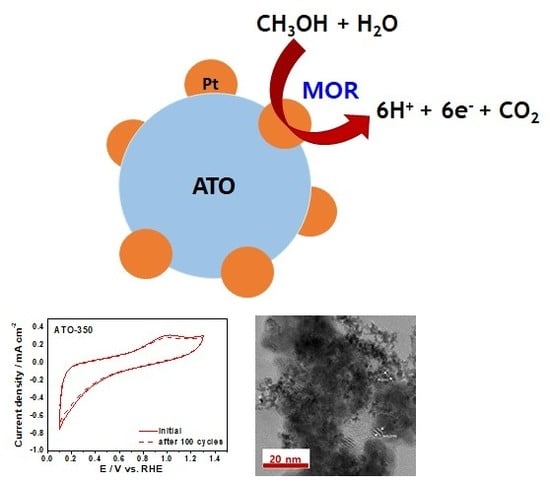
Effect of Sb-Doped SnO2 Nanostructures on Electrocatalytic Performance of a Pt Catalyst for Methanol Oxidation Reaction
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of ATO Supported Pt Catalysts
3.2. Structural Analysis of ATO Supported Pt Catalysts
3.3. Electrochemical Analysis of ATO Supported Pt Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, Y.Y.; Kong, W.Q.; Yin, Q.Y.; Du, L.X.; Zheng, Y.T.; Kong, D.S. Platinum catalysts promoted by in doped SnO2 support for methanol electrooxidation in alkaline electrolyte. J. Power Sources 2014, 252, 156–163. [Google Scholar] [CrossRef]
- Amani, M.; Kazemeini, M.; Hamedanian, M.; Pahlavanzadeh, H.; Gharibi, H. Investigation of methanol oxidation on a highly active and stable Pt-Sn electrocatalyst supported on carbon-polyaniline composite for application in a passive direct methanol fuel cell. Mater. Res. Bull. 2015, 68, 166–178. [Google Scholar] [CrossRef]
- Kwak, D.H.; Lee, Y.W.; Lee, K.H.; Park, A.R.; Moon, J.S.; Park, K.W. One-step synthesis of hexapod pt nanoparticles deposited on carbon black for improved methanol electrooxidation. Int. J. Electrochem. Sci. 2013, 8, 5102–5107. [Google Scholar]
- You, D.J.; Kwon, K.; Pak, C.; Chang, H. Platinum-antimony tin oxide nanoparticle as cathode catalyst for direct methanol fuel cell. Catal. Today 2009, 146, 15–19. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Y.; Shan, Y.; Huang, Z. One-Pot and Facile Fabrication of Hierarchical Branched Pt-Cu Nanoparticles as Excellent Electrocatalysts for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 5998–6003. [Google Scholar] [CrossRef]
- Chang, J.; Feng, L.; Jiang, K.; Xue, H.; Cai, W.B.; Liu, C.; Xing, W. Pt-CoP/C as an alternative PtRu/C catalyst for direct methanol fuel cells. J. Mater. Chem. A 2016, 4, 18607–18613. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiao, J.; Hu, J.; Song, F.; Huo, D.; Yuan, J.; Shen, J.; Niu, L.; Wang, A.-j. PtIr alloy nanowire assembly on carbon cloth as advanced anode catalysts for methanol oxidation. Int. J. Hydrogen Energy 2019, 44, 20336–20344. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, Q.; Qu, K.; Guo, L.; Hu, H.; Yang, Z.; Cai, W.; Cheng, H. Decorated PtRu Electrocatalyst for Concentrated Direct Methanol Fuel Cells. ChemCatChem 2019, 11, 1238–1243. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Jiang, L.; Wu, Z.; Liu, D.J.; Zhuang, L.; Ma, C.; et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Long, Z.; Gong, L.; Sun, Y.; Li, Y.; Xu, P.; Zhang, X.; Ge, J.; Liu, C.; Ma, S.; Jin, Z. In-situ precise electrocatalytic behaviors of Pt/C and PtRu/C for methanol oxidation of DMFCs via the designed micro-MEA. Int. J. Hydrogen Energy 2018, 43, 12413–12419. [Google Scholar] [CrossRef]
- Kang, Y.S.; Jo, S.; Choi, D.; Kim, J.Y.; Park, T.; Yoo, S.J. Pt-Sputtered Ti Mesh Electrode for Polymer Electrolyte Membrane Fuel Cells. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 271–279. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Qi, J.; Jiang, L.; Jing, M.; Tang, Q.; Sun, G. Preparation of Pt/C via a polyol process - Investigation on carbon support adding sequence. Int. J. Hydrogen Energy 2011, 36, 10490–10501. [Google Scholar] [CrossRef]
- Elezovic, N.R.; Radmilovic, V.R.; Krstajic, N.V. Platinum nanocatalysts on metal oxide based supports for low temperature fuel cell applications. RSC Adv. 2016, 6, 6788–6801. [Google Scholar] [CrossRef]
- Wang, J.; Yin, G.; Shao, Y.; Zhang, S.; Wang, Z.; Gao, Y. Effect of carbon black support corrosion on the durability of Pt/C catalyst. J. Power Sources 2007, 171, 331–339. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Hamelin, J. Electrochemical Durability of Carbon Nanostructures as Catalyst Support for PEMFCs. J. Electrochem. Soc. 2009, 156, B210. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, S.B.; Lee, Y.W.; Kim, D.Y.; Han, S.B.; Roh, B.; Hwang, I.; Park, K.W. Core-shell nanostructure electrodes for improved electrocatalytic properties in methanol electrooxidation. Appl. Catal. B Environ. 2012, 111–112, 200–207. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Glökler, C.; Arenas, A.O.; Jörissen, L. Sb doped SnO2 as a stable cathode catalyst support for low temperature polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2017, 42, 27950–27961. [Google Scholar] [CrossRef]
- Oh, H.S.; Oh, J.G.; Haam, S.; Arunabha, K.; Roh, B.; Hwang, I.; Kim, H. On-line mass spectrometry study of carbon corrosion in polymer electrolyte membrane fuel cells. Electrochem. Commun. 2008, 10, 1048–1051. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Guo, L.; Liu, H. Effects of carbon corrosion on mass transfer losses in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 4699–4705. [Google Scholar] [CrossRef]
- Maass, S.; Finsterwalder, F.; Frank, G.; Hartmann, R.; Merten, C. Carbon support oxidation in PEM fuel cell cathodes. J. Power Sources 2008, 176, 444–451. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Sui, X.; Gu, D. An efficient antimony doped tin oxide and carbon nanotubes hybrid support of Pd catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2014, 39, 5678–5688. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, I.S.; Cho, Y.H.; Jung, D.S.; Jung, N.; Park, H.Y.; Sung, Y.E. Electrocatalytic activity and stability of Pt supported on Sb-doped SnO2 nanoparticles for direct alcohol fuel cells. J. Catal. 2008, 258, 143–152. [Google Scholar] [CrossRef]
- Fang, L.; Luo, X.; Saipanya, S.; Huang, X.; Li, F. Preparation of Pt/ATO catalysts for electrocatalysis in direct alcohol fuel cell. Chiang Mai J. Sci. 2016, 43, 169–175. [Google Scholar]
- Guo, D.J. Electrooxidation of ethanol on novel multi-walled carbon nanotube supported platinum-antimony tin oxide nanoparticle catalysts. J. Power Sources 2011, 196, 679–682. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Mao, Z.; Wang, N. Preparation of nanometer-sized SnO2 by the fusion method. Mater. Lett. 2007, 61, 1205–1209. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L. Synthesis and characterization of antimony-doped tin oxide (ATO) nanoparticles. Inorg. Chem. Commun. 2004, 7, 91–93. [Google Scholar] [CrossRef]
- Yin, M.; Xu, J.; Li, Q.; Jensen, J.O.; Huang, Y.; Cleemann, L.N.; Bjerrum, N.J.; Xing, W. Highly active and stable Pt electrocatalysts promoted by antimony-doped SnO2 supports for oxygen reduction reactions. Appl. Catal. B Environ. 2014, 144, 112–120. [Google Scholar] [CrossRef]
- Cavaliere, S.; Jiménez-Morales, I.; Ercolano, G.; Savych, I.; Jones, D.; Rozière, J. Highly Stable PEMFC Electrodes Based on Electrospun Antimony-Doped SnO2. ChemElectroChem 2015, 2, 1966–1973. [Google Scholar] [CrossRef]
- Xu, J.; Aili, D.; Li, Q.; Pan, C.; Christensen, E.; Jensen, J.O.; Zhang, W.; Liu, G.; Wang, X.; Bjerrum, N.J. Antimony doped tin oxide modified carbon nanotubes as catalyst supports for methanol oxidation and oxygen reduction reactions. J. Mater. Chem. A 2013, 1, 9737–9745. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Pan, C.; Li, Y.; Ma, Y.; Zhao, X.; Zhang, Q. Platinum—Antimony doped tin oxide nanoparticles supported on carbon black as anode catalysts for direct methanol fuel cells. J. Power Sources 2011, 196, 6228–6231. [Google Scholar] [CrossRef]
- López Morales, F.; Zayas, T.; Contreras, O.E.; Salgado, L. Effect of Sn precursor on the synthesis of SnO2 and Sb-doped SnO2 particles via polymeric precursor method. Front. Mater. Sci. 2013, 7, 387–395. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, B.; Zhang, X.; Jia, J.; Yi, G. Effect of calcining temperature and time on the characteristics of Sb-doped SnO 2 nanoparticles synthesized by the sol-gel method. Particuology 2012, 10, 365–370. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Cavaliere, S.; Dupont, M.; Jones, D.; Rozière, J. On the stability of antimony doped tin oxide supports in proton exchange membrane fuel cell and water electrolysers. Sustain. Energy Fuels 2019, 3, 1526–1535. [Google Scholar] [CrossRef]
- Sladkevich, S.; Mikhaylov, A.A.; Prikhodchenko, P.V.; Tripol’skaya, T.A.; Lev, O. Antimony tin oxide (ATO) nanoparticle formation from H2O2 solutions: A new generic film coating from basic solutions. Inorg. Chem. 2010, 49, 9110–9112. [Google Scholar] [CrossRef]
- Mao, W.; Xiong, B.; Li, Q.; Zhou, Y.; Yin, C.; Liu, Y.; He, C. Influences of defects and Sb valence states on the temperature dependent conductivity of Sb doped SnO2 thin films. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2015, 379, 1946–1950. [Google Scholar] [CrossRef]
- Liu, S.M.; Ding, W.Y.; Chai, W.P. Influence of Sb doping on crystal structure and electrical property of SnO2 nanoparticles prepared by chemical coprecipitation. Phys. B Condens. Matter 2011, 406, 2303–2307. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, H.; Liang, J.; Peng, F.; Yu, H.; Yang, J. Effects of RuO2 content in Pt/RuO2/CNTs nanocatalyst on the electrocatalytic oxidation performance of methanol. Cuihua Xuebao / Chinese J. Catal. 2008, 29, 1093–1098. [Google Scholar] [CrossRef]
- Matin, M.A.; Lee, E.; Kim, H.; Yoon, W.S.; Kwon, Y.U. Rational syntheses of core-shell Fe@(PtRu) nanoparticle electrocatalysts for the methanol oxidation reaction with complete suppression of CO-poisoning and highly enhanced activity. J. Mater. Chem. A 2015, 3, 17154–17164. [Google Scholar] [CrossRef]
- Timperman, L.; Alonso-Vante, N. Oxide Substrate Effect Toward Electrocatalytic Enhancement of Platinum and Ruthenium-Selenium Catalysts. Electrocatalysis 2011, 2, 181–191. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Cavaliere, S.; Jones, D.; Rozière, J. Strong metal-support interaction improves activity and stability of Pt electrocatalysts on doped metal oxides. Phys. Chem. Chem. Phys. 2018, 20, 8765–8772. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.; Kasian, O.; Mingers, A.M.; Mayrhofer, K.J.J.; Cherevko, S. Stability limits of tin-based electrocatalyst supports. Sci. Rep. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Dubau, L.; Maillard, F.; Chatenet, M.; Cavaliere, S.; Jiménez-Morales, I.; Mosdale, A.; Mosdale, R. Durability of alternative metal oxide supports for application at a proton-exchange membrane fuel cell cathode—Comparison of antimony- And niobium-doped tin oxide. Energies 2020, 13, 403. [Google Scholar] [CrossRef]
- Han, S.B.; Mo, Y.H.; Lee, Y.S.; Lee, S.G.; Park, D.H.; Park, K.W. Mesoporous iridium oxide/Sb-doped SnO2 nanostructured electrodes for polymer electrolyte membrane water electrolysis. Int. J. Hydrogen Energy 2019, 45, 1409–1416. [Google Scholar] [CrossRef]
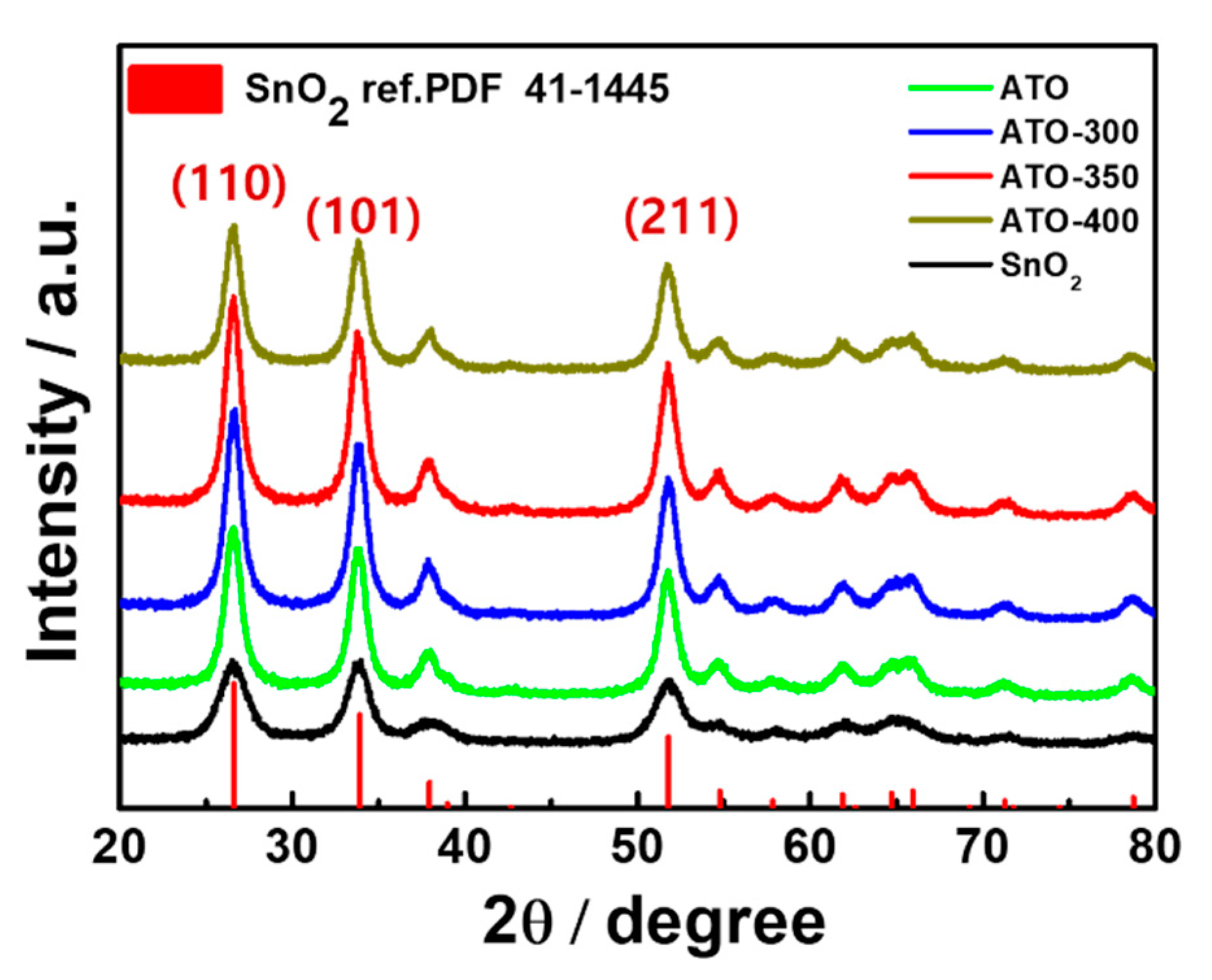
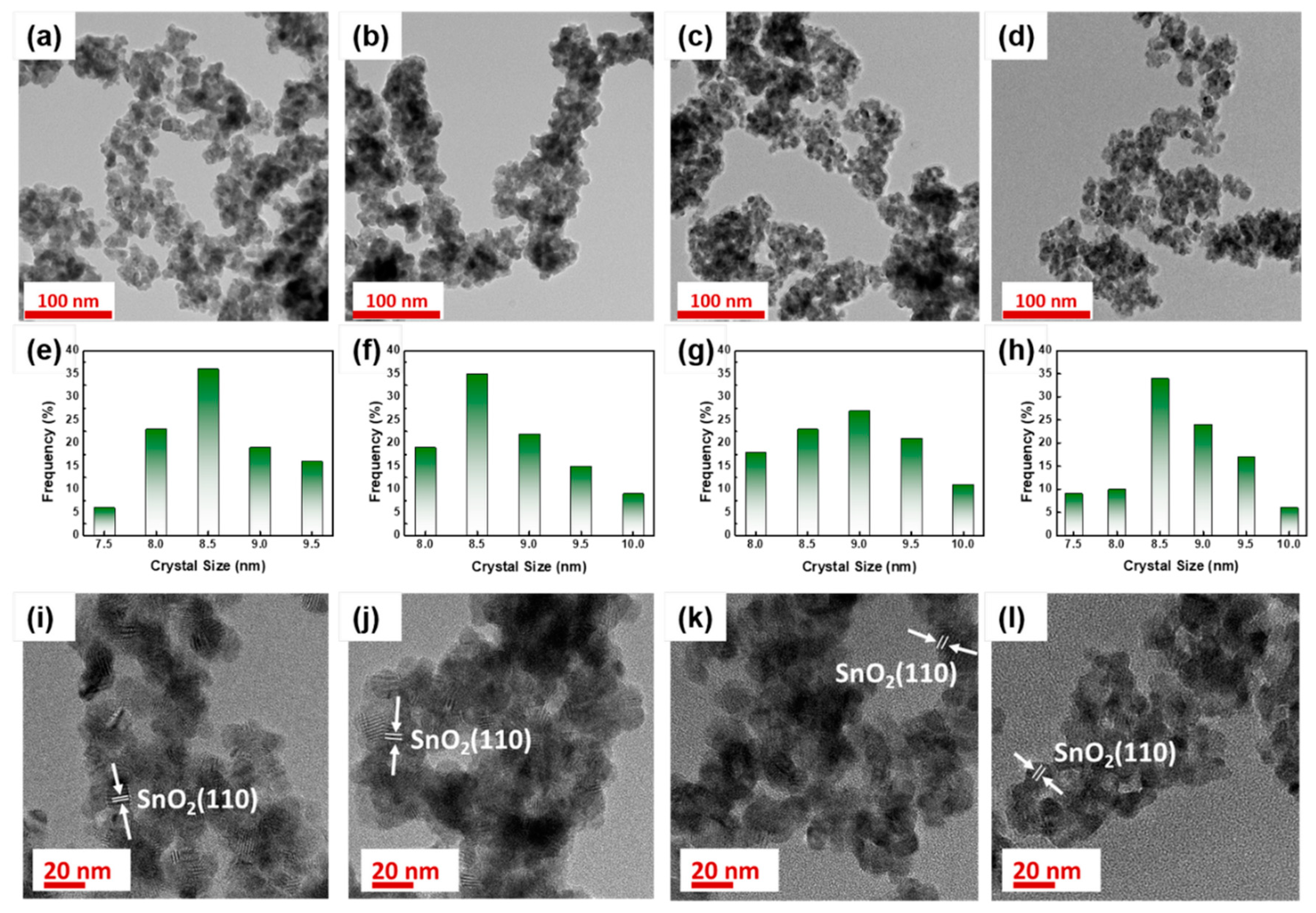
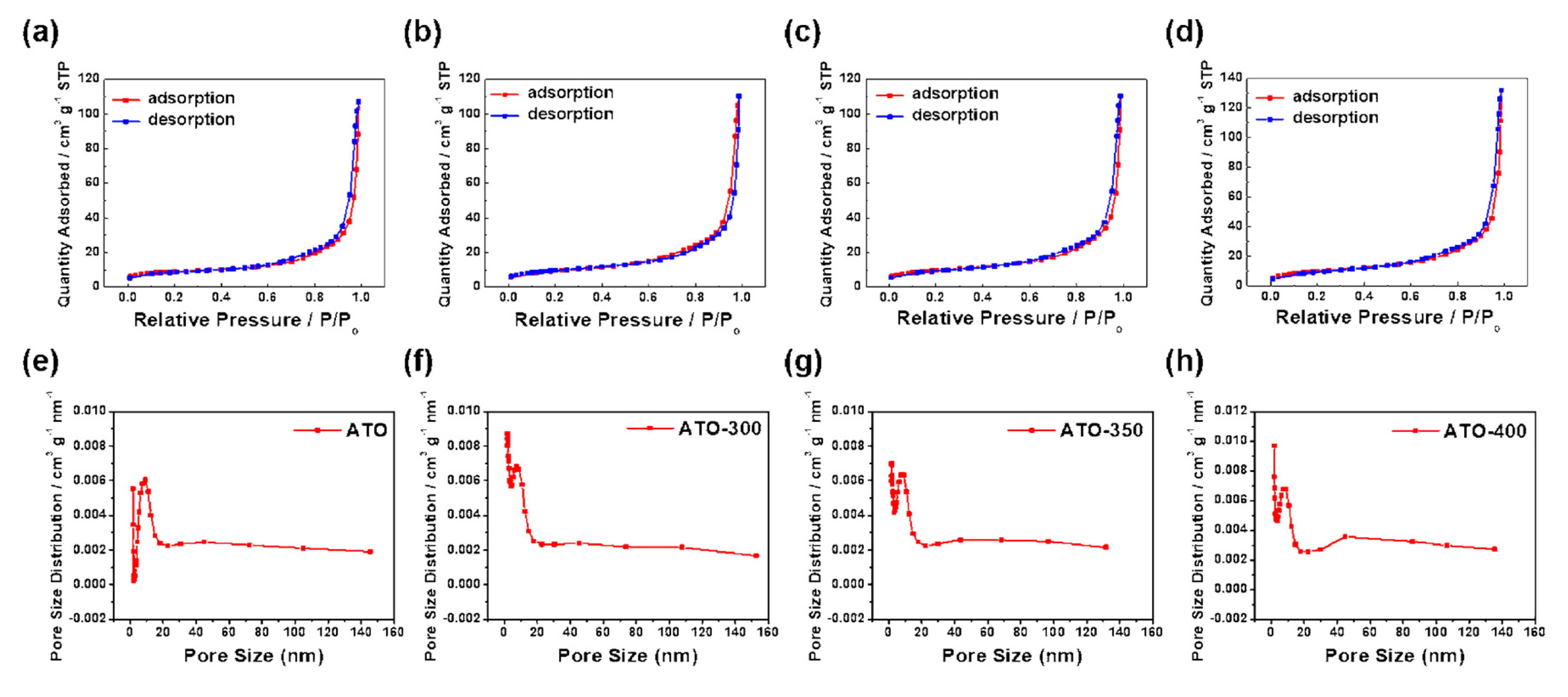

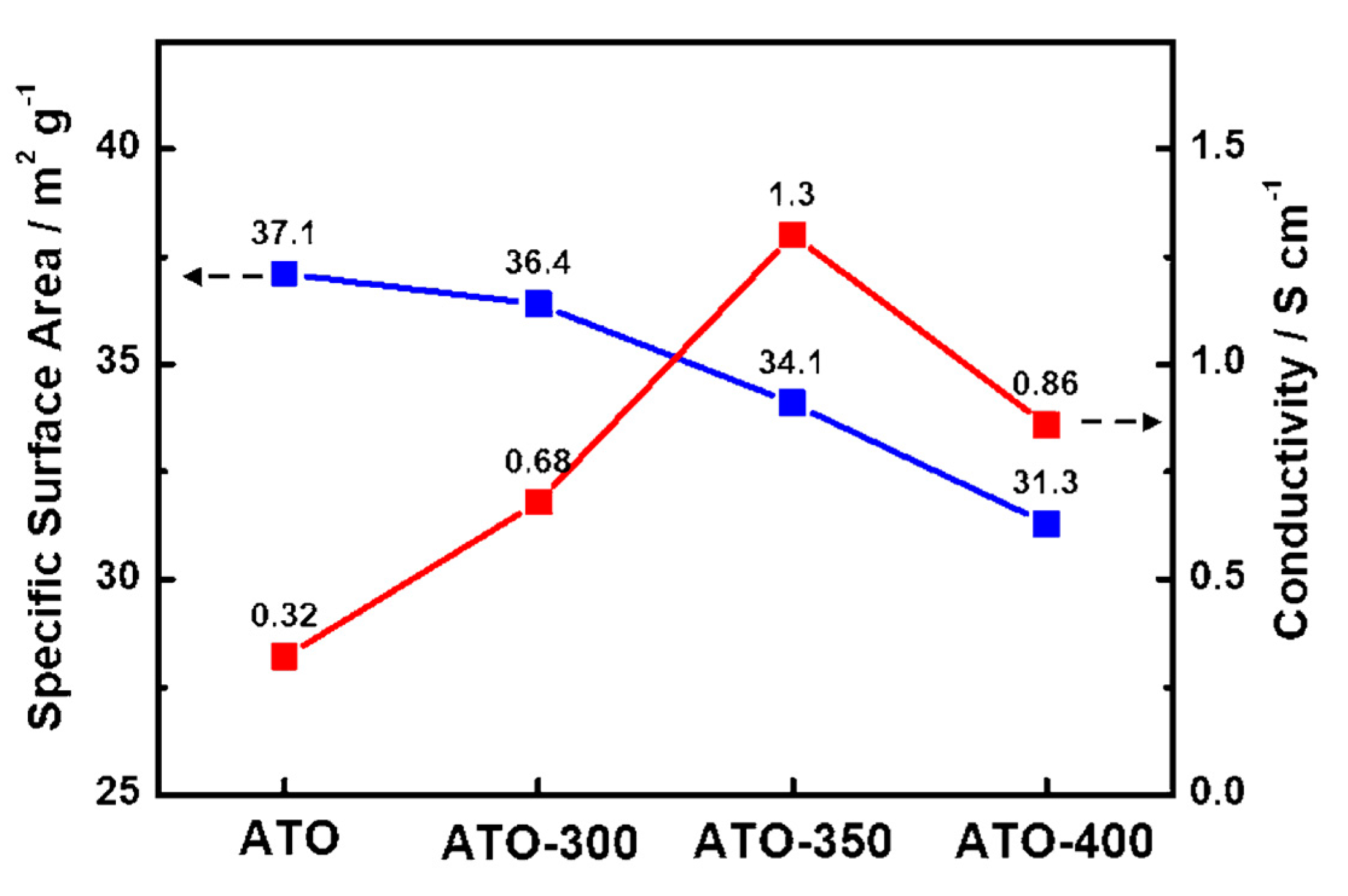

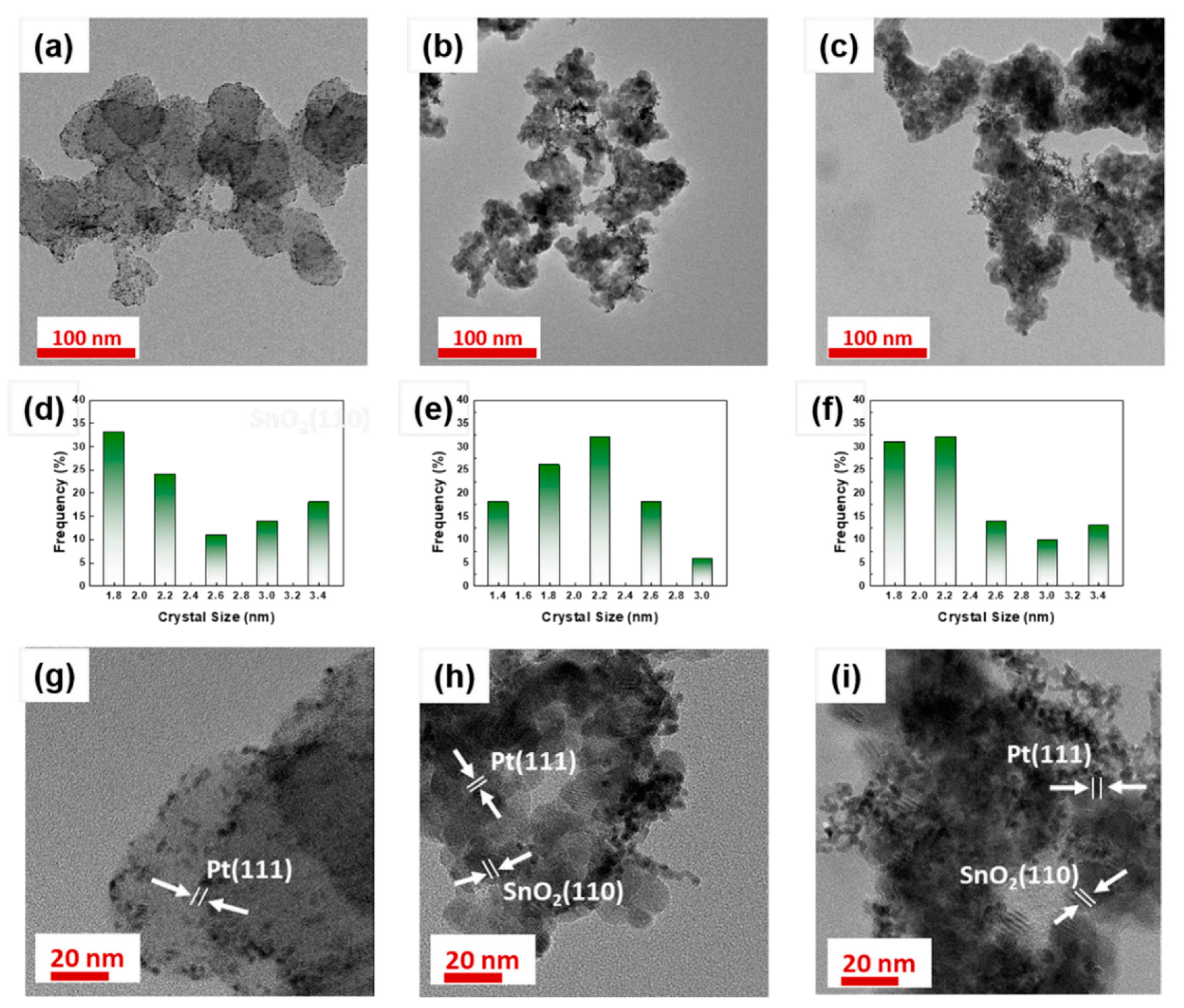

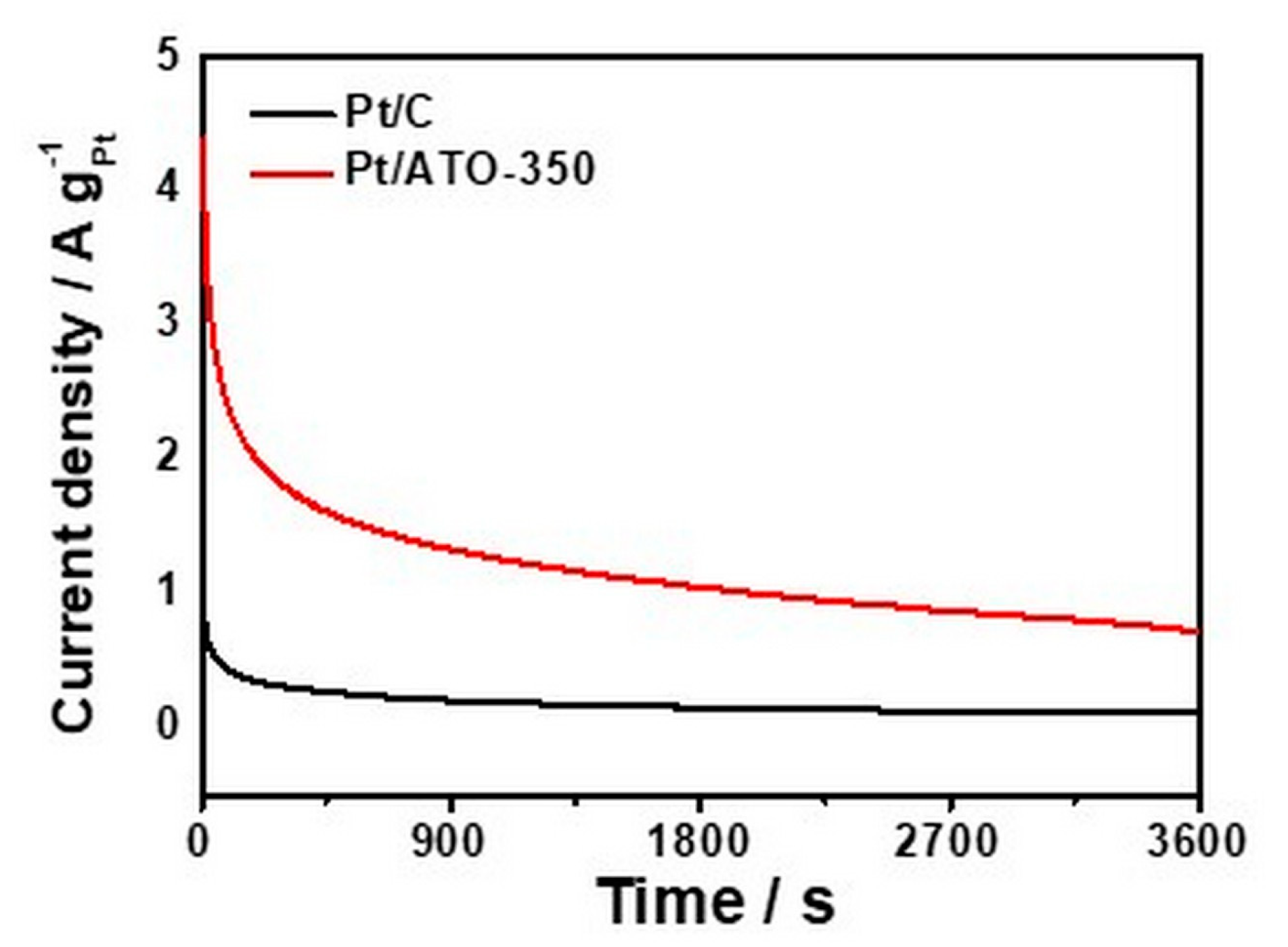


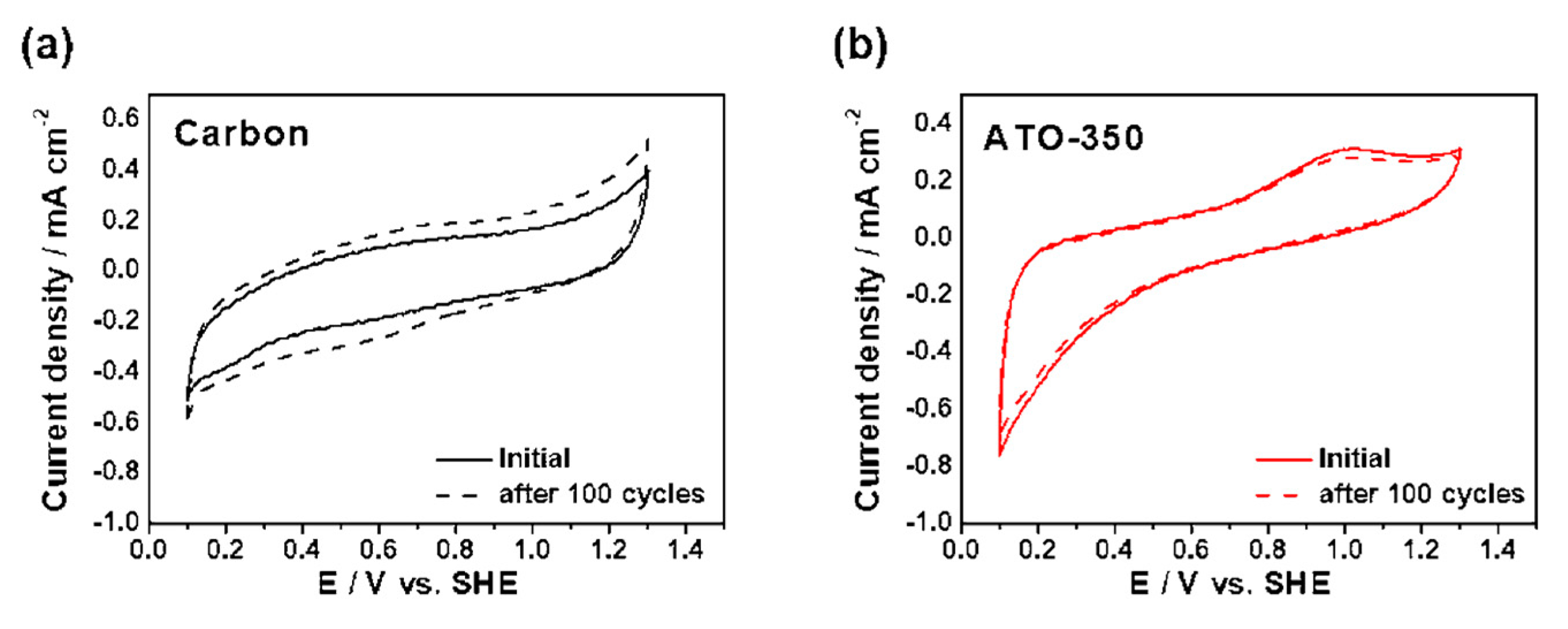
| Sample | Specific Surface Area (m2 g−1) | Pore Size (nm) | Crystalline Size (nm) |
|---|---|---|---|
| ATO | 37 | 19.4 | 8.6 |
| ATO-300 | 36 | 20.0 | 8.8 |
| ATO-350 | 34 | 21.2 | 8.9 |
| ATO-400 | 31 | 22.5 | 8.7 |
| Sample | Conductivity (S cm−1) | |
|---|---|---|
| ATO | 48% | 0.32 |
| ATO-300 | 49% | 0.68 |
| ATO-350 | 66% | 1.30 |
| ATO-400 | 64% | 0.86 |
| Sample | Current Density (A cmGEO−2) | Current Density (A cmEASA−2) | Current Density (A gPt−1) | EASA (m2 gPt−1) |
|---|---|---|---|---|
| Pt/C | 0.26 | 0.09 | 0.39 | 9.5 |
| Pt/ATO | 0.22 | 0.90 | 0.27 | 4.3 |
| Pt/ATO-350 | 0.47 | 0.52 | 0.58 | 18.6 |
| Sample | Condition | Mass Activity (A gPt−1@0.65 V) | Ref. |
|---|---|---|---|
| Pt/ATO-350 | 0.5 M H2SO4 + 2 M CH3OH 50 mV s−1 | 0.58 | In this study |
| Pt/SnO2 | 0.5 M KOH + 2 M CH3OH 20 mV s−1 | 0.30 | 7 |
| Pt/InSnO2 | 0.5 M KOH + 2 M CH3OH 20 mV s−1 | 0.41 | 7 |
| Pt/SbSnO2 | 0.5 M H2SO4 + 2 M CH3OH 50 mV s−1 | 0.64 | 24 |
| Pt/RuO2/CNT | 1 M HClO4 + 1 M CH3OH 100 mVs−1 | 0.49 | 35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-G.; Han, S.-B.; Lee, W.-J.; Park, K.-W. Effect of Sb-Doped SnO2 Nanostructures on Electrocatalytic Performance of a Pt Catalyst for Methanol Oxidation Reaction. Catalysts 2020, 10, 866. https://doi.org/10.3390/catal10080866
Lee S-G, Han S-B, Lee W-J, Park K-W. Effect of Sb-Doped SnO2 Nanostructures on Electrocatalytic Performance of a Pt Catalyst for Methanol Oxidation Reaction. Catalysts. 2020; 10(8):866. https://doi.org/10.3390/catal10080866
Chicago/Turabian StyleLee, Seul-Gi, Sang-Beom Han, Woo-Jun Lee, and Kyung-Won Park. 2020. "Effect of Sb-Doped SnO2 Nanostructures on Electrocatalytic Performance of a Pt Catalyst for Methanol Oxidation Reaction" Catalysts 10, no. 8: 866. https://doi.org/10.3390/catal10080866
APA StyleLee, S.-G., Han, S.-B., Lee, W.-J., & Park, K.-W. (2020). Effect of Sb-Doped SnO2 Nanostructures on Electrocatalytic Performance of a Pt Catalyst for Methanol Oxidation Reaction. Catalysts, 10(8), 866. https://doi.org/10.3390/catal10080866