Conversion of Xylose to Furfural over Lignin-Based Activated Carbon-Supported Iron Catalysts
Abstract
1. Introduction
2. Results and Discussion
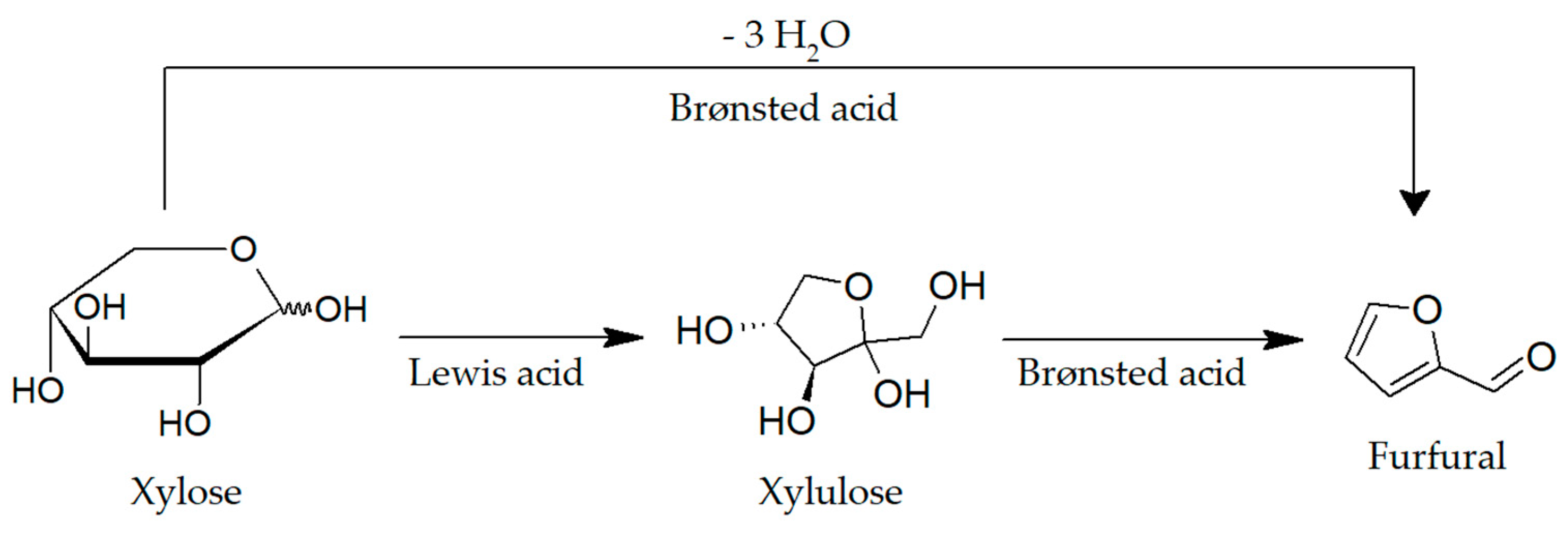
2.1. Preliminary Studies
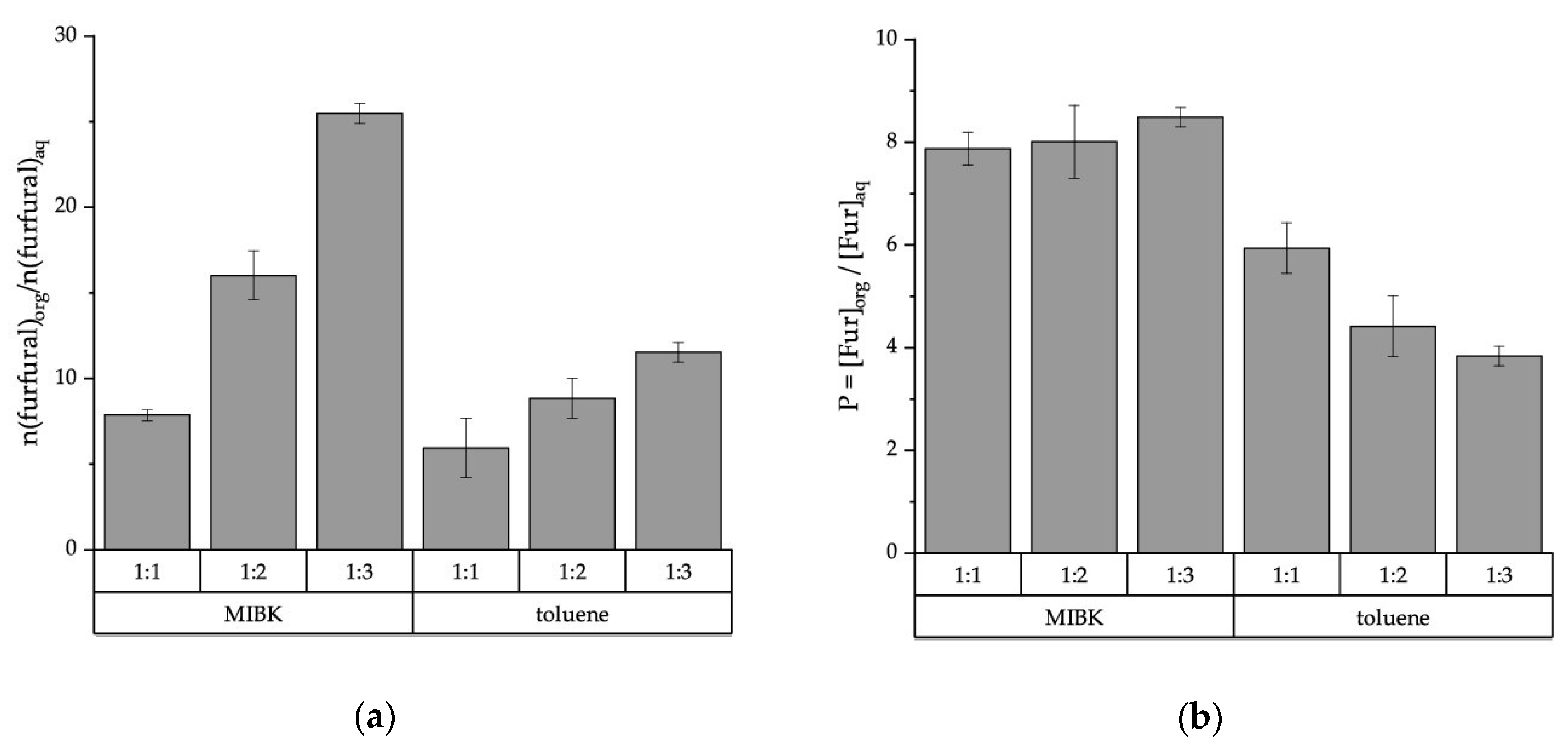
2.1.1. Furfural Partitioning in Biphasic Reaction System
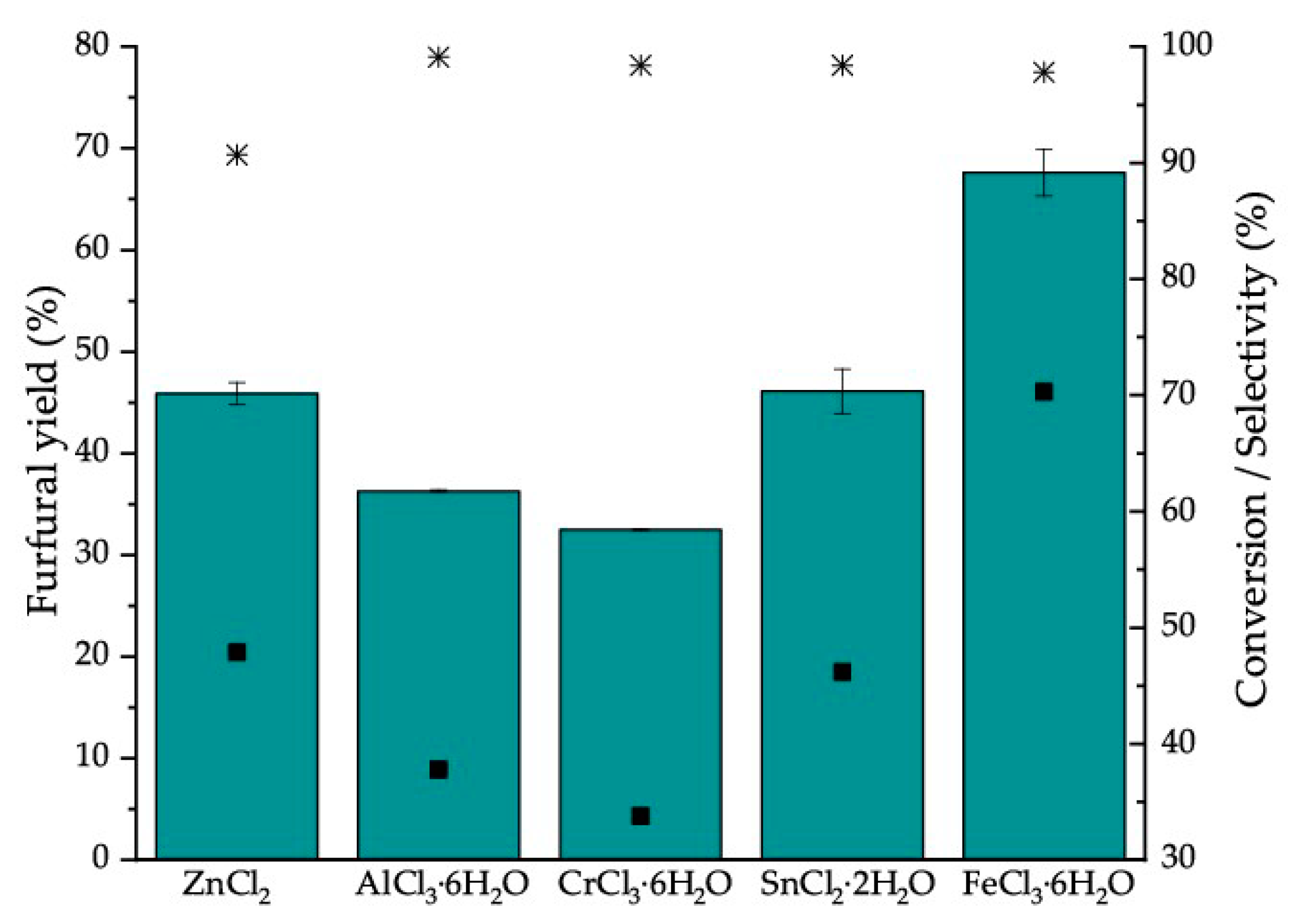
2.1.2. Furfural Production Using Homogeneous Catalysts
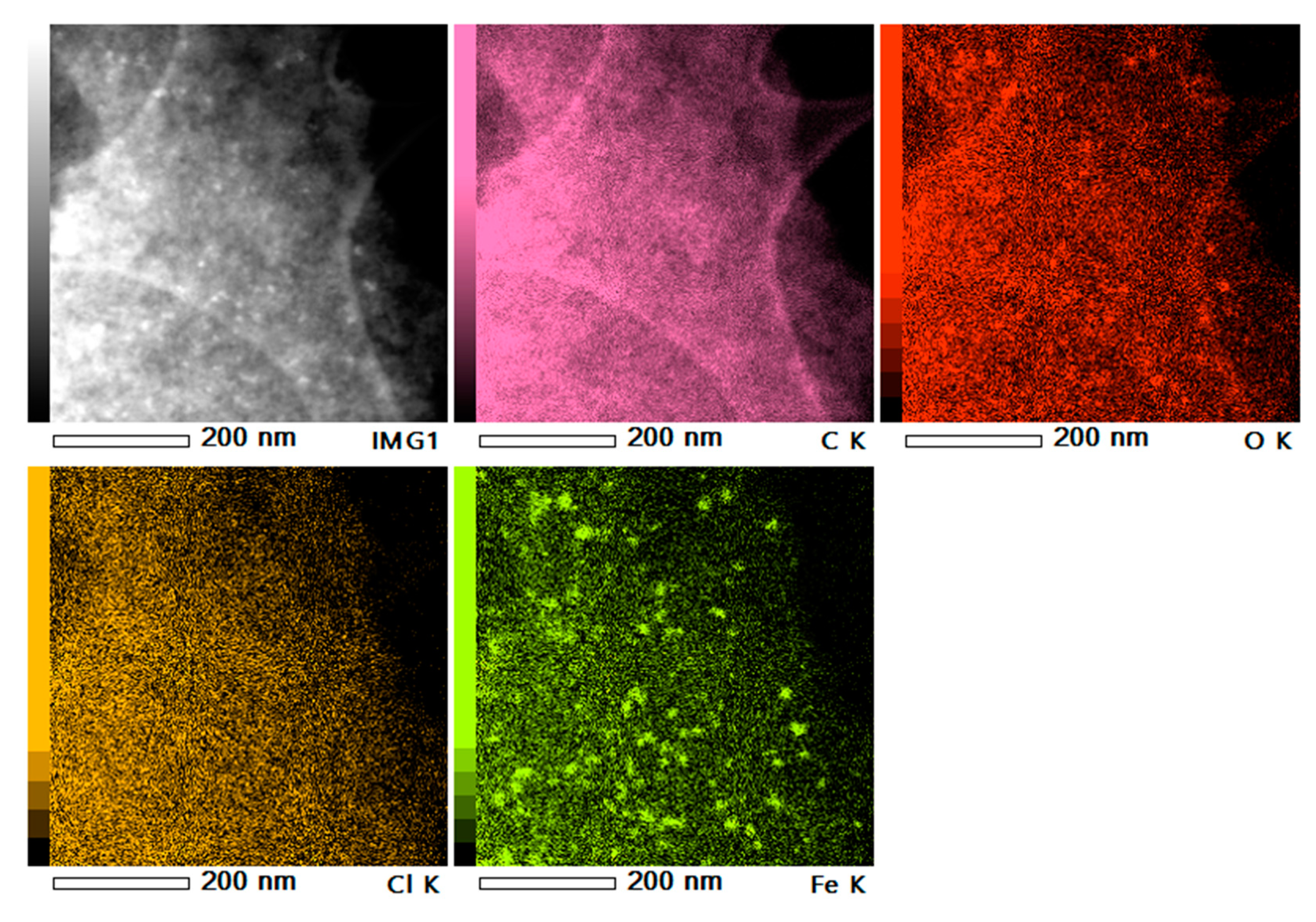
2.2. Preparation and Characterization of Heterogeneous Catalysts
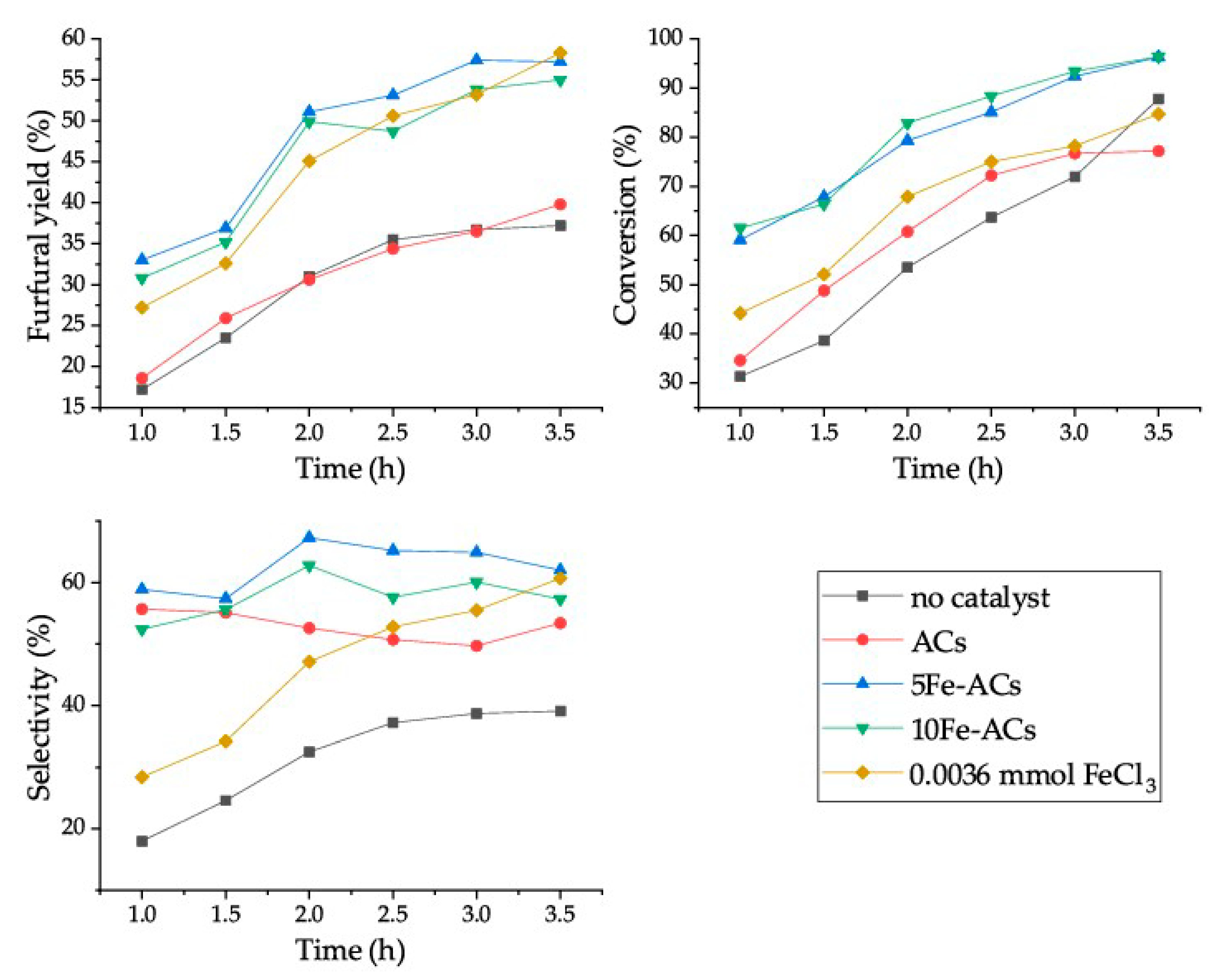
2.3. Furfural Production Using Heterogeneous Catalysts
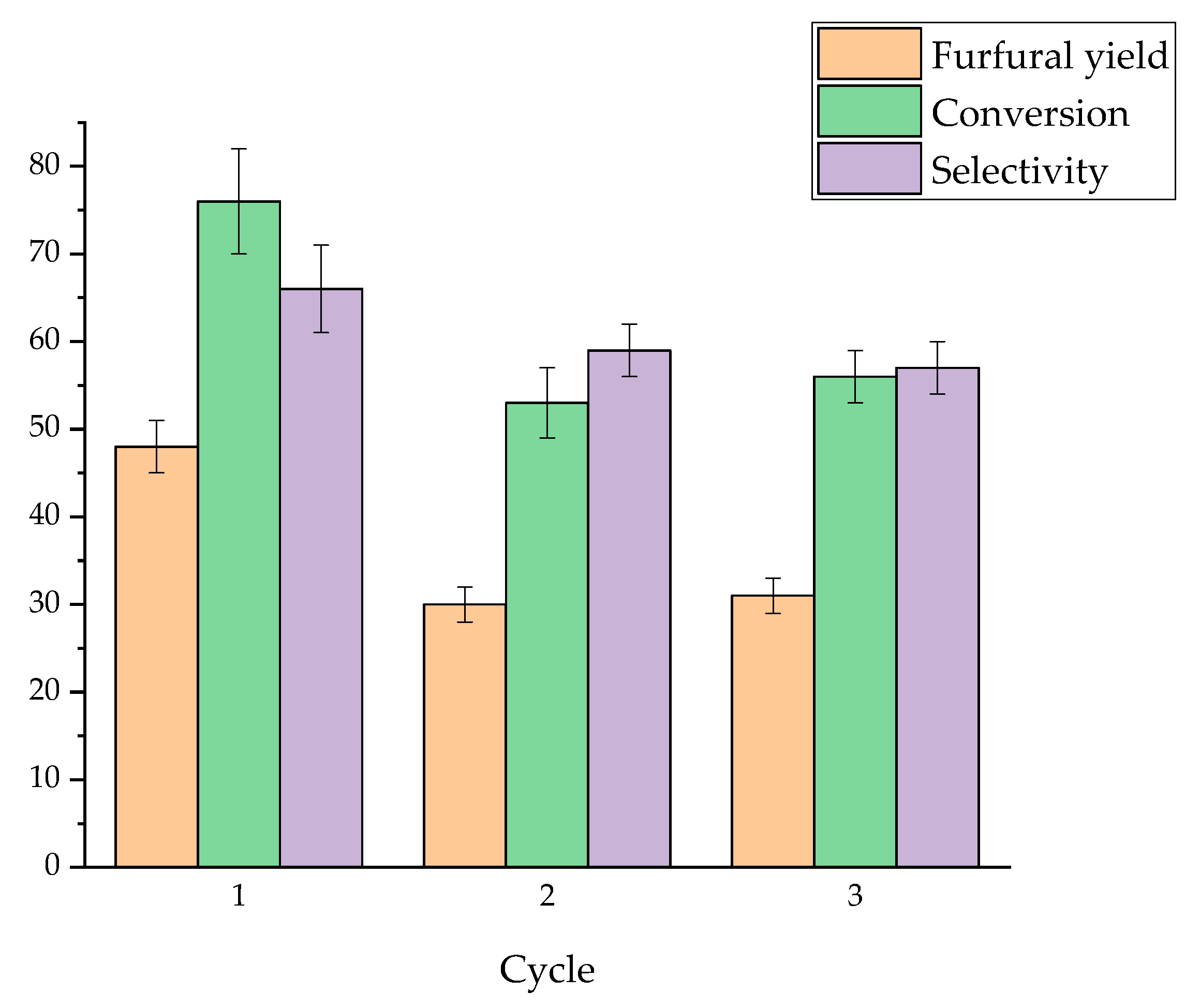
2.4. Recycling Experiments
3. Materials and Methods
3.1. Materials
3.2. Furfural Partitioning in Biphasic Reactor System
3.3. Catalyst Preparation and Characterization
3.4. Furfural Production from Xylose
3.5. Catalyst Recycling
3.6. Analytical Methods for Conversion Studies
3.7. Equations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I–Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Kottke, R.H. Furan derivatives. In Encyclopedia of Chemical Technology; Othmer, K., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; Volume 12, pp. 259–286. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar] [CrossRef]
- Win, D. Furfural–Gold from Garbage. Au J. Technol. 2005, 8, 185–190. [Google Scholar]
- Zeitsch, K. The Chemistry and Technology of Furfural and its Many By–Products; Elsevier: Amsterdam, The Netherlands, 2000; pp. 36–74. [Google Scholar]
- Dashtban, M.; Gilbert, A.; Fatehi, P. Production of furfural: Overview and challenges. J. Sci. Technol. For. Prod. Process 2012, 2, 44–53. [Google Scholar]
- Yemis, O.; Mazza, G. Catalytic Performances of Various Solid Catalysts and Metal Halides for Microwave–Assisted Hydrothermal Conversion of Xylose, Xylan, and Straw to Furfural. Waste Biomass Valori. 2019, 10, 1343–1353. [Google Scholar] [CrossRef]
- Le Guenic, S.; Delbecq, F.; Ceballos, C.; Len, C. Microwave–assisted dehydration of D–xylose into furfural by diluted inexpensive inorganic salts solution in a biphasic system. J. Mol. Catal. A Chem. 2015, 410, 1–7. [Google Scholar] [CrossRef]
- Liu, C.; Wyman, C.E. The enhancement of xylose monomer and xylotriose degradation by inorganic salts in aqueous solutions at 180 °C. Carbohydr. Res. 2006, 341, 2550–2556. [Google Scholar] [CrossRef]
- Ershova, O.; Nieminen, K.; Sixta, H. The Role of Various Chlorides on Xylose Conversion to Furfural: Experiments and Kinetic Modeling. Chem. Cat. Chem. 2017, 9, 3031–3040. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of Sugars and Biomass to Furans Using Heterogeneous Catalysts in Biphasic Solvent Systems. Chem. Cat. Chem. 2018, 10, 4819–4830. [Google Scholar] [CrossRef]
- Da Costa Lopez, A.M.; Morais, A.R.C.; Łukasik, R.M.; Fang, Z.; Smith, R.L., Jr.; Qi, X. Sustainable Catalytic Strategies for C5-Sugars and Biomass Hemicellulose Conversion Towards Furfural Production. In Production of Platform Chemicals from Sustainable Resources; Springer Nature: Singapore, 2017; Volume 7, pp. 45–80. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Liu, S. Efficient Production of Furan Derivatives from a Sugar Mixture by Catalytic. Process. Energ. Fuel 2012, 26, 4560–4567. [Google Scholar] [CrossRef]
- Chareonlimkun, A.; Champreda, V.; Shotipruk, A.; Laosiripojana, N. Reactions of C5 and C6-sugars, cellulose, and lignocellulose under hot compressed water (HCW) in the presence of heterogeneous acid catalysts. Fuel 2010, 89, 2873–2880. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Li, P.; Yi, H.; Yang, M.; Wang, G. Catalytic conversion of xylose to furfural over the solid acid SO42-/ZrO2–Al2O3/SBA-15 catalysts. Carbohydr. Res. 2011, 346, 480–487. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Mesoporous silica–supported 12–tungstophosphoric acid catalysts for the liquid phase dehydration of d-xylose. Micropor. Mesopor. Mater. 2006, 94, 214–225. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Conner, W.C., Jr.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose Isomerization to Xylulose and its Dehydration to Furfural in Aqueous Media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural Using Lewis and Brønsted Acid Catalysts in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Mazzotta, M.G.; Gupta, D.; Saha, B.; Patra, A.K.; Bhaumik, A.; Abu-Omar, M.M. Efficient Solid Acid Catalyst Containing Lewis and Brønsted Acid Sites for the Production of Furfurals. ChemSusChem 2014, 7, 2342–2350. [Google Scholar] [CrossRef]
- Russo, P.A.; Lima, S.; Rebuttini, V.; Pillinger, M.; Willinger, M.; Pinna, N.; Valente, A.A. Microwave-assisted coating of carbon nanostructures with titanium dioxide for the catalytic dehydration of d-xylose into furfural. RSC Adv. 2013, 3, 2595–2603. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez–Serrano, V. Preparation of activated carbon-metal oxide hybrid catalysts: Textural characterization. Fuel Process. Technol. 2014, 126, 95–103. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez–Serrano, V. Preparation and Microstructural Characterization of Activated Carbon–Metal Oxide Hybrid Catalysts: New Insights into Reaction Paths. J. Mater. Sci. Technol. 2015, 31, 806–814. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of Activated Carbon-SnO2, TiO2, and WO3 Catalysts. Study by FT-IR Spectroscopy. Ind. Eng. Chem. Res. 2016, 55, 5200–5206. [Google Scholar] [CrossRef]
- Mittal, A.; Black, S.K.; Vinzant, T.B.; O’Brien, M.; Tucker, M.P.; Johnson, D.K. Production of Furfural from Process-Relevant Biomass-Derived Pentoses in a Biphasic Reaction System. ACS Sustain. Chem. Eng. 2017, 5, 5694–5701. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Peyron, D.; Duhamet, J.; Rivalier, P. Selective preparation of furfural from xylose over microporous solid acid catalysts. Ind. Crops Prod. 1998, 7, 95–99. [Google Scholar] [CrossRef]
- Brouwer, T.; Blahusiak, M.; Babic, K.; Schuur, B. Reactive extraction and recovery of levulinic acid, formic acid and furfural from aqueous solutions containing sulphuric acid. Sep. Purif. Technol. 2017, 185, 186–195. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wang, P.; Li, Y. Production of furfural from xylose, xylan and corncob in gamma-valerolactone using FeCl3·6H2O as catalyst. Bioresour. Technol. 2014, 151, 355–360. [Google Scholar] [CrossRef]
- Vom Stein, T.; Grande, P.M.; Leitner, W.; Dominguez de Maria, P. Iron-Catalyzed Furfural Production in Biobased Biphasic Systems: From Pure Sugars to Direct Use of Crude Xylose Effluents as Feedstock. ChemSusChem 2011, 4, 1592–1594. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Wang, J.; Hu, Q. Furfural production from dehydration of xylose catalyzed by chromium chloride in biphasic system. Chem. Eng. 2014, 3, 54–58. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.; Abu–Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5–hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Marcotullio, G.; Jong, W.D. Chloride ions enhance furfural formation from D–xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739–1746. [Google Scholar] [CrossRef]
- Varila, T.; Bergna, D.; Lahti, R.; Romar, H.; Hu, T.; Lassi, U. Activated carbon production from peat using ZnCl2: Characterization and applications. Bioresources 2017, 12, 8078–8092. [Google Scholar] [CrossRef]
- Lahti, R.; Bergna, D.; Romar, H.; Tuuttila, T.; Hu, T.; Lassi, U. Physico–chemical properties and use of waste biomass-derived activated carbons. Chem. Eng. Trans. 2017, 57. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoelectrons de la degradation superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 45. [Google Scholar]
- Li, J.; Liu, J.; Zhou, H.; Fu, Y. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Nitrogen-Doped Carbon-Supported Iron Catalysts. ChemSusChem 2016, 9, 1339–1347. [Google Scholar] [CrossRef]
- Barroso–Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Activated carbon surface chemistry: Changes upon impregnation with Al(III), Fe(III) and Zn(II)-metal oxide catalyst precursors from NO3− aqueous solutions. Arab. J. Chem. 2019, 12, 3963–3976. [Google Scholar] [CrossRef]
- Xiong, Y.; Tong, Q.; Shan, W.; Xing, Z.; Wang, Y.; Wen, S.; Lou, Z. Arsenic transformation and adsorption by iron hydroxide/manganese dioxide doped straw activated carbon. Appl. Surf. Sci. 2017, 416, 618–627. [Google Scholar] [CrossRef]
- Pakuła, M.; Biniak, S.; Świa̧tkowski, A. Chemical and Electrochemical Studies of Interactions between Iron(III) Ions and an Activated Carbon Surface. Langmuir 1998, 14, 3082–3089. [Google Scholar] [CrossRef]
- Chang, Q.; Lin, W.; Ying, W. Preparation of iron-impregnated granular activated carbon for arsenic removal from drinking water. J. Hazard. Mater. 2010, 184, 515–522. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave–Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Antal, M.J.; Leesomboon, T.; Mok, W.S.; Richards, G.N. Mechanism of formation of 2-furaldehyde from d-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Védrine, J.C. Heterogeneous Catalyst Preparation. In Basic Principles in Applied Catalysis; Baerns, M., Ed.; Springer: Berlin, Germany, 2004; Volume 75, pp. 215–257. [Google Scholar] [CrossRef]
- Jüntgen, H. Activated carbon as catalyst support: A review of new research results. Fuel 1986, 65, 1436–1446. [Google Scholar] [CrossRef]
- Matatov-Meytal, Y.I.; Sheintuch, M. Catalytic Abatement of Water Pollutants. Ind. Eng. Chem. Res. 1998, 37, 309–326. [Google Scholar] [CrossRef]
- Kraemer, S.M. Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci. 2004, 66, 3–18. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, X.; Lin, Q.; Zhang, X.; Meng, L.; Sun, R.; Xin, F.; Ren, J. New Understandings of the Relationship and Initial Formation Mechanism for Pseudo–lignin, Humins, and Acid–Induced Hydrothermal Carbon. J. Agric. Food Chem. 2018, 66, 11981–11989. [Google Scholar] [CrossRef] [PubMed]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Sumerskii, I.V.; Krutov, S.M.; Zarubin, M.Y. Humin-like substances formed under the conditions of industrial hydrolysis of wood. Russ. J. Appl. Chem. 2010, 83, 320–327. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Dehydration of xylose into furfural over micro–mesoporous sulfonic acid catalysts. J. Catal. 2005, 229, 414–423. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, E.G.; Kim, S.B.; Park, E.D.; Kim, S.W. Fabrication of sulfonic acid modified mesoporous silica shells and their catalytic performance with dehydration reaction of d–xylose into furfural. Micropor. Mesopor. Mater. 2011, 144, 134–139. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A new analysis method for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- U.S. EPA. Method 3051A (SW–846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; U.S. EPA: Washington, DC, USA, 2007. [Google Scholar]
- Boehm, H.P. Chemical Identification of Surface Groups. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Elsevier Academic Press: San Diego, CA, USA, 1966; Volume 16, pp. 179–274. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm titration revisited (part i): Practical aspects for achieving a high precision in quantifying oxygen-containing surface groups on carbon materials. Carbon 2018, 4, 21. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Theriault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part, I. CO2 expulsion and endpoint determination. Carbon 2010, 48, 1252. [Google Scholar] [CrossRef]
- Oickle, A.M.; Goertzen, S.L.; Hopper, K.R.; Abdalla, Y.O.; Andreas, H.A. Standardization of the Boehm titration: Part II. Method of agitation, effect of filtering and dilute titrant. Carbon 2010, 48, 3313–3322. [Google Scholar] [CrossRef]

| Sample | Activation Method | Other Treatment | Metal Precursor | Initial Fe (wt%) | Measured Metal (wt%) | |
|---|---|---|---|---|---|---|
| Zn | Fe | |||||
| ACs | Steam (H2O) | - | - | - | 0.01 | 0.06 |
| 5Fe-ACs | Steam (H2O) | - | FeCl3 | 5 | - | 4.0 |
| 10Fe-ACs | Steam (H2O) | - | FeCl3 | 10 | - | 9.2 |
| ACz | ZnCl2 | - | - | - | 8.2 | 0.08 |
| 5FeNO3-ACz | ZnCl2 | - | FeNO3 | 5 | 4.6 | 5.0 |
| 5Fe-ACz | ZnCl2 | - | FeCl3 | 5 | 3.8 | 4.5 |
| ACzN | ZnCl2 | HNO3 | - | - | 0.07 | 0.06 |
| 5Fe-ACzN | ZnCl2 | HNO3 | FeCl3 | 5 | 0.08 | 5.5 |
| Entry | Sample | BET | DFT | |||
|---|---|---|---|---|---|---|
| BET SA (m2/g) | Avg. Pore Diam. (nm) | Total Pore Volume (cm3/g) | Mesopores (cm3/g) | Micropores (cm3/g) | ||
| 1 | ACs | 760 | 2.90 | 0.47 | 0.26 | 0.21 |
| 2 | 5Fe-ACs | 455 | 3.44 | 0.34 | 0.23 | 0.11 |
| 3 | 10Fe-ACs | 380 | 3.15 | 0.26 | 0.16 | 0.10 |
| 4 | ACz | 1470 | 2.29 | 0.72 | 0.31 | 0.41 |
| 5 | 5Fe-ACz | 1000 | 2.28 | 0.48 | 0.19 | 0.29 |
| 6 | 5FeNO3-ACz | 948 | 2.16 | 0.45 * | 0.15 | 0.29 |
| 7 | ACzN | 1091 | 2.15 | 0.49 | 0.16 | 0.33 |
| 8 | 5Fe-ACzN | 790 | 2.07 | 0.35 | 0.10 | 0.25 |
| Sample | XPS a | ||||
|---|---|---|---|---|---|
| Total C-% from C1s | Total O-% from O1s | Total Fe-% from Fe2p | Total Cl-% from Cl2p | Total Acidic Groups (mmol/g) b | |
| ACs | 96.8 | 3.0 | nd | nd | 0.07 |
| 5Fe-ACs | 93.7 | 4.1 | 0.6 | 1.5 | 1.77 |
| 10Fe-ACs | 86.2 | 9.6 | 2.4 | 1.6 | 1.95 |
| Entry | Catalyst | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| 1 | - | 12 | 18 | 12 |
| 2 | ACz | 28 | 82 | 36 |
| 3 | 5FeNO3-ACz | 23 | 91 | 27 |
| 4 | 5Fe-ACz | 32 | 66 | 51 |
| Time | 160 °C | 170 °C | 180 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Y (%) | C (%) | S (%) | Y (%) | C (%) | S (%) | Y (%) | C (%) | S (%) | |
| 1 | 25 | 50 | 54 | 36 | 82 | 46 | 38 | 97 | 41 |
| 1.5 | 32 | 66 | 51 | 44 | 93 | 49 | 39 | 99 | 41 |
| 2 | 38 | 79 | 51 | 50 | 96 | 55 | - | - | - |
| 2.5 | 38 | 85 | 48 | 44 | 98 | 48 | - | - | - |
| 3 | 47 | 89 | 55 | - | - | - | - | - | - |
| 4 | 47 | 95 | 56 | - | - | - | - | - | - |
| 5 | 48 | 98 | 54 | - | - | - | - | - | - |
| Entry | Catalyst | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| 1 | ACzN | 14 | 21 | 71 |
| 2 | 5Fe-ACzN | 22 | 47 | 50 |
| 3 | ACs | 14 | 19 | 81 |
| 4 | 5Fe-ACs | 25 | 36 | 72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanen, A.; Kupila, R.; Lappalainen, K.; Kärkkäinen, J.; Hu, T.; Lassi, U. Conversion of Xylose to Furfural over Lignin-Based Activated Carbon-Supported Iron Catalysts. Catalysts 2020, 10, 821. https://doi.org/10.3390/catal10080821
Rusanen A, Kupila R, Lappalainen K, Kärkkäinen J, Hu T, Lassi U. Conversion of Xylose to Furfural over Lignin-Based Activated Carbon-Supported Iron Catalysts. Catalysts. 2020; 10(8):821. https://doi.org/10.3390/catal10080821
Chicago/Turabian StyleRusanen, Annu, Riikka Kupila, Katja Lappalainen, Johanna Kärkkäinen, Tao Hu, and Ulla Lassi. 2020. "Conversion of Xylose to Furfural over Lignin-Based Activated Carbon-Supported Iron Catalysts" Catalysts 10, no. 8: 821. https://doi.org/10.3390/catal10080821
APA StyleRusanen, A., Kupila, R., Lappalainen, K., Kärkkäinen, J., Hu, T., & Lassi, U. (2020). Conversion of Xylose to Furfural over Lignin-Based Activated Carbon-Supported Iron Catalysts. Catalysts, 10(8), 821. https://doi.org/10.3390/catal10080821







