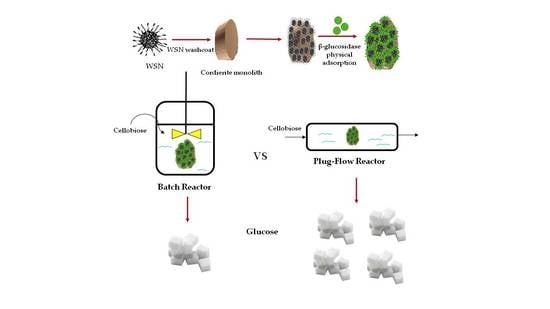
Immobilization of β-Glucosidase over Structured Cordierite Monoliths Washcoated with Wrinkled Silica Nanoparticles
Abstract
:1. Introduction
2. Results
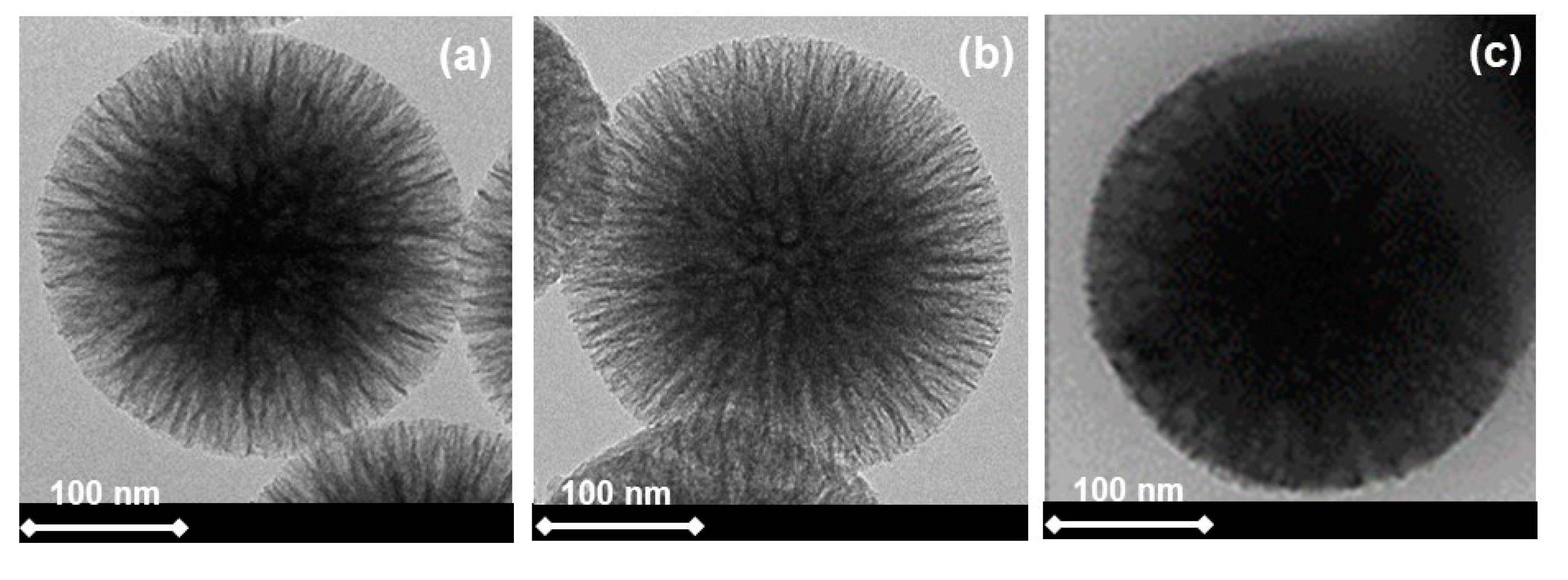
2.1. Textural and Morphological Characterizations
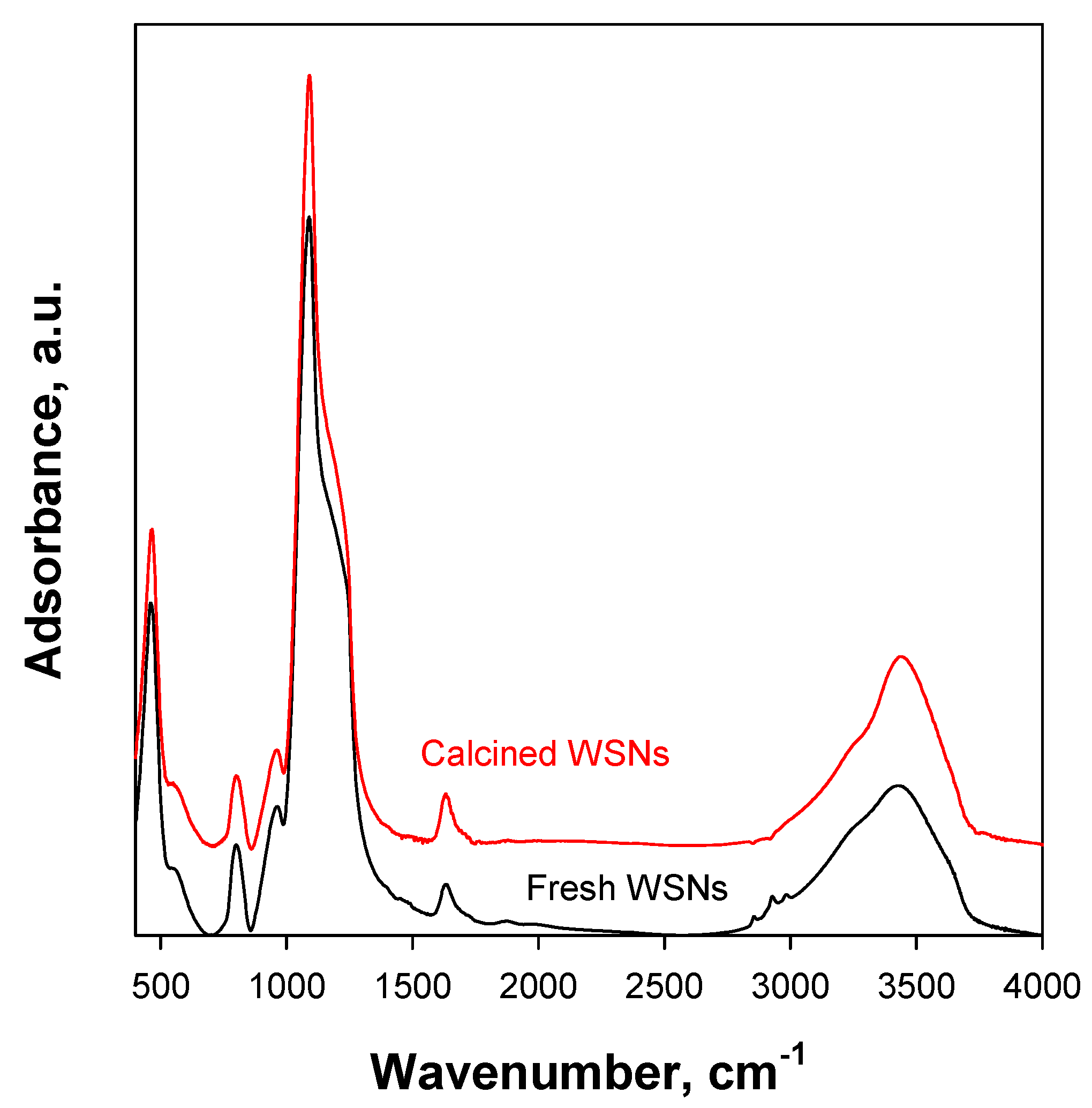
2.2. FTIR and TG Analysis
2.3. Scanning Electron Microscopy of WSN Monolith
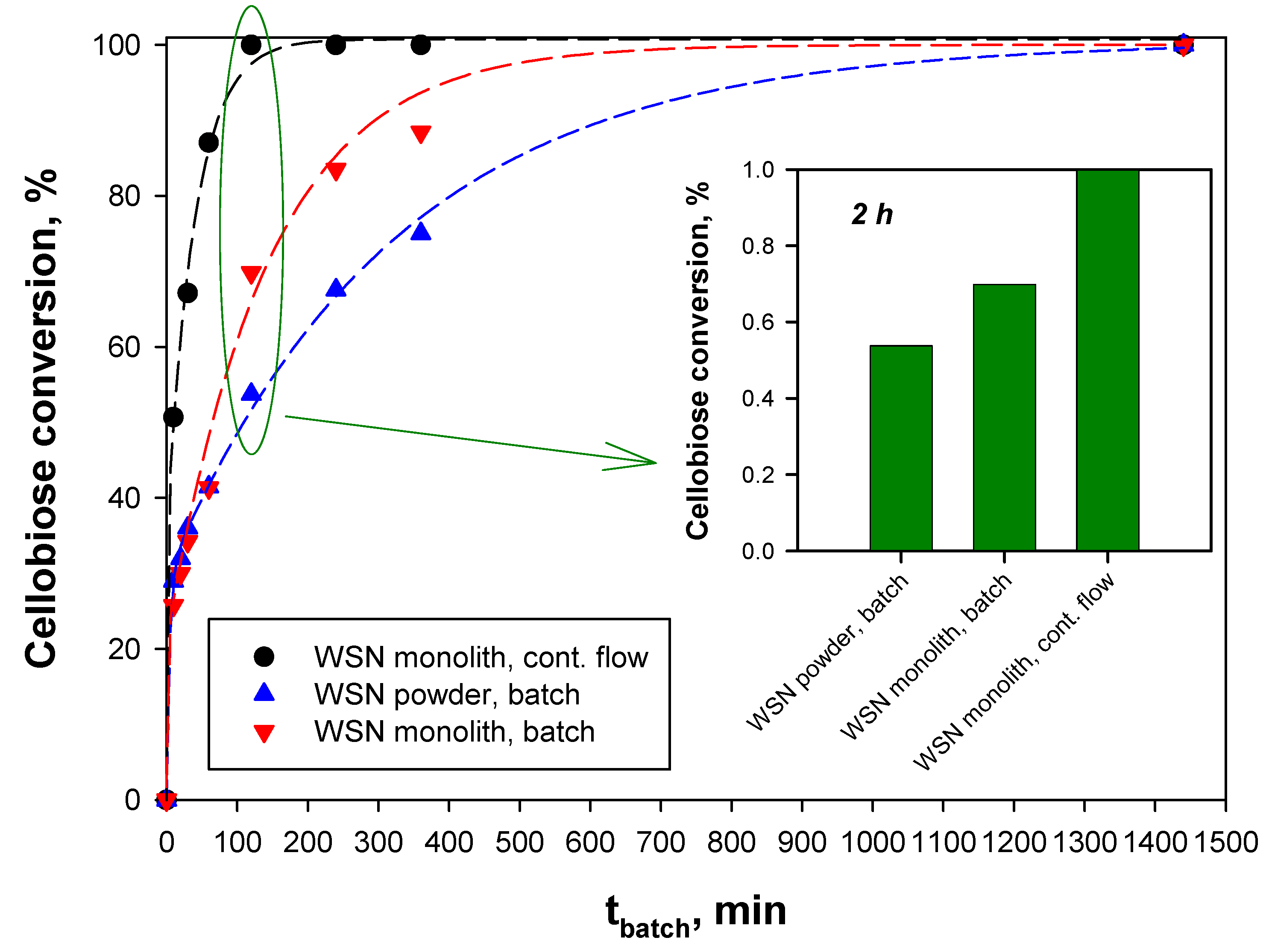
2.4. Reaction Tests
3. Materials and Methods
3.1. WSNs Synthesis
3.2. Monolith Preparation
3.3. Immobilization Procedure
3.4. Materials Characterizations
3.5. Batch Reactor Setup
3.6. Continuous Flow Reactor Setup
3.7. Glucose Analysis, Determination of Kinetic Constants and of Mass Transfer Limitations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Javed, M.R.; Buthe, A.; Rashid, M.H.; Wang, P. Cost-efficient entrapment of β-glucosidase in nanoscale latex and silicone polymeric thin films for use as stable biocatalysts. Food Chem. 2016, 190, 1078–1085. [Google Scholar] [CrossRef]
- Miyazaki, M.; Honda, T.; Yamaguchi, H.; Briones, M.P.P.; Maeda, H. Enzymatic processing in microfluidic reactors. Biotechnol. Genet. Eng. Rev. 2008, 25, 405–428. [Google Scholar] [CrossRef]
- Seo, T.Y.; Eum, K.W.; Han, S.O.; Kim, S.W.; Kim, J.H.; Song, K.H.; Choe, J. Immobilized cell microchannel bioreactor for evaluating fermentation characteristics of mixed substrate consumption and product formation. Process Biochem. 2012, 47, 1011–1015. [Google Scholar] [CrossRef]
- Lévesque, F.; Seeberger, P.H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem-Int. Ed. 2012, 51, 1706–1709. [Google Scholar] [CrossRef]
- Song, J.; Imanaka, H.; Imamura, K.; Kajitani, K.; Nakanishi, K. Development of a highly efficient indigo dyeing method using indican with an immobilized β-glucosidase from Aspergillus niger. J. Biosci. Bioeng. 2010, 110, 281–287. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Nidetzky, B. Coated-wall microreactor for continuous biocatalytic transformations using immobilized enzymes. Biotechnol. J. 2009, 4, 98–107. [Google Scholar] [CrossRef]
- Barsan, M.M.; David, M.; Florescu, M.; Ţugulea, L.; Brett, C.M.A. A new self-assembled layer-by-layer glucose biosensor based on chitosan biopolymer entrapped enzyme with nitrogen doped graphene. Bioelectrochemistry 2014, 99, 46–52. [Google Scholar] [CrossRef]
- Kecskemeti, A.; Gaspar, A. Preparation and characterization of a packed bead immobilized trypsin reactor integrated into a PDMS microfluidic chip for rapid protein digestion. Talanta 2017, 166, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl) triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Malecha, K.; Remiszewska, E.; Pijanowska, D.G. Surface modification of low and high temperature co-fired ceramics for enzymatic microreactor fabrication. Sens. Actuators B Chem. 2014, 190, 873–880. [Google Scholar] [CrossRef]
- Křenková, J.; Foret, F. Immobilized microfluidic enzymatic reactors. Electrophoresis 2004, 25, 3550–3563. [Google Scholar] [CrossRef]
- Schwarz, A.; Thomsen, M.S.; Nidetzky, B. Enzymatic synthesis of β-glucosylglycerol using a continuous-flow microreactor containing thermostable β-glycoside hydrolase CelB immobilized on coated microchannel walls. Biotechnol. Bioeng. 2009, 103, 865–872. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, Y.; Zhuang, W.; Li, G.; Jiang, M.; Zhang, H. Improving the performance of immobilized β-glucosidase using a microreactor. J. Biosci. Bioeng. 2018, 125, 377–384. [Google Scholar] [CrossRef]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the Eumelanin-Silica Interface in Chemically Engineered Bulk Hybrid Nanoparticles for Targeted Subcellular Antioxidant Protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol-Gel Sci. Technol. 2014, 73, 54–61. [Google Scholar] [CrossRef]
- Díaz, J.F.; Balkus, K.J. Enzyme immobilization in MCM-41 molecular sieve. J. Mol. Catal. B Enzym. 1996, 2, 115–126. [Google Scholar] [CrossRef]
- Hartono, S.B.; Qiao, S.Z.; Liu, J.; Jack, K.; Ladewig, B.P.; Hao, Z.; Lu, G.Q.M. Functionalized mesoporous silica with very large pores for cellulase immobilization. J. Phys. Chem. C 2010, 114, 8353–8362. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent immobilization of β-glucosidase into mesoporous silica nanoparticles from anhydrous acetone enhances its catalytic performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Califano, V.; Costantini, A.; Silvestri, B.; Venezia, V.; Cimino, S.; Sannino, F. The effect of pore morphology on the catalytic performance of β-glucosidase immobilized into mesoporous silica. Pure Appl. Chem. 2019, 91, 1583–1592. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled silica nanoparticles: Efficient matrix for β-glucosidase immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. Structuring CuO/CeO2 catalyst as option to improve performance towards CO-PROX. Top. Catal. 2016, 59, 1371–1382. [Google Scholar] [CrossRef]
- Zhang, Q.; Shore, L.; Farrauto, R.J. Selective CO oxidation over a commercial PROX monolith catalyst for hydrogen fuel cell applications. Int. J. Hydrogen Energy 2012, 37, 10874–10880. [Google Scholar] [CrossRef]
- Lisi, L.; Pirone, R.; Russo, G.; Stanzione, V. Cu-ZSM5 based monolith reactors for no decomposition. Chem. Eng. J. 2009, 154, 341–347. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Li, K.; Yu, X.; Wei, Y.; Liu, J.; Peng, Y.; Li, Y.; Chen, Y. Highly active monolith catalysts of LaKCoO3 perovskite-type complex oxide on alumina-washcoated diesel particulate filter and the catalytic performances for the combustion of soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- Gómez, L.E.; Sollier, B.M.; Lacoste, A.M.; Miró, E.E.; Boix, A.V. Hydrogen purification for fuel cells through CO preferential oxidation using PtCu/Al2O3 structured catalysts. J. Environ. Chem. Eng. 2019, 7, 103376. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Ashraf, M.A.; Pino, L.; Specchia, S. Syngas production by steam and oxy-steam reforming of biogas on monolith-supported CeO2-based catalysts. Int. J. Hydrogen Energy 2018, 43, 11731–11744. [Google Scholar] [CrossRef]
- Landi, G.; Lisi, L.; Pirone, R.; Tortorelli, M.; Russo, G. NO decomposition over La-doped Cu-ZSM5 monolith under adsorption-reaction conditions. Appl. Catal. A Gen. 2013, 464–465, 61–67. [Google Scholar] [CrossRef]
- Du, X.; He, J. Hierarchically mesoporous silica nanoparticles: Extraction, amino-functionalization, and their multipurpose potentials. Langmuir 2011, 27, 2972–2979. [Google Scholar] [CrossRef]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302, 110203. [Google Scholar] [CrossRef]
- Bloisi, F.; Califano, V.; Perretta, G.; Nasti, L.; Aronne, A.; Di Girolamo, R.; Auriemma, F.; De Rosa, C.; Vicari, L.R.M. Lipase immobilization for catalytic applications obtained using fumed silica deposited with MAPLE technique. Appl. Surf. Sci. 2016, 374, 346–352. [Google Scholar] [CrossRef]
- Pan, D.; Yuan, P.; Zhao, L.; Liu, N.; Zhou, L.; Wei, G.; Zhang, J.; Ling, Y.; Fan, Y.; Wei, B.; et al. New understanding and simple approach to synthesize highly hydrothermally stable and ordered mesoporous materials. Chem. Mater. 2009, 21, 5412–5425. [Google Scholar] [CrossRef]
- Cimino, S.; Landi, G.; Lisi, L.; Russo, G. Development of a dual functional structured catalyst for partial oxidation of methane to syngas. Catal. Today 2005, 105, 718–723. [Google Scholar] [CrossRef]
- Illanes, A. Enzyme Biocatalysis: Principles and Applications; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Liu, X.; Meng, X.Y.; Xu, Y.; Dong, T.; Zhang, D.Y.; Guan, H.X.; Zhuang, Y.; Wang, J. Enzymatic synthesis of 1-caffeoylglycerol with deep eutectic solvent under continuous microflow conditions. Biochem. Eng. J. 2019, 142, 41–49. [Google Scholar] [CrossRef]
- Muderrisoglu, C.; Sargin, S.; Yesil-Celiktas, O. Application of β-glucuronidase-immobilised silica gel formulation to microfluidic platform for biotransformation of β-glucuronides. Biotechnol. Lett. 2018, 40, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.S.; Lee, J.K. Tunable synthesis of hierarchical mesoporous silica nanoparticles with radial wrinkle structure. Langmuir 2012, 28, 12341–12347. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.A. Methods of Enzymatic Analysis; Elsevier: Berlin, Germany, 1974. [Google Scholar]

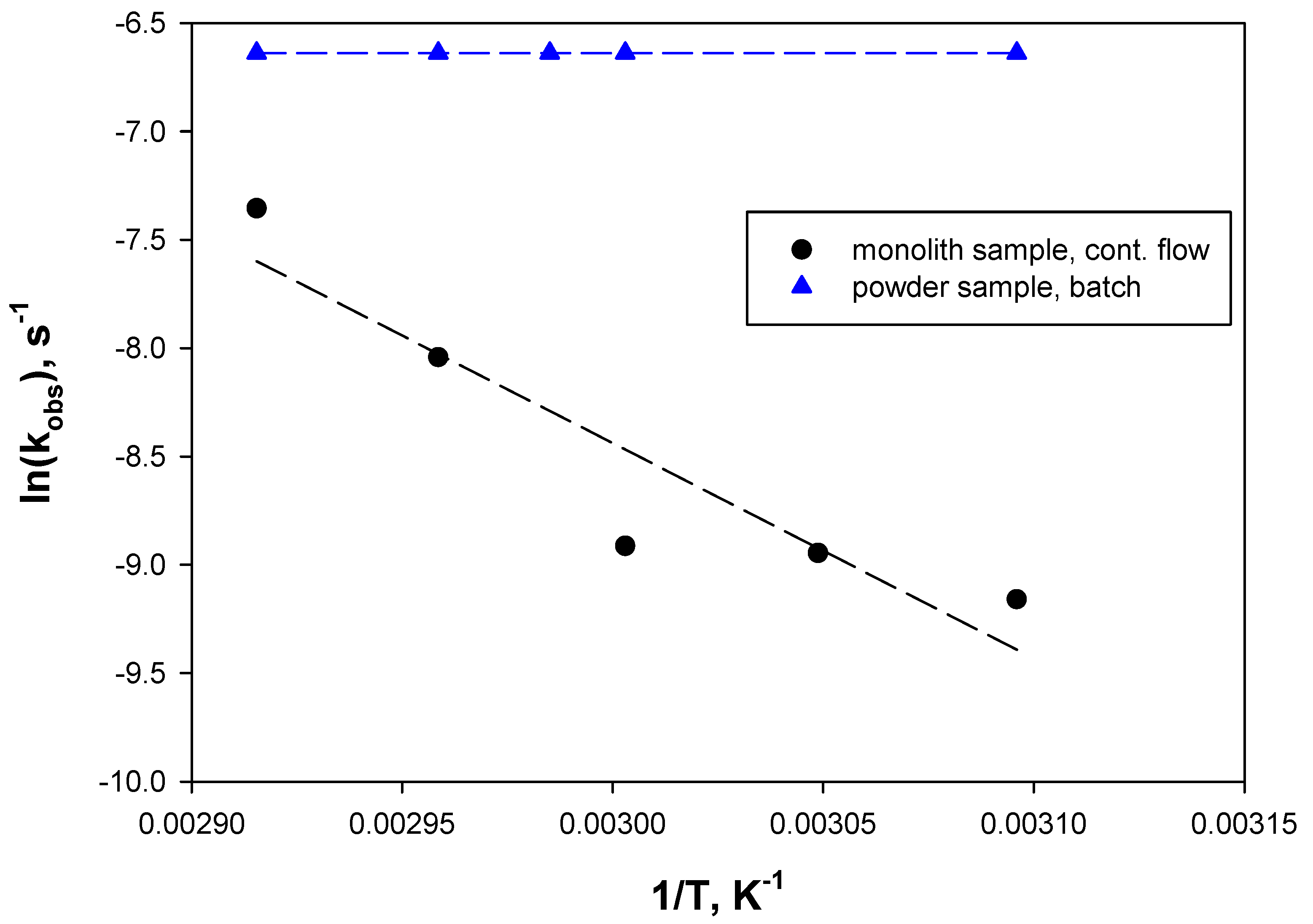
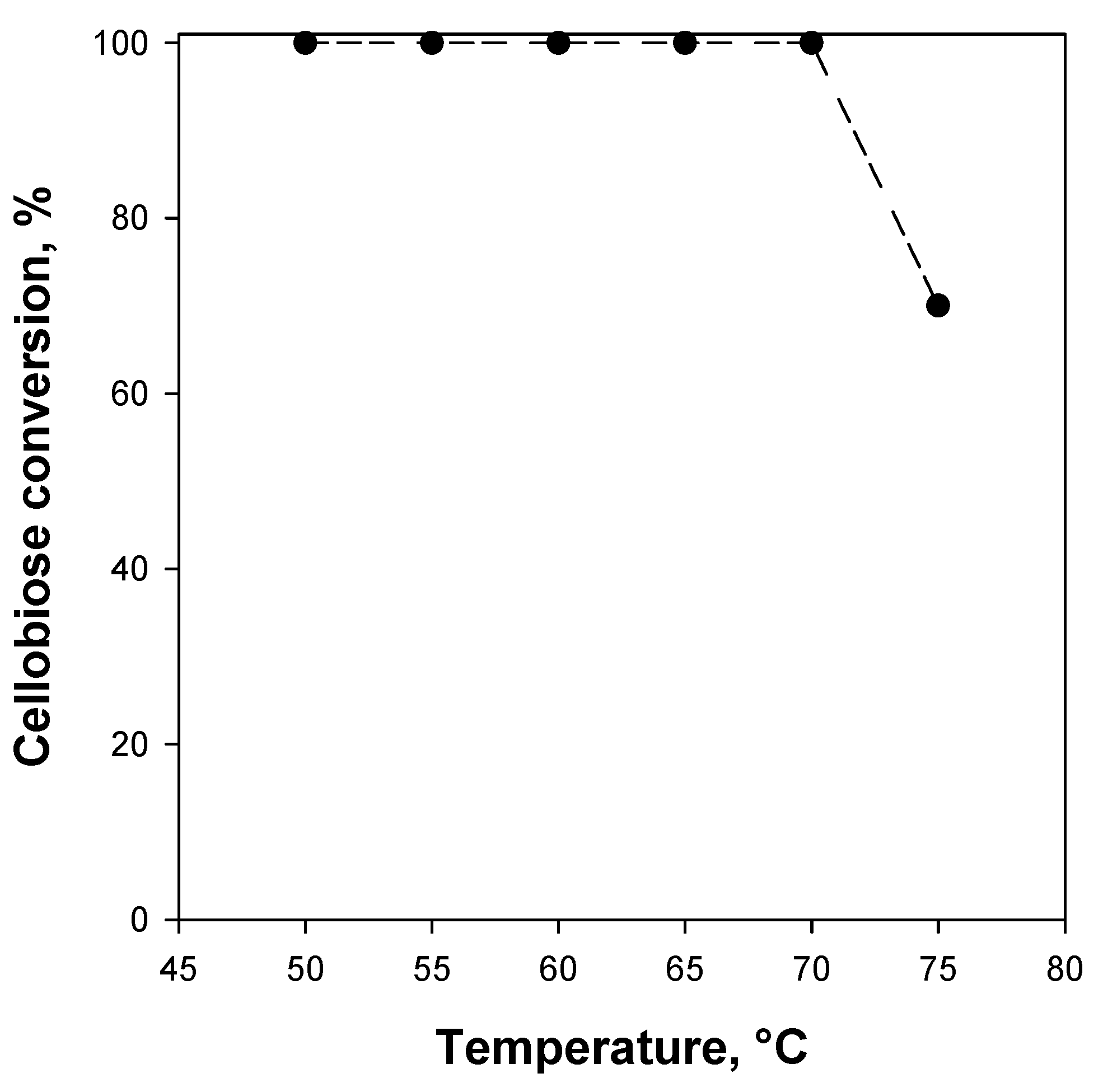
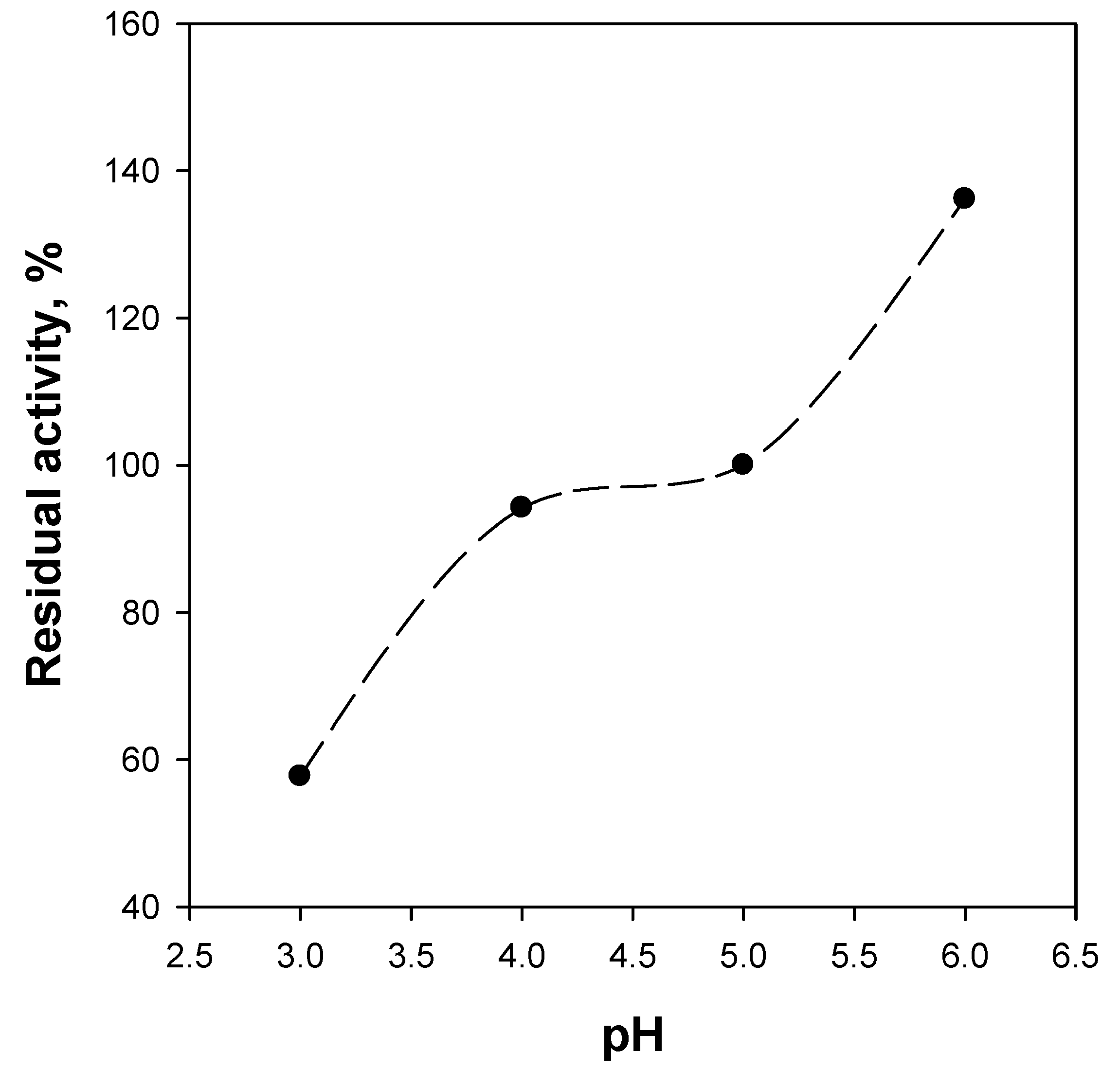
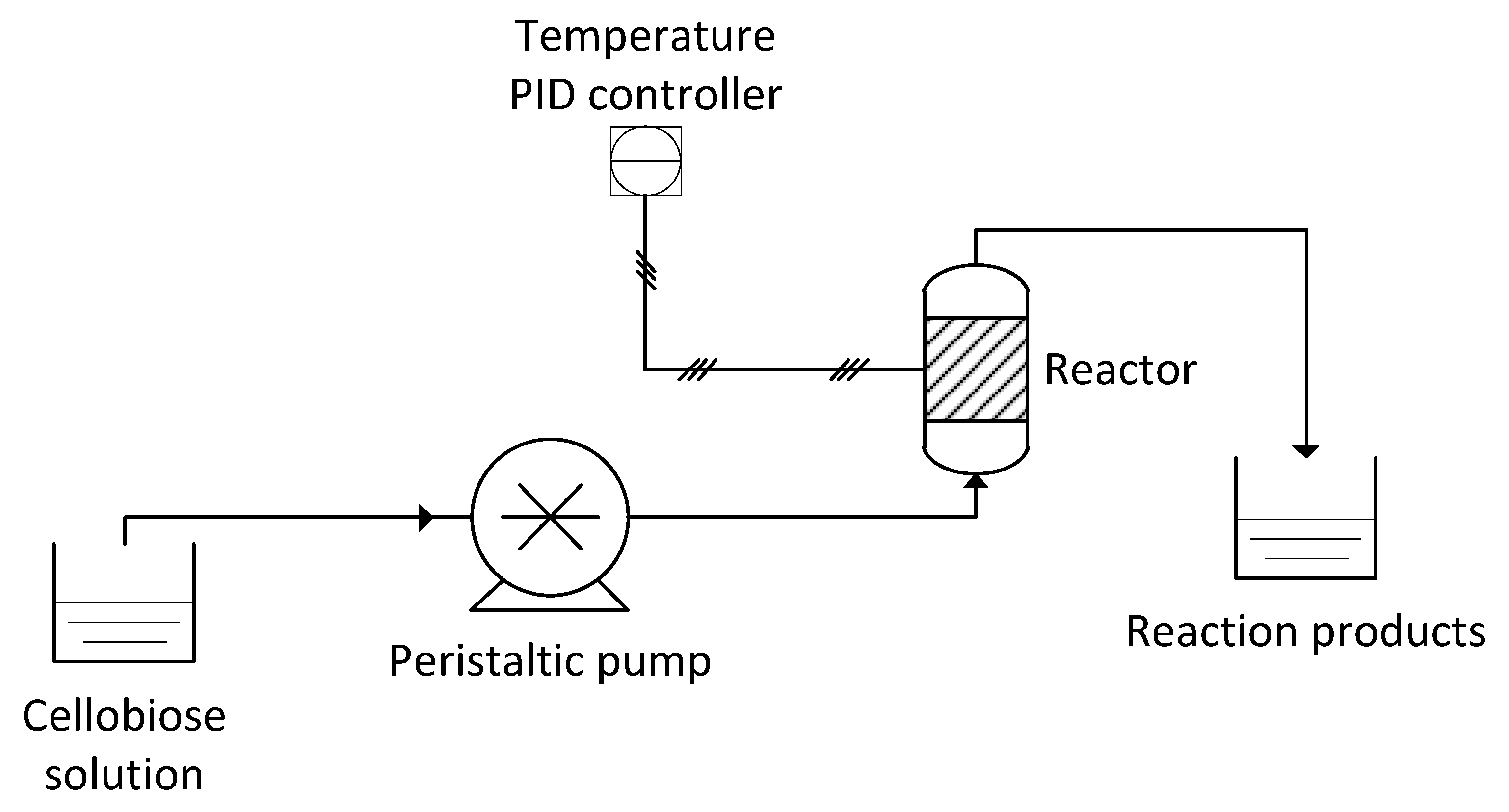
| Kinetic Constants | Free BG [20] | BG/WSNs Powder | BG/WSNs/Monolith Batch | BG/WSNs/Monolith Continuous |
|---|---|---|---|---|
| KM (mM) | 5.4 | 4.3 | 4.2 | 0.23 |
| Vmax (μmol/min∗mg) | 43 | 41 | 42 | 41 |
| - | ΔHi, kJ/mol | νi | cc, mol/m3 | cp, kJ/(kg·K) | ρ, kg/m3 |
|---|---|---|---|---|---|
| Cellobiose | –5401.50 | –1 | 4.67 | - | - |
| Water | –285.85 | –1 | - | - | - |
| Glucose | –1273.30 | 2 | - | - | - |
| Mixture | - | - | - | 4.186 | 1000 |
| Parameter | Units | Value |
|---|---|---|
| Fc | mol m–3 s–1 | |
| k, kobs | s–1 | |
| km | m s–1 | |
| a | m−1 | |
| l, dWSN | m | 0.001, 20 × 10–9 |
| Cb, Cs | mol m–3 | |
| r | mol m–3 s–1 | |
| τ, t | s | |
| x | ||
| d | m | 0.001 |
| D | m2 s–1 | 4.5 × 10–10 |
| ρ | kg m–3 | 1000 |
| u | m s–1 | |
| μ | kg m–1 s–1 | 5.47 × 10–4 |
| δ | m | 4.00 × 10–6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venezia, V.; Costantini, A.; Landi, G.; Di Benedetto, A.; Sannino, F.; Califano, V. Immobilization of β-Glucosidase over Structured Cordierite Monoliths Washcoated with Wrinkled Silica Nanoparticles. Catalysts 2020, 10, 889. https://doi.org/10.3390/catal10080889
Venezia V, Costantini A, Landi G, Di Benedetto A, Sannino F, Califano V. Immobilization of β-Glucosidase over Structured Cordierite Monoliths Washcoated with Wrinkled Silica Nanoparticles. Catalysts. 2020; 10(8):889. https://doi.org/10.3390/catal10080889
Chicago/Turabian StyleVenezia, Virginia, Aniello Costantini, Gianluca Landi, Almerinda Di Benedetto, Filomena Sannino, and Valeria Califano. 2020. "Immobilization of β-Glucosidase over Structured Cordierite Monoliths Washcoated with Wrinkled Silica Nanoparticles" Catalysts 10, no. 8: 889. https://doi.org/10.3390/catal10080889
APA StyleVenezia, V., Costantini, A., Landi, G., Di Benedetto, A., Sannino, F., & Califano, V. (2020). Immobilization of β-Glucosidase over Structured Cordierite Monoliths Washcoated with Wrinkled Silica Nanoparticles. Catalysts, 10(8), 889. https://doi.org/10.3390/catal10080889