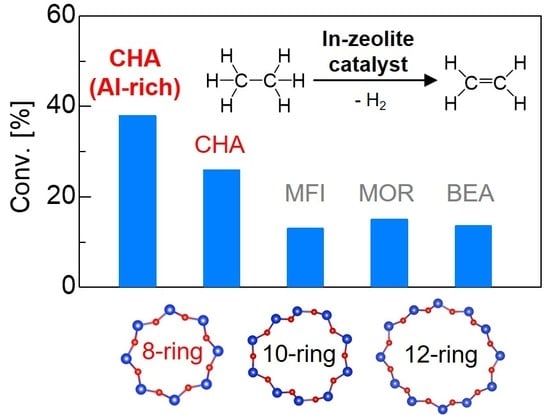
In-Exchanged CHA Zeolites for Selective Dehydrogenation of Ethane: Characterization and Effect of Zeolite Framework Type
Abstract
1. Introduction
2. Results
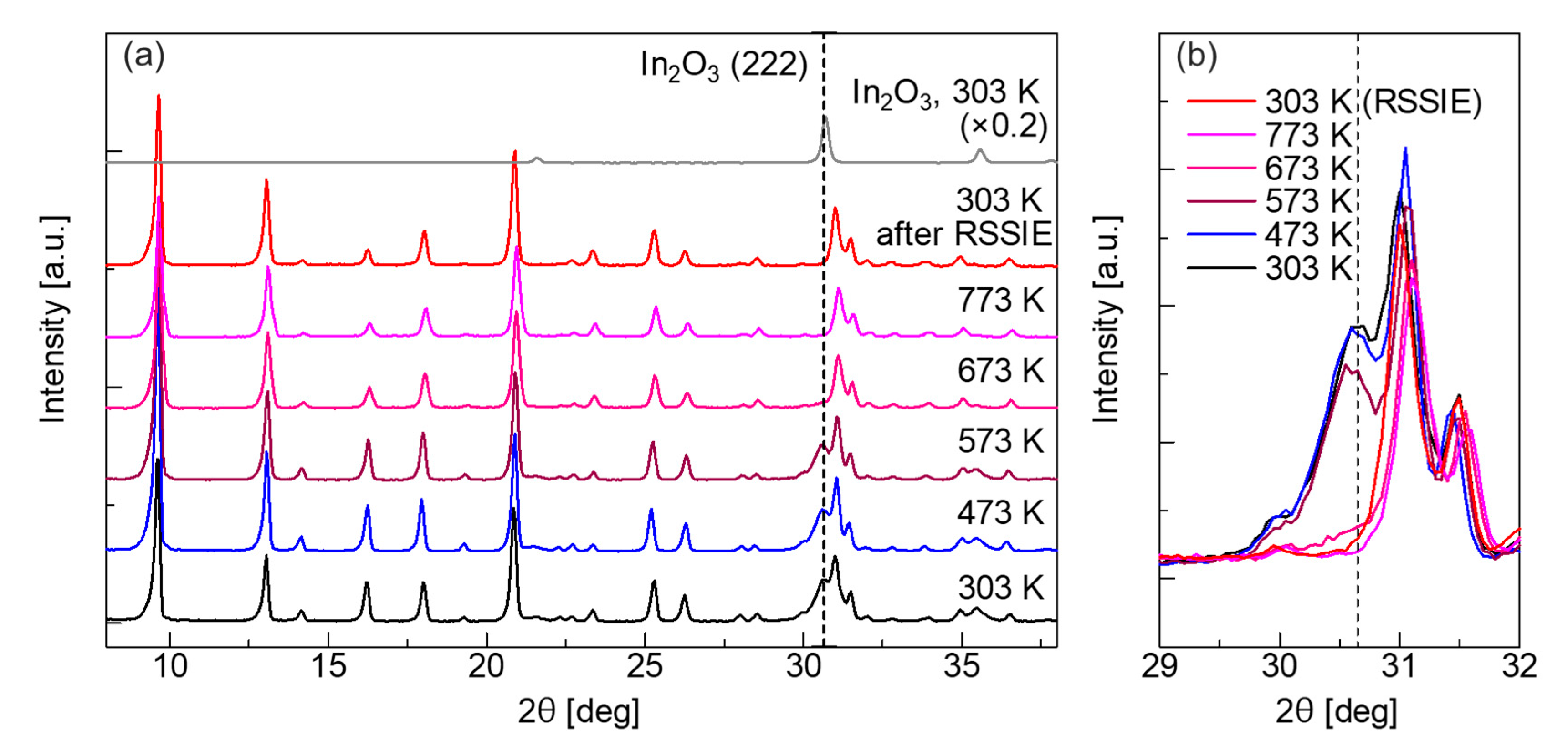
2.1. XRD
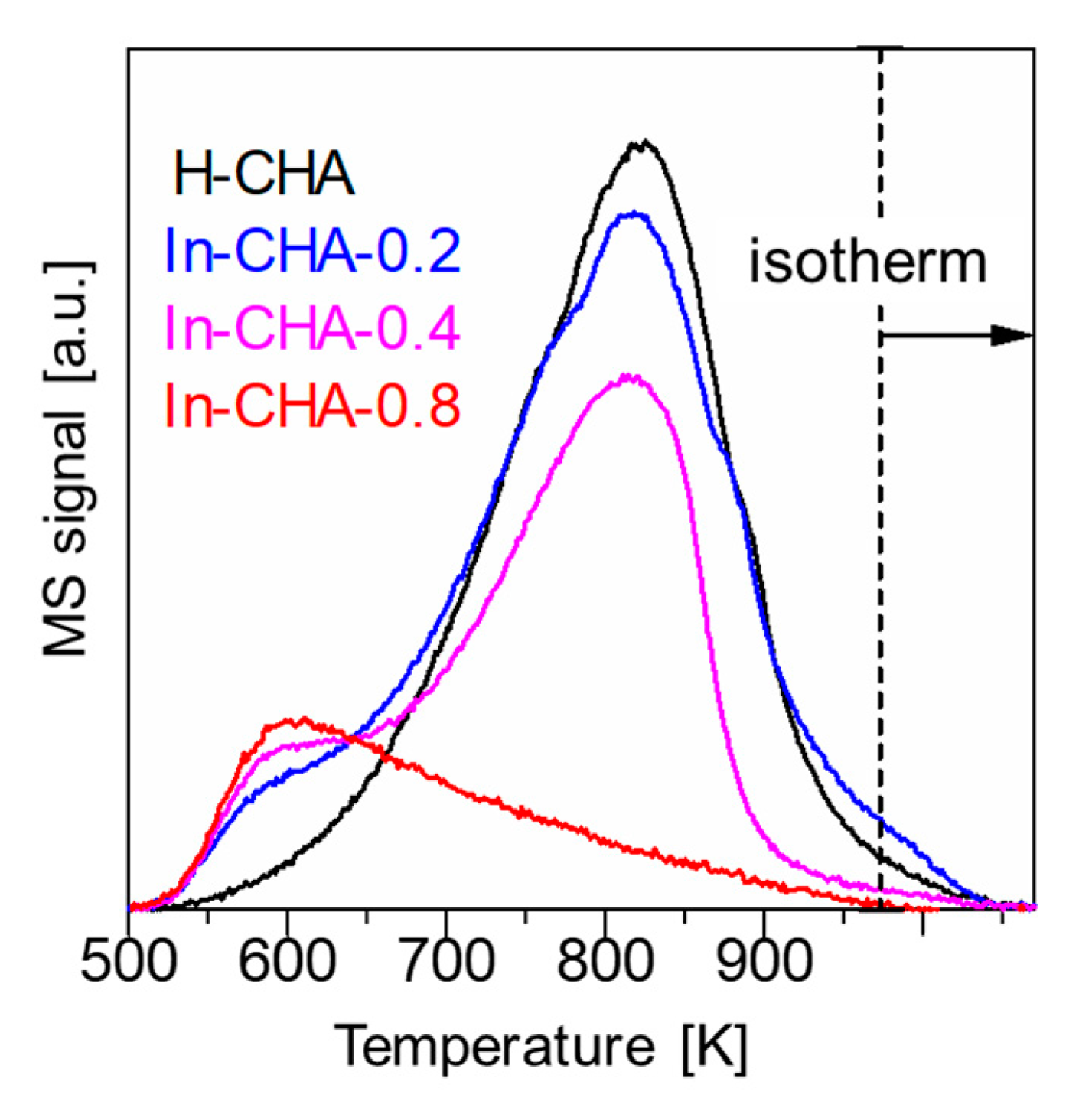
2.2. NH3-TPD
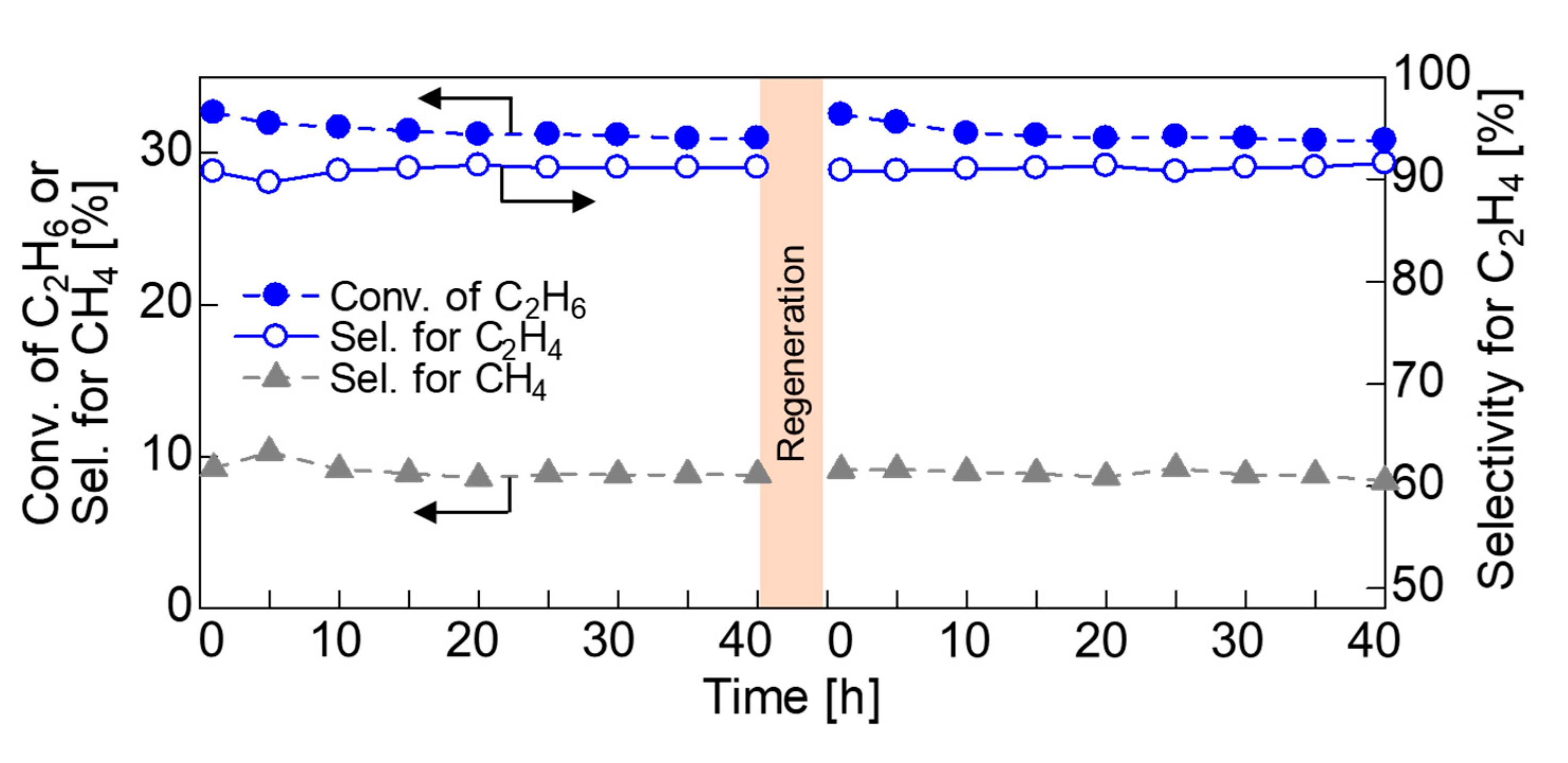
2.3. Effect of Zeolite Host upon Ethane Dehydrogenation Catalyzed by In-Exchanged Zeolites
3. Materials and Methods
3.1. Catalyst Preparation
3.2. XRD
3.3. NH3-TPD
3.4. Catalytic Tests
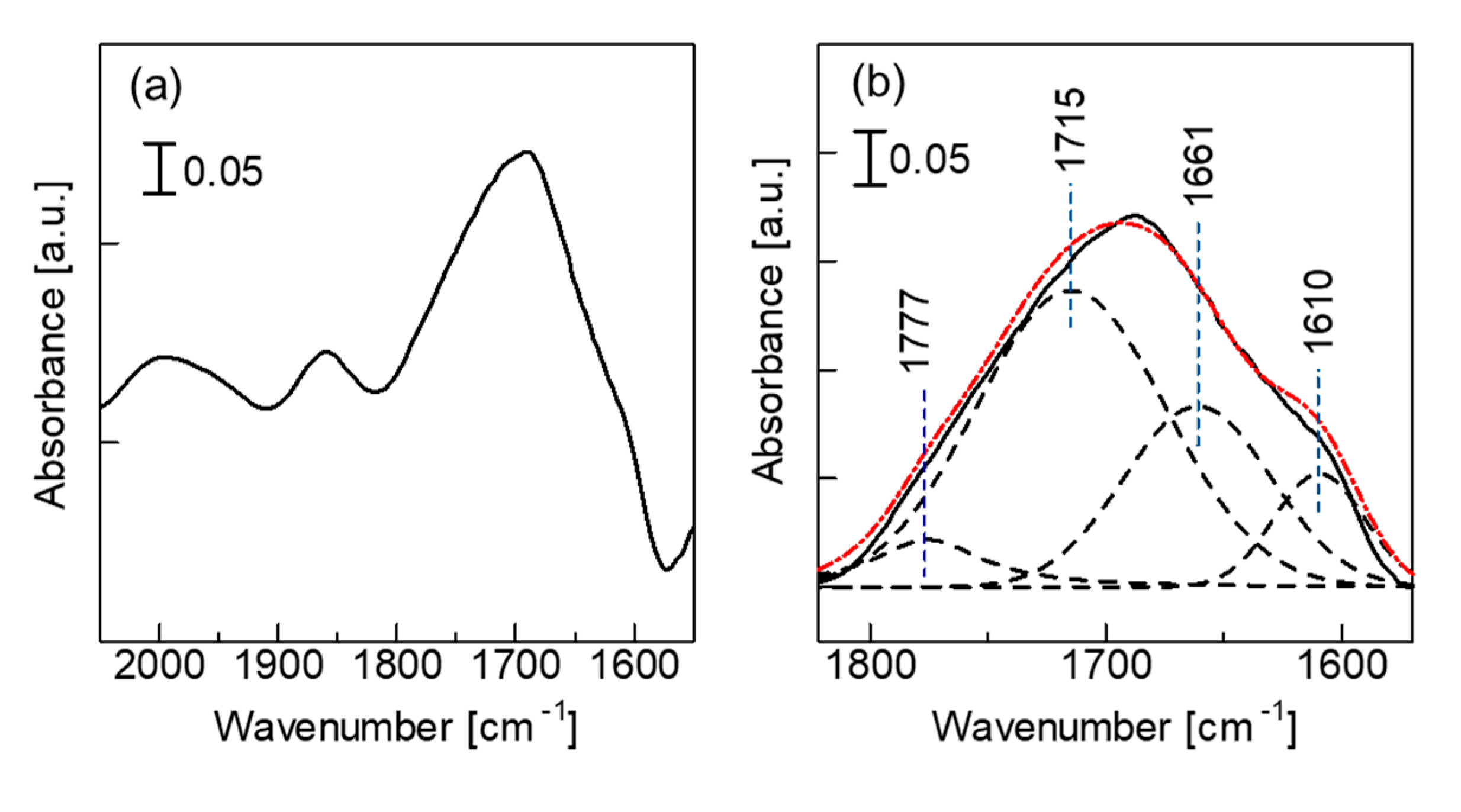
3.5. In-Situ FTIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulamanti, A.; Moya, J.A. Production Costs of the Chemical Industry in the EU and Other Countries: Ammonia, Methanol and Light Olefins. Renew. Sustain. Energy Rev. 2017, 68, 1205–1212. [Google Scholar] [CrossRef]
- Siirola, J.J. The Impact of Shale Gas in the Chemical Industry. AIChE J. 2014, 7, 810–819. [Google Scholar] [CrossRef]
- Grace Chan, K.Y.; Inal, F.; Senkan, S. Suppression of Coke Formation in the Steam Cracking of Alkanes: Ethane and Propane. Ind. Eng. Chem. Res. 2002, 37, 901–907. [Google Scholar] [CrossRef]
- Saito, H.; Sekine, Y. Catalytic Conversion of Ethane to Valuable Products through Non-Oxidative Dehydrogenation and Dehydroaromatization. RSC Adv. 2020, 10, 21427–21453. [Google Scholar] [CrossRef]
- James, O.O.; Mandal, S.; Alele, N.; Chowdhury, B.; Maity, S. Lower Alkanes Dehydrogenation: Strategies and Reaction Routes to Corresponding Alkenes. Fuel Process. Technol. 2016, 149, 239–255. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Galvita, V.; Siddiqi, G.; Sun, P.; Bell, A.T. Ethane Dehydrogenation on Pt/Mg(Al)O and PtSn/Mg(Al)O Catalysts. J. Catal. 2010, 271, 209–219. [Google Scholar] [CrossRef]
- Wegener, E.C.; Wu, Z.; Tseng, H.T.; Gallagher, J.R.; Ren, Y.; Diaz, R.E.; Ribeiro, F.H.; Miller, J.T. Structure and Reactivity of Pt–In Intermetallic Alloy Nanoparticles: Highly Selective Catalysts for Ethane Dehydrogenation. Catal. Today 2018, 299, 146–153. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, K.; Wang, C.; Ma, L.; Zhang, Q.; Liu, Q.; Chen, L.; Chen, L.; Zhang, Q.; Tian, Z. The Properties and Catalytic Performance of PtSn/Mg(x-Ga)AlO Catalysts for Ethane Dehydrogenation. RSC Adv. 2017, 7, 22836–22844. [Google Scholar] [CrossRef]
- Wu, Z.; Wegener, E.C.; Tseng, H.T.; Gallagher, J.R.; Harris, J.W.; Diaz, R.E.; Ren, Y.; Ribeiro, F.H.; Miller, J.T. Pd-In Intermetallic Alloy Nanoparticles: Highly Selective Ethane Dehydrogenation Catalysts. Catal. Sci. Technol. 2016, 6, 6965–6976. [Google Scholar] [CrossRef]
- Shi, X.; Ji, S.; Li, C. Oxidative Dehydrogenation of Ethane with CO2 over Novel Cr/SBA-15/Al2O3/FeCrAl Monolithic Catalysts. Energy Fuels 2008, 22, 3631–3638. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Ikenaga, N.O.; Suzuki, T.; Kobayashi, T.; Nishitani-Gamo, M.; Ando, T. The Role of Chemisorbed Oxygen on Diamond Surfaces for the Dehydrogenation of Ethane in the Presence of Carbon Dioxide. J. Phys. Chem. B 2003, 107, 4048–4056. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X. Oxidative Dehydrogenation of Ethane to Ethylene by Carbon Dioxide over Cr/TS-1 Catalysts. Catal. Commun. 2006, 7, 633–638. [Google Scholar] [CrossRef]
- Nakagawa, K.; Okamura, M.; Ikenaga, N.; Suzuki, T.; Kobayashi, T. Dehydrogenation of Ethane over Gallium Oxide in the Presence of Carbon Dioxide. Chem. Commun. 1998, 3, 1025–1026. [Google Scholar] [CrossRef]
- Lei, T.; Cheng, Y.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Silica-Doped TiO2 as Support of Gallium Oxide for Dehydrogenation of Ethane with CO2. Fuel Process. Technol. 2018, 177, 246–254. [Google Scholar] [CrossRef]
- Cejka, J.; Corma, A.; Zones, S. (Eds.) Zeolites and Catalysis: Synthesis, Reactions and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Kröcher, O.; Nachtegaal, M.; Ferri, D. Time-Resolved Copper Speciation during Selective Catalytic Reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. From Metal-Supported Oxides to Well-Defined Metal Site Zeolites: The next Generation of Passive NOx Adsorbers for Low-Temperature Control of Emissions from Diesel Engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Battiston, A.A.; Bitter, J.H.; Koningsberger, D.C. Reactivity of Binuclear Fe Complexes in Over-Exchanged Fe/ZSM5, Studied by in Situ XAFS Spectroscopy 2. Selective Catalytic Reduction of NO with Isobutane. J. Catal. 2003, 218, 163–177. [Google Scholar] [CrossRef]
- Kosinov, N.; Liu, C.; Hensen, E.J.M.; Pidko, E.A. Engineering of Transition Metal Catalysts Confined in Zeolites. Chem. Mater. 2018, 30, 3177–3198. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cao, W.; Bruijnincx, P.C.A.; Lin, L.; Wang, A.; Zhang, T. Zeolite-Supported Metal Catalysts for Selective Hydrodeoxygenation of Biomass-Derived Platform Molecules. Green Chem. 2019, 21, 3744–3768. [Google Scholar] [CrossRef]
- Harris, J.W.; Liao, W.C.; Di Iorio, J.R.; Henry, A.M.; Ong, T.C.; Comas-Vives, A.; Copéret, C.; Gounder, R. Molecular Structure and Confining Environment of Sn Sites in Single-Site Chabazite Zeolites. Chem. Mater. 2017, 29, 8824–8837. [Google Scholar] [CrossRef]
- Ichihashi, H.; Sato, H. The Development of New Heterogeneous Catalytic Processes for the Production of ε-Caprolactam. Appl. Catal. A Gen. 2001, 221, 359–366. [Google Scholar] [CrossRef]
- Nakamura, K.; Okuda, A.; Ohta, K.; Matsubara, H.; Okumura, K.; Yamamoto, K.; Itagaki, R.; Suganuma, S.; Tsuji, E.; Katada, N. Direct Methylation of Benzene with Methane Catalyzed by Co/MFI Zeolite. ChemCatChem 2018, 10, 3806–3812. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J.A. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and Beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent Advances of Pore System Construction in Zeolite-Catalyzed Chemical Industry Processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local Environment and Nature of Cu Active Sites in Zeolite-Based Catalysts for the Selective Catalytic Reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Gounder, R.; Iglesia, E. The Roles of Entropy and Enthalpy in Stabilizing Ion-Pairs at Transition States in Zeolite Acid Catalysis. Acc. Chem. Res. 2012, 45, 229–238. [Google Scholar] [CrossRef]
- Mahyuddin, M.H.; Staykov, A.; Shiota, Y.; Miyanishi, M.; Yoshizawa, K. Roles of Zeolite Confinement and Cu-O-Cu Angle on the Direct Conversion of Methane to Methanol by [Cu2(μ-O)]2+-Exchanged AEI, CHA, AFX, and MFI Zeolites. ACS Catal. 2017, 7, 3741–3751. [Google Scholar] [CrossRef]
- Lusardi, M.; Chen, T.T.; Kale, M.; Kang, J.H.; Neurock, M.; Davis, M.E. Carbonylation of Dimethyl Ether to Methyl Acetate over SSZ-13. ACS Catal. 2020, 10, 842–851. [Google Scholar] [CrossRef]
- Otomo, R.; Yokoi, T.; Tatsumi, T. Synthesis of Isosorbide from Sorbitol in Water over High-Silica Aluminosilicate Zeolites. Appl. Catal. A Gen. 2015, 505, 28–35. [Google Scholar] [CrossRef]
- Goto, D.; Harada, Y.; Furumoto, Y.; Takahashi, A.; Fujitani, T.; Oumi, Y.; Sadakane, M.; Sano, T. Conversion of Ethanol to Propylene over HZSM-5 Type Zeolites Containing Alkaline Earth Metals. Appl. Catal. A Gen. 2010, 383, 89–95. [Google Scholar] [CrossRef]
- Arudra, P.; Bhuiyan, T.I.; Akhtar, M.N.; Aitani, A.M.; Al-Khattaf, S.S.; Hattori, H. Silicalite-1 as Efficient Catalyst for Production of Propene from 1-Butene. ACS Catal. 2014, 4, 4205–4214. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, J.; Xu, C.; Shen, B. Alkali-Treatment of ZSM-5 Zeolites with Different SiO2/Al2O3 Ratios and Light Olefin Production by Heavy Oil Cracking. Fuel Process. Technol. 2011, 92, 414–420. [Google Scholar] [CrossRef]
- Wan, Z.; Wu, W.; Li, G.K.; Wang, C.; Yang, H.; Zhang, D. Effect of SiO2/Al2O3 Ratio on the Performance of Nanocrystal ZSM-5 Zeolite Catalysts in Methanol to Gasoline Conversion. Appl. Catal. A Gen. 2016, 523, 312–320. [Google Scholar] [CrossRef]
- Chen, N.Y.; Yan, T.Y. M2 Forming a Process for Aromatization of Light Hydrocarbons. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 151–155. [Google Scholar] [CrossRef]
- Seddon, D. Paraffin Oligomerisation to Aromatics. Catal. Today 1990, 6, 351–372. [Google Scholar] [CrossRef]
- Al-Zahrani, S.M. Catalytic Conversion of LPG to High-Value Aromatics: The Current State of the Art and Future Predictions. Dev. Chem. Eng. Miner. Process. 1988, 6, 101–120. [Google Scholar] [CrossRef]
- Fricke, R.; Kosslick, H.; Lischke, G.; Richter, M. Incorporation of Gallium into Zeolites: Syntheses, Properties and Catalytic Application. Chem. Rev. 2000, 100, 2303–2405. [Google Scholar] [CrossRef]
- Ono, Y.; Kitagawa, H.; Sendoda, Y. Transformation of Lower Alkanes into Aromatic Hydrocarbons over ZSM-5 Zeolites. J. Jpn. Pet. Inst. 1987, 30, 77–88. [Google Scholar] [CrossRef]
- Dooley, K.M.; Chang, C.; Price, G.L. Effects of Pretreatments on State of Gallium and Aromatization Activity of Gallium/ZSM-5 Catalysts. Appl. Catal. A Gen. 1992, 84, 17–30. [Google Scholar] [CrossRef]
- Frash, M.V.; van Santen, R.A. Activation of Small Alkanes in Ga-Exchanged Zeolites: A Quantum Chemical Study of Ethane Dehydrogenation. J. Phys. Chem. A 2000, 104, 2468–2475. [Google Scholar] [CrossRef]
- Meitzner, G.D.; Iglesia, E.; Baumgartner, J.E.; Huang, E.S. The Chemical State of Gallium in Working Alkane Dehydrocyclodimerization Catalysts. In Situ Gallium K-Edge X-ray Absorption Spectroscopy. J. Catal. 1993, 140, 209–225. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Subbotina, I.R.; van Santen, R.A.; Hensen, E.J.M. DRIFTS Study of the Chemical State of Modifying Gallium Ions in Reduced Ga/ZSM-5 Prepared by Impregnation. I. Observation of Gallium Hydrides and Application of CO Adsorption as Molecular Probe for Reduced Gallium Ions. J. Catal. 2004, 227, 263–269. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and Kinetics of Propane Dehydrogenation and Cracking over Ga/H-MFI Prepared via Vapor-Phase Exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2019, 141, 1614–1627. [Google Scholar] [CrossRef]
- Schreiber, M.W.; Plaisance, C.P.; Baumgärtl, M.; Reuter, K.; Jentys, A.; Bermejo-Deval, R.; Lercher, J.A. Lewis-Brønsted Acid Pairs in Ga/H-ZSM-5 to Catalyze Dehydrogenation of Light Alkanes. J. Am. Chem. Soc. 2018, 140, 4849–4859. [Google Scholar] [CrossRef] [PubMed]
- Uslamin, E.A.; Saito, H.; Sekine, Y.; Hensen, E.J.M.; Kosinov, N. Different Mechanisms of Ethane Aromatization over Mo/ZSM-5 and Ga/ZSM-5 Catalysts. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Sooknoi, T. Tunable Activity of [Ga]HZSM-5 with H2 Treatment: Ethane Dehydrogenation. Catal. Commun. 2014, 45, 63–68. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Kinage, A.K.; Choudhary, T.V. Low-Temperature Nonoxidative Activation of Methane over H-Galloaluminosilicate (MFI) Zeolite. Science 1997, 275, 1286–1288. [Google Scholar] [CrossRef]
- Solt, H.; Lónyi, F.; Mihályi, R.M.; Valyon, J.; Gutierrez, L.B.; Miro, E.E. A Mechanistic Study of the Solid-State Reactions of H-Mordenite with Indium (0) and Indium (III) Oxide. J. Phys. Chem. C 2008, 112, 19423–19430. [Google Scholar] [CrossRef]
- Price, G.L.; Kanazirev, V.; Dooley, K.M.; Hart, V.I. On the Mechanism of Propane Dehydrocyclization over Cation-Containing, Proton-Poor MFI Zeolite. J. Catal. 1998, 173, 17–27. [Google Scholar] [CrossRef]
- Hart, V.I.; Bryant, M.B.; Butler, L.G.; Wu, X.; Dooley, K.M. Proton-Poor, Gallium- and Indium-Loaded Zeolite Dehydrogenation Catalysts. Catal. Lett. 1998, 53, 111–118. [Google Scholar] [CrossRef]
- Baba, T.; Abe, Y.; Nomoto, K.; Inazu, K.; Echizen, T.; Ishikawa, A.; Murai, K. Catalytic Transformation of Methane over In-Loaded ZSM-5 Zeolite in the Presence of Ethene. J. Phys. Chem. B 2005, 109, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Z.; Yasumura, S.; Wu, X.; Huang, M.; Liu, C.; Toyao, T.; Shimizu, K. Isolated Indium Hydrides in CHA Zeolites: Speciation and Catalysis for Nonoxidative Dehydrogenation of Ethane. J. Am. Chem. Soc. 2020, 142, 4820–4832. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.K.; Mihályi, R.M.; Minchev, C.; Neinska, Y.; Kanazirev, V. Study of the Reductive Solid-State Ion Exchange of Indium into an NH4-Beta Zeolite. Microporous Mesoporous Mater. 1996, 7, 333–341. [Google Scholar] [CrossRef]
- Maeno, Z.; Yasumura, S.; Liu, C.; Toyao, T.; Kon, K.; Nakayama, A.; Hasegawa, J.; Shimizu, K. Experimental and Theoretical Study of Multinuclear Indium-Oxo Clusters in CHA Zeolite for CH4 Activation at Room Temperature. Phys. Chem. Chem. Phys. 2019, 21, 13415–13427. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Abdala, P.M.; Stoian, D.; Huang, X.; Willinger, M.G.; Fedorov, A.; Müller, C.R. Structural Evolution and Dynamics of an In2O3 Catalyst for CO2 Hydrogenation to Methanol: An Operando XAS-XRD and In Situ TEM Study. J. Am. Chem. Soc. 2019, 141, 13497–13505. [Google Scholar] [CrossRef]
- Zhu, Q.; Kondo, J.N.; Tatsumi, T.; Inagaki, S.; Ohnuma, R.; Kubota, Y.; Shimodaira, Y.; Kobayashi, H.; Domen, K. A Comparative Study of Methanol to Olefin over CHA and MTF Zeolites. J. Phys. Chem. C 2007, 111, 5409–5415. [Google Scholar] [CrossRef]
- Blakeman, P.G.; Burkholder, E.M.; Chen, H.Y.; Collier, J.E.; Fedeyko, J.M.; Jobson, H.; Rajaram, R.R. The Role of Pore Size on the Thermal Stability of Zeolite Supported Cu SCR Catalysts. Catal. Today 2014, 231, 56–63. [Google Scholar] [CrossRef]
- Villamaina, R.; Liu, S.; Nova, I.; Tronconi, E.; Ruggeri, M.P.; Collier, J.; York, A.; Thompsett, D. Speciation of Cu Cations in Cu-CHA Catalysts for NH3-SCR: Effects of SiO2/Al2O3 Ratio and Cu-Loading Investigated by Transient Response Methods. ACS Catal. 2019, 9, 8916–8927. [Google Scholar] [CrossRef]
- Ward, J.W. A Spectroscopic Study of the Surface of Zeolite Y. II. Infrared Spectra of Structural Hydroxyl Groups and Adsorbed Water on Alkali, Alkaline Earth, and Rare Earth Ion-Exchanged Zeolites. J. Phys. Chem. 1968, 72, 4211–4223. [Google Scholar] [CrossRef]
- Duncan, T.M.; Vaughan, R.W. The Adsorption of Formic Acid on Y Zeolites: An Infrared Absorbance Study. J. Catal. 1981, 471, 469–471. [Google Scholar] [CrossRef]

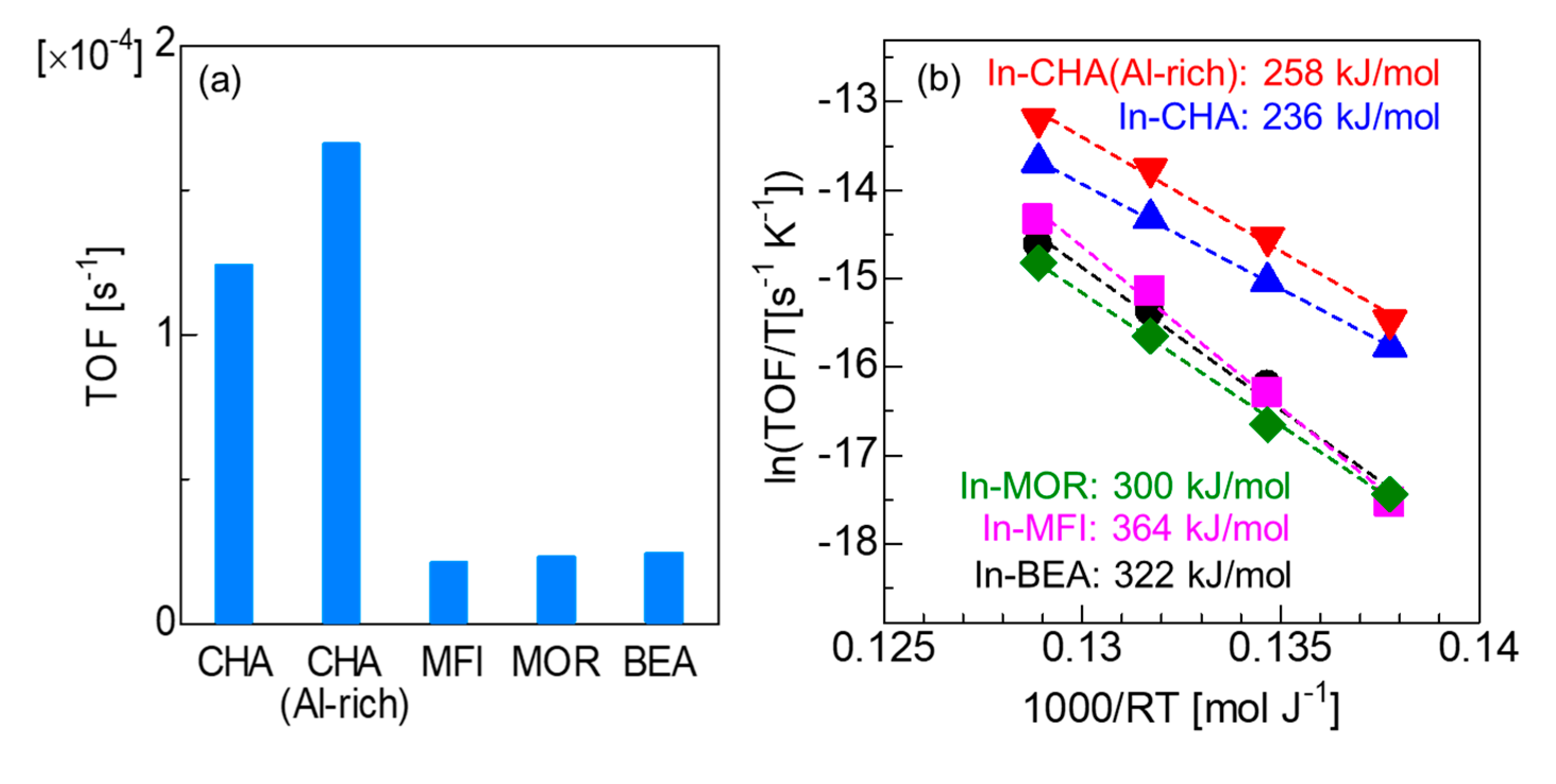

| Entry | Catalyst | SiO2/Al2O3 | In/Al | Conv. [%] a | Sel. [%] a | Carbon Balance [%] a |
|---|---|---|---|---|---|---|
| 1 | In-MFI | 22.3 | 0.8 | 12.9 | 99.2 | 99 |
| 2 | In-MOR | 20.0 | 0.8 | 14.9 | 97.6 | 99 |
| 3 | In-BEA | 25.0 | 0.8 | 13.5 | 97.5 | 94 |
| 4 | In-CHA(Al-rich) | 13.7 | 0.8 | 37.9 | 96.6 | 98 |
| 5 | In-CHA b | 22.3 | 0.8 | 25.9 | 96.1 | 99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeno, Z.; Wu, X.; Yasumura, S.; Toyao, T.; Kanda, Y.; Shimizu, K.-i. In-Exchanged CHA Zeolites for Selective Dehydrogenation of Ethane: Characterization and Effect of Zeolite Framework Type. Catalysts 2020, 10, 807. https://doi.org/10.3390/catal10070807
Maeno Z, Wu X, Yasumura S, Toyao T, Kanda Y, Shimizu K-i. In-Exchanged CHA Zeolites for Selective Dehydrogenation of Ethane: Characterization and Effect of Zeolite Framework Type. Catalysts. 2020; 10(7):807. https://doi.org/10.3390/catal10070807
Chicago/Turabian StyleMaeno, Zen, Xiaopeng Wu, Shunsaku Yasumura, Takashi Toyao, Yasuharu Kanda, and Ken-ichi Shimizu. 2020. "In-Exchanged CHA Zeolites for Selective Dehydrogenation of Ethane: Characterization and Effect of Zeolite Framework Type" Catalysts 10, no. 7: 807. https://doi.org/10.3390/catal10070807
APA StyleMaeno, Z., Wu, X., Yasumura, S., Toyao, T., Kanda, Y., & Shimizu, K.-i. (2020). In-Exchanged CHA Zeolites for Selective Dehydrogenation of Ethane: Characterization and Effect of Zeolite Framework Type. Catalysts, 10(7), 807. https://doi.org/10.3390/catal10070807