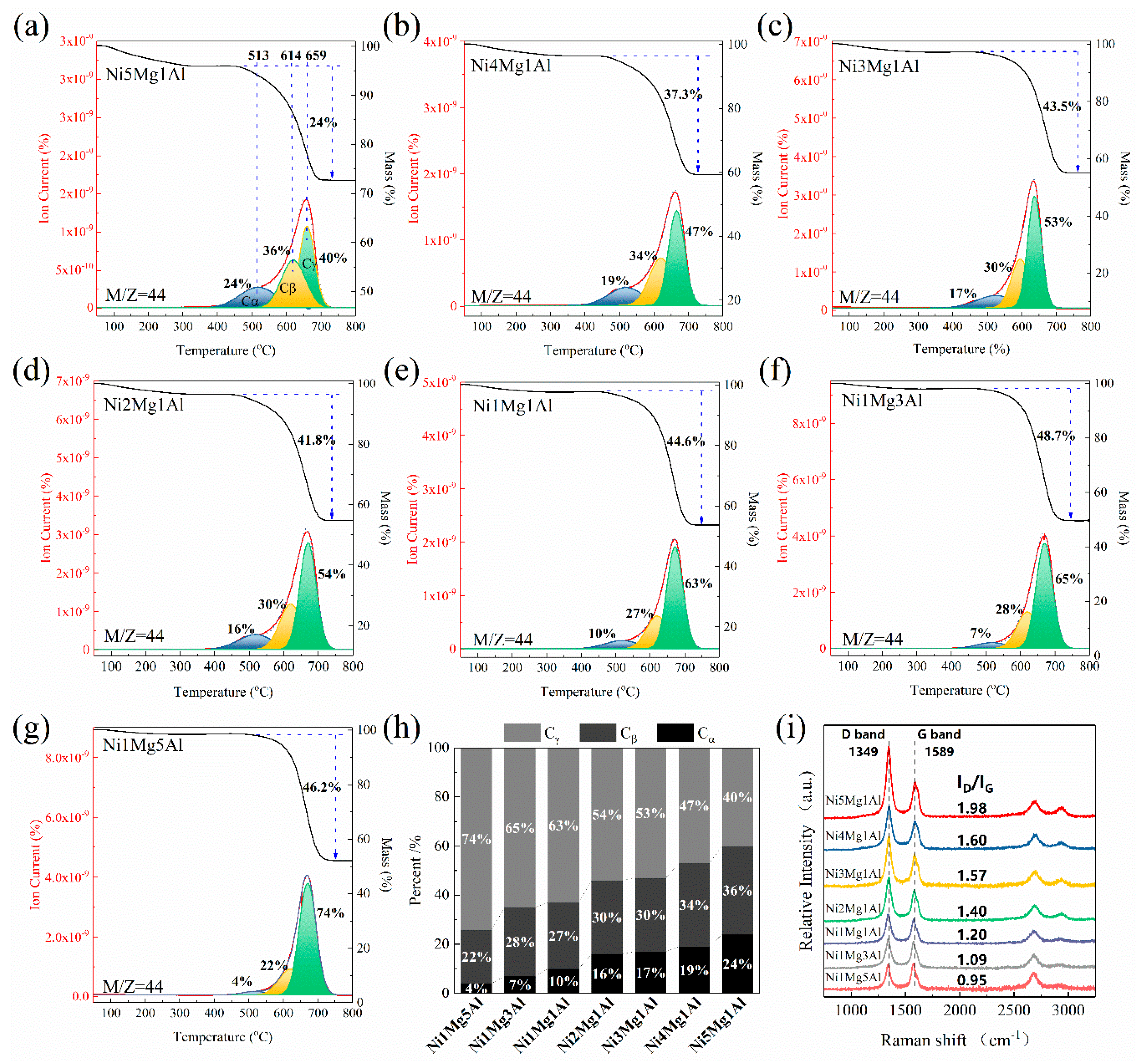
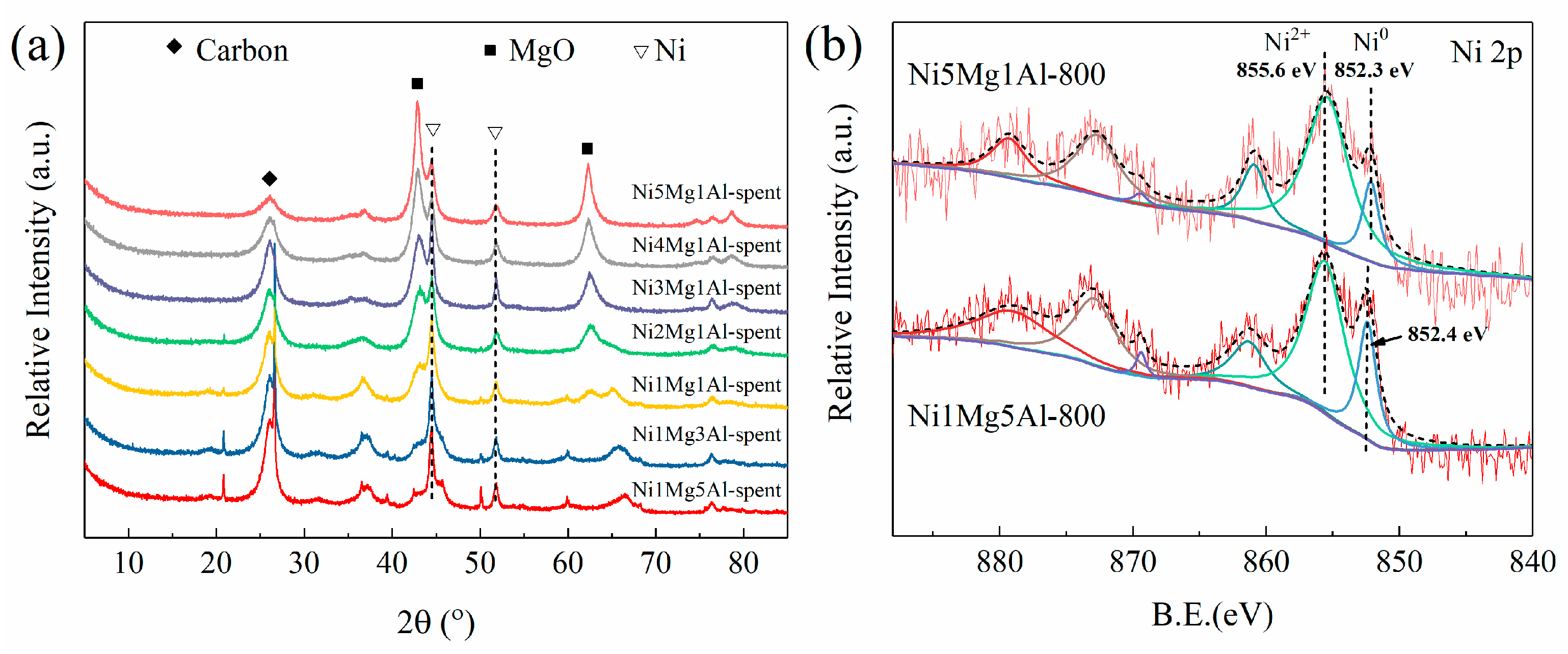
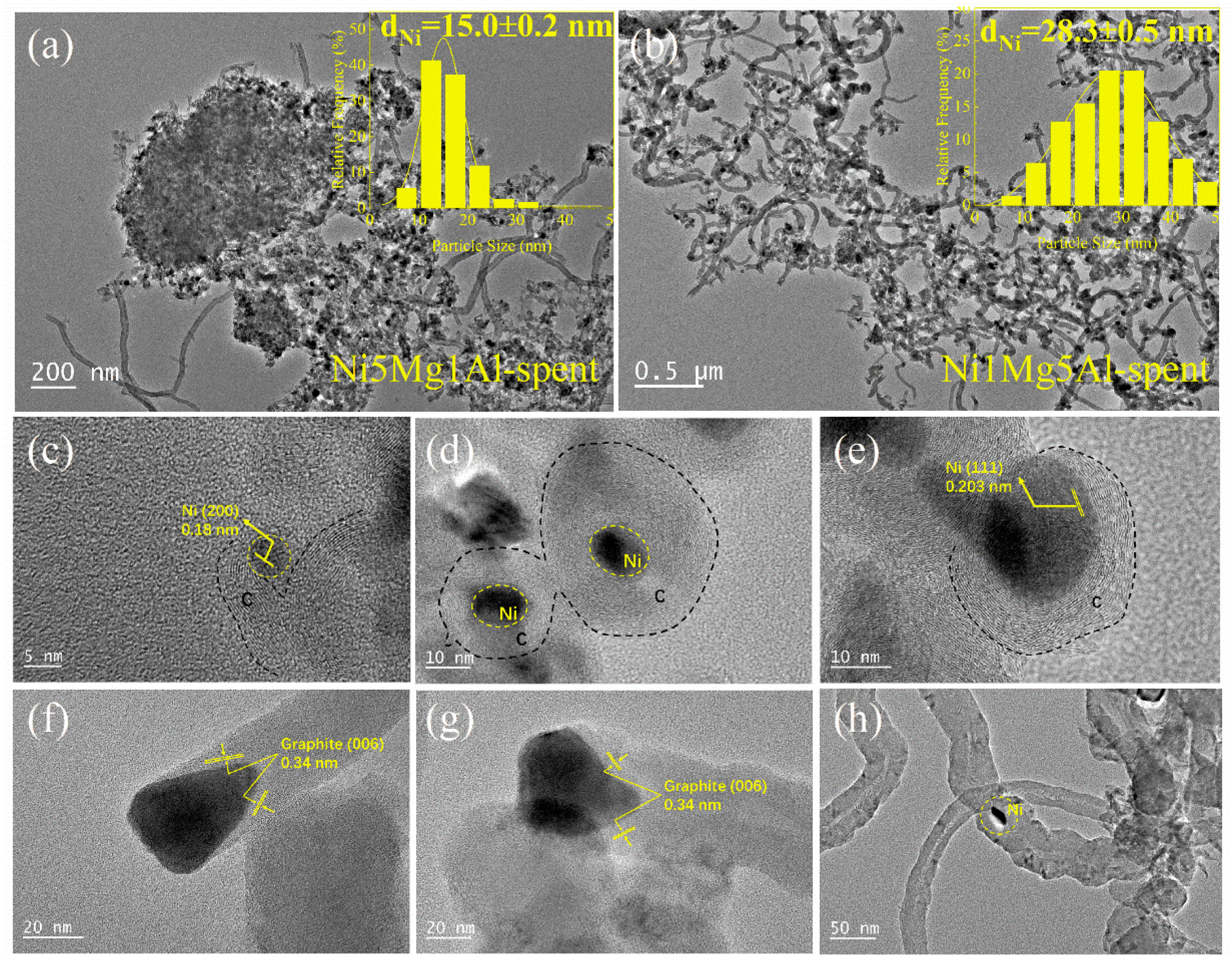
2.3. Characterization of Spent Catalysts
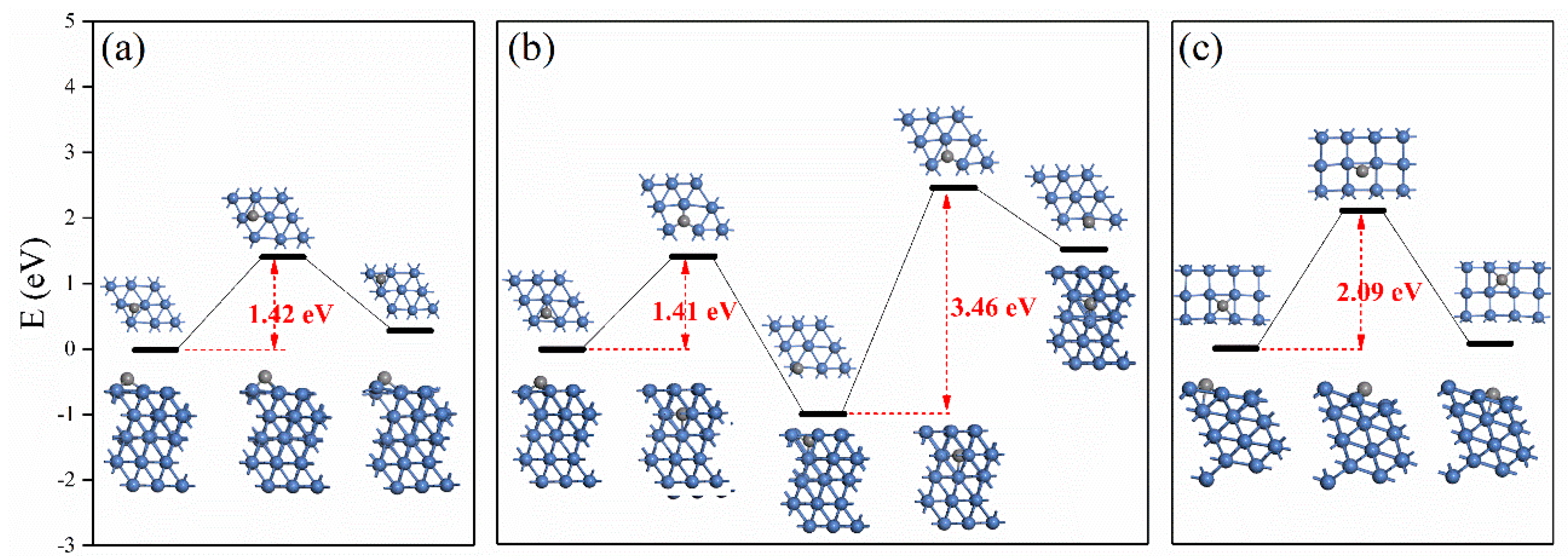
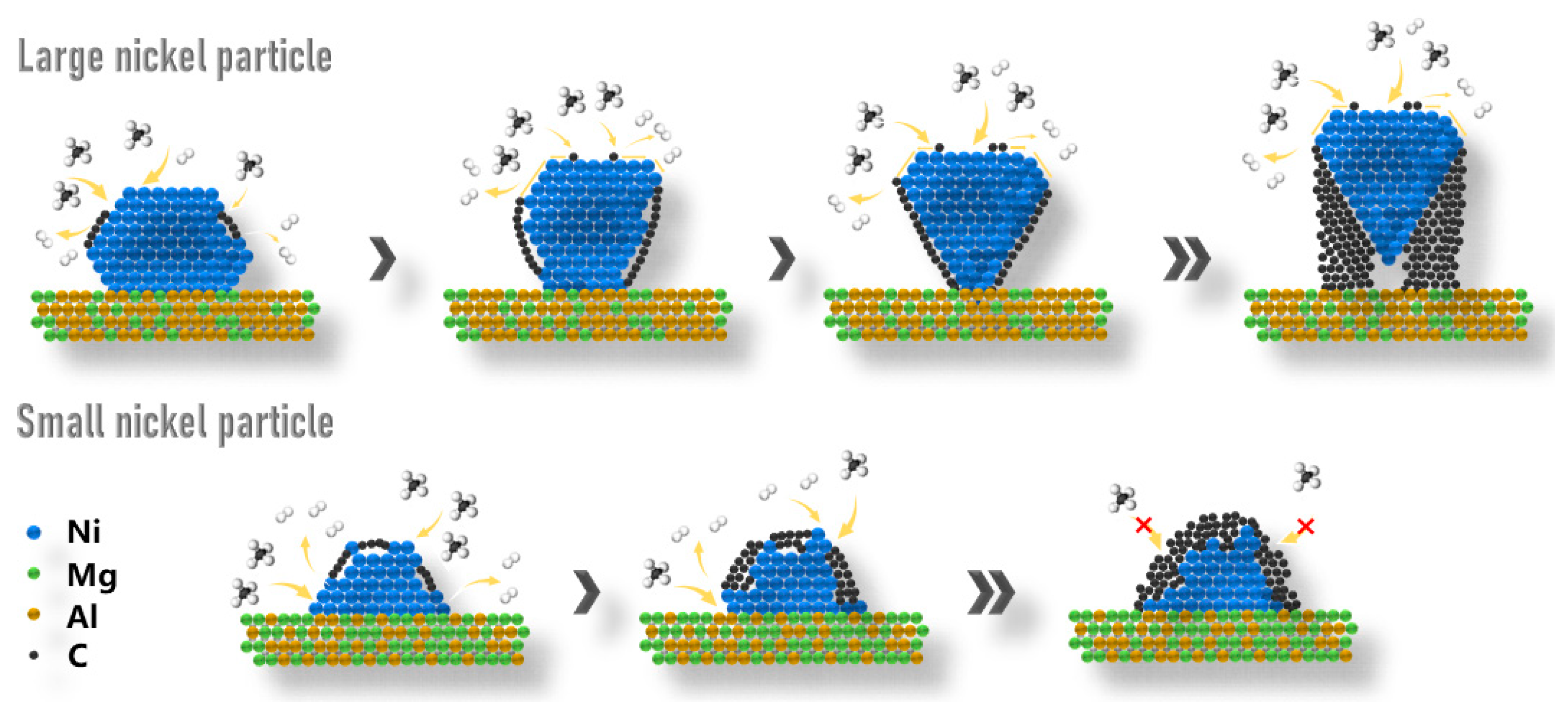
2.4. Discussions
3. Experimental Section
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Characterization Techniques
3.4. Catalytic Evaluation
3.5. Density Functional Theory Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.Q.; Wu, X.D.; Guo, J.; Meng, J.; Li, C. Global overview for energy use of the world economy: Household-consumption-based accounting based on the world input-output database (WIOD). Energy Econ. 2019, 81, 835–847. [Google Scholar] [CrossRef]
- De_Richter, R.; Caillol, S. Fighting global warming: The potential of photocatalysis against CO2, CH4, N2O, CFCs, tropospheric O3, BC and other major contributors to climate change. J. Photochem. Photobiol. C 2011, 12, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.-M.; Zhou, L. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: A review. Int. J. Hydrog. Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Klötzer, B.; Mayr, L.; Rameshan, R.; Zemlyanov, D.; Bernardi, J.; Föttinger, K.; Rupprechter, G. Surface modification processes during methane decomposition on Cu-promoted Ni–ZrO2 catalysts. Catal. Sci. Technol. 2015, 5, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Kutteri, D.A.; Wang, I.W.; Samanta, A.; Li, L.; Hu, J. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3d transition metal catalysts. Catal. Sci. Technol. 2018, 8, 858–869. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrog. Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Zhang, T.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl. Catal. A Gen. 1998, 167, 161–172. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Gac, W.; Denis, A.; Borowiecki, T.; Kępiński, L. Methane decomposition over Ni–MgO–Al2O3 catalysts. Appl. Catal. A Gen. 2009, 357, 236–243. [Google Scholar] [CrossRef]
- Aiello, R.; Fiscus, J.E.; Loye, H.-C.z.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over Ni/SiO2: Catalyst deactivation and regeneration. Appl. Catal. A Gen. 2000, 192, 227–234. [Google Scholar] [CrossRef]
- Rahman, M.S.; Croiset, E.; Hudgins, R.R. Catalytic Decomposition of Methane for Hydrogen Production. Top. Catal. 2006, 37, 137–145. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Órfão, J.J.M.; Cunha, A.F. Hydrogen production via methane decomposition on Raney-type catalysts. Int. J. Hydrogen. Energy 2010, 35, 9795–9800. [Google Scholar] [CrossRef]
- Ammendola, P.; Chirone, R.; Ruoppolo, G.; Russo, G. Production of hydrogen from thermo-catalytic decomposition of methane in a fluidized bed reactor. Chem. Eng. J. 2009, 154, 287–294. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Tang, X.; Xu, Y.; Shen, W. Hydrogen production from methane decomposition over Ni/CeO2 catalysts. Catal. Commun. 2006, 7, 380–386. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Chen, D.; Christensen, K.O.; Ochoa-Fernández, E.; Yu, Z.; Tøtdal, B.; Latorre, N.; Monzón, A.; Holmen, A. Synthesis of carbon nanofibers: Effects of Ni crystal size during methane decomposition. J. Catal. 2005, 229, 82–96. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Kuvshinov, G.G.; Plyasova, L.M. New Nickel Catalysts for the Formation of Filamentous Carbon in the Reaction of Methane Decomposition. J. Catal. 1999, 187, 77–84. [Google Scholar] [CrossRef]
- García-Sancho, C.; Guil-Lópeza, R.; Pascual, L.; Maireles-Torres, P.; Navarro, R.M.; Fierro, J.L.G. Optimization of nickel loading of mixed oxide catalyst ex-hydrotalcite for H2 production by methane decomposition. Appl. Catal. A Gen. 2017, 548, 71–82. [Google Scholar] [CrossRef]
- Sikander, U.; Samsudin, M.F.; Sufian, S.; KuShaari, K.; Kait, C.F.; Naqvi, S.R.; Chen, W.H. Tailored hydrotalcite-based Mg-Ni-Al catalyst for hydrogen production via methane decomposition: Effect of nickel concentration and spinel-like structures. Int. J. Hydrog. Energy 2019, 44, 14424–14433. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [Green Version]
- Świrk, K.; Motak, M.; Grzybek, T.; Ronning, M.; Costa, P.D. Effect of low loading of yttrium on Ni-based layered double hydroxides in CO2 reforming of CH4. React. Kinet. Mech. Catal. 2019, 126, 611–628. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Wang, N.; Chen, B.; Xie, X.; Wang, Q.; Huang, L. Auto-thermal reforming of acetic acid over hydrotalcites-derived co-based catalyst: A stable and anti-coking Co/Sr-Alx-O catalyst. Appl. Catal. B Environ. 2020, 267, 118370. [Google Scholar] [CrossRef]
- Meza-Fuentes, E.; Rodriguez-Ruiz, J.; Solano-Polo, C.; Rangel, M.C.; Faro, A. Monitoring the structural and textural changes of Ni-Zn-Al hydrotalcites under heating. Thermochim. Acta 2020, 687, 178594. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Chen, B.; Zhang, Z.; Shi, C. Effect of Mg/Al ratio of NiMgAl mixed oxide catalyst derived from hydrotalcite for carbon dioxide reforming of methane. Catal. Today 2016, 264, 163–170. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Biogas reforming of carbon dioxide to syngas production over Ni-Mg-Al catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar] [CrossRef]
- Broda, M.; Kierzkowska, A.M.; Baudouin, D.; Imtiaz, Q.; Copéret, C.; Müller, C.R. Sorbent-Enhanced Methane Reforming over a Ni–Ca-Based, Bifunctional Catalyst Sorbent. ACS. Catal. 2012, 2, 1635–1646. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of composition and thermal pretreatment on properties of Ni–Mg–Al catalysts for CO2 reforming of methane. Appl. Catal. A Gen. 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg–Al hydrotalcite-like anionic clay. J. Catal. 2004, 221, 43–54. [Google Scholar] [CrossRef]
- González, A.R.; Asencios, Y.J.O.; Assaf, E.M.; Assaf, J.M. Dry reforming of methane on Ni–Mg–Al nano-spheroid oxide catalysts prepared by the sol–gel method from hydrotalcite-like precursors. Appl. Surf. Sci. 2013, 280, 876–887. [Google Scholar] [CrossRef]
- Cruz, I.O.; Ribeiro, N.F.P.; Aranda, D.A.G.; Souza, M.M.V.M. Hydrogen production by aqueous-phase reforming of ethanol over nickel catalysts prepared from hydrotalcite precursors. Catal. Commun. 2008, 9, 2606–2611. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Coke- and sintering-resistant monolithic catalysts derived from in situ supported hydrotalcite-like films on Al wires for dry reforming of methane. Nanoscale 2013, 5, 2659–2663. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Gao, R.; Huang, L.; Shi, L.; Zhang, J. Design of modular catalysts derived from NiMgAl-LDH@m-SiO2 with dual confinement effects for dry reforming of methane. Chem. Commun. 2013, 49, 6770–6772. [Google Scholar] [CrossRef]
- Montañez, M.K.; Molina, R.; Moreno, S. Nickel catalysts obtained from hydrotalcites by coprecipitation and urea hydrolysis for hydrogen production. Int. J. Hydrog. Energy 2014, 39, 8225–8237. [Google Scholar] [CrossRef]
- Li, M.; Fan, G.; Qin, H.; Li, F. Investigation of the Structure and Catalytic Performance of Highly Dispersed Ni-Based Catalysts for the Growth of Carbon Nanostructures. Ind. Eng. Chem. Res. 2012, 51, 11892–11900. [Google Scholar] [CrossRef]
- Mile, B.; Stirling, D.; Zammitt, M.A.; Lovell, A.; Webb, M. The location of nickel oxide and nickel in silica-supported catalysts: Two forms of “NiO” and the assignment of temperature-programmed reduction profiles. J. Catal. 1988, 114, 217–229. [Google Scholar] [CrossRef]
- Mangnus, P.J.; Bos, A.; Moulijn, J.A. Temperature-Programmed Reduction of Oxidic and Sulfidic Alumina-Supported NiO, WO3, and NiO-WO3 Catalysts. J. Catal. 1994, 146, 437–448. [Google Scholar] [CrossRef]
- Feng, X.-D.; Feng, J.; Li, W.-Y. CO2 reforming of CH4 over a highly active and stable NiMgAl catalyst. Int. J. Hydrog. Energy 2017, 42, 3036–3042. [Google Scholar] [CrossRef]
- Sun, Y.; Collins, M.; French, D.; McEvoy, S.; Hart, G.; Stein, W. Investigation into the mechanism of NiMg(Ca)bAlcOx catalytic activity for production of solarised syngas from carbon dioxide reforming of methane. Fuel 2013, 105, 551–558. [Google Scholar] [CrossRef]
- Duprez, D.; DeMicheli, M.C.; Marecot, P.; Barbier, J.; Ferretti, O.A.; Ponzi, E.N. Deactivation of steam-reforming model catalysts by coke formation: I. Kinetics of the Formation of Filamentous Carbon in the Hydrogenolysis of cyclopentane on Ni/Al2O3 Catalysts. J. Catal. 1990, 124, 324–335. [Google Scholar] [CrossRef]
- Bengaard, H.S.; Nørskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R. Steam Reforming and Graphite Formation on Ni Catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-Doped Ni/SBA-16 Catalysts for Dry Reforming of Methane. ACS. Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Bu, K.; Kuboon, S.; Deng, J.; Li, H.; Yan, T.; Chen, G.; Shi, L.; Zhang, D. Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts. Appl. Catal. B Environ. 2019, 252, 86–97. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni nanoparticles in CeZrO2 as stable catalyst for dry reforming of methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Silva, C.G.; Passos, F.B.; da Silva, V.T. Influence of the support on the activity of a supported nickel-promoted molybdenum carbide catalyst for dry reforming of methane. J. Catal. 2019, 375, 507–518. [Google Scholar] [CrossRef]
- Van Hardeveld, R.; Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969, 15, 189–230. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; el Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg(Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B Environ. 2017, 201, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Utilization of greenhouse gases through carbon dioxide reforming of methane over Ni–Co/MgO–ZrO2: Preparation, characterization and activity studies. Appl. Catal. B Environ. 2010, 100, 365–377. [Google Scholar] [CrossRef]
- Guevara, J.C.; Wang, J.A.; Chen, L.F.; Valenzuela, M.A.; Salas, P.; García-Ruiz, A.; Toledo, J.A.; Cortes-Jácome, M.A.; Angeles-Chavez, C.; Novaro, O. Ni/Ce-MCM-41 mesostructured catalysts for simultaneous production of hydrogen and nanocarbon via methane decomposition. Int. J. Hydrog. Energy 2010, 35, 3509–3521. [Google Scholar] [CrossRef]
- He, C.; Zhao, N.; Shi, C.; Du, X.; Li, J. Carbon nanotubes and onions from methane decomposition using Ni/Al catalysts. Mater. Chem. Phys. 2006, 97, 109–115. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Feng, X.; Chen, C.; Lin, D.; Liu, J.; Chen, X.; Liu, Y.; Yang, C.; Shan, H. NiMgAlMo catalyst derived from a guest-host MoO42− mediated layered double hydroxide: High performance for the methane decomposition reaction. Appl. Catal. A Gen. 2020, 597, 117551. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Lu, M.; Chen, C.; Li, D.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ni catalysts prepared from Ni–Mg–Al layered double hydroxides: Influence of Ni loadings. Fuel 2015, 162, 271–280. [Google Scholar] [CrossRef]
- Dupuis, A.-C. The catalyst in the CCVD of carbon nanotubes—A review. Prog. Mater. Sci. 2005, 50, 929–961. [Google Scholar] [CrossRef]
- Abild-Pedersen, F.; Nørskov, J.K.; Rostrup-Nielsen, J.R.; Sehested, J.; Helveg, S. Mechanisms for catalytic carbon nanofiber growth studied by ab initio density functional theory calculations. Phys. Rev. B 2006, 73, 115419. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methane and carbon deposition via Ni-CeO2-x interaction. Appl. Catal. B Environ. 2018, 237, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Fu, Y.; Kong, W.; Pan, B.; Yuan, C.; Cai, F.; Zhu, H.; Zhang, J.; Sun, Y. Dually Confined Ni Nanoparticles by Room-Temperature Degradation of AlN for Dry Reforming of Methane. Appl. Catal. B Environ. 2020, 227, 118921. [Google Scholar] [CrossRef]
- Helveg, S.; López-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef]
- Rao, R.; Sharma, R.; Abild-Pedersen, F.; Nørskov, J.K.; Harutyunyan, A.R. Insights into carbon nanotube nucleation: Cap formation governed by catalyst interfacial step flow. Sci. Rep. UK 2014, 4, 6510. [Google Scholar] [CrossRef]
- Gao, J.; Yip, J.; Zhao, J.; Yakobson, B.I.; Ding, F. Correction to Graphene Nucleation on Transition Metal Surface: Structure Transformation and Role of the Metal Step Edge. J. Am. Chem. Soc. 2012, 134, 9534. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Barber, M.A.; Harris, P.S.; Feates, F.S.; Waite, R.J. Nucleation and growth of carbon deposits from the nickel catalyzed decomposition of acetylene. J. Catal. 1972, 26, 51–62. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Trimm, D.L. Mechanisms of carbon formation on nickel-containing catalysts. J. Catal. 1977, 48, 155–165. [Google Scholar] [CrossRef]
- Baird, T.; Fryer, J.R.; Grant, B. Carbon formation on iron and nickel foils by hydrocarbon pyrolysis—Reactions at 700 °C. Carbon 1974, 12, 591–602. [Google Scholar] [CrossRef]
- Hofmann, S.; Ducati, C.; Robertson, J.; Kleinsorge, B. Low-temperature growth of carbon nanotubes by plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2003, 83, 135–137. [Google Scholar] [CrossRef]
- Li, J.; Croiset, E.; Ricardez-Sandoval, L. Methane dissociation on Ni (100), Ni (111), and Ni (553): A comparative density functional theory study. J. Mol. Catal. A Chem. 2012, 365, 103–114. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, J.; Guo, J.; Liang, D.; Lou, H.; Zheng, X. Deactivation of Ni catalysts during methane autothermal reforming with CO2 and O2 in a fluidized-bed reactor. J. Catal. 2007, 250, 331–341. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Dam, A.H.; Xiao, L.; Qi, Y.; Niu, J.; Yang, J.; Zhu, Y.-A.; Holmen, A.; Chen, D. Understanding effects of Ni particle size on steam methane reforming activity by combined experimental and theoretical analysis. Catal. Today 2019, in press. [Google Scholar] [CrossRef]
- Wu, J.; Helveg, S.; Ullmann, S.; Peng, Z.; Bell, A.T. Growth of encapsulating carbon on supported Pt nanoparticles studied by in situ TEM. J. Catal. 2016, 338, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Fischer, S.; Karplus, M. Conjugate peak refinement: An algorithm for finding reaction paths and accurate transition states in systems with many degrees of freedom. Chem. Phys. Lett. 1992, 194, 252–261. [Google Scholar] [CrossRef]
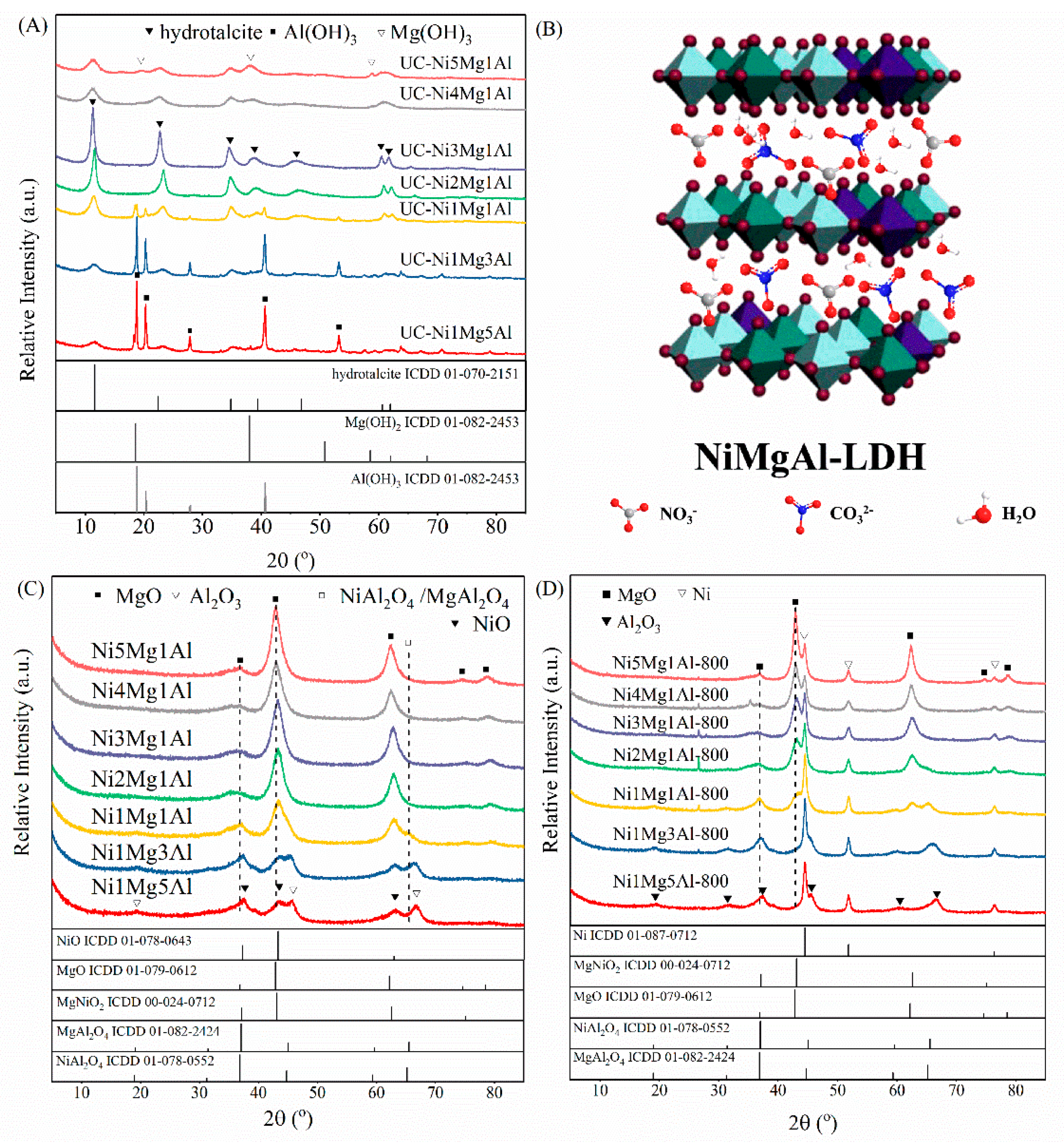
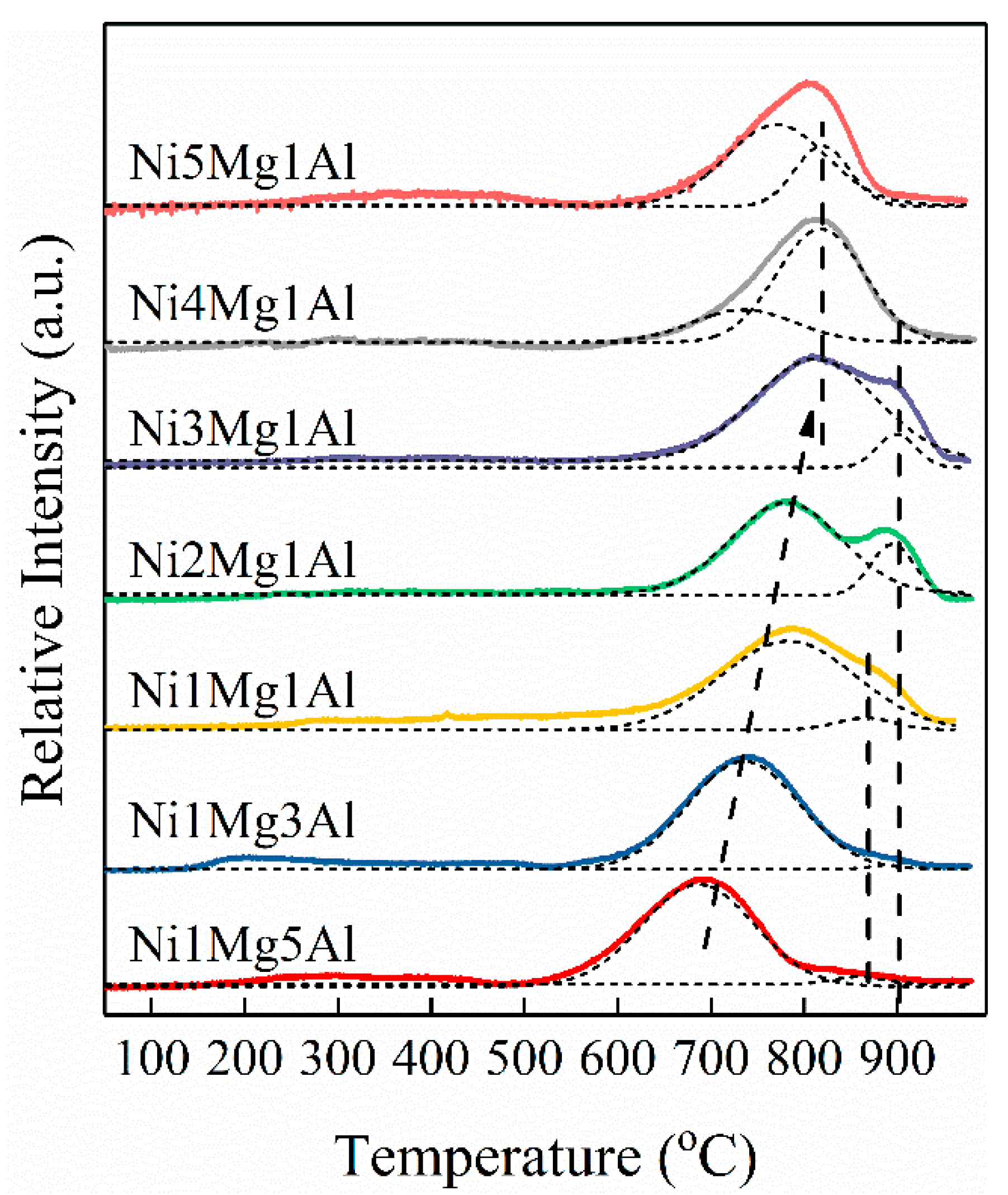
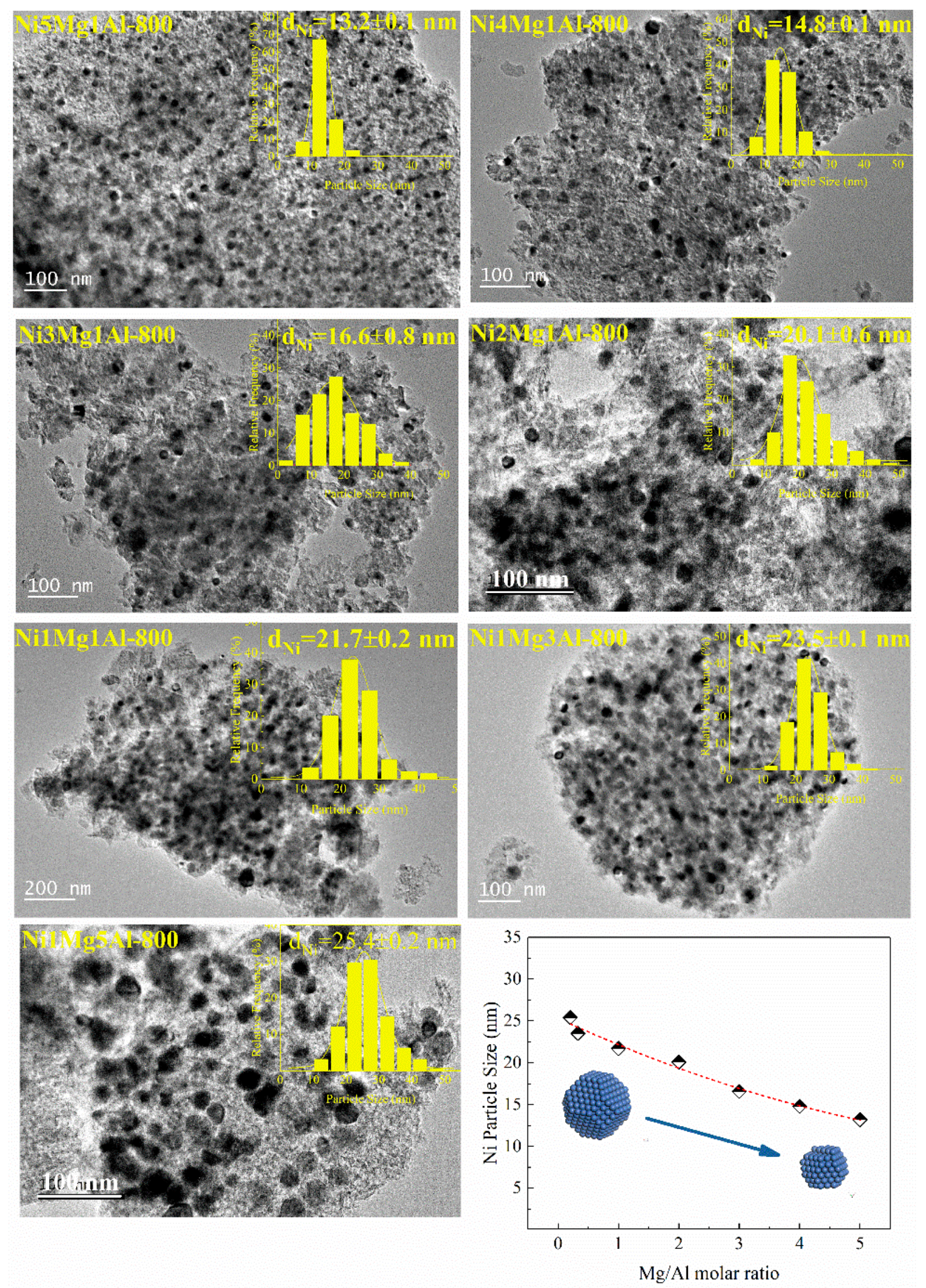
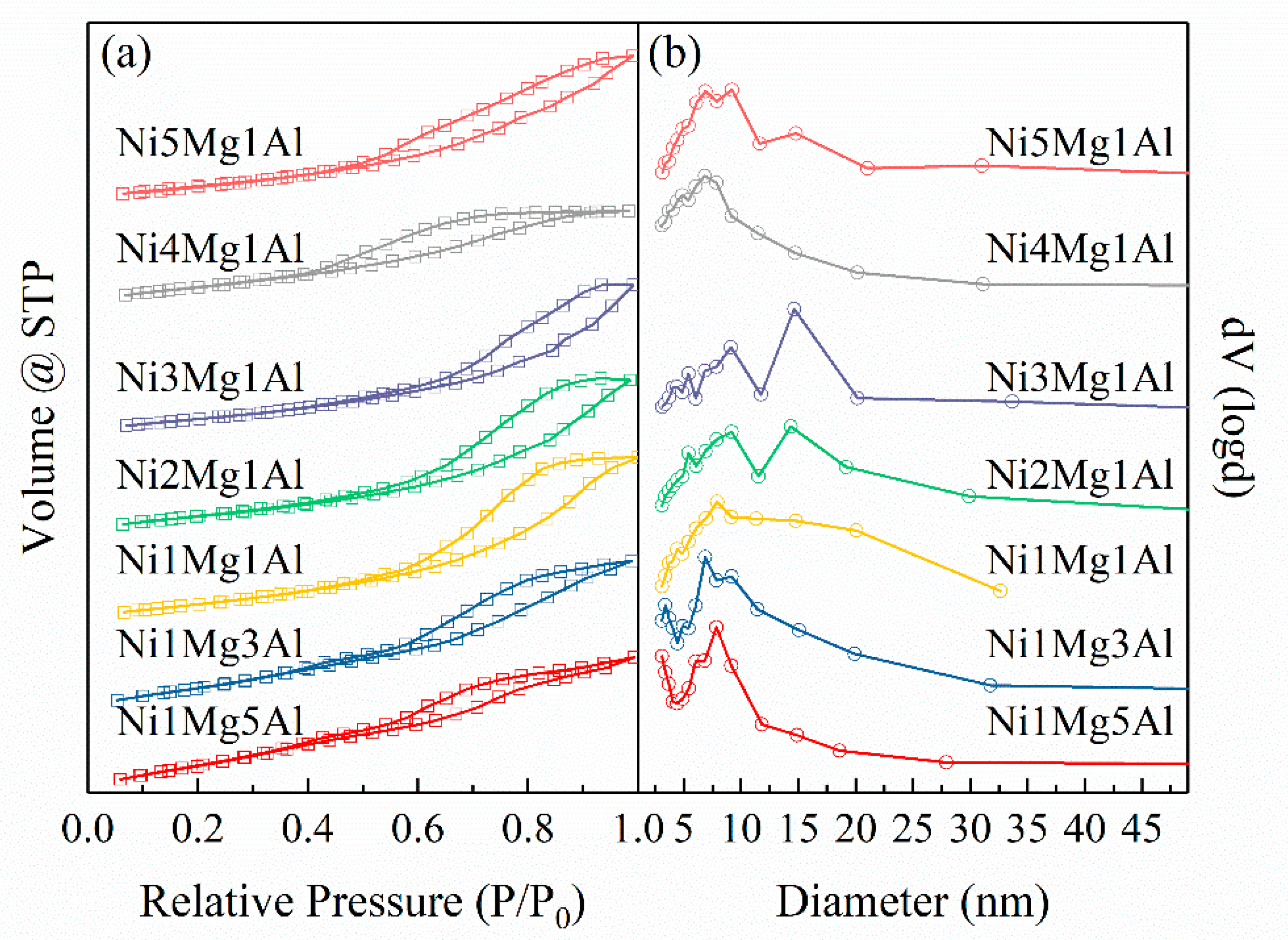
| Catalysts | For UC-NixMgyAl Samples | Mg2+/Al3+ b | Ni b (wt%) | For NixMgyAl-800 Samples | |||||
|---|---|---|---|---|---|---|---|---|---|
| Feeding Mg2+/Al3+ a | M3+/(M3+ + M2+) | BET Surface Area (m2/g) | Pore Volume (mL/g) | Mean Pore Size (nm) | Reduced Ni0 Particle size c (nm) | Used Ni0 Particle Size c (nm) | |||
| Ni5Mg1Al | 5.00 | 0.142 | 4.29 | 12.5 | 212.5 | 0.43 | 6.1 | 11.5 | 12.6 |
| Ni4Mg1Al | 4.00 | 0.167 | 3.43 | 11.9 | 223.1 | 0.29 | 3.7 | 12.3 | 14.5 |
| Ni3Mg1Al | 3.00 | 0.200 | 2.51 | 13.4 | 204.2 | 0.44 | 4.1 | 13.9 | 16.1 |
| Ni2Mg1Al | 2.00 | 0.250 | 1.76 | 12.5 | 219.3 | 0.45 | 5.4 | 15.1 | 17.7 |
| Ni1Mg1Al | 1.00 | 0.333 | 0.94 | 12.6 | 230.5 | 0.45 | 3.7 | 18.7 | 20.1 |
| Ni1Mg3Al | 0.33 | 0.600 | 0.36 | 12.6 | 284.7 | 0.45 | 3.4 | 20.6 | 22.4 |
| Ni1Mg5Al | 0.20 | 0.714 | 0.24 | 12.6 | 319.4 | 0.41 | 3.1 | 21.5 | 25.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction. Catalysts 2020, 10, 890. https://doi.org/10.3390/catal10080890
Liang W, Yan H, Chen C, Lin D, Tan K, Feng X, Liu Y, Chen X, Yang C, Shan H. Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction. Catalysts. 2020; 10(8):890. https://doi.org/10.3390/catal10080890
Chicago/Turabian StyleLiang, Wei, Hao Yan, Chen Chen, Dong Lin, Kexin Tan, Xiang Feng, Yibin Liu, Xiaobo Chen, Chaohe Yang, and Honghong Shan. 2020. "Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction" Catalysts 10, no. 8: 890. https://doi.org/10.3390/catal10080890
APA StyleLiang, W., Yan, H., Chen, C., Lin, D., Tan, K., Feng, X., Liu, Y., Chen, X., Yang, C., & Shan, H. (2020). Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction. Catalysts, 10(8), 890. https://doi.org/10.3390/catal10080890





