Catalytic Pyrolysis as a Technology to Dispose of Herbal Medicine Waste
Abstract
1. Introduction
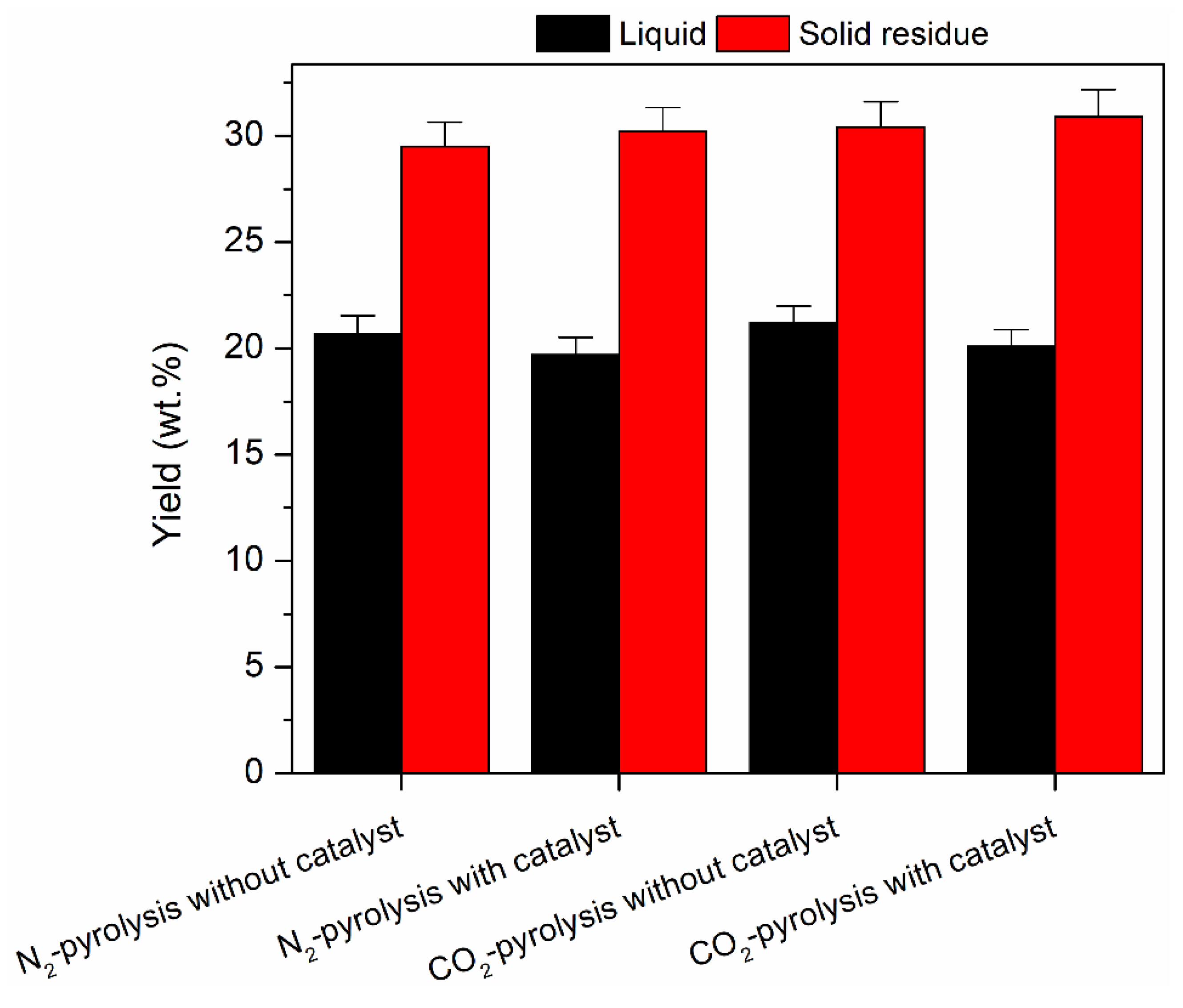
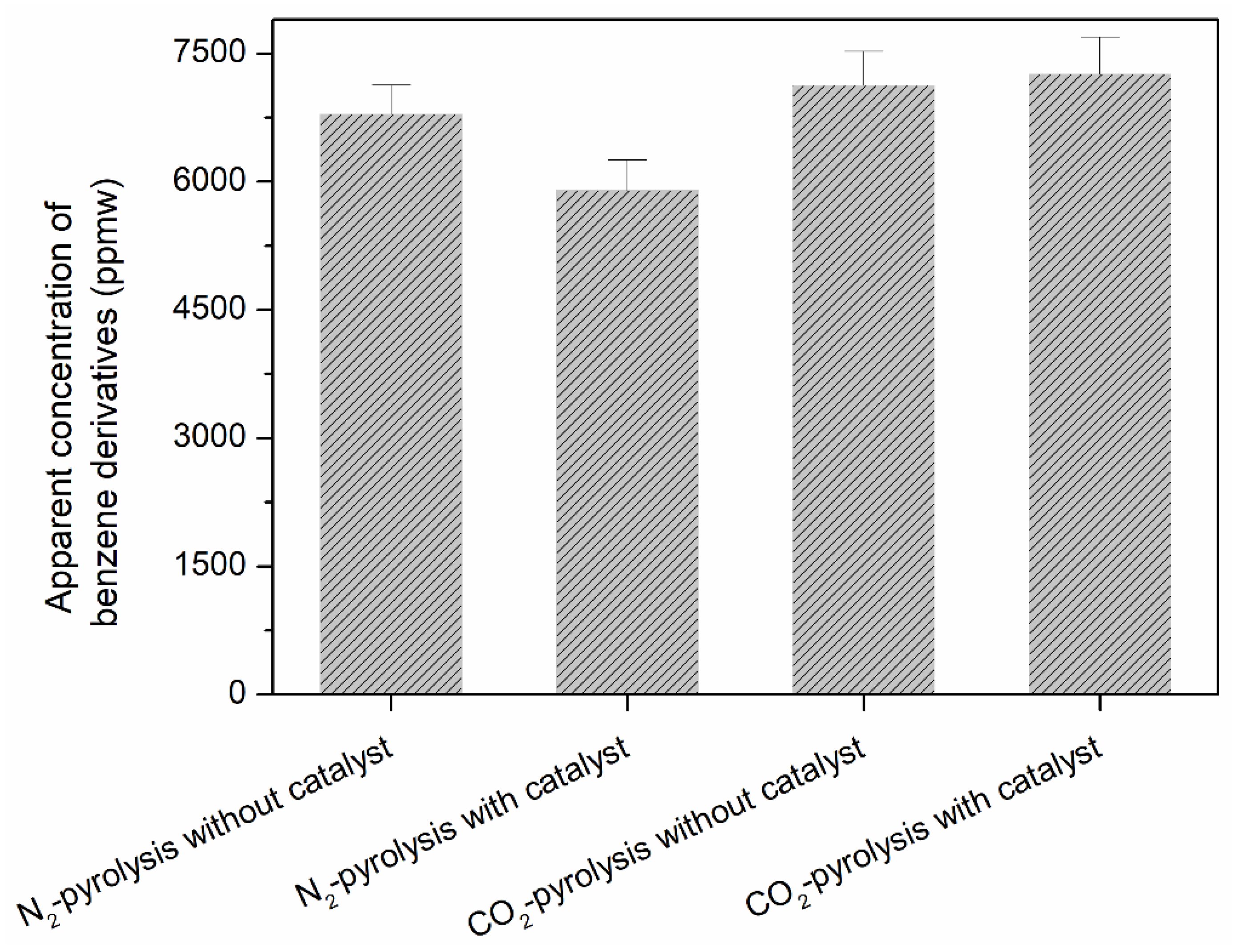
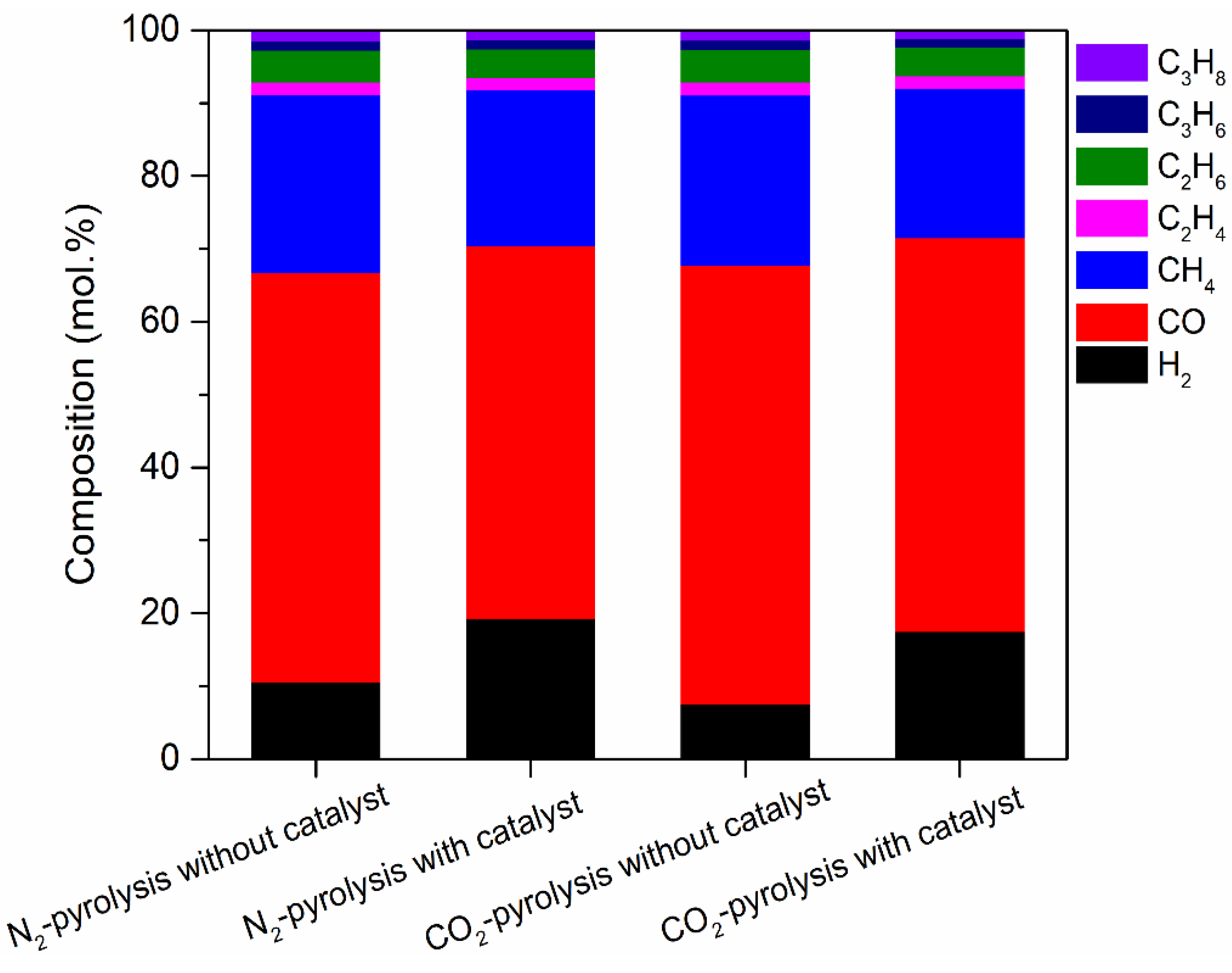
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Characteristic Analysis
3.3. Pyrolysis Experiments and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Traditional Medicine Strategy 2002–2005; (WHO/EDM/TRM/2002.1); WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Hexa Research. Herbal Medicine Market Size and Forecast, By Product (Tablets & Capsules, Powders, Extracts), By Indication (Digestive Disorders, Respiratory Disorders, Blood Disorders), And Trend Analysis, 2014–2024. 2017. Available online: https://www.hexaresearch.com/research-report/global-herbal-medicine-market (accessed on 14 April 2020).
- MarketWatch. Herbal Medicine Market Research Reports 2019. Global Industry Size, Share, Emerging Trends, Growth Boosted By Demand and Advanced Technology till 2023. 2019. Available online: https://www.marketwatch.com/press-release/herbal-medicine-market-research-reports-2019-global-industry-size-share-emerging-trends-growth-boosted-by-demand-and-advanced-technology-till-2023-2019-2010-2002 (accessed on 14 April 2020).
- Mi, T.; Yu, X.-M. Study on fluidization characteristics of chinese herbal medicine waste in a fluidized bed reactor. Appl. Mech. Mater. 2012, 138, 952–957. [Google Scholar] [CrossRef]
- Soetrisnanto, D.; Christwardana, M.; Hadiyanto, H. Application of phytoremediation for herbal medicine waste and its utilization for protein production. Reaktor 2012, 14, 129–134. [Google Scholar] [CrossRef]
- Chen, M.; Tang, R.; Fu, G.; Xu, B.; Zhu, P.; Qiao, S.; Chen, X.; Xu, B.; Qin, Y.; Lu, C.; et al. Association of exposure to phenols and idiopathic male infertility. J. Hazard. Mater. 2013, 250, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, J.; Sun, C. Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potential polluted river basin in China. Int. J. Environ. Res. Public Health 2017, 14, 1140. [Google Scholar] [CrossRef]
- Ali, M.; Duba, K.S.; Kalamdhad, A.S.; Bhatia, A.; Khursheed, A.; Kazmi, A.A.; Ahmed, N. High rate composting of herbal pharmaceutical industry solid waste. Water Sci. Technol. 2012, 65, 1817–1825. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.W.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Andrew Lin, K.-Y.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, H.W.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Ha, J.-M.; Park, Y.-K. Catalytic fast co-pyrolysis of organosolv lignin and polypropylene over in-situ red mud and ex-situ HZSM-5 in two-step catalytic micro reactor. Appl. Surf. Sci. 2020, 511, 145521. [Google Scholar] [CrossRef]
- Lam, S.S.; Wan Mahari, W.A.; Ok, Y.S.; Peng, W.; Chong, C.T.; Ma, N.L.; Chase, H.A.; Liew, Z.; Yusup, S.; Kwon, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sust. Energ. Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, E.E.; Park, Y.-K. Recent advances in the catalytic pyrolysis of microalgae. Catal. Today 2019. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Lam, K.L.; Hui, C.W. Charcoal production via multistage pyrolysis. Chin. J. Chem. Eng. 2012, 20, 455–460. [Google Scholar] [CrossRef]
- Alonso, M.Z.; Tran, K.-Q.; Wang, L.; Skreiberg, Ø. A kinetic study on simultaneously boosting the mass and fixed-carbon yield of charcoal production via atmospheric carbonization. Energy Proc. 2017, 120, 333–340. [Google Scholar] [CrossRef]
- Demirbas, A.; Ahmad, W.; Alamoudi, R.; Sheikh, M. Sustainable charcoal production from biomass. Energy Sources Part A 2016, 38, 1882–1889. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Liew, R.K.; Osman, M.S.; Lee, C.L.; Chuah, J.H.; Park, Y.-K.; Lam, S.S. Microwave steam activation, an innovative pyrolysis approach to convert waste palm shell into highly microporous activated carbon. J. Environ. Manag. 2019, 236, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Su, M.H.; Nam, W.L.; Thoo, D.S.; Ng, C.M.; Liew, R.K.; Yuh Yek, P.N.; Ma, N.L.; Nguyen Vo, D.V. Microwave pyrolysis with steam activation in producing activated carbon for removal of herbicides in agricultural surface water. Ind. Eng. Chem. Res. 2019, 58, 695–703. [Google Scholar] [CrossRef]
- Azwar, E.; Wan Mahari, W.A.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrog. Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Jung, J.-M.; Oh, J.-I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.; Lee, J. Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J. Hazard. Mater. 2020, 392, 122464. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Kwon, E.E.; Lee, J. Effect of carbon dioxide on thermal treatment of food waste as a sustainable disposal method. J. CO2 Util. 2020, 36, 76–81. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J. Pyrolysis of food waste over a Pt catalyst in CO2 atmosphere. J. Hazard. Mater. 2020, 393, 122449. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Current catalytic processes for biomass conversion. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 29–52. [Google Scholar]
- Kim, S.; Tsang, Y.F.; Kwon, E.E.; Lin, K.-Y.A.; Lee, J. Recently developed methods to enhance stability of heterogeneous catalysts for conversion of biomass-derived feedstocks. Korean J. Chem. Eng. 2019, 36, 1–11. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, E.E.; Kim, Y.T.; Jung, S.; Kim, H.J.; Huber, G.W.; Lee, J. Recent advances in hydrodeoxygenation of biomass-derived oxygenates over heterogeneous catalysts. Green Chem. 2019, 21, 3715–3743. [Google Scholar] [CrossRef]
- Jenkins, N.; Ekanayake, J. Bioenergy. In Renewable Energy Engineering; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- NCBI. National Center for Biotechnology Information. PubChem Database. Catechol, CID=289. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Catechol#datasheet=LCSS (accessed on 18 April 2020).
- NCBI. National Center for Biotechnology Information. PubChem Database. Hydroquinone, CID=785. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydroquinone#datasheet=LCSS (accessed on 18 April 2020).
- NCBI. National Center for Biotechnology Information. PubChem Database. Indole, CID=798. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indole#datasheet=LCSS (accessed on 18 April 2020).
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y.; Uzoejinwa, B.B.; Cao, B.; He, Z.; Wang, Q.; Xu, S. Pyrolysis mechanisms of typical seaweed polysaccharides. J. Anal. Appl. Pyrol. 2017, 124, 373–383. [Google Scholar] [CrossRef]
- Yanishlieva, N.; Schiller, H.; Marinova, E. Autoxidation of sitosterol. II: Main products formed at ambient and high temperature treatment with oxygen. Riv. Ital. Sostanze Grasse 1980, 57, 572–576. [Google Scholar]
- Cert, A.; Lanzón, A.; Carelli, A.A.; Albi, T.; Amelotti, G. Formation of stigmasta-3,5-diene in vegetable oils. Food Chem. 1994, 49, 287–293. [Google Scholar] [CrossRef]
- Ye, J.-C.; Chang, W.-C.; Hsieh, D.J.-Y.; Hsiao, M.-W. Extraction and analysis of β-sitosterol in herbal medicines. J. Med. Plant Res. 2010, 4, 522–527. [Google Scholar]
- Greensfelder, B.S.; Voge, H.H.; Good, G.M. Catalytic and thermal cracking of pure hydrocarbons: Mechanisms of reaction. Ind. Eng. Chem. 1949, 41, 2573–2584. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.; Wimble, J.M.; Lucci, F.R.; Lee, S.; Michaelides, A.; Flytzani-Stephanopoulos, M.; Stamatakis, M.; Sykes, E.C.H. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat. Chem. 2018, 10, 325. [Google Scholar] [CrossRef]
- Cho, S.-H.; Lee, S.S.; Jung, S.; Park, Y.-K.; Lin, K.-Y.A.; Lee, J.; Kwon, E.E. Carbon dioxide-cofeeding pyrolysis of pine sawdust over nickle-based catalyst for hydrogen production. Energy Convers. Manag. 2019, 201, 112140. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.-E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar]
- Choi, D.; Oh, J.-I.; Baek, K.; Lee, J.; Kwon, E.E. Compositional modification of products from co-pyrolysis of chicken manure and biomass by shifting carbon distribution from pyrolytic oil to syngas using CO2. Energy 2018, 153, 530–538. [Google Scholar] [CrossRef]
- Cho, S.-H.; Lee, J.; Kim, K.-H.; Jeon, Y.J.; Kwon, E.E. Carbon dioxide assisted co-pyrolysis of coal and ligno-cellulosic biomass. Energy Convers. Manag. 2016, 118, 243–252. [Google Scholar] [CrossRef]
- Lee, J.; Yang, X.; Cho, S.-H.; Kim, J.-K.; Lee, S.S.; Tsang, D.C.W.; Ok, Y.S.; Kwon, E.E. Pyrolysis process of agricultural waste using CO2 for waste management, energy recovery, and biochar fabrication. Appl. Energy 2017, 185, 214–222. [Google Scholar] [CrossRef]

| Proximate analysis (wt %) | |
| Moisture | 7.7 |
| Volatile matter | 80.4 |
| Fixed matter | 9.3 |
| Ash | 2.6 |
| Ultimate analysis (wt %) | |
| C | 46.6 |
| H | 6.1 |
| O | 45.8 |
| N | 1.5 |
| S | N.D. |
| Component analysis (wt %) | |
| Cellulose | 26.1 |
| Hemicellulose | 19.2 |
| Lignin | 23.5 |
| Pt loading (wt %) | 5 |
| Pt dispersion (%) | 49.4 |
| Average Pt particle size a (nm) | 2.4 |
| Pt particle size b (nm) | 2.27 |
| Specific surface area (m2 g−1) | 1608.3 |
| Average pore diameter (nm) | 4.7 |
| Total pore volume (cm3 g−1) | 0.98 |
| Entry | Chemical Name | Chemical Formula | MW | Chemical Structure | Apparent Concentration a (ppm, Weight Basis) | |||
|---|---|---|---|---|---|---|---|---|
| N2 without Catalyst | N2 with Catalyst | CO2 without Catalyst | CO2 with Catalyst | |||||
| 1 | 1,2-Benzenediol | C6H6O2 | 110.11 | 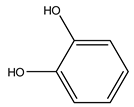 | 0 | 0 | 180 | 170 |
| 2 | 1,4-Benzenediol | C6H6O2 | 110.11 | 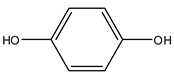 | 0 | 0 | 300 | 270 |
| 3 | 2,3-Benzopyrrole | C8H7N | 117.15 | 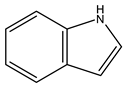 | 72 | 75 | 87 | 65 |
| 4 | Benzo[h]quinoline | C13H9N | 179.22 |  | 99 | 130 | 90 | 91 |
| 5 | 2,3-Dihydro-2-(1-methylethenyl)-7H-furo [3,2-g][1]benzopyran-7-one | C14H12O3 | 228.24 |  | 140 | 86 | 120 | 260 |
| 6 | (S)-7,8-Dihydro-7-hydroxy-8,8-dimethyl-2H,6H-benzo(1,2-b:5,4-b’)dipyran-2-one | C14H14O4 | 246.26 | 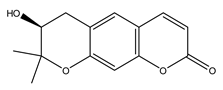 | 200 | 91 | 260 | 270 |
| 7 | (E)-4-(2,2-Dimethyl-2H-chromen-3-yl)but-3-en-2-one | C15H16O2 | 228.29 |  | 390 | 340 | 410 | 410 |
| 8 | 2-(1-Hydroxy-1-methylethyl)-2,3-dihydrofuro[3,2-g] chromen-7-one | C14H14O4 | 246.26 | 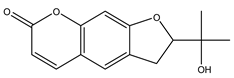 | 160 | 140 | 150 | 120 |
| 9 | 4,4,9-Trimethylnaphtho[1,2-b]furan-5(4H)-one | C15H14O2 | 226.27 |  | 640 | 580 | 640 | 970 |
| 10 | 2H,8H-Benzo[1,2-b:5,4-b’]dipyran-2-one | C12H8O3 | 200.19 |  | 340 | 260 | 380 | 450 |
| 11 | N-isopropyl-1,6-dimethyl-2-methylene-1,2-dihydroquinolin-5-amine | C15H20N2 | 228.33 | 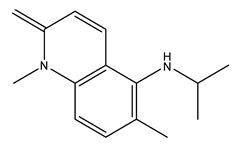 | 190 | 130 | 290 | 260 |
| 12 | 2-Methyl-2-phenyl-1,2,3,7-tetrahydro-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-amine | C11H14N6 | 230.27 | 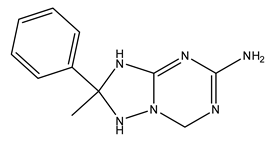 | 3800 | 3300 | 3500 | 3300 |
| Entry | Chemical Name | Chemical Formula | MW | Chemical Structure | Apparent Concentration a (ppm, Weight Basis) | |||
|---|---|---|---|---|---|---|---|---|
| N2 without Catalyst | N2 with Catalyst | CO2 without Catalyst | CO2 with Catalyst | |||||
| 1 | Caprolactam | C6H11NO | 113.16 | 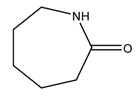 | 0 | 0 | 230 | 50 |
| 2 | Pyrrolo [1,2,a] pyrazine-1,4-dione | C7H4N2O2 | 148.12 | 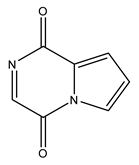 | 76 | 41 | 120 | 100 |
| 3 | 3,9 Diazatricyclo [7.3.0.0(3,7)] dodecan-2,8-dione | C10H14N2O2 | 194.23 |  | 210 | 150 | 280 | 250 |
| 4 | Palmitic acid | C16H32O2 | 256.42 | 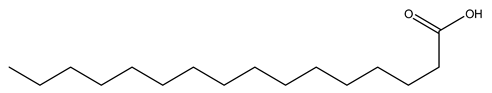 | 340 | 270 | 200 | 260 |
| 5 | Americanolide D | C15H20O2 | 232.32 | 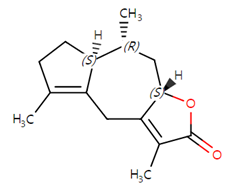 | 150 | 130 | 190 | 210 |
| 6 | 9,12-Octadecadienoic acid | C18H32O2 | 280.45 | 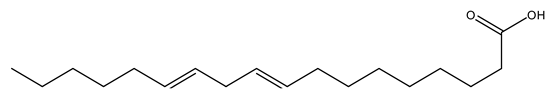 | 81 | 85 | 81 | 75 |
| 7 | 9-Octadecenoic acid | C18H34O2 | 282.46 | 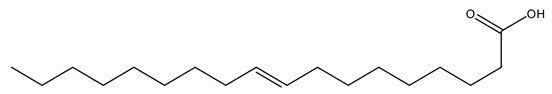 | 960 | 760 | 530 | 370 |
| 8 | Stearic acid | C18H36O2 | 284.48 | 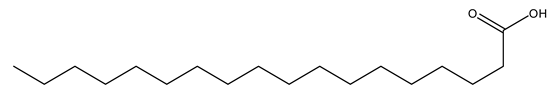 | 160 | 190 | 100 | 140 |
| 9 | 1-Hexadecene | C16H32 | 224.43 |  | 99 | 100 | 0 | 0 |
| 10 | 9-Octadecenamide | C18H35NO | 281.48 | 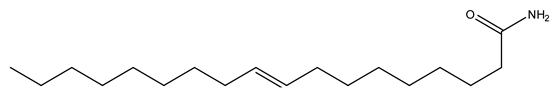 | 190 | 64 | 0 | 0 |
| 11 | 3-Methyl-but-2-enoic acid | C5H8O2 | 100.12 | 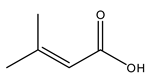 | 2100 | 1700 | 1800 | 1200 |
| 12 | Tetracosa-2,6,10,14,18,22-hexaene | C24H38 | 326.56 |  | 99 | 110 | 0 | 0 |
| 13 | Eicosane | C20H42 | 282.55 |  | 120 | 170 | 73 | 59 |
| 14 | Stigmastan-3,5-diene | C29H48 | 396.69 |  | 760 | 830 | 720 | 590 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Kim, S.; Kim, J.; Shin, G.-A.; Lee, C.-G.; Jung, S.; Lee, J. Catalytic Pyrolysis as a Technology to Dispose of Herbal Medicine Waste. Catalysts 2020, 10, 826. https://doi.org/10.3390/catal10080826
Lee Y, Kim S, Kim J, Shin G-A, Lee C-G, Jung S, Lee J. Catalytic Pyrolysis as a Technology to Dispose of Herbal Medicine Waste. Catalysts. 2020; 10(8):826. https://doi.org/10.3390/catal10080826
Chicago/Turabian StyleLee, Younghyun, Soosan Kim, Jisu Kim, Gwy-Am Shin, Chang-Gu Lee, Seungho Jung, and Jechan Lee. 2020. "Catalytic Pyrolysis as a Technology to Dispose of Herbal Medicine Waste" Catalysts 10, no. 8: 826. https://doi.org/10.3390/catal10080826
APA StyleLee, Y., Kim, S., Kim, J., Shin, G.-A., Lee, C.-G., Jung, S., & Lee, J. (2020). Catalytic Pyrolysis as a Technology to Dispose of Herbal Medicine Waste. Catalysts, 10(8), 826. https://doi.org/10.3390/catal10080826







