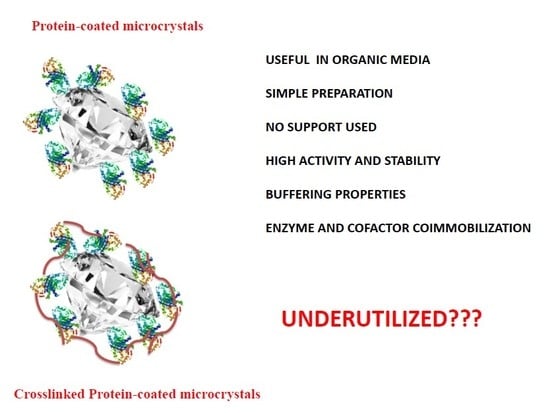
Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy
Abstract
1. Introduction: Enzyme Biocatalysis
2. Enzyme Immobilization
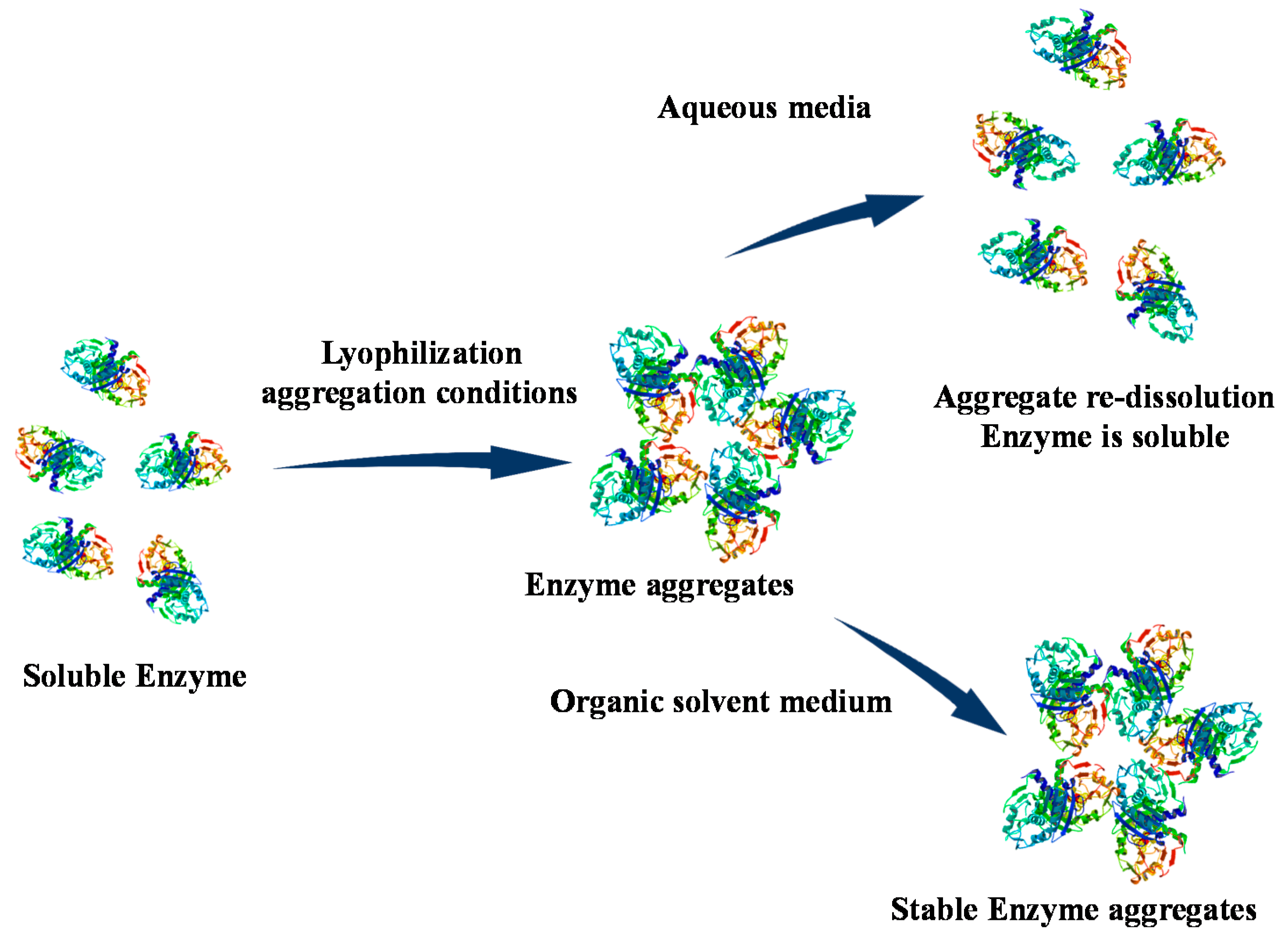
3. Use of Enzyme in Organic Solvents with Low Water Activity
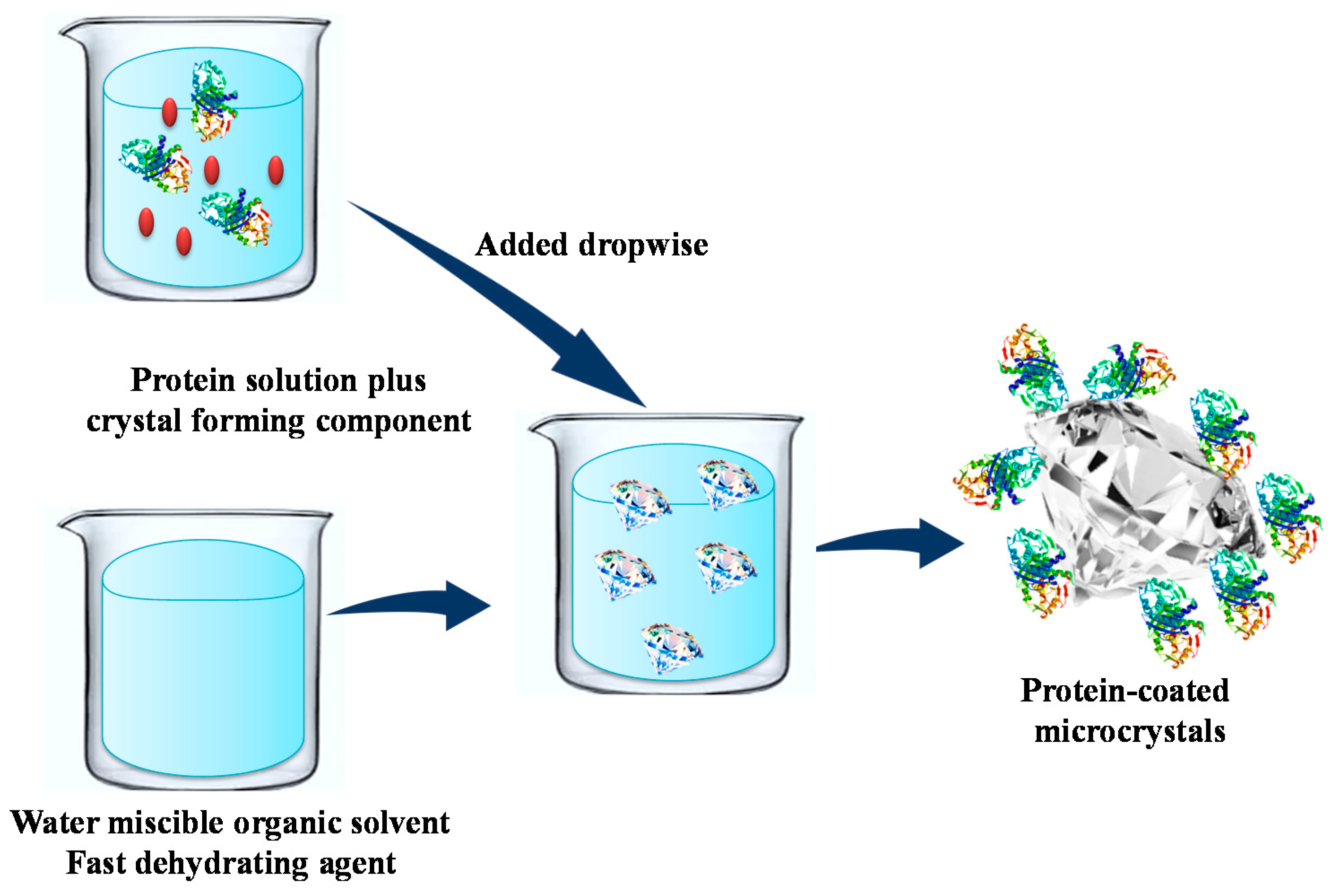
4. Immobilization of Enzyme by Protein-Coated Microcrystals (PCMCs)
4.1. Chemical Modification of PCMCs
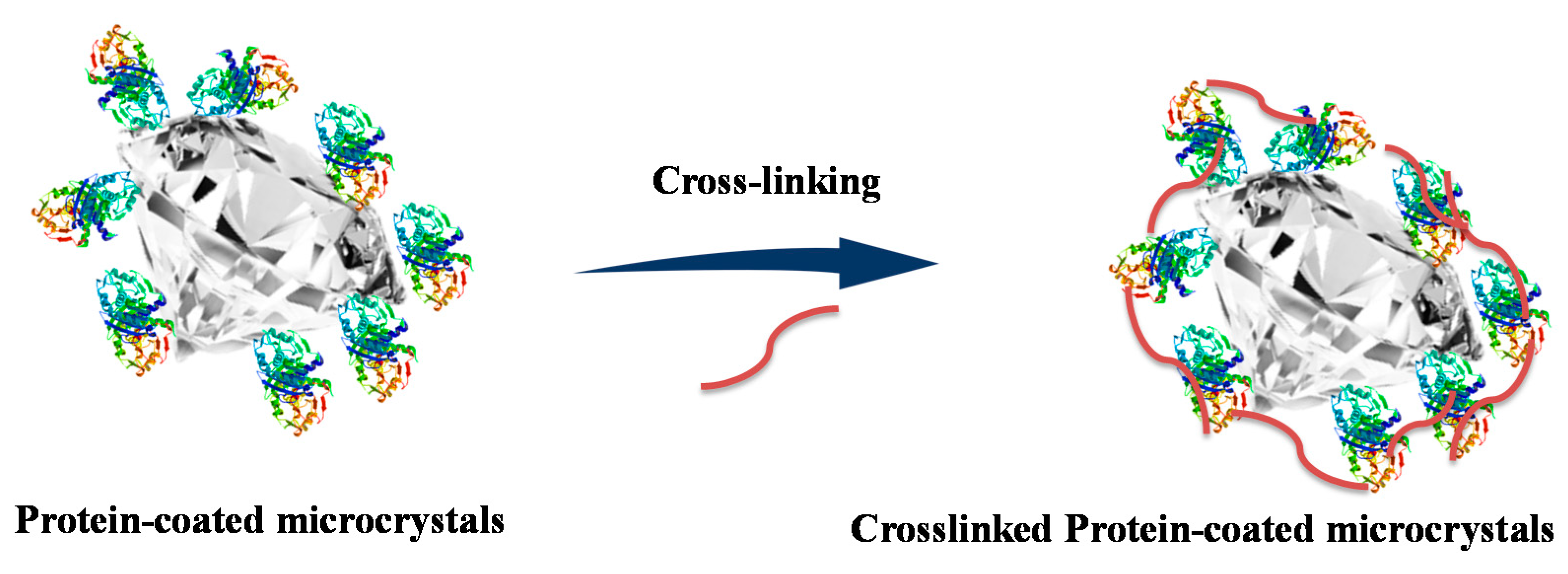
4.2. Chemically Crosslinked PCMCs
4.3. Modification of the Enzyme before PCMCs Production
4.4. Enzyme Co-immobilization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowie, J.U.; Sherkhanov, S.; Korman, T.P.; Valliere, M.A.; Opgenorth, P.H.; Liu, H. Synthetic biochemistry: The bio-inspired cell-free approach to commodity chemical production. Trends Biotechnol. 2020, 38, 766–778. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Schoemaker, H.E. Dispelling the myths—Biocatalysis in industrial synthesis. Science 2003, 299, 1694–1697. [Google Scholar] [CrossRef]
- Ferrer, M.; Golyshina, O.; Beloqui, A.; Golyshin, P.N. Mining enzymes from extreme environments. Curr. Opin. Microbiol. 2007, 10, 207–214. [Google Scholar] [CrossRef]
- Ferrer, M.; Beloqui, A.; Timmis, K.N.; Golyshin, P.N. Metagenomics for mining new genetic resources of microbial communities. J. Mol. Microbiol. Biotechnol. 2009, 16, 109–123. [Google Scholar] [CrossRef]
- Ferrer, M.; Martinezabarca, F.; Golyshin, P. Mining genomes and ‘metagenomes’ for novel catalysts. Curr. Opin. Biotechnol. 2005, 16, 588–593. [Google Scholar] [CrossRef]
- Ren, C.; Wen, X.; Mencius, J.; Quan, S. Selection and screening strategies in directed evolution to improve protein stability. Bioresour. Bioprocess. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Romero, P.A.; Arnold, F.H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 2009, 10, 866–876. [Google Scholar] [CrossRef]
- Spicer, C.D.; Davis, B.G. Selective chemical protein modification. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J.L. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Chalker, J.M.; Bernardes, G.J.L.; Lin, Y.A.; Davis, B.G. Chemical modification of proteins at cysteine: Opportunities in chemistry and biology. Chem. Asian J. 2009, 4, 630–640. [Google Scholar] [CrossRef]
- Gunnoo, S.B.; Madder, A. Chemical protein modification through cysteine. ChemBioChem 2016, 17, 529–553. [Google Scholar] [CrossRef]
- Sakamoto, S.; Hamachi, I. Recent progress in chemical modification of proteins. Anal. Sci. 2019, 35, 5–27. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.; Martínez-Martínez, M.; Alonso, S.; Bargiela, R.; Coscolín, C.; Golyshin, P.N.; Guallar, V.; Ferrer, M. Rational engineering of multiple active sites in an ester hydrolase. Biochemistry 2018, 57, 2245–2255. [Google Scholar] [PubMed]
- Alonso, S.; Santiago, G.; Cea-Rama, I.; Fernandez-Lopez, L.; Coscolín, C.; Modregger, J.; Ressmann, A.K.; Martínez-Martínez, M.; Marrero, H.; Bargiela, R.; et al. Genetically engineered proteins with two active sites for enhanced biocatalysis and synergistic chemo-and biocatalysis. Nat. Catal. 2020, 3, 319–328. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as an emergent tool in the design of biocatalysts: Mechanism of reaction and applications. Catalysts 2019, 9, 1035. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical modification in the design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Bernal, C.; Rodríguez, K.; Martínez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef]
- Gupta, M.N.; Roy, I. Enzymes in organic media: Forms, functions and applications. Eur. J. Biochem. 2004, 271, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.-E.; von Langermann, J.; Kragl, U. Recent developments in biocatalysis in multiphasic ionic liquid reaction systems. Biophys. Rev. 2018, 10, 901–910. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Garcia-Verdugo, E.; Sanchez-Gomez, G.; Vaultier, M.; Burguete, M.I.; Luis, S.V. Sponge-like ionic liquids: A new platform for green biocatalytic chemical processes. Green Chem. 2015, 17, 3706–3717. [Google Scholar] [CrossRef]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef]
- Lozano, P.; Nieto, S.; Serrano, J.; Perez, J.; Sanchez-Gomez, G.; García-Verdugo, E.; Luis, S. Flow biocatalytic processes in ionic liquids and supercritical fluids. Mini Rev. Org. Chem. 2017, 14, 65–74. [Google Scholar] [CrossRef]
- Karmee, S.K.; Casiraghi, L.; Greiner, L. Technical aspects of biocatalysis in non-CO2-based supercritical fluids. Biotechnol. J. 2008, 3, 104–111. [Google Scholar] [CrossRef]
- Tan, J.-N.; Dou, Y. Deep eutectic solvents for biocatalytic transformations: Focused lipase-catalyzed organic reactions. Appl. Microbiol. Biotechnol. 2020, 104, 1481–1496. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Paul, C.E. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019, 293, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Müller, C.R.; Schrebler, R.; Carlesi, C.; de María, P.D. Deep eutectic solvents for organocatalysis, biotransformations, and multistep organocatalyst/enzyme combinations. ChemCatChem 2016, 8, 1020–1027. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Bilal, M.; Cui, J.; Iqbal, H.M.N. Tailoring enzyme microenvironment: State-of-the-art strategy to fulfill the quest for efficient bio-catalysis. Int. J. Biol. Macromol. 2019, 130, 186–196. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Gupta, M.N.; Perwez, M.; Sardar, M. Protein crosslinking: Uses in chemistry, biology and biotechnology. Biocatal. Biotransform. 2020, 38, 178–201. [Google Scholar] [CrossRef]
- Dal Magro, L.; Kornecki, J.F.; Klein, M.P.; Rodrigues, R.C.; Fernandez-Lafuente, R. Pectin lyase immobilization using the glutaraldehyde chemistry increases the enzyme operation range. Enzyme Microb. Technol. 2020, 132, 109397. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, L.; Kornecki, J.F.; Klein, M.P.; Rodrigues, R.C.; Fernandez-Lafuente, R. Optimized immobilization of polygalacturonase from Aspergillus niger following different protocols: Improved stability and activity under drastic conditions. Int. J. Biol. Macromol. 2019, 138, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Fernández-Lafuente, R.; Hernández-Jústiz, O.; Mateo, C.; Terreni, M.; Fernández-Lorente, G.; Moreno, M.A.; Alonso, J.; García-López, J.L.; Guisan, J.M. Biotransformations catalyzed by multimeric enzymes: Stabilization of tetrameric ampicillin acylase permits the optimization of ampicillin synthesis under dissociation conditions. Biomacromolecules 2001, 2, 95–104. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Gomes, M.D.; Woodley, J.M. Considerations when measuring biocatalyst performance. Molecules 2019, 24, 3573. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Müller, F.; Torger, B.; Allertz, P.J.; Jähnichen, K.; Keßler, S.; Müller, M.; Simon, F.; Salchert, K.; Mäurer, H.; Pospiech, D. Multifunctional crosslinkable itaconic acid copolymers for enzyme immobilization. Eur. Polym. J. 2018, 102, 47–55. [Google Scholar] [CrossRef]
- Pollak, A.; Blumenfeld, H.; Wax, M.; Baughn, R.L.; Whitesides, G.M. Enzyme immobilization by condensation copolymerization into crosslinked polyacrylamide gels. J. Am. Chem. Soc. 1980, 102, 6324–6336. [Google Scholar] [CrossRef]
- Johansson, A.-C.; Mosbach, K. Acrylic copolymers as matrices for the immobilization of enzymes. Biochim. Biophys. Acta BBA Enzymol. 1974, 370, 339–347. [Google Scholar] [CrossRef]
- Abraham, T.E.; Joseph, J.R.; Bindhu, L.B.V.; Jayakumar, K.K. Crosslinked enzyme crystals of glucoamylase as a potent catalyst for biotransformations. Carbohydr. Res. 2004, 339, 1099–1104. [Google Scholar] [CrossRef]
- Navia, M.A.; St Clair, N.L.; Griffith, J.P. Crosslinked enzyme crystals (CLECsTM) as immobilized enzyme particles. In Studies in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1993; Volume 47, pp. 63–73. ISBN 978-0-444-89372-7. [Google Scholar]
- Wu, X.; Hou, M.; Ge, J. Metal-organic frameworks and inorganic nanoflowers: A type of emerging inorganic crystal nanocarrier for enzyme immobilization. Catal. Sci. Technol. 2015, 5, 5077–5085. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; Ocsoy, I. A new generation approach in enzyme immobilization: Organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzyme Microb. Technol. 2016, 93, 105–112. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Schoevaart, R.; Van Langen, L.M. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransform. 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Kreiner, M.; Parker, M.C.; Moore, B.D. Enzyme-coated micro-crystals: A 1-step method for high activity biocatalyst preparation. Chem. Commun. 2001, 12, 1096–1097. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Enzyme-catalyzed processes in organic solvents. Proc. Natl. Acad. Sci. USA 1985, 82, 3192–3196. [Google Scholar] [CrossRef]
- Kirchner, G.; Scollar, M.P.; Klibanov, A.M. Resolution of racemic mixtures via lipase catalysis in organic solvents. J. Am. Chem. Soc. 1985, 107, 7072–7076. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Substrate specificity of enzymes in organic solvents vs. water is reversed. J. Am. Chem. Soc. 1986, 108, 2767–2768. [Google Scholar] [CrossRef]
- Klibanov, A.M. Enzyme-catalyzed processes in organic solvents. Ann. N. Y. Acad. Sci. 1987, 501, 129. [Google Scholar] [CrossRef]
- Kilbanov, A.M. Enzymes that work in organic-solvents. Chemtech 1986, 16, 354–359. [Google Scholar]
- Klibanov, A.M. Enzymatic catalysis in anhydrous organic solvents. Trends Biochem. Sci. 1989, 14, 141–144. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Enzymatic catalysis in nonaqueous solvents. J. Biol. Chem. 1988, 263, 3194–3201. [Google Scholar]
- Zaks, A.; Klibanov, A.M. The effect of water on enzyme action in organic media. J. Biol. Chem. 1988, 263, 8017–8021. [Google Scholar]
- Russell, A.J.; Klibanov, A.M. Enzymes in organic solvents. Biochem. Soc. Trans. 1989, 17, 1145. [Google Scholar] [CrossRef]
- Klibanov, A.M. Asymmetric transformations catalyzed by enzymes in organic solvents. Acc. Chem. Res. 1990, 23, 114–120. [Google Scholar] [CrossRef]
- Fitzpatrick, P.A.; Klibanov, A.M. How can the solvent affect enzyme enantioselectivity? J. Am. Chem. Soc. 1991, 113, 3166–3171. [Google Scholar] [CrossRef]
- Narayan, V.S.; Klibanov, A.M. Are water-immiscibility and apolarity of the solvent relevant to enzyme efficiency? Biotechnol. Bioeng. 1993, 41, 390–393. [Google Scholar] [CrossRef]
- Wescott, C.R.; Klibanov, A.M. The solvent dependence of enzyme specificity. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1994, 1206, 1–9. [Google Scholar] [CrossRef]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Empie, M.W.; Gross, A. Enzyme assisted transformations in organic media. In Annual Reports in Medicinal Chemistry; Allen, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 23, pp. 305–313. ISBN 978-0-12-040523-7. [Google Scholar]
- Mozhaev, V.V.; Berezin, I.V.; Martinek, K.; Nosoh, Y. Structure-stability relationship in proteins: Fundamental tasks and strategy for the development of stabilized enzyme catalysts for biotechnolog. Crit. Rev. Biochem. 1988, 23, 235–281. [Google Scholar] [CrossRef] [PubMed]
- Deetz, J.S.; Rozzell, J.D. Enzyme-catalysed reactions in non-aqueous media. Trends Biotechnol. 1988, 6, 15–19. [Google Scholar] [CrossRef]
- Arnold, F.H. Protein design for non-aqueous solvents. Protein Eng. Des. Sel. 1988, 2, 21–25. [Google Scholar] [CrossRef]
- Dordick, J.S. Patents and literature biocatalysis in nonaqueous media. Appl. Biochem. Biotechnol. 1988, 19, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Khmelnitsky, Y.L.; Levashov, A.V.; Klyachko, N.L.; Martinek, K. Engineering biocatalytic systems in organic media with low water content. Enzyme Microb. Technol. 1988, 10, 710–724. [Google Scholar] [CrossRef]
- Dordick, J.S. Enzymatic catalysis in monophasic organic solvents. Enzyme Microb. Technol. 1989, 11, 194–211. [Google Scholar] [CrossRef]
- Chen, C.-S.; Sih, C.J. General aspects and optimization of enantioselective biocatalysis in organic solvents: The use of lipases [New Synthetic Methods (76)]. Angew. Chem. Int. Ed. Engl. 1989, 28, 695–707. [Google Scholar] [CrossRef]
- Tramper, J.; Laane, C. Tailoring the medium and reactor for biocatalysis. Chemtech 1990, 20, 502–506. [Google Scholar]
- Faber, K. A rationale to explain the catalytic activity of solid enzymes in organic solvents. J. Mol. Catal. 1991, 65, L49–L51. [Google Scholar] [CrossRef]
- Van Erp, S.H.; Kamenskaya, E.O.; Khmelnitsky, Y.L. The effect of water content and nature of organic solvent on enzyme activity in low-water media: A quantitative description. Eur. J. Biochem. 1991, 202, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Hartsough, D.S.; Merz, K.M. Protein flexibility in aqueous and nonaqueous solutions. J. Am. Chem. Soc. 1992, 114, 10113–10116. [Google Scholar] [CrossRef]
- Gorman, L.A.S.; Dordick, J.S. Organic solvents strip water off enzymes. Biotechnol. Bioeng. 1992, 39, 392–397. [Google Scholar] [CrossRef]
- Halling, P.J. Biocatalysis in multi-phase reaction mixtures containing organic liquids. Biotechnol. Adv. 1987, 5, 47–84. [Google Scholar] [CrossRef]
- Halling, P. Organic liquids and biocatalysts: Theory and practice. Trends Biotechnol. 1989, 7, 50–52. [Google Scholar] [CrossRef]
- Gupta, M.N. Methods in Non-Aqueous Enzymology; Birkhäuser Basel: Basel, Switzerland, 2000; ISBN 978-3-7643-6109-9. [Google Scholar]
- Koskinen, A.; Klibanov, A. Enzymatic Reactions in Organic Media; Springer Science+Business Media: Dordrecht, The Netherlands, 1996; ISBN 978-0-7514-0259-9. [Google Scholar]
- Vulfson, E.N.; Halling, P.J. Enzymes in nonaqueous solvents-methods and protocols. In Methods and Protocols; Humana Press: Totowa, NJ, USA, 2001. [Google Scholar]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W. H. Freeman: New York, NY, USA, 2017; ISBN 978-0-7167-4339-2. [Google Scholar]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water dynamics in the hydration shells of biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Bose Majumdar, A.; Kim, I.J.; Na, H. Effect of solvent on protein structure and dynamics. Phys. Biol. 2020, 17, 036006. [Google Scholar] [CrossRef]
- Maurer, M.; Oostenbrink, C. Water in protein hydration and ligand recognition. J. Mol. Recognit. 2019, 32, e2810. [Google Scholar] [CrossRef]
- Petersson, A.E.V.; Adlercreutz, P.; Mattiasson, B. A water activity control system for enzymatic reactions in organic media. Biotechnol. Bioeng. 2007, 97, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kara, S.; Liese, A. Process considerations for the application of enzymes. In Industrial Enzyme Applications; Vogel, A., May, O., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 71–94. ISBN 978-3-527-81378-0. [Google Scholar]
- Lousa, D.; Baptista, A.M.; Soares, C.M. A molecular perspective on nonaqueous biocatalysis: Contributions from simulation studies. Phys. Chem. Chem. Phys. 2013, 15, 13723. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.M. What is remembered and why? Nature 1995, 374, 596. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Meyer, J.D.; Kendrick, B.S.; Manning, M.C.; Carpenter, J.F. Effect of secondary structure on the activity of enzymes suspended in organic solvents. Arch. Biochem. Biophys. 1996, 334, 406–414. [Google Scholar] [CrossRef]
- Noritomi, H.; Almarsson, Ö.; Barletta, G.L.; Klibanov, A.M. The influence of the mode of enzyme preparation on enzymatic enantioselectivity in organic solvents and its temperature dependence. Biotechnol. Bioeng. 2000, 51, 95–99. [Google Scholar] [CrossRef]
- Ke, T.; Klibanov, A.M. On enzymatic activity in organic solvents as a function of enzyme history. Biotechnol. Bioeng. 1998, 57, 746–750. [Google Scholar] [CrossRef]
- Rueda, A.J.V.; Monzon, A.M.; Ardanaz, S.M.; Iglesias, L.E.; Parisi, G. Large scale analysis of protein conformational transitions from aqueous to non-aqueous media. BMC Bioinform. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Nie, G.; Cai, W.; Yao, Z.; Zhu, Z.; Zhu, X.; Zheng, Z.; Yue, W. Changing enzymatic conformation in organic media with pH buffer lyophilized powder. Catal. Commun. 2015, 65, 62–65. [Google Scholar] [CrossRef]
- Staahl, M.; Jeppsson-Wistrand, U.; Maansson, M.O.; Mosbach, K. Induced stereo-and substrate selectivity of bioimprinted α-chymotrypsin in anhydrous organic media. J. Am. Chem. Soc. 1991, 113, 9366–9368. [Google Scholar] [CrossRef]
- Mosbach, K.; Ramström, O. The emerging technique of molecular imprinting and its future impact on biotechnology. Nat. Biotechnol. 1996, 14, 163–170. [Google Scholar] [CrossRef]
- Maruyama, T.; Nakajima, M.; Uchikawa, S.; Nabetani, H.; Furusaki, S.; Seki, M. Oil-water interfacial activation of lipase for interesterification of triglyceride and fatty acid. J. Am. Oil Chem. Soc. 2000, 77, 1121. [Google Scholar] [CrossRef]
- Mukherjee, J.; Gupta, M.N. Molecular bioimprinting of lipases with surfactants and its functional consequences in low water media. Int. J. Biol. Macromol. 2015, 81, 544–551. [Google Scholar] [CrossRef]
- Yang; Zhang Surfactant imprinting hyperactivated immobilized lipase as efficient biocatalyst for biodiesel production from waste cooking oil. Catalysts 2019, 9, 914. [CrossRef]
- Matsumoto, M.; Hasegawa, Y. Enzymatic kinetics of solvent-free esterification with bio-imprinted lipase. Chem. Biochem. Eng. Q. 2020, 33, 495–499. [Google Scholar] [CrossRef]
- Nie, G.; Zheng, Z.; Jin, W.; Gong, G.; Wang, L. Development of a tannase biocatalyst based on bio-imprinting for the production of propyl gallate by transesterification in organic media. J. Mol. Catal. B Enzym. 2012, 78, 32–37. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhao, G.; Wang, P.; Wang, L.; Wu, H.; Fang, X.; Sun, X.; Wu, X.; Zheng, Z. Enhancing the performance of a phospholipase A1 for oil degumming by bio-imprinting and immobilization. J. Mol. Catal. B Enzym. 2016, 123, 122–131. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; He, Y.; Cui, G.; Abdulrazaq, M.A.; Yan, Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine-glutaraldehyde dendrimer modified magnetic nanoparticles. Chem. Eng. J. 2018, 351, 258–268. [Google Scholar] [CrossRef]
- Mukherjee, J.; Gupta, M.N. Enhancing the catalytic efficiency of subtilisin for transesterification by dual bioimprinting. Tetrahedron Lett. 2015, 56, 4397–4401. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Moore, B.D.; Parker, M.C.; Halling, P.J.; Partridge, J. Rapid Dehydration of Proteins. U.S. Patent US20020168414A1, 21 March 2006. [Google Scholar]
- Quirós, M.; Parker, M.-C.; Turner, N.J. Tuning lipase enantioselectivity in organic media using solid-state buffers. J. Org. Chem. 2001, 66, 5074–5079. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, M.; Parker, M.C. High-activity biocatalysts in organic media: Solid-state buffers as the immobilisation matrix for protein-coated microcrystals. Biotechnol. Bioeng. 2004, 87, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, M.; Fernandes, J.F.A.; O’Farrell, N.; Halling, P.J.; Parker, M.-C. Stability of protein-coated microcrystals in organic solvents. J. Mol. Catal. B Enzym. 2005, 33, 65–72. [Google Scholar] [CrossRef]
- Kreiner, M.; Parker, M.-C. Protein-coated microcrystals for use in organic solvents: Application to oxidoreductases. Biotechnol. Lett. 2005, 27, 1571–1577. [Google Scholar] [CrossRef]
- Klibanov, A.M. Asymmetric enzymatic oxidoreductions in organic solvents. Curr. Opin. Biotechnol. 2003, 14, 427–431. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Benítez-Mateos, A.I.; López-Gallego, F. Co-immobilized phosphorylated cofactors and enzymes as self-sufficient heterogeneous biocatalysts for chemical processes. Angew. Chem. Int. Ed. 2017, 56, 771–775. [Google Scholar] [CrossRef]
- O’Farrell, N.; Kreiner, M.; Moore, B.D.; Parker, M.-C. A rapid and direct method for the determination of active site accessibility in proteins based on ESI-MS and active site titrations. Biotechnol. Bioeng. 2006, 95, 767–771. [Google Scholar] [CrossRef]
- Kumari, V.; Shah, S.; Gupta, M.N. Preparation of biodiesel by lipase-catalyzed transesterification of high free fatty acid containing oil from Madhuca indica. Energy Fuels 2007, 21, 368–372. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Kinetic resolution of (±)-1-phenylethanol in [Bmim][PF6] using high activity preparations of lipases. Bioorg. Med. Chem. Lett. 2007, 17, 921–924. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Varandani, D.; Mehta, B.; Gupta, M.N. A high performance lipase preparation: Characterization and atomic force microscopy. J. Nanosci. Nanotechnol. 2007, 7, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.; Gupta, M.N. Optimising biocatalyst design for obtaining high transesterification activity by α-chymotrypsin in non-aqueous media. Chem. Cent. J. 2008, 2, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, Y.; Carnell, A.J. A practical chemo-enzymatic synthesis of homochiral bicyclo[2.2.2]octane-2,5-dione. J. Org. Chem. 2010, 75, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Gupta, G.N.; Vamsikrishnan, M.; Khare, S.K. Protein-coated microcrystals of Pseudomonas aeruginosa PseA lipase. Appl. Biochem. Biotechnol. 2008, 151, 160–166. [Google Scholar] [CrossRef]
- Wu, J.C.; Yang, J.X.; Zhang, S.H.; Chow, Y.; Talukder, M.M.R.; Choi, W.J. Activity, stability and enantioselectivity of lipase-coated microcrystals of inorganic salts in organic solvents. Biocatal. Biotransform. 2009, 27, 283–289. [Google Scholar] [CrossRef]
- Raita, M.; Champreda, V.; Laosiripojana, N. Biocatalytic ethanolysis of palm oil for biodiesel production using microcrystalline lipase in tert-butanol system. Process Biochem. 2010, 45, 829–834. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, L.; Liu, Y.; Zhang, X.; Yan, Y. Lipase-coated K2SO4 micro-crystals: Preparation, characterization, and application in biodiesel production using various oil feedstocks. Bioresour. Technol. 2012, 110, 224–231. [Google Scholar] [CrossRef]
- Kazlauskas, S.; Kiriliauskaitė, V.; Kalėdienė, L.; Bendikienė, V. High performance protein-coated microcrystals of Rhizomucor miehei lipase: Preparation and application for organic synthesis. Appl. Biochem. Biotechnol. 2015, 176, 321–332. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Xu, L.; Ke, C.; Zhang, R.; Yan, Y. Protein-coated microcrystals from Candida rugosa lipase: Its immobilization, characterization, and application in resolution of racemic ibuprofen. Appl. Biochem. Biotechnol. 2015, 177, 36–47. [Google Scholar] [CrossRef]
- Yildirim, D.; Toprak, A.; Alagöz, D.; Tukel, S.S. Protein-coated microcrystals of Prunus armeniaca hydroxynitrile lyase: An effective and recyclable biocatalyst for synthesis of (R)-mandelonitrile. Chem. Pap. 2019, 73, 185–193. [Google Scholar] [CrossRef]
- Yildirim, D.; Baran, E.; Ates, S.; Yazici, B.; Tukel, S.S. Improvement of activity and stability of Rhizomucor miehei lipase by immobilization on nanoporous aluminium oxide and potassium sulfate microcrystals and their applications in the synthesis of aroma esters. Biocatal. Biotransform. 2019, 37, 210–223. [Google Scholar] [CrossRef]
- König, C.; Bechtold-Peters, K.; Baum, V.; Schultz-Fademrecht, T.; Bassarab, S.; Steffens, K.-J. Development of a pilot-scale manufacturing process for protein-coated microcrystals (PCMC): Mixing and precipitation—Part I. Eur. J. Pharm. Biopharm. 2012, 80, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.; Hidalgo, A.; Bornscheuer, U.T. Immobilization of enzymes in microtiter plate scale. Biotechnol. J. 2006, 1, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Pagani, G.; Ubiali, D.; Fernández-Lafuente, R.; Mateo, C.; Guisán, J.M. Modulation of penicillin acylase properties via immobilization techniques: One-pot chemoenzymatic synthesis of cephamandole from cephalosporin C. Bioorg. Med. Chem. Lett. 2001, 11, 2429–2432. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fuentes, M.; Guisan, J.M.; Fernández-Lafuente, R. Modulation of Mucor miehei lipase properties via directed immobilization on different hetero-functional epoxy resins. J. Mol. Catal. B Enzym. 2003, 21, 201–210. [Google Scholar] [CrossRef]
- Martins, A.B.; Friedrich, J.L.R.; Cavalheiro, J.C.; Garcia-Galan, C.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene–divinylbenzene beads. Bioresour. Technol. 2013, 134, 417–422. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernandez-Lorente, G.; Pedroche, J.; Fernandez-Lafuente, R.; Guisan, J.M.; Tam, A.; Daminati, M. Epoxy sepabeads: A novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernandez-Lafuente, R.; Guisan, J.M. Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb. Technol. 2000, 26, 509–515. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazu, V.; López-Gallego, F.; Pessela, B.C.C.; Hidalgo, A.; Fernández-Lorente, G.; Fernández-Lafuente, R.; et al. Glyoxyl agarose: Afully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Zaak, H.; Peirce, S.; de Albuquerque, T.; Sassi, M.; Fernandez-Lafuente, R. Exploiting the versatility of aminated supports activated with glutaraldehyde to immobilize β-galactosidase from Aspergillus oryzae. Catalysts 2017, 7, 250. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.; Fernandez-Lafuente, R. Immobilization/stabilization of ficin extract on glutaraldehyde-activated agarose beads. Variables that control the final stability and activity in protein hydrolyses. Catalysts 2018, 8, 149. [Google Scholar] [CrossRef]
- De Andrades, D.; Graebin, N.G.; Kadowaki, M.K.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization and stabilization of different β-glucosidases using the glutaraldehyde chemistry: Optimal protocol depends on the enzyme. Int. J. Biol. Macromol. 2019, 129, 672–678. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; del Mar Yust, M.; et al. Characterization of supports activated with divinyl sulfone as a tool to immobilize and stabilize enzymes via multipoint covalent attachment. Application to chymotrypsin. RSC Adv. 2015, 5, 20639–20649. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; del Carmen Millán-Linares, M.; Millán-Linares, M.; Pedroche, J.; del Mar Yuste, M.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Bovine trypsin immobilization on agarose activated with divinylsulfone: Improved activity and stability via multipoint covalent attachment. J. Mol. Catal. B Enzym. 2015, 117, 38–44. [Google Scholar] [CrossRef]
- Bonomi, P.; Bavaro, T.; Serra, I.; Tagliani, A.; Terreni, M.; Ubiali, D. Modulation of the microenvironment surrounding the active site of penicillin G acylase immobilized on acrylic aarriers improves the enzymatic synthesis of cephalosporins. Molecules 2013, 18, 14349–14365. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, T.L.; Rueda, N.; dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Torres, R.; Barbosa, O.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Evaluation of divinylsulfone activated agarose to immobilize lipases and to tune their catalytic properties. Process Biochem. 2015, 50, 918–927. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzyme Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Khosravani, A.; Parker, M.-C.; Parton, R.; Coote, J. Formulation of the adenylate cyclase toxin of Bordetella pertussis as protein-coated microcrystals. Vaccine 2007, 25, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Asokanathan, C.; Kmiec, D.; Irvine, J.; Fleck, R.; Xing, D.; Moore, B.; Parton, R.; Coote, J. Protein coated microcrystals formulated with model antigens and modified with calcium phosphate exhibit enhanced phagocytosis and immunogenicity. Vaccine 2014, 32, 4234–4242. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Gonzalez, E.; Alfadhel, M.; Mane, P.; Ford, S.J.; Moore, B.D.; van der Walle, C.F. Bioprocessing of bacteriophages via rapid drying onto microcrystals. Biotechnol. Prog. 2012, 28, 540–548. [Google Scholar] [CrossRef]
- Wong, S.S.; Wong, L.-J.C. Chemical crosslinking and the stabilization of proteins and enzymes. Enzyme Microb. Technol. 1992, 14, 866–874. [Google Scholar] [CrossRef]
- Govardhan, C.P. Crosslinking of enzymes for improved stability and performance. Curr. Opin. Biotechnol. 1999, 10, 331–335. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Virgen-Ortíz, J.J.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers. Process Biochem. 2017, 54, 81–88. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzyme Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef]
- Gupta, M.N.; Shah, S.; Sharma, A. Preparation of Cross-Linked Protein Coated Microcrystals. Indian Patent IN 2006-DE2046, 18 September 2006. [Google Scholar]
- Shah, S.; Sharma, A.; Gupta, M.N. Cross-linked protein-coated microcrystals as biocatalysts in non-aqueous solvents. Biocatal. Biotransform. 2008, 26, 266–271. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Obtaining monoglycerides by esterification of glycerol with palmitic acid using some high activity preparations of Candida antarctica lipase B. Process Biochem. 2012, 47, 503–508. [Google Scholar] [CrossRef]
- Solanki, K.; Gupta, M.N.; Halling, P.J. Examining structure-activity correlations of some high activity enzyme preparations for low water media. Bioresour. Technol. 2012, 115, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Majumder, A.B.; Mukherjee, J.; Gupta, M.N. Decarboxylative aldol reaction catalysed by lipases and a protease in organic co-solvent mixtures and nearly anhydrous organic solvent media. Biocatal. Biotransform. 2012, 30, 399–408. [Google Scholar] [CrossRef]
- Raita, M.; Laothanachareon, T.; Champreda, V.; Laosiripojana, N. Biocatalytic esterification of palm oil fatty acids for biodiesel production using glycine-based cross-linked protein coated microcrystalline lipase. J. Mol. Catal. B Enzym. 2011, 73, 74–79. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Raita, M.; Laosiripojana, N.; Champreda, V. Biocatalytic methanolysis activities of cross-linked protein-coated microcrystalline lipase toward esterification/transesterification of relevant palm products. Enzyme Microb. Technol. 2015, 70, 28–34. [Google Scholar] [CrossRef]
- Gao, J.; Yin, L.; Feng, K.; Zhou, L.; Ma, L.; He, Y.; Wang, L.; Jiang, Y. Lipase immobilization through the combination of bioimprinting and cross-linked protein-coated microcrystal technology for biodiesel production. Ind. Eng. Chem. Res. 2016, 55, 11037–11043. [Google Scholar] [CrossRef]
- Borgstrom, B.; Erlanson, C. Pancreatic lipase and co-Lipase. Interactions and effects of bile salts and other detergents. Eur. J. Biochem. 1973, 37, 60–68. [Google Scholar] [CrossRef]
- Hermoso, J.; Pignol, D.; Kerfelec, B.; Crenon, I.; Chapus, C.; Fontecilla-Camps, J.C. Lipase activation by nonionic detergents: The crystal structure of the porcine lipase-colipase-tetraethylene glycol monooctyl ether complex. J. Biol. Chem. 1996, 271, 18007–18016. [Google Scholar] [CrossRef]
- Mogensen, J.E.; Sehgal, P.; Otzen, D.E. Activation, inhibition, and destabilization of Thermomyces lanuginosus lipase by detergents. Biochemistry 2005, 44, 1719–1730. [Google Scholar]
- Fernández-Lorente, G.; Palomo, J.M.; Mateo, C.; Munilla, R.; Ortiz, C.; Cabrera, Z.; Guisán, J.M.; Fernandez-Lafuente, R. Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules 2006, 7, 2610–2615. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Cabrera, Z.; Fernandez-Lafuente, R.; Guisán, J.M. Improved catalytic properties of immobilized lipases by the presence of very low concentrations of detergents in the reaction medium. Biotechnol. Bioeng. 2007, 97, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; de Oliveira, D. Nanomaterials for biocatalyst immobilization—State of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Ortiz, C.; Nazzoly, R.; Berenguer-Murcia, A.; Acosta, N.; Aranaz, I.; Civera, C.; Fernandez-Lafuente Fernandez-Lafuente, R.; Alcántara, A.R. Dextran aldehyde in biocatalysis: More than a mere immobilization system. Catalysts 2019, 9, 622. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; Fuentes, M.; Dellamora-Ortiz, G.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Dextran aldehyde coating of glucose oxidase immobilized on magnetic nanoparticles prevents its inactivation by gas bubbles. J. Mol. Catal. B Enzym. 2005, 32, 97–101. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Fuentes, M.; Fernández-Lafuente, R.; Guisán, J.M. Prevention of interfacial inactivation of enzymes by coating the enzyme surface with dextran-aldehyde. J. Biotechnol. 2004, 110, 201–207. [Google Scholar] [CrossRef]
- Solanki, K.; Gupta, M.N. A chemically modified lipase preparation for catalyzing the transesterification reaction in even highly polar organic solvents. Bioorg. Med. Chem. Lett. 2011, 21, 2934–2936. [Google Scholar] [CrossRef]
- Wheeldon, I.; Minteer, S.D.; Banta, S.; Barton, S.C.; Atanassov, P.; Sigman, M. Substrate channelling as an approach to cascade reactions. Nat. Chem. 2016, 8, 299–309. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial biocatalytic linear cascades for preparation of organic molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef] [PubMed]
- France, S.P.; Hepworth, L.J.; Turner, N.J.; Flitsch, S.L. Constructing biocatalytic cascades: In vitro and in vivo approaches to de novo multi-enzyme pathways. ACS Catal. 2017, 7, 710–724. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcantara, A.R.; Rodrigues, R.C.; Fernández-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. in press. [CrossRef]
- Mukherjee, J.; Gupta, M.N. Protein-coated microcrystals, combi-protein-coated microcrystals, and cross-linked protein-coated microcrystals of enzymes for use in low-water media. In Enzyme Stabilization and Immobilization; Minteer, S.D., Ed.; Springer: New York, NY, USA, 2017; Volume 1504, pp. 125–137. ISBN 978-1-4939-6497-0. [Google Scholar]
- Arana-Peña, S.; Carballares, D.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. One pot use of combilipases for full modification of oils and fats: Multifunctional and heterogeneous substrates. Catalysts 2020, 10, 605. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Tailoring multipurpose biocatalysts via protein engineering approaches: A review. Catal. Lett. 2019, 149, 2204–2217. [Google Scholar] [CrossRef]
- Suganthi, S.H.; Swathi, K.V.; Biswas, R.; Basker, S.; Ramani, K. Co-immobilization of multiple enzymes onto surface-functionalized magnetic nanoparticle for the simultaneous hydrolysis of multiple substrates containing industrial wastes. Appl. Nanosci. 2019, 9, 1439–1457. [Google Scholar] [CrossRef]
- Liang, H.; Jiang, S.; Yuan, Q.; Li, G.; Wang, F.; Zhang, Z.; Liu, J. Co-immobilization of multiple enzymes by metal coordinated nucleotide hydrogel nanofibers: Improved stability and an enzyme cascade for glucose detection. Nanoscale 2016, 8, 6071–6078. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Abián, O.; Pessela, B.C.C.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of penicillin G acylase and polyionic polymers: An easy methodology to prepare enzyme biocatalysts stable in organic media. Biomacromolecules 2004, 5, 852–857. [Google Scholar] [CrossRef]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzyme Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Hidalgo, A.; Alonso, N.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of enzymes and polyethyleneimine: A simple method to prepare stable and immobilized derivatives of glutaryl acylase. Biomacromolecules 2005, 6, 1839–1842. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Rocha-Martin, J.; Mateo, C.; Cava, F.; Berenguer, J.; Fernandez-Lafuente, R.; Guisan, J.M. Coating of soluble and immobilized enzymes with ionic polymers: Full stabilization of the quaternary structure of multimeric enzymes. Biomacromolecules 2009, 10, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Barbosa, O.; Fernandez-Lafuente, R. Stabilization of the hexameric glutamate dehydrogenase from Escherichia coli by cations and polyethyleneimine. Enzyme Microb. Technol. 2013, 52, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Tacias-Pascacio, V.; Russo, M.; Marzocchella, A.; Virgen-Ortíz, J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica lipase B (CALB) immobilized on octyl agarose by treatment with polyethyleneimine (PEI). Molecules 2016, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Illanes, A.; Pessela, B.C.C.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Encapsulation of crosslinked penicillin G acylase aggregates in lentikats: Evaluation of a novel biocatalyst in organic media: Evaluation of a novel biocatalyst in organic media. Biotechnol. Bioeng. 2004, 86, 558–562. [Google Scholar] [CrossRef]
- Nawawi, N.N.; Hashim, Z.; Rahman, R.A.; Murad, A.M.A.; Bakar, F.D.A.; Illias, R.M. Entrapment of porous cross-linked enzyme aggregates of maltogenic amylase from Bacillus lehensis G1 into calcium alginate for maltooligosaccharides synthesis. Int. J. Biol. Macromol. 2020, 150, 80–89. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; de María, P.D. Immobilized lipase-CLEA aggregates encapsulated in lentikats® as robust biocatalysts for continuous processes in deep eutectic solvents. J. Biotechnol. 2020, 310, 97–102. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, R.R.C.; dos Santos, J.C.S.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts 2020, 10, 891. https://doi.org/10.3390/catal10080891
Monteiro RRC, dos Santos JCS, Alcántara AR, Fernandez-Lafuente R. Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts. 2020; 10(8):891. https://doi.org/10.3390/catal10080891
Chicago/Turabian StyleMonteiro, Rodolpho R. C., José C. S. dos Santos, Andrés R. Alcántara, and Roberto Fernandez-Lafuente. 2020. "Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy" Catalysts 10, no. 8: 891. https://doi.org/10.3390/catal10080891
APA StyleMonteiro, R. R. C., dos Santos, J. C. S., Alcántara, A. R., & Fernandez-Lafuente, R. (2020). Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts, 10(8), 891. https://doi.org/10.3390/catal10080891