Elucidation of Water Promoter Effect of Proton Conductor in WGS Reaction over Pt-Based Catalyst: An Operando DRIFTS Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical and Textural Properties
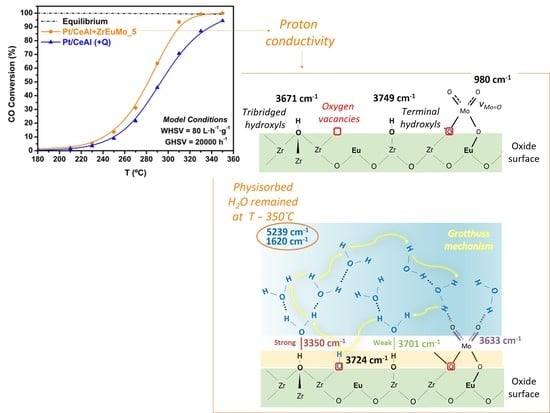
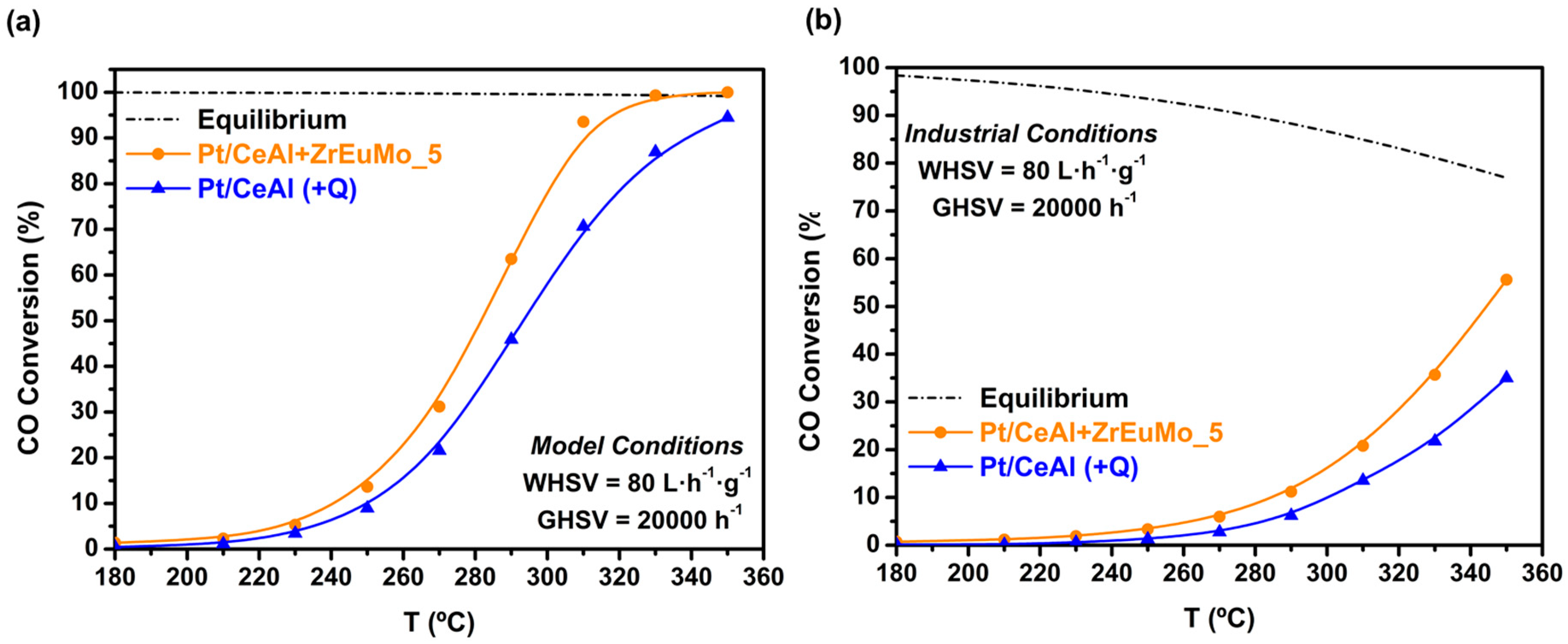
2.2. Catalytic Performance
2.3. Operando DRIFTS Study
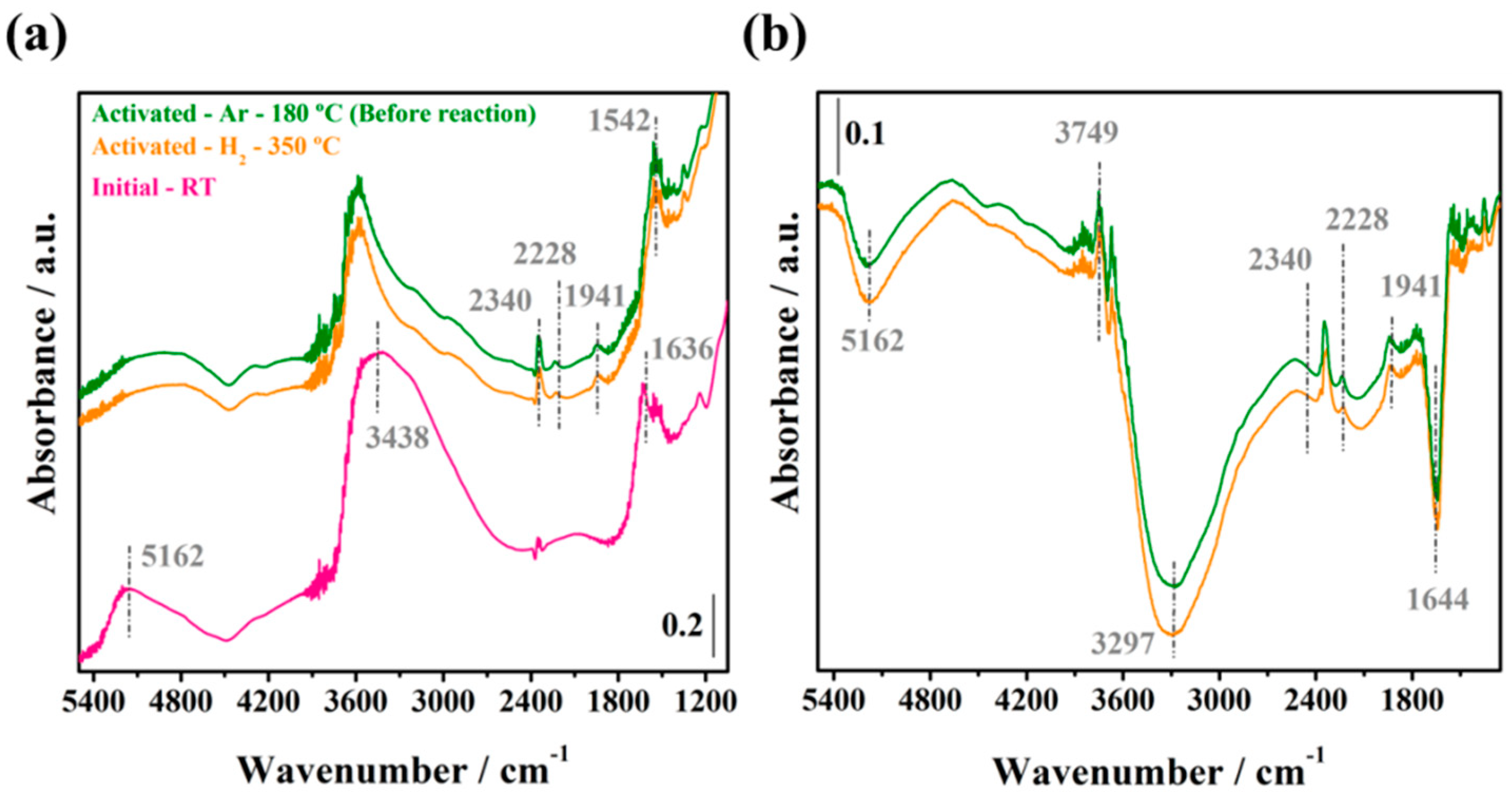
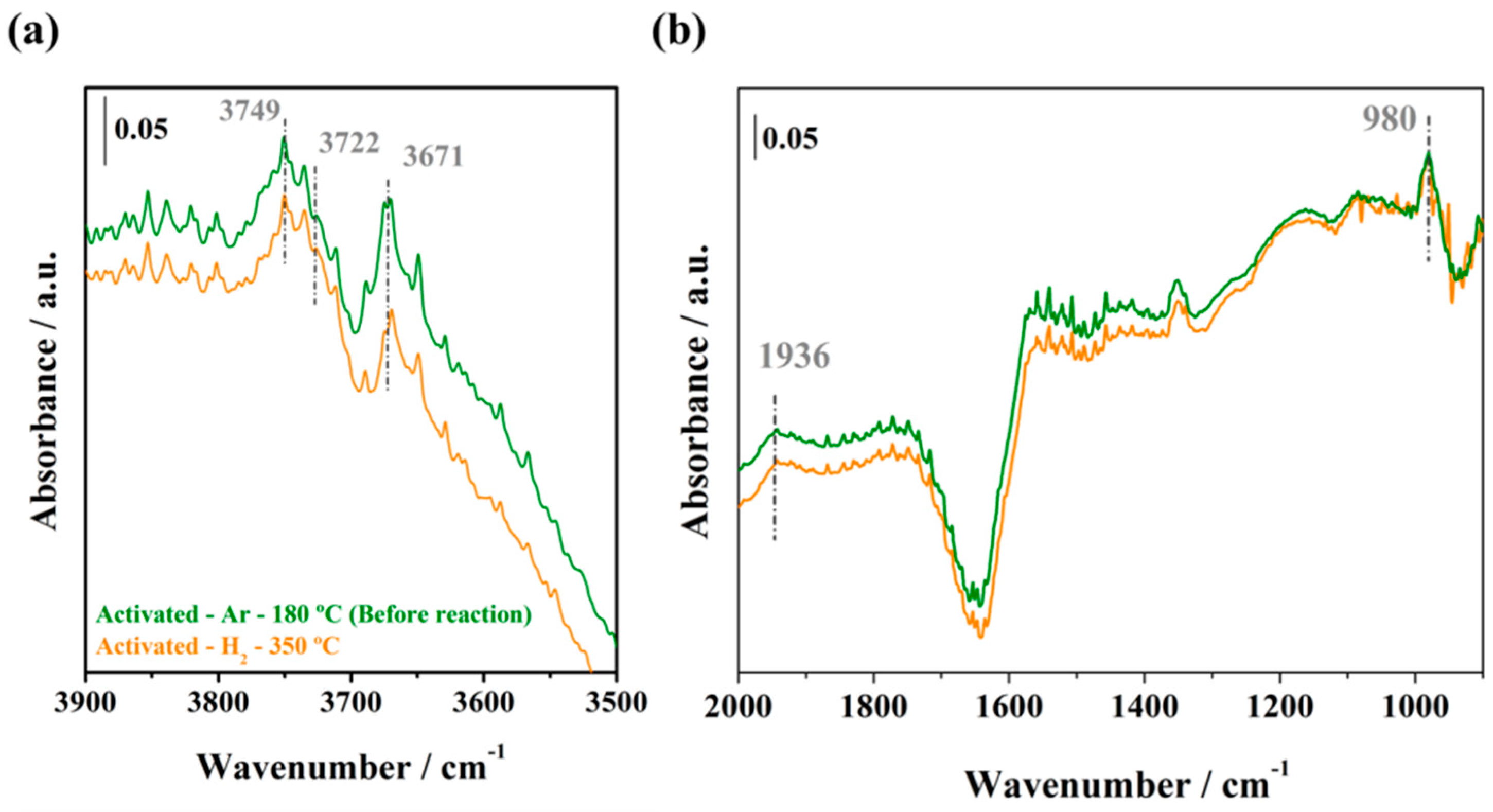
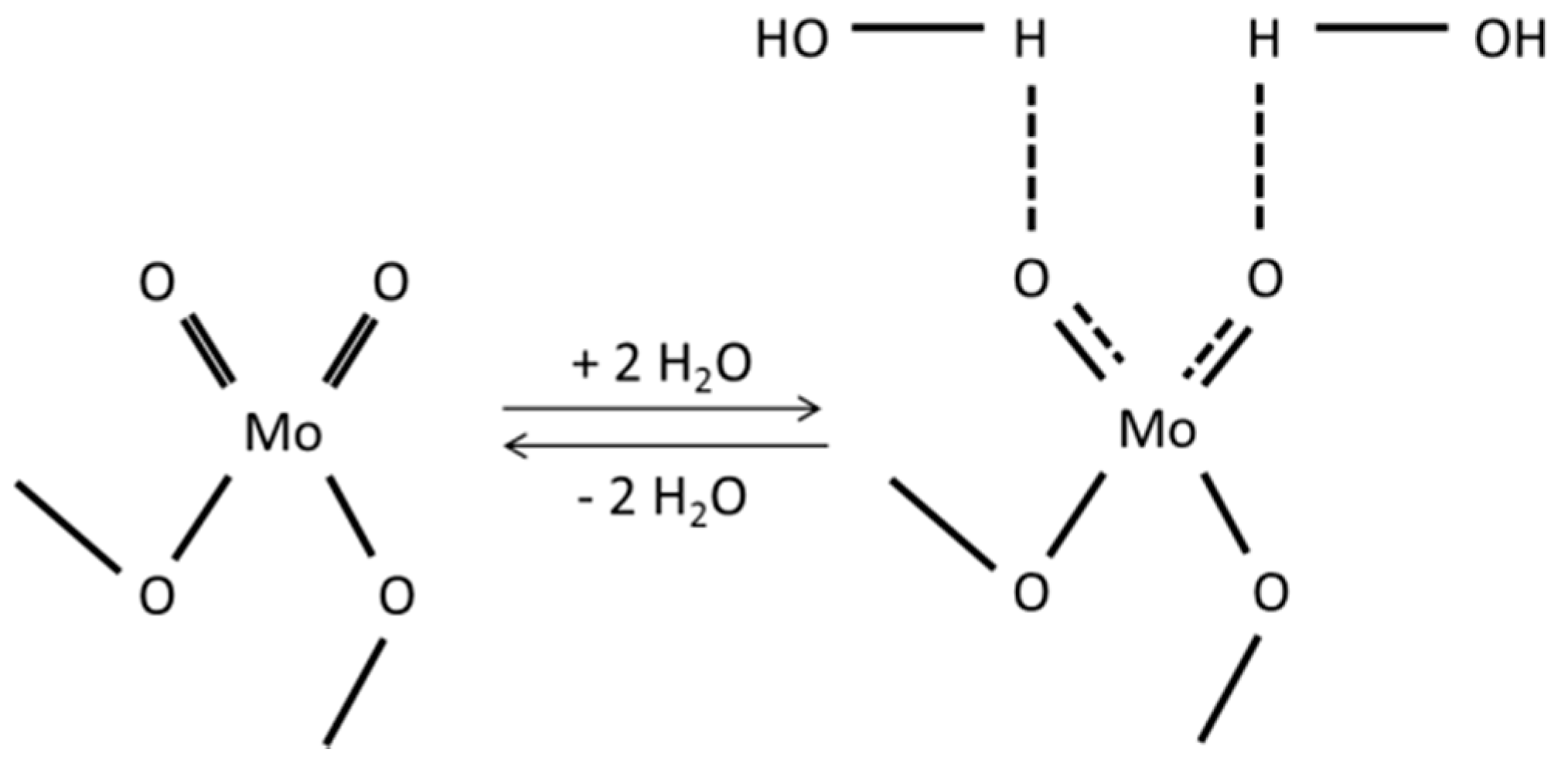
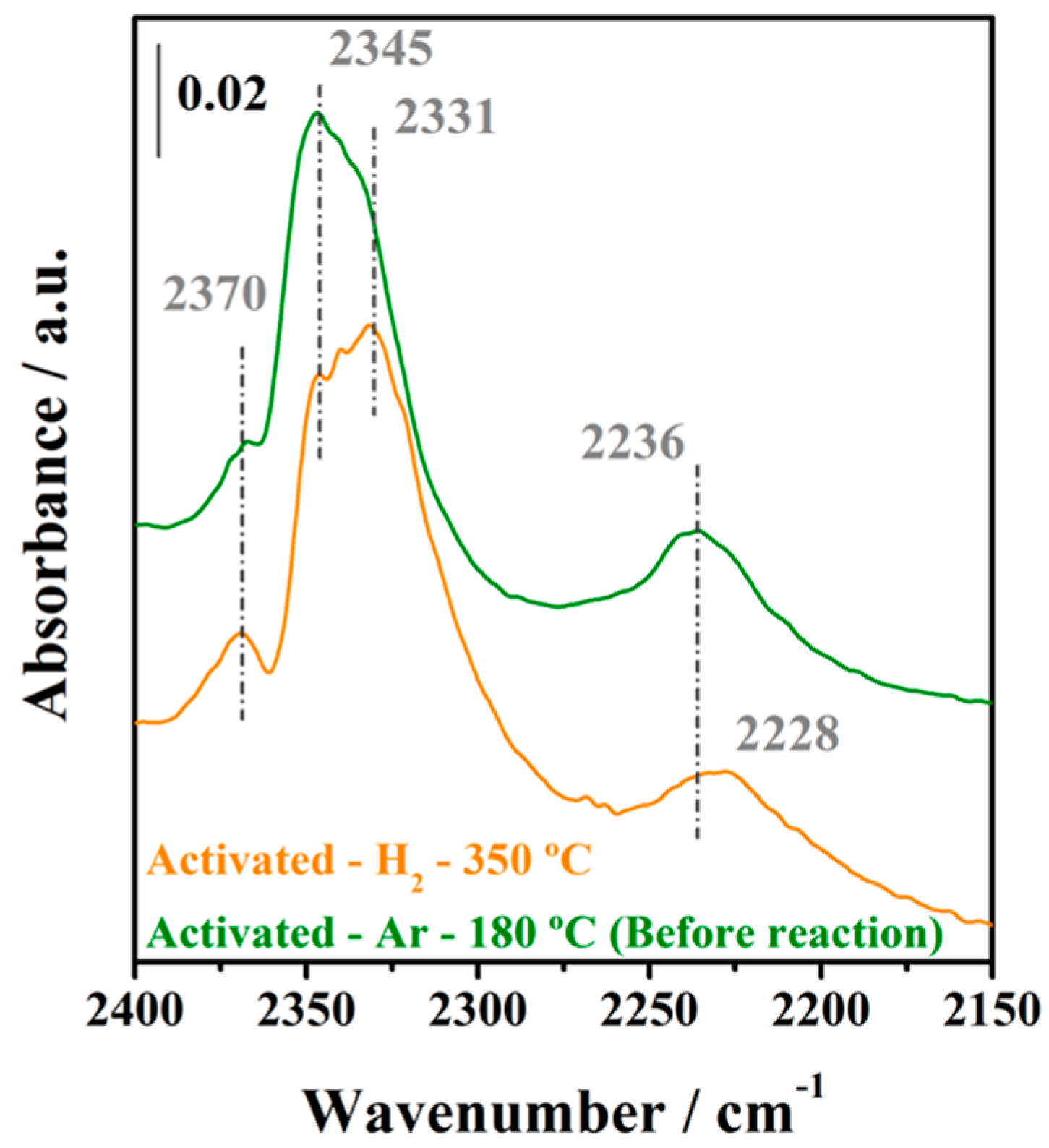
2.3.1. Initial Pretreatment of the Samples
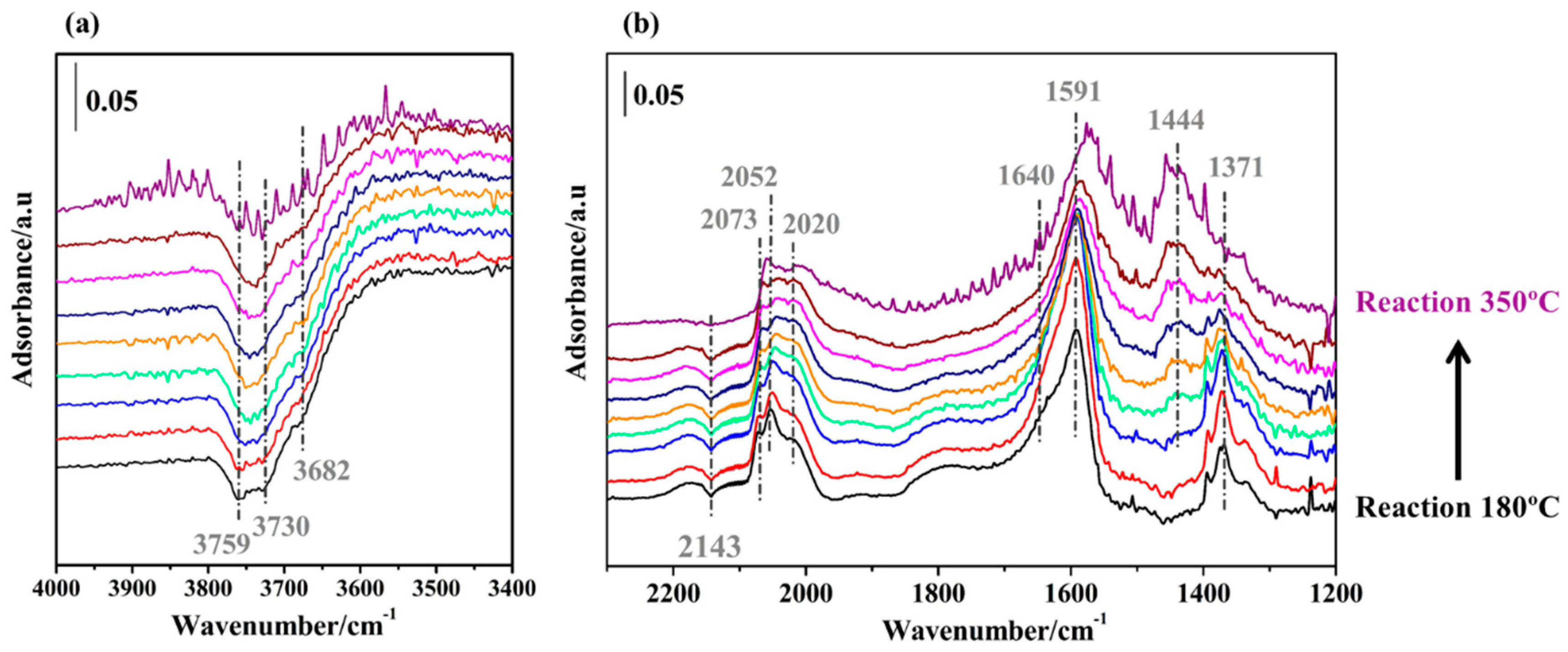
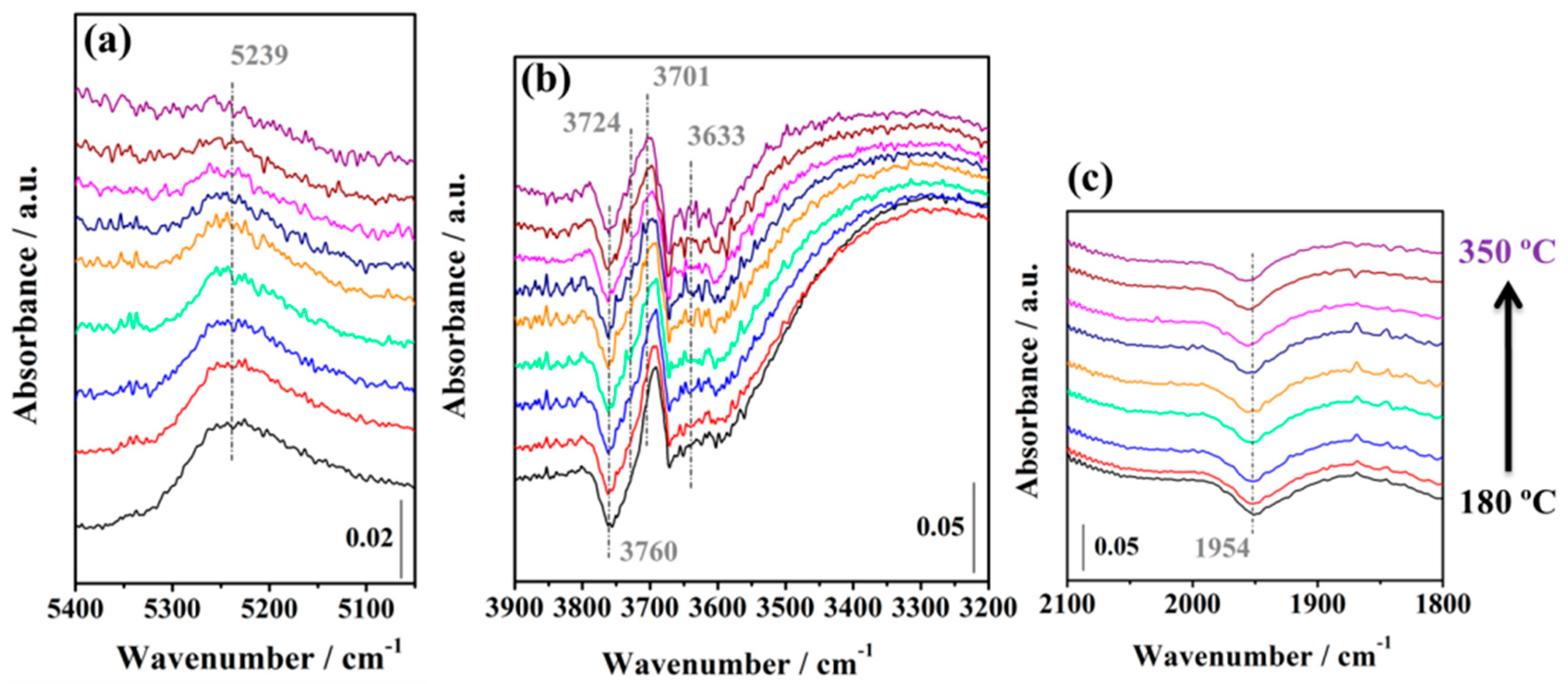
2.3.2. WGS Reaction under Model Conditions
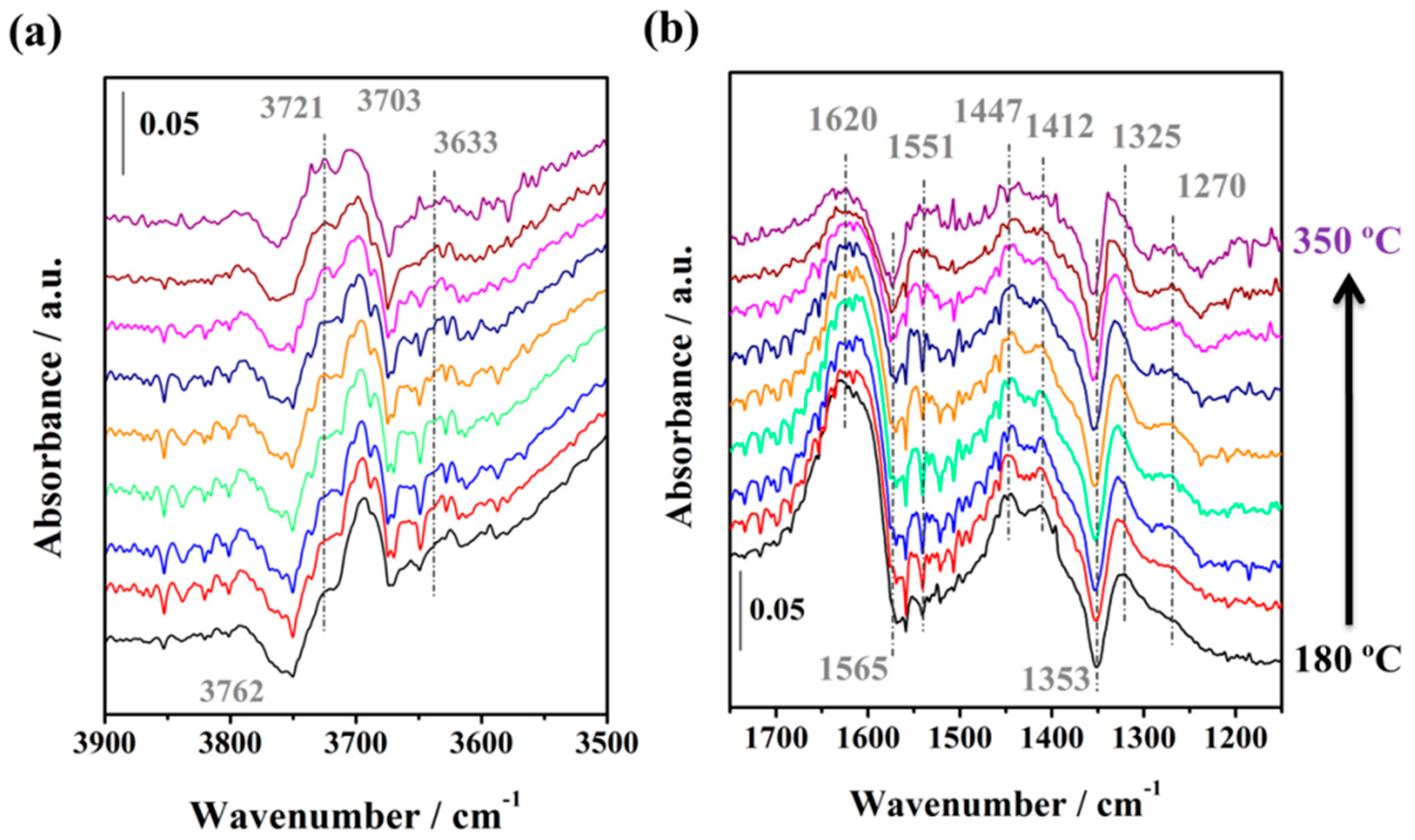
2.3.3. WGS Reaction under Industrial Conditions
3. Materials and Methods
3.1. Preparation of Materials
3.2. Characterization Techniques
3.3. Catalytic Activity Tests
3.4. Operando DRIFTS Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratnasamy, C.; Wagner, J.P. Water gas shift catalysis. Catal. Rev. Sci. Eng. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Liu, Y.; Ruettinger, W.; Ilinich, O.; Shore, L.; Giroux, T. Precious Metal catalysts supported on ceramic and metal monolithic structures for the hydrogen economy. Catal. Rev. Sci. Eng. 2007, 49, 141–196. [Google Scholar] [CrossRef]
- Andreeva, D.; Ivanov, I.; Ilieva, L.; Sobczak, J.W.; Avdeev, G.; Petrov, K. Gold based catalysts on ceria and ceria-alumina for WGS reaction (WGS Gold catalysts). Top. Catal. 2007, 44, 173–182. [Google Scholar] [CrossRef]
- Gonzalez Castaño, M.; Reina, T.R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Pt vs. Au in water-gas shift reaction. J. Catal. 2014, 314, 1–9. [Google Scholar] [CrossRef]
- Kam, R.; Scott, J.; Amal, R.; Selomulya, C. Pyrophoricity and stability of copper and platinum based water-gas shift catalysts during oxidative shut-down/start-up operation. Chem. Eng. Sci. 2010, 65, 6461–6470. [Google Scholar] [CrossRef]
- Luengnaruemitchai, A.; Osuwan, S.; Gulari, E. Comparative studies of low-temperature water-gas shift reaction over Pt/CeO2, Au/CeO2, and Au/Fe2O3 catalysts. Catal. Commun. 2003, 4, 215–221. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effect of morphological characteristics of TiO2-supported noble metal catalysts on their activity for the water-gas shift reaction. J. Catal. 2004, 225, 327–336. [Google Scholar] [CrossRef]
- Meunier, F.C.; Goguet, A.; Hardacre, C.; Burch, R.; Thompsett, D. Quantitative DRIFTS investigation of possible reaction mechanisms for the water-gas shift reaction on high-activity Pt- and Au-based catalysts. J. Catal. 2007, 252, 18–22. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the mechanism of low-temperature water gas shift reaction on copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Americanou, S.; Efstathiou, A.M. “Redox” vs “associative formate with -OH group regeneration” WGS reaction mechanism on Pt/CeO2: Effect of platinum particle size. J. Catal. 2011, 279, 287–300. [Google Scholar] [CrossRef]
- Saeidi, S.; Fazlollahi, F.; Najari, S.; Iranshahi, D.; Klemeš, J.J.; Baxter, L.L. Hydrogen production: Perspectives, separation with special emphasis on kinetics of WGS reaction: A state-of-the-art review. J. Ind. Eng. Chem. 2017, 49, 1–25. [Google Scholar] [CrossRef]
- Clay, J.P.; Greeley, J.P.; Ribeiro, F.H.; Delgass, W.N.; Schneider, W.F. DFT comparison of intrinsic WGS kinetics over Pd and Pt. J. Catal. 2014, 320, 106–117. [Google Scholar] [CrossRef]
- González-Castaño, M.; Ivanova, S.; Laguna, O.H.; Martínez, T.L.M.; Centeno, M.A.; Odriozola, J.A. Structuring Pt/CeO2/Al2O3 WGS catalyst: Introduction of buffer layer. Appl. Catal. B Environ. 2017, 200, 420–427. [Google Scholar] [CrossRef]
- Carter, J.H.; Hutchings, G.J. Recent advances in the gold-catalysed low-temperature water–gas shift reaction. Catalysts 2018, 8, 627. [Google Scholar] [CrossRef]
- Kugai, J.; Miller, J.T.; Guo, N.; Song, C. Role of metal components in Pd–Cu bimetallic catalysts supported on CeO2 for the oxygen-enhanced water gas shift. Appl. Catal. B Environ. 2011, 105, 306–316. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effect of the nature of the support on the catalytic performance of noble metal catalysts for the water-gas shift reaction. Catal. Today 2006, 112, 49–52. [Google Scholar] [CrossRef]
- Plata, J.J.; Graciani, J.; Evans, J.; Rodriguez, J.A.; Sanz, J.F. Cu deposited on CeOx-Modified TiO2(110): Synergistic effects at the metal-oxide interface and the mechanism of the WGS reaction. ACS Catal. 2016, 6, 4608–4615. [Google Scholar] [CrossRef]
- Vignatti, C.; Avila, M.S.; Apesteguía, C.R.; Garetto, T.F. Catalytic and DRIFTS study of the WGS reaction on Pt-based catalysts. Int. J. Hydrog. Energy 2010, 35, 7302–7312. [Google Scholar] [CrossRef]
- García-Moncada, N.; González-Castaño, M.; Ivanova, S.; Centeno, M.Á.; Romero-Sarria, F.; Odriozola, J.A. New concept for old reaction: Novel WGS catalyst design. Appl. Catal. B Environ. 2018, 238, 1–5. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Rabenau, A.; Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Malavasi, L.; Fisher, C.A.J.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370–4387. [Google Scholar] [CrossRef] [PubMed]
- García-Moncada, N.; Bobadilla, L.F.; Poyato, R.; López-Cartes, C.; Romero-Sarria, F.; Centeno, M.Á.; Odriozola, J.A. A direct in situ observation of water-enhanced proton conductivity of Eu-doped ZrO2: Effect on WGS reaction. Appl. Catal. B Environ. 2018, 231, 343–356. [Google Scholar] [CrossRef]
- Morozov, V.; Arakcheeva, A.; Redkin, B.; Sinitsyn, V.; Khasanov, S.; Kudrenko, E.; Raskina, M.; Lebedev, O.; Van Tendeloo, G. Na2/7Gd4/7MoO4: A modulated scheelite-type structure and conductivity properties. Inorg. Chem. 2012, 51, 5313–5324. [Google Scholar] [CrossRef]
- El Khal, H.; Cordier, A.; Batis, N.; Siebert, E.; Georges, S.; Steil, M.C. Effect of porosity on the electrical conductivity of LAMOX materials. Solid State Ion. 2017, 304, 75–84. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Pavone, M.; Ritzmann, A.M.; Carter, E.A. Oxide ion transport in Sr2Fe1.5Mo0.5O(6-δ), a mixed ion-electron conductor: New insights from first principles modeling. Phys. Chem. Chem. Phys. 2013, 15, 6250–6259. [Google Scholar] [CrossRef]
- Aguadero, A.; Perez-Coll, D.; Alonso, J.A.; Skinner, S.J.; Kilner, J.A. A new family of Mo-doped SrCoO3-delta perovskites for application in reversible solid state electrochemical cells. Chem. Mater. 2012, 24, 2655–2663. [Google Scholar] [CrossRef]
- Vega-Castillo, J.E.; Ravella, U.K.; Corbel, G.; Lacorre, P.; Caneiro, A. Thermodynamic stability, structural and electrical characterization of mixed ionic and electronic conductor La2Mo2O8.96. Dalton Trans. 2012, 41, 7266–7271. [Google Scholar] [CrossRef]
- Amsif, M.; Magrasó, A.; Marrero-López, D.; Ruiz-Morales, J.C.; Canales-Vázquez, J.; Núñez, P. Mo-substituted lanthanum tungstate La28-yW4+yO54+delta: A competitive mixed electron-proton conductor for gas separation membrane applications. Chem. Mater. 2012, 24, 3868–3877. [Google Scholar] [CrossRef]
- Chippindale, A.M.; Cheetham, A.K. Chapter 3. The oxide chemistry of molybdenum. In Molybdenum: An Outline of its Chemistry and Uses; Braithwaite, E.R., Haber, J., Eds.; Elsevier Science B.V: Amsterdam, The Netherlands, 1994; pp. 146–184. [Google Scholar]
- Pan, Q.; Huang, L.; Li, Z.; Han, J.; Zhao, N.; Xie, Y.; Li, X.; Liu, M.; Wang, X.; Liu, J.M. A first-principles study on the hydrogenation of acetone on HxMoO3 surface. Int. J. Hydrog. Energy 2019, 44, 10443–10452. [Google Scholar] [CrossRef]
- Fei, S.; Xia, K.; Tian, X.; Mei, P.; Yan, H.; Cheng, H. Fabrication of ordered mesoporous MoO3 for olefin catalytic hydrogenation. Int. J. Hydrog. Energy 2016, 41, 5652–5660. [Google Scholar] [CrossRef]
- Schöllhorn, R.; Kuhlmann, R.; Besenhard, J.O. Topotactic redox reactions and ion exchange of layered MoO3 bronzes. Mater. Res. Bull. 1976, 11, 83–90. [Google Scholar] [CrossRef]
- Adams, S. CDW superstructures in hydrogen molybdenum bronzes HxMoO3. J. Solid State Chem. 2000, 149, 75–87. [Google Scholar] [CrossRef]
- Bollapragada, P.K.S. Chemical Transformations Using Tungsten and Molybdenum Hydrogen Bronzes. Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2003. [Google Scholar]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.-C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3·xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Pajonk, G.M.; Teichner, S.J.; Germain, J.E. (Eds.) Spillover of Adsorbed Species; Elsevier Science Publishers B.V: Amsterdam, The Netherlands, 1983; Volume 17, ISBN 9780444422248. [Google Scholar]
- García-Moncada, N.; Jurado, L.; Martínez-Tejada, L.M.; Romero-sarria, F.; Odriozola, J.A. Boosting water activation determining-step in WGS reaction on structured catalyst by Mo-doping. Catal. Today 2020. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Doped Aluminas, Silica-Aluminas, Mixed Metal Oxides, Hydrotalcites; Sasol Performance Chemicals. Available online: https://docplayer.net/64892116-Doped-aluminas-silica-aluminas-mixed-metal-oxides-hydrotalcites.html (accessed on 20 June 2020).
- Phatak, A.A.; Koryabkina, N.; Rai, S.; Ratts, J.L.; Ruettinger, W.; Farrauto, R.J.; Blau, G.E.; Delgass, W.N.; Ribeiro, F.H. Kinetics of the water-gas shift reaction on Pt catalysts supported on alumina and ceria. Catal. Today 2007, 123, 224–234. [Google Scholar] [CrossRef]
- Phatak, A.A.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Density functional theory comparison of water dissociation steps on Cu, Au, Ni, Pd, and Pt. J. Phys. Chem. C 2009, 113, 7269–7276. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Mei, D.; Li, J.; Rousseau, R. DFT+U study on the localized electronic states and their potential role during H2O dissociation and CO oxidation processes on CeO2 (111) surface. J. Phys. Chem. C 2013, 117, 23082–23089. [Google Scholar] [CrossRef]
- Guild, C.J.; Vovchok, D.; Kriz, D.A.; Bruix, A.; Hammer, B.; Llorca, J.; Xu, W.; El-Sawy, A.; Biswas, S.; Rodriguez, J.A.; et al. Water-Gas-Shift over metal-free nanocrystalline ceria: An experimental and theoretical study. ChemCatChem 2017, 9, 1373–1377. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO 2 -based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Andonova, S.; Ok, Z.A.; Ozensoy, E.; Hadjiivanov, K. Effects induced by interaction of the Pt/CeO x /ZrO x /γ-Al 2 O 3 ternary mixed oxide DeNO x catalyst with hydrogen. Catal. Today 2019. [Google Scholar] [CrossRef]
- Romero-Sarria, F.; Garcia-Dali, S.; Palma, S.; Jimenez-Barrera, E.M.; Oliviero, L.; Bazin, P.; Odriozola, J.A. The role of carbon overlayers on Pt-based catalysts for H2-cleanup by CO-PROX. Surf. Sci. 2016, 648, 84–91. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Andreeva, D. FTIR study of low-temperature water-gas shift reaction on gold/ceria catalyst. Appl. Catal. A Gen. 2003, 252, 385–397. [Google Scholar] [CrossRef]
- Köck, E.-M.; Kogler, M.; Klötzer, B.; Noisternig, M.F.; Penner, S. Structural and electrochemical properties of physisorbed and chemisorbed water layers on the ceramic oxides Y2O3, YSZ, and ZrO2. ACS Appl. Mater. Interfaces 2016, 8, 16428–16443. [Google Scholar] [CrossRef]
- Heracleous, E.; Lemonidou, A.A.; Lercher, J.A. Mechanistic features of the ethane oxidative dehydrogenation by in situ FTIR spectroscopy over a MoO3/Al2O3 catalyst. Appl. Catal. A Gen. 2004, 264, 73–80. [Google Scholar] [CrossRef]
- Daturi, M.; Binet, C.; Bernal, S.; Pérez Omil, J.A.; Lavalley, J.C. FTIR study of defects produced in ZrO2 samples by thermal treatment. Residual species into cavities and surface defects. J. Chem. Soc. Faraday Trans. 1998, 94, 1143–1147. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Fitzgerald, S.A.; Schloss, J.M.; Pierce, C.J.; Thompson, B.; Rowsell, J.L.C.; Yu, K.; Schmidt, J.R. Insights into the anomalous vibrational frequency shifts of CO2 adsorbed to metal sites in microporous frameworks. J. Phys. Chem. C 2015, 119, 5293–5300. [Google Scholar] [CrossRef]
- Bazin, P.; Saur, O.; Lavalley, J.C.; Daturi, M.; Blanchard, G. FT-IR study of CO adsorption on Pt/CeO2: Characterisation and structural rearrangement of small Pt particles. Phys. Chem. Chem. Phys. 2005, 7, 187–194. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Gonzalez, I.D.; Navarro, R.M.; Fierro, J.L.G.; Efstathiou, A.M. Effects of reaction temperature and support composition on the mechanism of water–gas shift reaction over supported-Pt catalysts. J. Phys. Chem. C 2011, 115, 11595–11610. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Dionysiou, D.D.; Efstathiou, A.M. Mechanistic studies of the water–gas shift reaction over Pt/CexZr1–xO2 catalysts: The effect of Pt particle size and Zr dopant. ACS Catal. 2012, 2, 2729–2742. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.C.; Tibiletti, D.; Breen, J.P.; Burch, R. Spectrokinetic investigation of reverse water-gas-shift reaction intermediates over a Pt/CeO2 catalyst. J. Phys. Chem. B 2004, 108, 20240–20246. [Google Scholar] [CrossRef]
- Schreiter, N.; Kirchner, J.; Kureti, S. A DRIFTS and TPD study on the methanation of CO2 on Ni/Al2O3 catalyst. Catal. Commun. 2020, 140, 105988. [Google Scholar] [CrossRef]
- García-Moncada, N.; Groppi, G.; Beretta, A.; Romero-Sarria, F.; Odriozola, J.A. Metal micro-monoliths for the kinetic study and the intensification of the water gas shift reaction. Catalysts 2018, 8, 594. [Google Scholar] [CrossRef]
- Cerrato, G.; Bordiga, S.; Barbera, S.; Morterra, C. Surface characterization of monoclinic ZrO2. I Morphology, FTIR spectral features, and computer modelling. Appl. Surf. Sci. 1997, 115, 53–65. [Google Scholar] [CrossRef]
- Du, X.; Dong, L.; Li, C.; Liang, Y.; Chen, Y. Diffuse reflectance infrared fourier transform and raman spectroscopic studies of MoO dispersed on CeO support. Langmuir 1999, 15, 1693–1697. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. {IR} study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Köck, E.-M.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In situ FT-IR spectroscopic study of CO2 and CO adsorption on Y2O3, ZrO2, and Yttria-Stabilized ZrO2. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef] [PubMed]
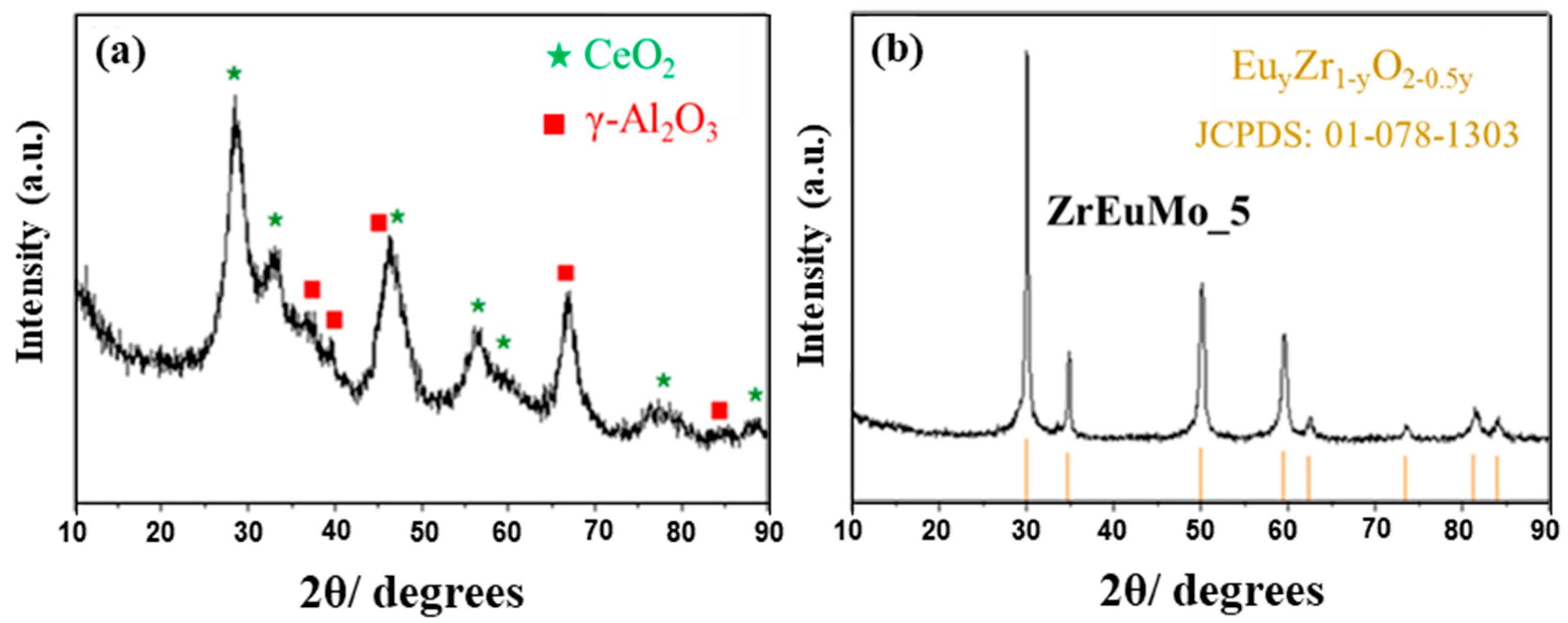
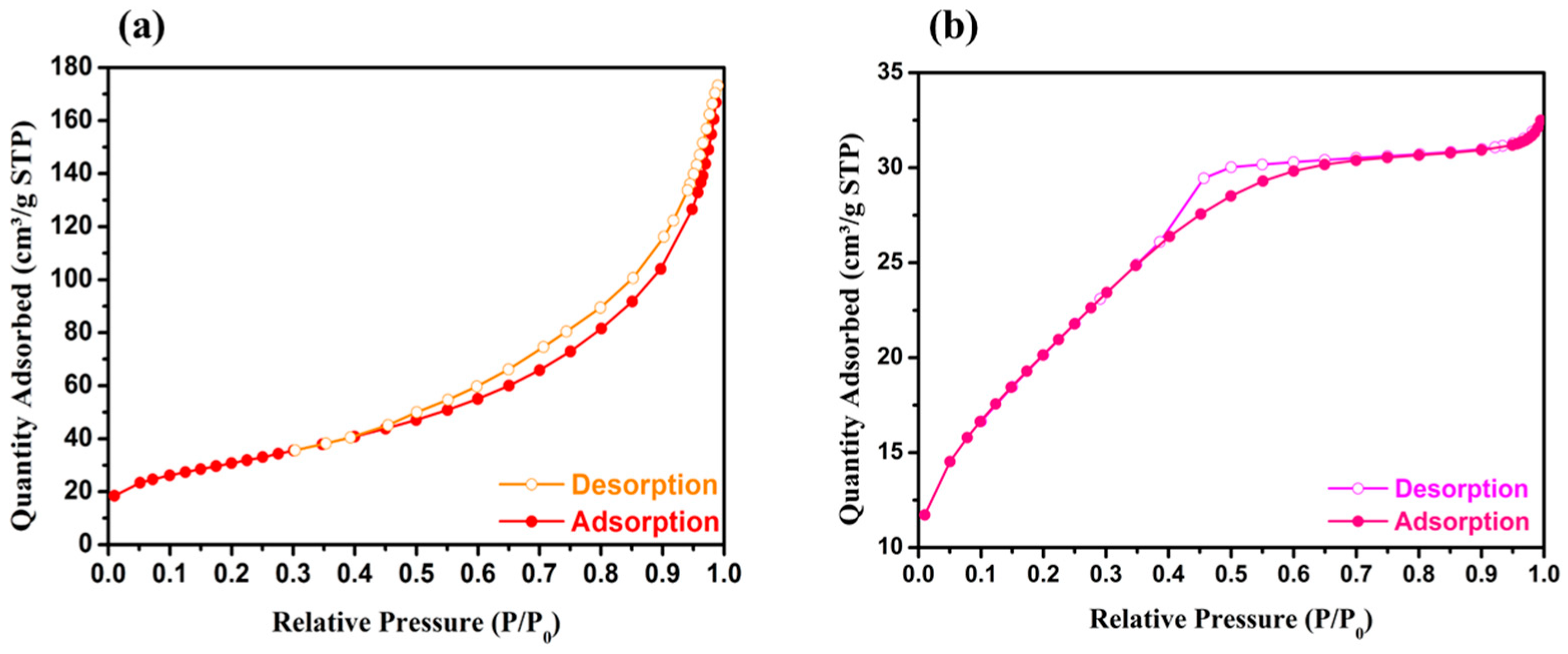
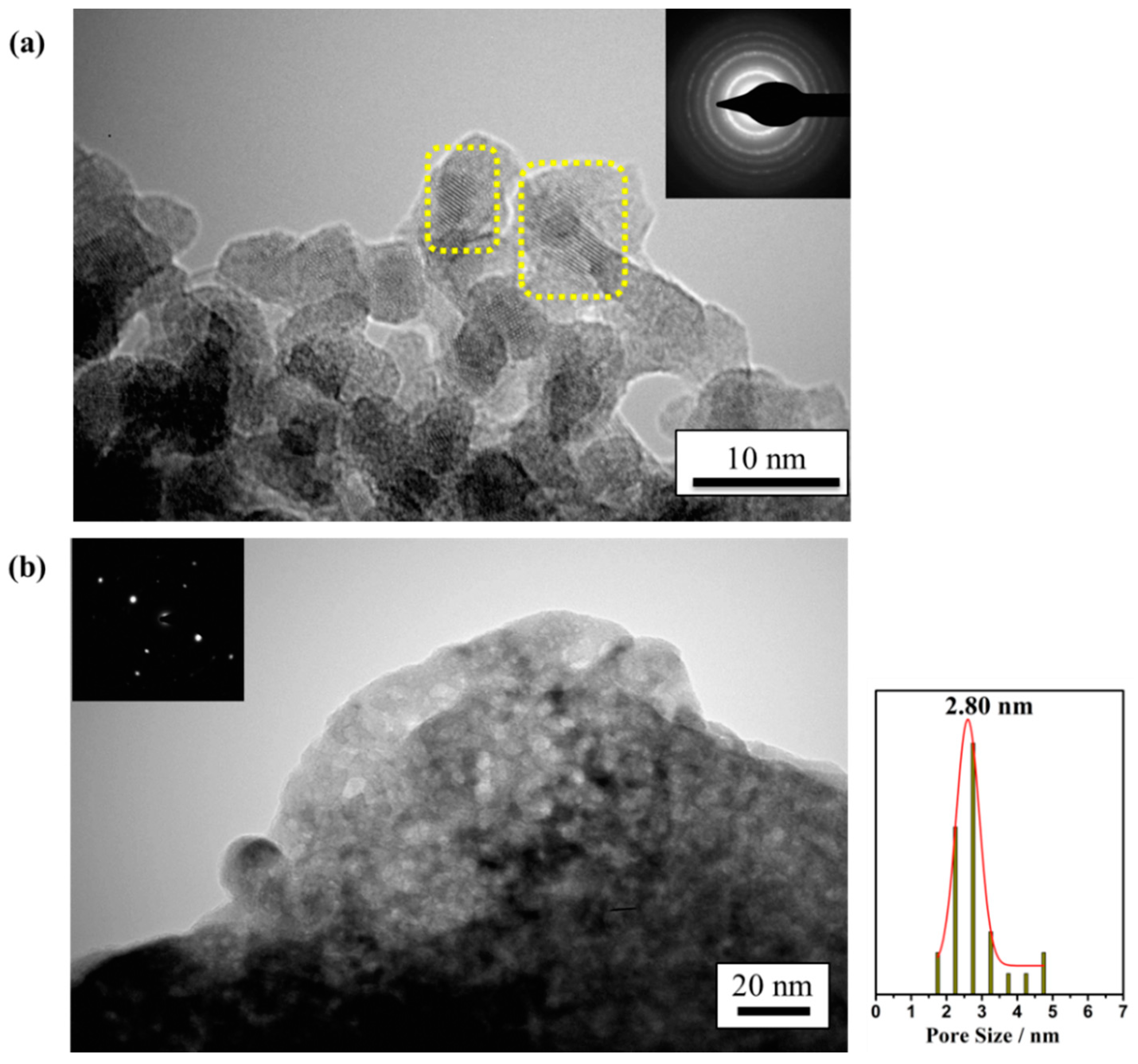


| Sample | SBET (m2 g−1) | Vp (cm3 g−1) | Dp (nm) | Chemical Composition a (wt.%) |
|---|---|---|---|---|
| Pt/CeAl | 110 | 0.26 | 8.84 | 1.8% Pt (2%) 18.8% CeO2 (19.6%) 79.4% Al2O3 (78.4%) |
| ZrEuMo_5 | 74 | 0.06 | 2.4 | 58.4% Zr (60.9%) 11.9% Eu (10.7%) 4.5% Mo (3.6%) 25.2% O (26.8%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado, L.; García-Moncada, N.; Bobadilla, L.F.; Romero-Sarria, F.; Odriozola, J.A. Elucidation of Water Promoter Effect of Proton Conductor in WGS Reaction over Pt-Based Catalyst: An Operando DRIFTS Study. Catalysts 2020, 10, 841. https://doi.org/10.3390/catal10080841
Jurado L, García-Moncada N, Bobadilla LF, Romero-Sarria F, Odriozola JA. Elucidation of Water Promoter Effect of Proton Conductor in WGS Reaction over Pt-Based Catalyst: An Operando DRIFTS Study. Catalysts. 2020; 10(8):841. https://doi.org/10.3390/catal10080841
Chicago/Turabian StyleJurado, Lole, Nuria García-Moncada, Luis F. Bobadilla, Francisca Romero-Sarria, and José A. Odriozola. 2020. "Elucidation of Water Promoter Effect of Proton Conductor in WGS Reaction over Pt-Based Catalyst: An Operando DRIFTS Study" Catalysts 10, no. 8: 841. https://doi.org/10.3390/catal10080841
APA StyleJurado, L., García-Moncada, N., Bobadilla, L. F., Romero-Sarria, F., & Odriozola, J. A. (2020). Elucidation of Water Promoter Effect of Proton Conductor in WGS Reaction over Pt-Based Catalyst: An Operando DRIFTS Study. Catalysts, 10(8), 841. https://doi.org/10.3390/catal10080841