Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane
Abstract
1. Introduction
2. The Processes of Hydrogen Production on NH3BH3 (AB)
2.1. The Methods of Producing Hydrogen from NH3BH3(AB)
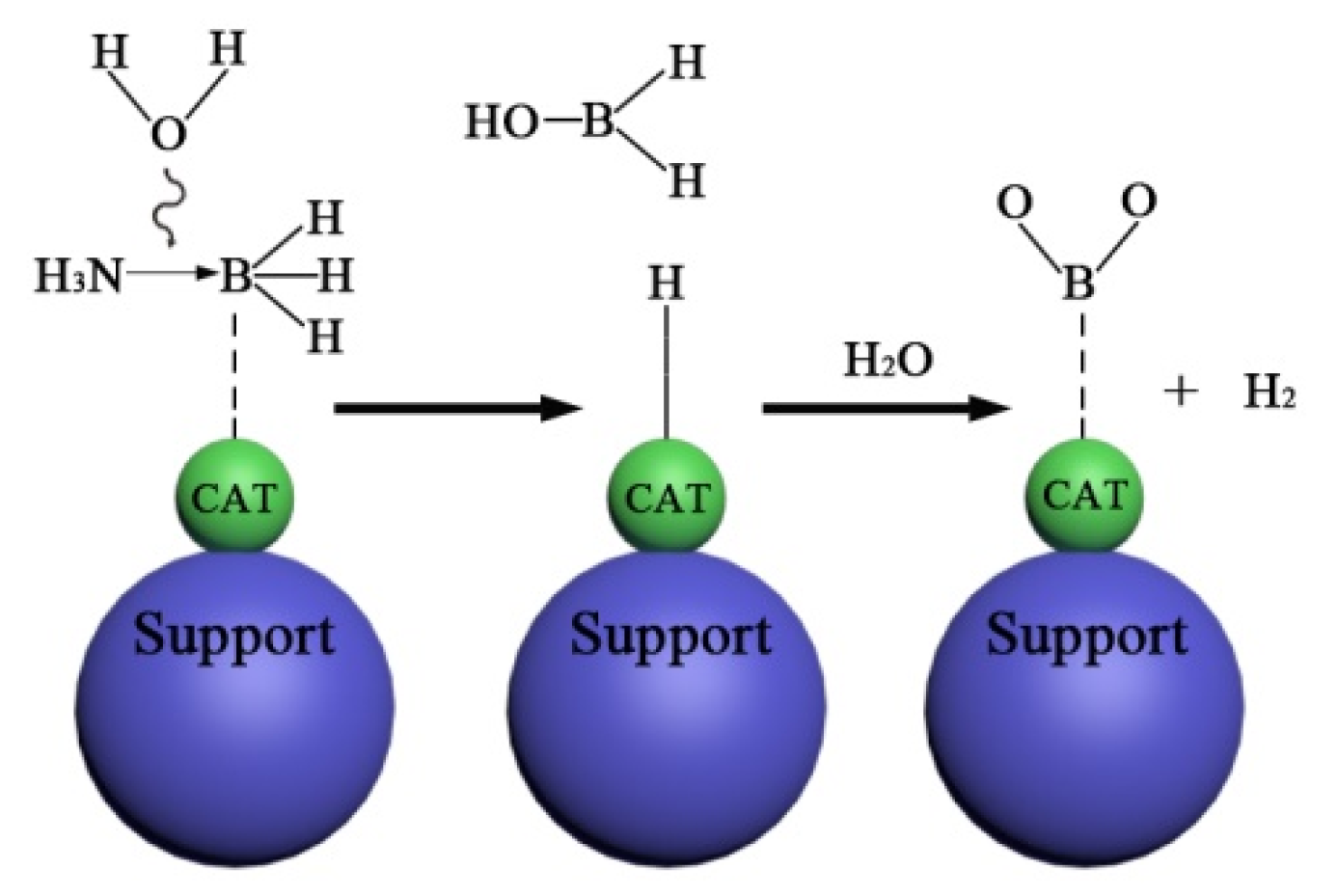
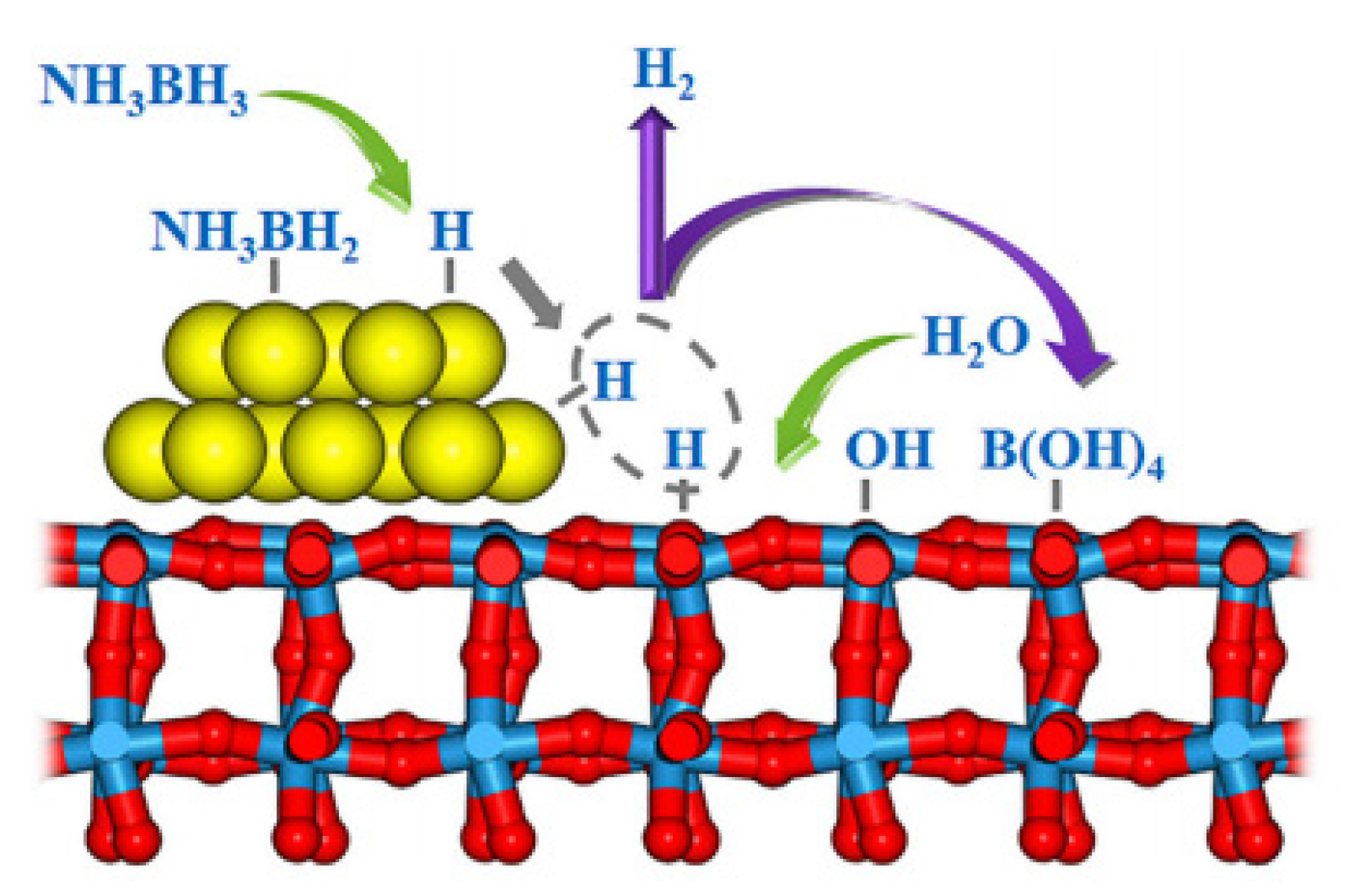
2.2. The Hydrolysis Mechanism of NH3BH3(AB)
3. The Development of Catalysts for NH3BH3 (AB) Dehydrogenation
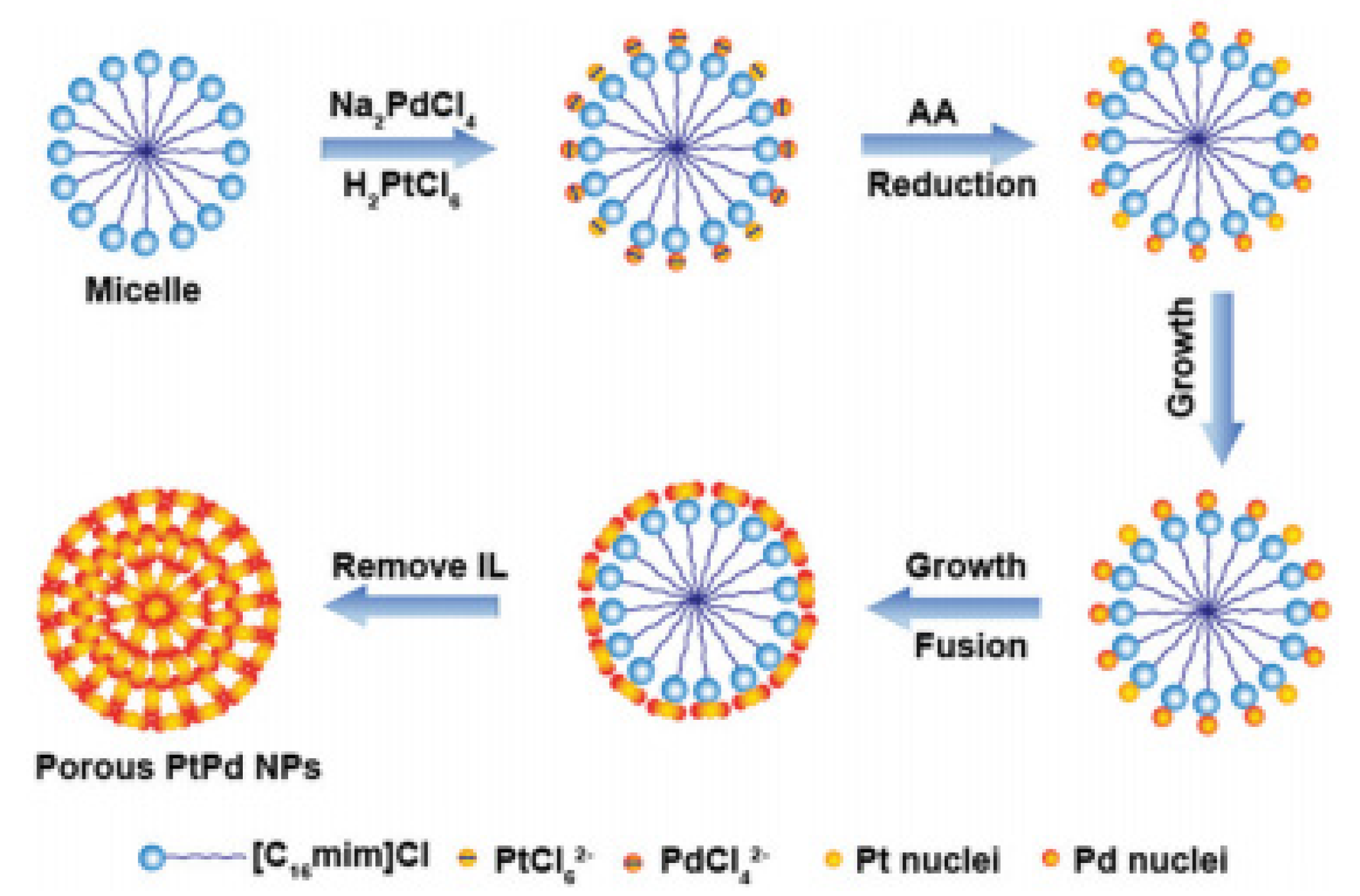
3.1. Noble Metal Catalysts
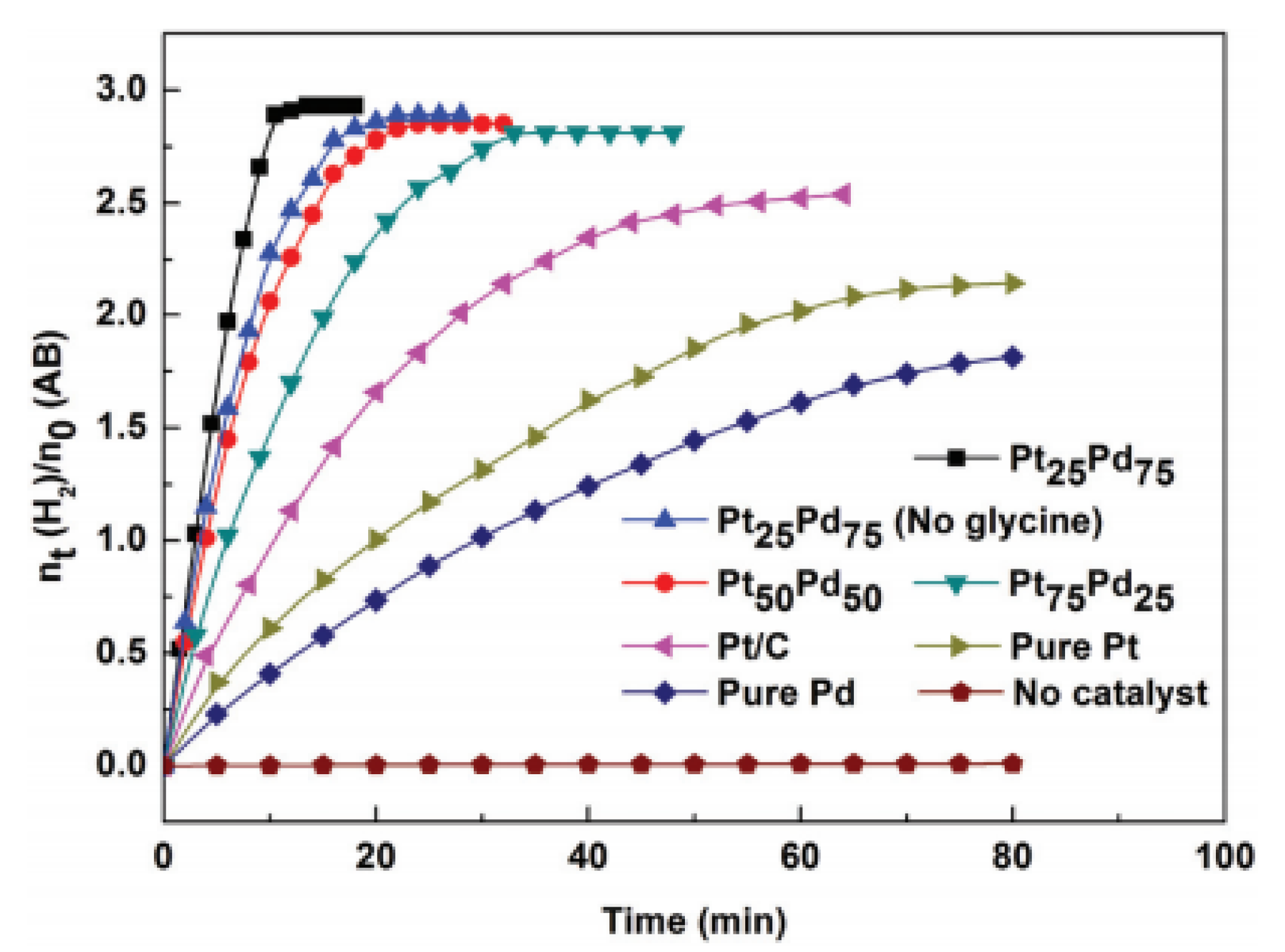
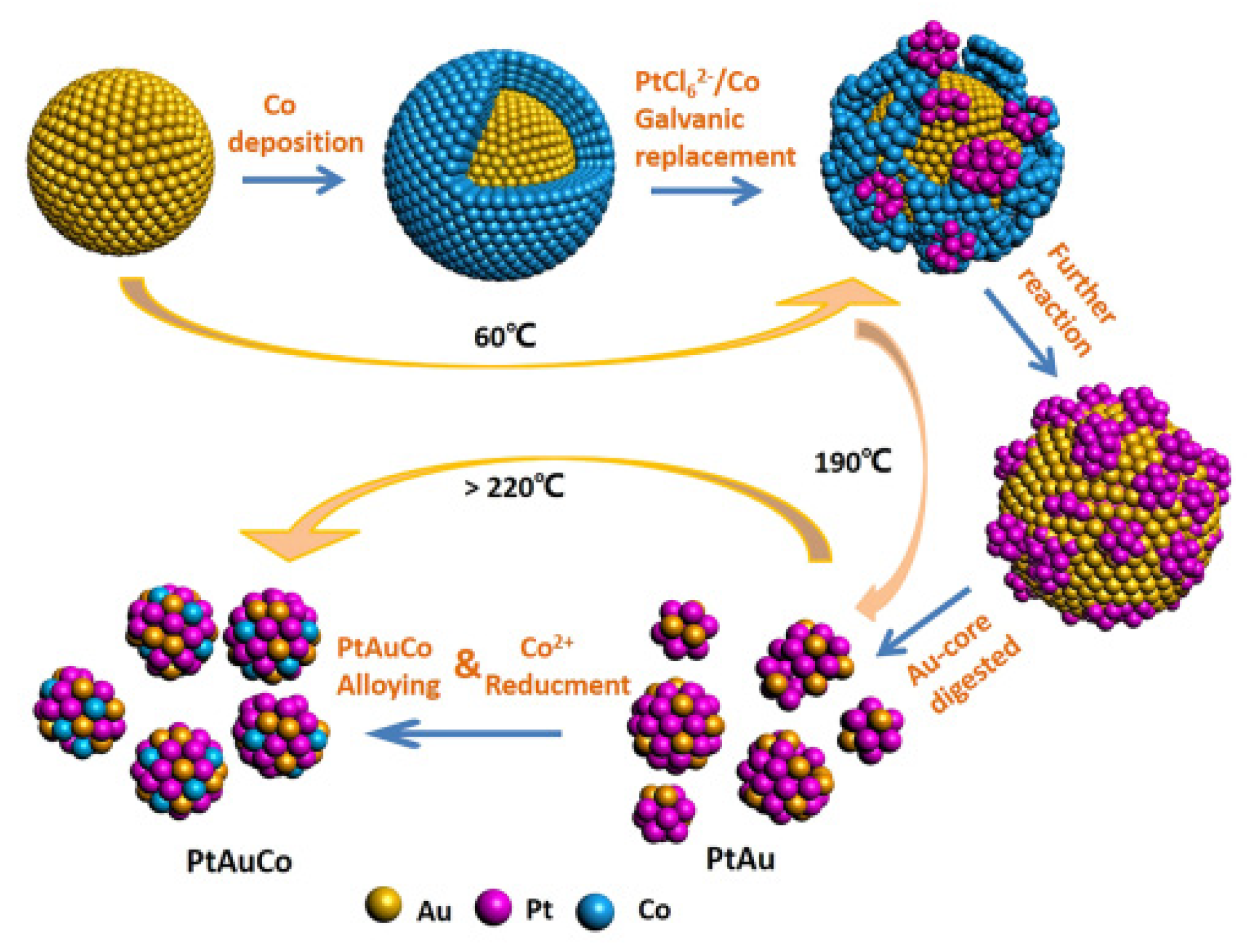
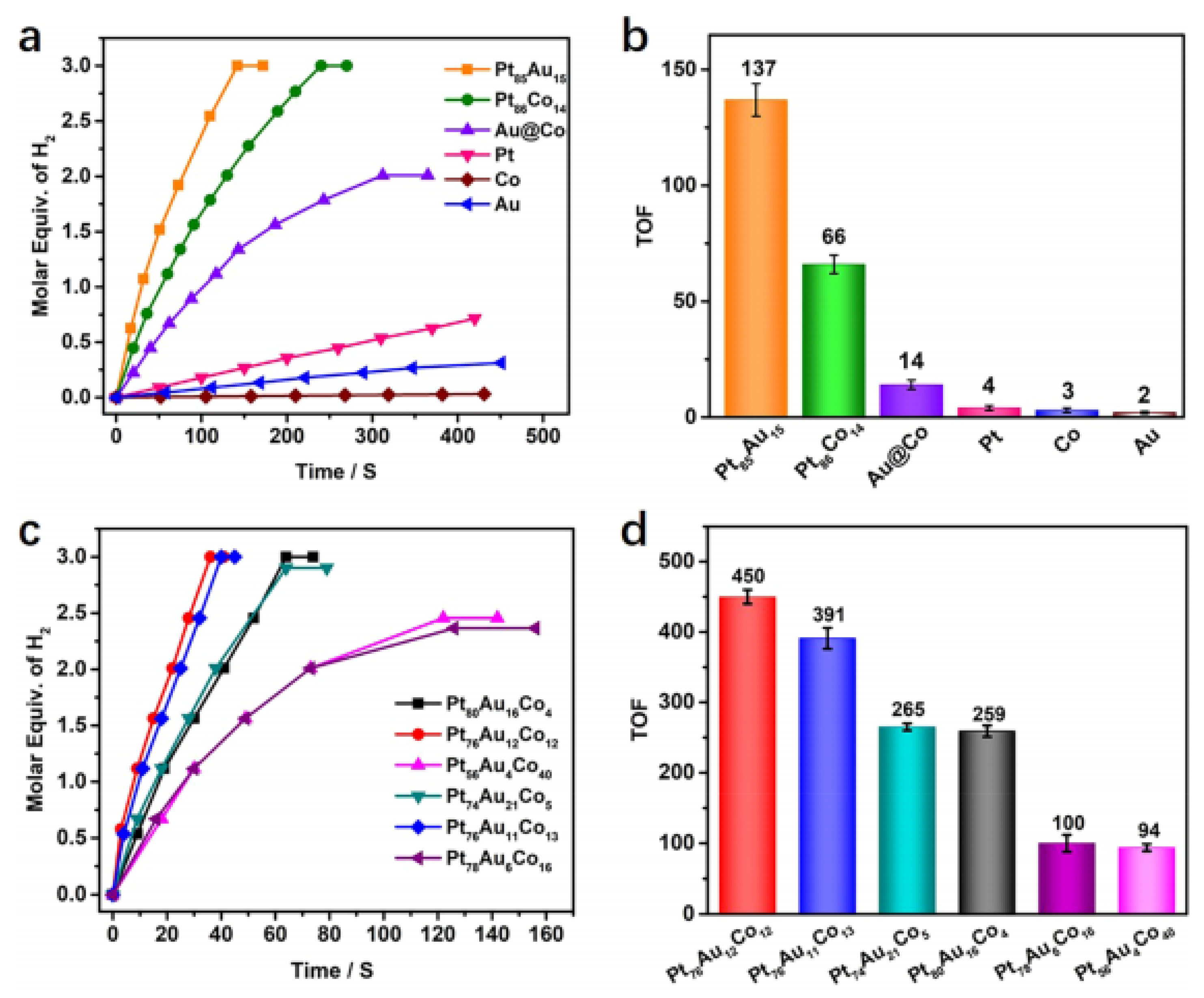
3.2. Noble Metal and Non-Precious Metal Composite Catalysts
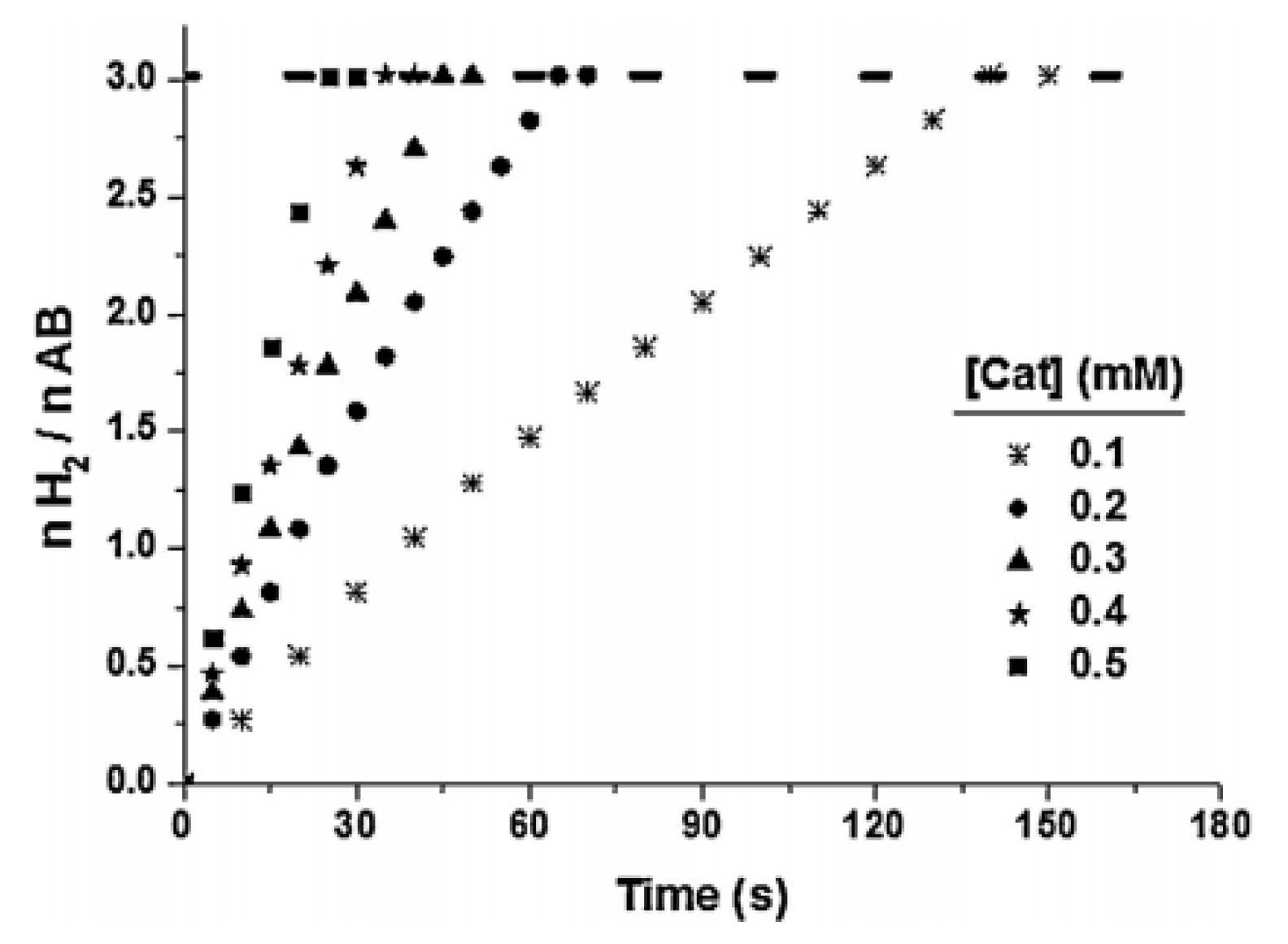
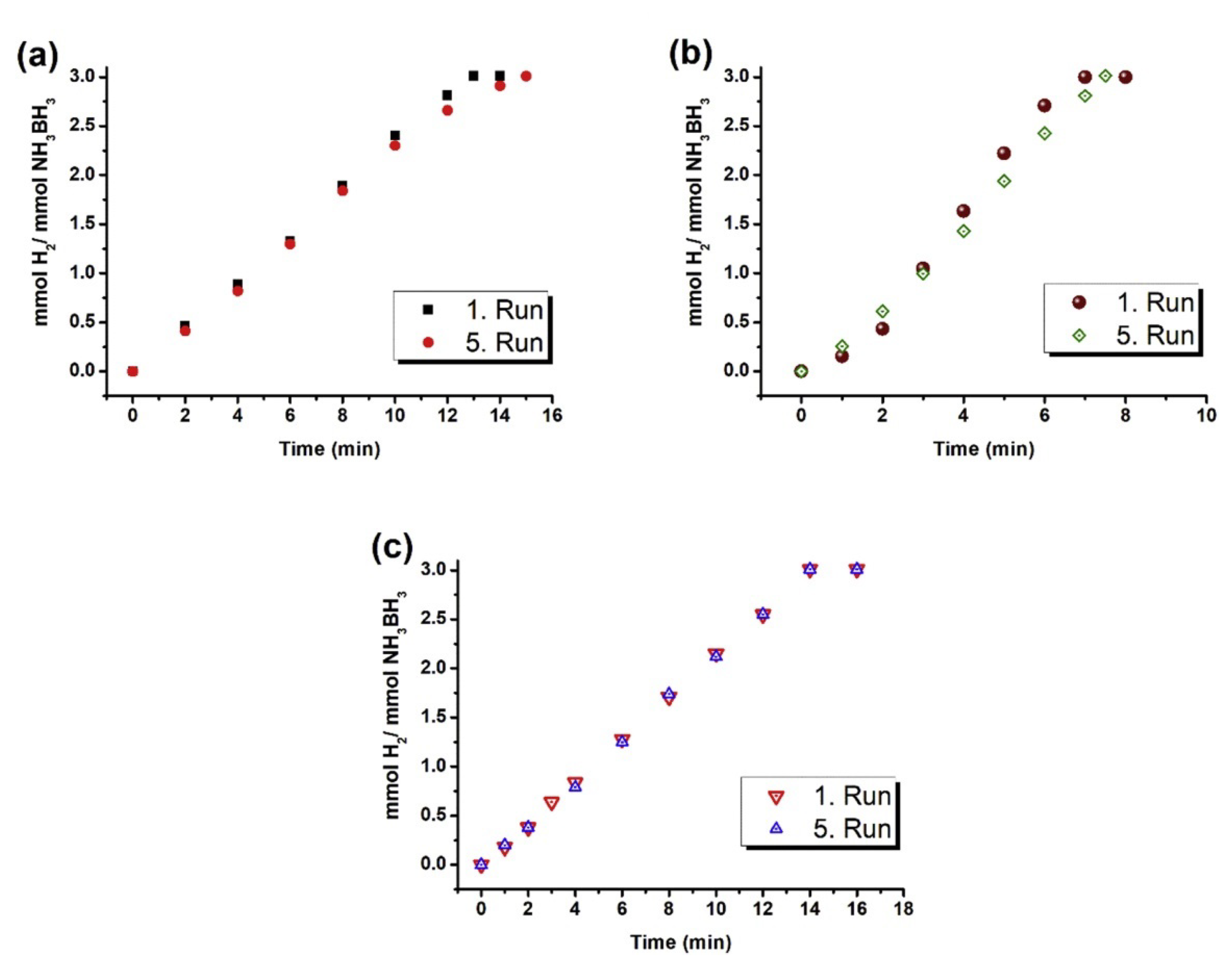
3.3. Catalytic Activities of Supported Metal Catalysts in NH3BH3(AB) Hydrolysis
3.3.1. Graphene Material Supported Metal Catalysts
3.3.2. Carbon Material Supported Metal Catalysts
3.3.3. Carbon Nanotubes Material Supported Metal Catalysts
3.3.4. Silicon Dioxide Material Supported Metal Catalysts
3.3.5. Cerium Dioxide Material Supported Metal Catalysts
3.3.6. Titanium Dioxide Material Supported Metal Catalysts
4. Direction of Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adeniran, B.; Mokaya, R. Compactivation: A mechanochemical approach to carbons with superior porosity and exceptional performance for hydrogen and CO2 storage. Nano Energy 2015, 16, 173–185. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, L.; Xu, L.X.; Wan, C.; An, Y.; Ye, M.F. Recent Developments of Effective Catalysts for Hydrogen Storage Technology Using N-Ethylcarbazole. Catalysts 2020, 10, 648. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Ali, N.; Hussain, A.; Ahmed, R.; Wang, M.K.; Zhao, C.; Haq, B.U.; Fu, Y.Q. Advances in nanostructured thin film materials for solar cell applications. Renew. Sustain. Energy Rev. 2016, 59, 726–737. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Sun, L.; Xu, L.X.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L.; Yang, Y.R. Boosting visible-light-driven hydrogen evolution from formic acid over AgPd/2D g-C3N4 nanosheets Mott-Schottky photocatalyst. Chem. Eng. J. 2020, 396, 125229. [Google Scholar]
- Liu, Q.B.; Zhang, S.J.; Liao, J.Y.; Feng, K.J.; Zheng, Y.Y.; Pollet, B.G.; Li, H. CuCo2O4 nanoplate film as a low-cost, highly active and durable catalyst towards the hydrolytic dehydrogenation of ammonia borane for hydrogen production. J. Power Sources 2017, 355, 191–198. [Google Scholar] [CrossRef]
- Wan, C.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L. Fabrication of CeO2 nanotube supported Pt catalyst encapsulated with silica for high and stable performance. Chem. Commun. 2015, 51, 9785–9788. [Google Scholar] [CrossRef]
- Wan, C.; An, Y.; Xu, G.H.; Kong, W.J. Study of catalytic hydrogenation of N-ethylcarbazole over ruthenium catalyst. Int. J. Hydrog. Energy 2012, 37, 13092–13096. [Google Scholar] [CrossRef]
- Wan, C.; Sun, L.; Xu, L.X.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L.; Yang, Y.R. Novel NiPt alloy nanoparticle decorated 2D layered g-C3N4 Nanosheets: A highly efficient catalyst for hydrogen generation from hydrous hydrazine. J. Mater. Chem. A 2019, 7, 8798–8804. [Google Scholar] [CrossRef]
- Li, X.F.; Ma, X.F.; Zhang, J.; Akiyama, E.; Wang, Y.F.; Song, X.L. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. 2020, 33, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liu, L.; Lu, S.; Xu, L.X.; An, Y.; Wan, C. Facile Fabrication of NiPt/CNTs as an Efficient Catalyst for Hydrogen Production from Hydrous Hydrazine. ChemistrySelect 2019, 4, 10494–10500. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, L.; Du, L.; An, Y.; Wan, C. Palladium Supported on Carbon Nanotubes as a High-Performance Catalyst for the Dehydrogenation of Dodecahydro-N-ethylcarbazole. Catalysts 2018, 8, 638. [Google Scholar] [CrossRef]
- Attia, N.F.; Lee, S.M.; Kim, H.J.; Geckeler, K.E. Nanoporous polypyrrole: Preparation and hydrogen storage properties. Int. J. Energy Res. 2014, 38, 466–476. [Google Scholar] [CrossRef]
- Oumellal, Y.; Courty, M.; Rougier, A.; Nazri, G.A.; Aymard, L. Electrochemical reactivity of magnesium hydride toward lithium: New synthesis route of nano-particles suitable for hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 5852–5857. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrog. Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrog. Energy 2019, 23, 11901–11919. [Google Scholar] [CrossRef]
- Yao, F.; Li, X.; Wan, C.; Xu, L.X.; An, Y.; Ye, M.F.; Lei, Z. Highly efficient hydrogen release from formic acid using a graphitic carbon nitride-supported AgPd nanoparticle catalyst. Appl. Surf. Sci. 2017, 426, 605–611. [Google Scholar] [CrossRef]
- Semiz, L. Hydrogen generation from ammonia borane by chemically dealloyed platinum nanoparticles. React. Kinet. Mech. Catal. 2020, 129, 205–218. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.; Manners, L. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef]
- Yao, Q.L.; Lu, Z.H.; Huang, W.; Chen, X.S.; Zhu, J. High Pt-like activity of the Ni–Mo/graphene catalyst for hydrogen evolution from hydrolysis of ammonia borane. J. Mater. Chem. A 2016, 4, 8579–8583. [Google Scholar] [CrossRef]
- Sutton, A.D.; Burrell, A.K.; Dixon, D.A.; Garner, E.B.; Gordon, J.C.; Nakagawa, T.; Ott, K.C.; Robinson, P.; Vasiliu, M. Regeneration of ammonia borane spent fuel by direct reaction with hydrazine and liquid ammonia. Science 2011, 331, 1426–1429. [Google Scholar] [CrossRef]
- Smythe, N.C.; Gordon, J.C. Ammonia borane as a hydrogen carrier: Dehydrogenation and regeneration. Eur. J. Inorg. Chem. 2010, 509–521. [Google Scholar] [CrossRef]
- Sun, Q.M.; Wang, N.; Bai, R.S.; Hui, Y.; Zhang, T.J.; Do, D.A.; Zhang, P.; Song, L.J.; Miao, S.; Yu, J.H. Synergetic Effect of Ultrasmall Metal Clusters and Zeolites Promoting Hydrogen Generation. Adv. Sci. 2019, 6, 1802350. [Google Scholar] [CrossRef]
- Amali, A.J.; Aranishi, K.; Uchida, T.; Xu, Q. PdPt Nanocubes: A High-Performance Catalyst for Hydrolytic Dehydrogenation of Ammonia Borane. Part. Part. Syst. Charact. 2013, 30, 888–892. [Google Scholar] [CrossRef]
- Roy, B.; Hajari, A.; Kumar, V.; Manna, J.; Sharma, P. Kinetic model analysis and mechanistic correlation of ammonia borane thermolysis under dynamic heating conditions. Int. J. Hydrog. Energy 2018, 43, 10386–10395. [Google Scholar] [CrossRef]
- Yan, J.M.; Zhang, X.B.; Shioyama, H.; Xu, Q. Room temperature hydrolytic dehydrogenation of ammonia borane catalyzed by Co nanoparticles. J. Power Sources 2010, 195, 1091–1094. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Sun, D.H.; Mazumder, V.; Metin, O.; Sun, S.H. Hethanolysis of ammonia borane by CoPd nanoparticles. ACS Catal. 2012, 2, 1290–1295. [Google Scholar] [CrossRef]
- Marder, T.B. Will we soon be fueling our automobiles with ammonia–borane? Angew. Chem. Int. Ed. 2007, 46, 8116–8118. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.Y.; Shin, Y.S.; Wang, C.M.; Li, X.H.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. Int. Ed. 2005, 44, 3578–3582. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhu, G.Z.; Lu, G.Q.; Qiu, S.L.; Yao, X.D. Ammonia borane confined by a metal− organic framework for chemical hydrogen storage: Enhancing kinetics and eliminating ammonia. J. Am. Chem. Soc. 2010, 132, 1490–1491. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.H.; Baker, R.T.; Matus, M.H.; Grant, D.J.; Dixon, D.A. Acid initiation of ammonia–borane dehydrogenation for hydrogen storage. Angew. Chem. Int. Ed. 2007, 46, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Li, H.L.; Zhang, W.B.; Zhao, X.; Qiu, J.X.; Li, A.W.; Zheng, X.S.; Hu, Z.P.; Si, R.; Zeng, J. Supported rhodium catalysts for ammonia–borane hydrolysis: Dependence of the catalytic activity on the highest occupied state of the single rhodium atoms. Angew. Chem. Int. Ed. 2017, 56, 4712–4718. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B–N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef]
- Diwan, M.; Diakov, V.; Shafirovich, E.; Varma, A. Noncatalytic hydrothermolysis of ammonia borane. Int. J. Hydrog. Energy 2008, 33, 1135–1141. [Google Scholar] [CrossRef]
- Diwan, M.; Hanna, D.; Varma, A. Method to release hydrogen from ammonia borane for portable fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 577–584. [Google Scholar] [CrossRef]
- Demirci, U.B.; Akdim, O.; Miele, P. Ten-year efforts and a no-go recommendation for sodium borohydride for on-board automotive hydrogen storage. Int. J. Hydrog. Energy 2009, 34, 2638–2645. [Google Scholar] [CrossRef]
- Hu, M.G.; Geanangel, R.A.; Wendlandt, W.W. The thermal decomposition of ammonia borane. Thermochim. Acta 1978, 23, 249–255. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ikarashi, Y.; Isobe, S.; Hino, S.; Ohnuki, S. Ammonia borane–metal alanate composites: Hydrogen desorption properties and decomposition processes. RSC Adv. 2014, 4, 20626–20631. [Google Scholar] [CrossRef]
- Dong, H.L.; Berke, H. A mild and efficient rhenium-catalyzed transfer hydrogenation of terminal olefins using alcoholysis of amine–borane adducts as a reducing system. J. Organomet. Chem. 2011, 696, 1803–1808. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Z.J.; Xu, D.D.; Li, Y.; Wang, M.M.; Xia, L.M.; Shu-Ping, L. In-Situ Formed Amorphous Co Nanoparticles for Efficiently Catalytic Hydrogen Production from the Methanolysis of Ammonia Borane. Chin. J. Inorg. Chem. 2019, 35, 141–148. [Google Scholar]
- Yu, C.; Fu, J.J.; Muzzio, M.; Shen, T.L.; Su, D.; Zhu, J.J.; Sun, S.H. CuNi nanoparticles assembled on graphene for catalytic methanolysis of ammonia borane and hydrogenation of nitro/nitrile compounds. Chem. Mater. 2017, 29, 1413–1418. [Google Scholar] [CrossRef]
- Özhava, D.; Kılıçaslan, N.Z.; Özkar, S. PVP-stabilized nickel (0) nanoparticles as catalyst in hydrogen generation from the methanolysis of hydrazine borane or ammonia borane. Appl. Catal. B Environ. 2015, 162, 573–582. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Karkamkar, A.; Hess, N.J.; Bowden, M.; Rassat, S.; Zheng, F.; Kenneth, R.; Autrey, T. The effects of chemical additives on the induction phase in solid-state thermal decomposition of ammonia borane. Chem. Mater. 2008, 20, 5332–5336. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Segovia, J.J.; Martín, Á. Improvement of the kinetics of hydrogen release from ammonia borane confined in silica aerogel. Microporous Mesoporous Mater. 2017, 237, 189–200. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Kam, L.; Trerise, R.; Williams, T.J. Ruthenium-catalyzed ammonia borane dehydrogenation: Mechanism and utility. Acc. Chem. Res. 2017, 50, 86–95. [Google Scholar] [CrossRef]
- Gil-San-Millan, R.; Grau-Atienza, A.; Johnson, D.T.; Rico-Francés, S.; Serrano, E.; Linares, N.; Garcia-Martinez, J. Improving hydrogen production from the hydrolysis of ammonia borane by using multifunctional catalysts. Int. J. Hydrog. Energy 2018, 43, 17100–17111. [Google Scholar] [CrossRef]
- Du, X.Q.; Yang, C.L.; Zeng, X.; Wu, T.; Zhou, Y.H.; Cai, P.; Cheng, G.Z.; Luo, W. Amorphous NiP supported on rGO for superior hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2017, 42, 14181–14187. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xiao, X.; Wu, Y.; An, Y.; Xu, L.X.; Wan, C. Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride. Catalysts 2019, 9, 1009. [Google Scholar] [CrossRef]
- Kim, S.K.; Han, W.S.; Kim, T.J.; Kim, T.Y.; Nam, S.W.; Mitoraj, M.; Piekos, L.; Michalak, A.; Hwang, S.J.; Kang, S.O. Palladium catalysts for dehydrogenation of ammonia borane with preferential B−H activation. J. Am. Chem. Soc. 2010, 132, 9954–9955. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.M.; Abutaleb, A.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun CoCr7C3-supported C nanofibers: Effective, durable, and chemically stable catalyst for H2 gas generation from ammonia borane. Mol. Catal. 2017, 434, 32–38. [Google Scholar] [CrossRef]
- Figen, A.K.; Filiz, B.C. Polymeric and metal oxide structured nanofibrous composites fabricated by electrospinning as highly efficient hydrogen evolution catalyst. J. Colloid Interface Sci. 2019, 533, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Alpaydın, C.Y.; Gülbay, S.K.; Colpan, C.O. A review on the catalysts used for hydrogen production from ammonia borane. Int. J. Hydrog. Energy 2020, 45, 3414–3434. [Google Scholar] [CrossRef]
- Brockman, A.; Zheng, Y.; Gore, J. A study of catalytic hydrolysis of concentrated ammonia borane solutions. Int. J. Hydrog. Energy 2010, 35, 7350–7356. [Google Scholar] [CrossRef]
- Manna, J.; Akbayrak, S.; Özkar, S. Palladium (0) nanoparticles supported on polydopamine coated CoFe2O4 as highly active, magnetically isolable and reusable catalyst for hydrogen generation from the hydrolysis of ammonia borane. Appl. Catal. B Environ. 2017, 208, 104–115. [Google Scholar] [CrossRef]
- Xu, L.X.; Yao, F.; Luo, J.L.; Wan, C.; Ye, M.F.; Cui, P.; An, Y. Facile synthesis of amine-functionalized SBA-15-supported bimetallic Au–Pd nanoparticles as an efficient catalyst for hydrogen generation from formic acid. RSC Adv. 2017, 7, 4746–4752. [Google Scholar] [CrossRef]
- Xu, Q.; Chandra, M. A portable hydrogen generation system: Catalytic hydrolysis of ammonia–borane. J. Alloy. Compd. 2007, 446, 729–732. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts. J. Power Sources 2007, 168, 135–142. [Google Scholar] [CrossRef]
- Chen, W.Y.; Ji, J.; Duan, X.Z.; Qian, G.; Li, P.; Zhou, X.G.; Chen, D.; Yuan, W.K. Unique reactivity in Pt/CNT catalyzed hydrolytic dehydrogenation of ammonia borane. Chem. Commun. 2014, 50, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- Durap, F.; Zahmakıran, M.; Özkar, S. Water soluble laurate-stabilized ruthenium (0) nanoclusters catalyst for hydrogen generation from the hydrolysis of ammonia-borane: High activity and long lifetime. Int. J. Hydrog. Energy 2009, 34, 7223–7230. [Google Scholar] [CrossRef]
- Cao, N.; Luo, W.; Cheng, G.Z. One-step synthesis of graphene supported Ru nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2013, 38, 11964–11972. [Google Scholar] [CrossRef]
- Liang, H.Y.; Chen, G.Z.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D.L. In situ facile synthesis of ruthenium nanocluster catalyst supported on carbon black for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2012, 37, 17921–17927. [Google Scholar] [CrossRef]
- Du, C.; Ao, Q.; Cao, N.; Yang, L.; Luo, W.; Cheng, G.Z. Facile synthesis of monodisperse ruthenium nanoparticles supported on graphene for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2015, 40, 6180–6187. [Google Scholar] [CrossRef]
- Wen, L.; Su, J.; Wu, X.J.; Cai, P.; Luo, W.; Cheng, G.Z. Ruthenium supported on MIL-96: An efficient catalyst for hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 17129–17135. [Google Scholar] [CrossRef]
- Yang, K.Z.; Zhou, L.Q.; Yu, G.F.; Xiong, X.; Ye, M.L.; Li, Y.; Lu, D.; Pan, Y.X.; Chen, M.H.; Zhang, L.; et al. Ru nanoparticles supported on MIL-53 (Cr, Al) as efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 6300–6309. [Google Scholar] [CrossRef]
- Fan, G.Y.; Liu, Q.Q.; Tang, D.M.; Li, X.J.; Bi, J.; Gao, D.J. Nanodiamond supported Ru nanoparticles as an effective catalyst for hydrogen evolution from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 1542–1549. [Google Scholar] [CrossRef]
- Wu, Z.J.; Duan, Y.L.; Ge, S.H.; Yip, A.C.; Yang, F.; Li, Y.F.; Dou, T. Promoting hydrolysis of ammonia borane over multiwalled carbon nanotube-supported Ru catalysts via hydrogen spillover. Catal. Commun. 2017, 91, 10–15. [Google Scholar] [CrossRef]
- Yao, Q.L.; Shi, W.M.; Feng, G.; Lu, Z.H.; Zhang, X.L.; Tao, D.J.; Kong, D.J.; Chen, X.S. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2014, 257, 293–299. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tonbul, Y.; Özkar, S. Ceria supported rhodium nanoparticles: Superb catalytic activity in hydrogen generation from the hydrolysis of ammonia borane. Appl. Catal. B Environ. 2016, 198, 162–170. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Rhodium (0) nanoparticles supported on nanosilica: Highly active and long lived catalyst in hydrogen generation from the methanolysis of ammonia borane. Appl. Catal. B Environ. 2016, 181, 716–726. [Google Scholar] [CrossRef]
- Roy, S.; Pachfule, P.; Xu, Q. High Catalytic Performance of MIL-101-Immobilized NiRu Alloy Nanoparticles towards the Hydrolytic Dehydrogenation of Ammonia Borane. Eur. J. Inorg. Chem. 2016, 2016, 4353–4357. [Google Scholar] [CrossRef]
- Yao, Q.L.; Lu, Z.H.; Wang, Y.Q.; Chen, X.S.; Feng, G. Synergetic catalysis of non-noble bimetallic Cu–Co nanoparticles embedded in SiO2 nanospheres in hydrolytic dehydrogenation of ammonia borane. J. Phys. Chem. C 2015, 119, 14167–14174. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Edla, R.; Bazzanella, N.; Kothari, D.C.; Miotello, A. Ruthenium nanoparticles supported over carbon thin film catalyst synthesized by pulsed laser deposition for hydrogen production from ammonia borane. Appl. Catal. A Gen. 2015, 495, 23–29. [Google Scholar] [CrossRef]
- Park, J.W.; Lai, S.W.; Cho, S.O. Catalytic hydrogen generation from hydrolysis of ammonia borane using octahedral Au@ Pt nanoparticles. Int. J. Hydrog. Energy 2015, 40, 16316–16322. [Google Scholar] [CrossRef]
- Shen, J.F.; Yang, L.; Hu, K.; Luo, W.; Cheng, G.Z. Rh nanoparticles supported on graphene as efficient catalyst for hydrolytic dehydrogenation of amine boranes for chemical hydrogen storage. Int. J. Hydrog. Energy 2015, 40, 1062–1070. [Google Scholar] [CrossRef]
- Xia, B.Q.; Liu, C.; Wu, H.; Luo, W.; Cheng, G.Z. Hydrolytic dehydrogenation of ammonia borane catalyzed by metal-organic framework supported bimetallic RhNi nanoparticles. Int. J. Hydrog. Energy 2015, 40, 16391–16397. [Google Scholar] [CrossRef]
- Shang, N.Z.; Feng, C.; Gao, S.T.; Wang, C. Ag/Pd nanoparticles supported on amine-functionalized metal–organic framework for catalytic hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 944–950. [Google Scholar] [CrossRef]
- Rakap, M. The highest catalytic activity in the hydrolysis of ammonia borane by poly (N-vinyl-2-pyrrolidone)-protected palladium–rhodium nanoparticles for hydrogen generation. Appl. Catal. B Environ. 2015, 163, 129–134. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Herron, R.; Phillips, A.D. Towards an understanding of the beneficial effect of mesoporous materials on the dehydrogenation characteristics of NH3BH3. Appl. Catal. B Environ. 2017, 201, 182–188. [Google Scholar] [CrossRef]
- Zhang, T.R.; Yang, X.J.; Yang, S.Q.; Li, D.X.; Cheng, F.Y.; Tao, Z.L.; Chen, J. Silica hollow nanospheres as new nanoscaffold materials to enhance hydrogen releasing from ammonia borane. Phys. Chem. Chem. Phys. 2011, 13, 18592–18599. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Liu, N.; Hong, B.; Cui, P.; Cheng, D.G.; Chen, F.Q.; An, Y.; Wan, C. Nickel–platinum nanoparticles immobilized on graphitic carbon nitride as highly efficient catalyst for hydrogen release from hydrous hydrazine. RSC Adv. 2016, 6, 31687–31691. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Huang, W.Y.; Tsung, C.K.; Zhang, Y.W.; Somorjai, G.A. Structure sensitivity of carbon−nitrogen ring opening: Impact of platinum particle size from below 1 to 5 nm upon pyrrole hydrogenation product selectivity over monodisperse platinum nanoparticles loaded onto mesoporous silica. J. Am. Chem. Soc. 2008, 130, 14026–14027. [Google Scholar] [CrossRef] [PubMed]
- Tsung, C.K.; Kuhn, J.N.; Huang, W.Y.; Aliaga, C.; Hung, L.I.; Somorjai, G.A.; Yang, P. Sub-10 nm platinum nanocrystals with size and shape control: Catalytic study for ethylene and pyrrole hydrogenation. J. Am. Chem. Soc. 2009, 131, 5816–5822. [Google Scholar] [CrossRef]
- Arenz, M.; Mayrhofer, K.J.; Stamenkovic, V.; Blizanac, B.B.; Tomoyuki, T.; Ross, P.N.; Markovic, N.M. The effect of the particle size on the kinetics of CO electrooxidation on high surface area Pt catalysts. J. Am. Chem. Soc. 2005, 127, 6819–6829. [Google Scholar] [CrossRef] [PubMed]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.H.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef]
- Chin, Y.H.; Buda, C.; Neurock, M.; Iglesia, E. Reactivity of chemisorbed oxygen atoms and their catalytic consequences during CH4–O2 catalysis on supported Pt clusters. J. Am. Chem. Soc. 2011, 133, 15958–15978. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.S.; Zhao, C.C.; Wang, N.; Li, T.J.; Lu, W.W.; Wang, J.J. An aqueous synthesis of porous PtPd nanoparticles with reversed bimetallic structures for highly efficient hydrogen generation from ammonia borane hydrolysis. Nanoscale 2020, 12, 638–647. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.L.; Vara, M.; Hood, Z.D.; Luo, M.; Yang, T.H.; Bao, S.X.; Chi, M.F.; Xiao, P.; Zhang, Y.H.; et al. Quantitative analysis of the reduction kinetics responsible for the one-pot synthesis of Pd–Pt bimetallic nanocrystals with different structures. J. Am. Chem. Soc. 2016, 138, 12263–12270. [Google Scholar] [CrossRef]
- Käß, M.; Friedrich, A.; Drees, M.; Schneider, S. Ruthenium complexes with cooperative PNP ligands: Bifunctional catalysts for the dehydrogenation of ammonia–borane. Angew. Chem. Int. Ed. 2009, 48, 905–907. [Google Scholar] [CrossRef]
- Wen, L.; Zheng, Z.; Luo, W.; Cai, P.; Cheng, G.Z. Ruthenium deposited on MCM-41 as efficient catalyst for hydrolytic dehydrogenation of ammonia borane and methylamine borane. Chin. Chem. Lett. 2015, 26, 1345–1350. [Google Scholar] [CrossRef]
- Xu, C.X.; Su, J.X.; Xu, X.H.; Liu, P.P.; Zhao, H.J.; Tian, F.; Ding, Y. Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 2007, 129, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.B.; Shi, Y.Y.; Zhao, Z.; Yin, H.B.; Wei, Y.C.; Liu, J.; Kang, W.B.; Jiang, T.S.; Wang, A.L. Ultrasmall silver nanoparticles supported on silica and their catalytic performances for carbon monoxide oxidation. Catal. Commun. 2011, 12, 616–620. [Google Scholar] [CrossRef]
- Huang, L.; Zou, J.S.; Ye, J.Y.; Zhou, Z.Y.; Lin, Z.; Kang, X.W.; Jain, P.; Chen, S.W. Synergy between Plasmonic and Electrocatalytic Activation of Methanol Oxidation on Palladium–Silver Alloy Nanotubes. Angew. Chem. 2019, 131, 8886–8890. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Xu, W.Q.; Gu, W.L.; Zhu, C.Z.; Du, D.; Lin, Y.H. Polydopamine-Capped Bimetallic AuPt Hydrogels Enable Robust Biosensor for Organophosphorus Pesticide Detection. Small 2019, 15, 1900632. [Google Scholar] [CrossRef]
- Lv, H.; Sun, L.Z.; Zou, L.; Xu, D.D.; Yao, H.Q.; Liu, B. Size-dependent synthesis and catalytic activities of trimetallic PdAgCu mesoporous nanospheres in ethanol electrooxidation. Chem. Sci. 2019, 10, 1986–1993. [Google Scholar] [CrossRef]
- Abo-Hamed, E.; Pennycook, T.; Vaynzof, Y.; Toprakcioglu, C.; Koutsioubas, A.; Scherman, O.A. Highly active metastable ruthenium nanoparticles for hydrogen production through the catalytic hydrolysis of ammonia borane. Small 2014, 10, 3145–3152. [Google Scholar] [CrossRef]
- Wan, C.; An, Y.; Chen, F.Q.; Cheng, D.G.; Wu, F.Y.; Xu, G.H. Kinetics of N-ethylcarbazole hydrogenation over a supported Ru catalyst for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 7065–7069. [Google Scholar] [CrossRef]
- Du, J.; Cheng, F.Y.; Si, M.; Liang, J.; Tao, Z.L.; Chen, J. Nanoporous Ni-based catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2013, 38, 5768–5774. [Google Scholar] [CrossRef]
- Li, Y.; Dai, Y.; Tian, X.K. Controlled synthesis of monodisperse PdxSn100−x nanoparticles and their catalytic activity for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2015, 40, 9235–9243. [Google Scholar]
- Güngörmez, K.; Metin, Ö. Composition-controlled catalysis of reduced graphene oxide supported CuPd alloy nanoparticles in the hydrolytic dehydrogenation of ammonia borane. Appl. Catal. A Gen. 2015, 494, 22–28. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Liu, Q.X.; Zhou, M.; Mi, G.; Du, X.G. Hierarchically alloyed Pd–Cu microarchitecture with tunable shapes: Morphological engineering, and catalysis for hydrogen evolution reaction of ammonia borane. Int. J. Hydrog. Energy 2019, 44, 30226–30236. [Google Scholar] [CrossRef]
- Deka, J.R.; Saikia, D.; Chen, P.H.; Chen, K.T.; Kao, H.M.; Yang, Y.C. Palladium nanoparticles encapsulated in carboxylic acid functionalized periodic mesoporous organosilicas as efficient and reusable heterogeneous catalysts for hydrogen generation from ammonia borane. Materials Research Bulletin 2020, 125, 110786. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.G.; Man, T.T.; Wu, M.; Chen, C.C. Recent process and development of metal aminoborane. Chem. Asian J. 2013, 8, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Meng, X.F.; Wang, J.M.; Shang, N.Z.; Feng, T.; Gao, Z.Y.; Zhang, H.X.; Ding, X.L.; Gao, S.T.; Feng, C.; et al. Boron nitride supported NiCoP nanoparticles as noble metal-free catalyst for highly efficient hydrogen generation from ammonia borane. Int. J. Hydrog. Energy 2019, 44, 4764–4770. [Google Scholar] [CrossRef]
- Singh, A.K.; Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Yang, L.; Su, J.; Meng, X.Y.; Luo, W.; Cheng, G.Z. In situ synthesis of graphene supported Ag@CoNi core–shell nanoparticles as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane and methylamine borane. J. Mater. Chem. A 2013, 1, 10016–10023. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Chen, H.M.; Liu, R.S. Architecture of metallic nanostructures: Synthesis strategy and specific applications. J. Phys. Chem. C 2011, 115, 3513–3527. [Google Scholar] [CrossRef]
- Chen, S.W.; Yang, Y.Y. Magnetoelectrochemistry of gold nanoparticle quantized capacitance charging. J. Am. Chem. Soc. 2002, 124, 5280–5281. [Google Scholar] [CrossRef]
- Yang, X.J.; Cheng, F.Y.; Liang, J.; Tao, Z.L.; Chen, J. PtxNi1−x nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2009, 34, 8785–8791. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, W.W.; Yu, Y.S. Monodisperse PtCu alloy nanoparticles as highly efficient catalysts for the hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2018, 43, 14293–14300. [Google Scholar] [CrossRef]
- Chen, W.Y.; Fu, W.Z.; Qian, G.; Zhang, B.S.; Chen, D.; Duan, X.Z.; Zhou, X.G. Synergistic Pt-WO3 Dual Active Sites to Boost Hydrogen Production from Ammonia Borane. Iscience 2020, 23, 100922. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia borane as hydrogen storage materials. Int. J. Hydrog. Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Özkar, S. Transition metal nanoparticles in catalysis for the hydrogen generation from the hydrolysis of ammonia-borane. Top. Catal. 2013, 56, 1171–1183. [Google Scholar] [CrossRef]
- Özkar, S.; Finke, R.G. Nanocluster formation and stabilization fundamental studies: Ranking commonly employed anionic stabilizers via the development, then application, of five comparative criteria. J. Am. Chem. Soc. 2002, 124, 5796–5810. [Google Scholar] [CrossRef]
- Shylesh, S.; Schuenemann, V.; Thiel, W.R. Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef]
- Baig, R.N.; Varma, R.S. Magnetically retrievable catalysts for organic synthesis. Chem. Commun. 2013, 49, 752–770. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. Fast-growing field of magnetically recyclable nanocatalysts. Chem. Rev. 2014, 114, 6949–6985. [Google Scholar] [CrossRef]
- Akbayrak, S.; Çakmak, G.; Öztürk, T.; Özkar, S. Rhodium (0), Ruthenium (0) and Palladium (0) nanoparticles supported on carbon-coated iron: Magnetically isolable and reusable catalysts for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Yang, H.X.; Xu, C.X. Nanoporous Ru as highly efficient catalyst for hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 12714–12721. [Google Scholar] [CrossRef]
- Wei, Z.H.; Liu, Y.; Peng, Z.K.; Song, H.Q.; Liu, Z.Y.; Liu, B.Z.; Li, B.Z.; Yang, B.; Lu, S.Y. Cobalt-ruthenium nanoalloys parceled in porous nitrogen-doped graphene as highly efficient difunctional catalysts for hydrogen evolution reaction and hydrolysis of ammonia borane. ACS Sustain. Chem. Eng. 2019, 7, 7014–7023. [Google Scholar] [CrossRef]
- Guo, L.T.; Cai, Y.Y.; Ge, J.M.; Zhang, Y.N.; Gong, L.H.; Li, X.H.; Wang, K.X.; Ren, Q.Z.; Su, J.; Chen, J.S. Multifunctional Au–Co@CN nanocatalyst for highly efficient hydrolysis of ammonia borane. ACS Catal. 2015, 5, 388–392. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.H.; Wu, L.L.; Long, Y.; Li, J.; Song, S.Y.; Zhang, H.J. Tunable bimetallic Au–Pd@CeO2 for semihydrogenation of phenylacetylene by ammonia borane. Nanoscale 2019, 11, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fu, F.Y.; Yang, S.; Martinez Moro, M.; Ramirez, M.D.L.A.; Moya, S.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. Dramatic synergy in CoPt nanocatalysts stabilized by “Click” dendrimers for evolution of hydrogen from hydrolysis of ammonia borane. ACS Catal. 2018, 9, 1110–1119. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Qi, L.; Yang, H.X.; Xu, C.X. Hierarchical nanoporous platinum–copper alloy nanoflowers as highly active catalysts for the hydrolytic dehydrogenation of ammonia borane. J. Colloid Interface Sci. 2018, 513, 258–265. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.L.; Li, L.L.; Lin, J.; Yang, X.J.; Yu, C.; Liu, Z.Y.; Fang, Y.; Huang, Y.F.; Tang, C.C. CuCo binary metal nanoparticles supported on boron nitride nanofibers as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. J. Power Sources 2019, 431, 135–143. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Jiang, H.L.; Umegaki, T.; Akita, T.; Zhang, X.B.; Haruta, M.; Xu, Q. Bimetallic Au–Ni nanoparticles embedded in SiO2 nanospheres: Synergetic catalysis in hydrolytic dehydrogenation of ammonia borane. Chem. A Eur. J. 2010, 16, 3132–3137. [Google Scholar] [CrossRef]
- Rej, S.; Hsia, C.F.; Chen, T.Y.; Lin, F.C.; Huang, J.S.; Huang, M.H. Facet-Dependent and Light-Assisted Efficient Hydrogen Evolution from Ammonia Borane Using Gold–Palladium Core-Shell Nanocatalysts. Angew. Chem. Int. Ed. 2016, 55, 7222–7226. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.F.; Xi, Z.; Chen, Z.Z.; Guo, S.J.; Yu, Y.S.; Zhu, W.L.; Li, Q.; Zhang, X.; Pan, M.; Lu, G.; et al. A new core/shell NiAu/Au nanoparticle catalyst with Pt-like activity for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 5859–5862. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.P.; Su, D.; Adzic, R.R. Platinum-monolayer shell on AuNi0.5Fe nanoparticle core electrocatalyst with high activity and stability for the oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 14364–14366. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Chen, T.W.; Zhang, D.F.; Guo, L. PtNiAu trimetallic nanoalloys enabled by a digestive-assisted process as highly efficient catalyst for hydrogen generation. Nano Energy 2016, 23, 145–152. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Tonegawa, A.; Komatsu, M.; Wang, H.J.; Wang, L.; Nemoto, Y.; Suzuki, N.; Kuroda, K. Electrochemical synthesis of mesoporous Pt–Au binary alloys with tunable compositions for enhancement of electrochemical performance. J. Am. Chem. Soc. 2012, 134, 5100–5109. [Google Scholar] [CrossRef]
- Fu, L.L.; Zhang, D.F.; Yang, Z.; Chen, T.W.; Zhai, J. PtAuCo Trimetallic Nanoalloys as Highly Efficient Catalysts toward Dehydrogenation of Ammonia Borane. ACS Sustain. Chem. Eng. 2020, 8, 3734–3742. [Google Scholar] [CrossRef]
- Shui, J.L.; Chen, C.; Li, J.C. Evolution of nanoporous Pt–Fe alloy nanowires by dealloying and their catalytic property for oxygen reduction reaction. Adv. Funct. Mater. 2011, 21, 3357–3362. [Google Scholar] [CrossRef]
- Cui, C.H.; Gan, L.; Li, H.H.; Yu, S.H.; Heggen, M.; Strasser, P. Octahedral PtNi nanoparticle catalysts: Exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Li, N.; Yan, Y.; Lou, X.W.; Wang, X. One-pot synthesis of Pt-Co alloy nanowire assemblies with tunable composition and enhanced electrocatalytic properties. Angew. Chem. Int. Ed. 2015, 54, 3797–3801. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Luo, Z.M.; Chen, B.; Wei, C.; Zhao, J.; Chen, J.Z.; Zhang, X.; Lai, Z.C.; Fan, Z.X.; Tan, C.L.; et al. One-pot synthesis of highly anisotropic five-fold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv. Mater. 2016, 28, 8712–8717. [Google Scholar] [CrossRef]
- Bu, L.Z.; Shao, Q.; Bin, E.; Guo, J.; Yao, J.L.; Huang, X.Q. PtPb/PtNi intermetallic core/atomic layer shell octahedra for efficient oxygen reduction electrocatalysis. J. Am. Chem. Soc. 2017, 139, 9576–9582. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Ling, T.; Ma, T.Y.; Wang, H.; Hu, Z.P.; Zhou, Y.; Mao, J.; Du, X.W.; Jaroniec, M.; Qiao, S.Z. Atomically and electronically coupled Pt and CoO hybrid nanocatalysts for enhanced electrocatalytic performance. Adv. Mater. 2017, 29, 1604607. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Hu, M.; Wang, Q.; Fan, G.Y.; Wang, Y.; Zhang, Y.; Gao, D.J.; Bi, J. Hyper-cross-linked polymer supported rhodium: An effective catalyst for hydrogen evolution from ammonia borane. Dalton Trans. 2018, 47, 2561–2567. [Google Scholar] [CrossRef]
- Zhong, F.Y.; Wang, Q.; Xu, C.L.; Yang, Y.C.; Wang, Y.; Zhang, Y.; Gao, D.J.; Bi, J.; Fan, G. Ultrafine and highly dispersed Ru nanoparticles supported on nitrogen-doped carbon nanosheets: Efficient catalysts for ammonia borane hydrolysis. Appl. Surf. Sci. 2018, 455, 326–332. [Google Scholar] [CrossRef]
- Metin, Ö.; Kayhan, E.; Özkar, S.; Schneider, J.J. Palladium nanoparticles supported on chemically derived graphene: An efficient and reusable catalyst for the dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2012, 37, 8161–8169. [Google Scholar] [CrossRef]
- Lu, R.; Xu, C.L.; Wang, Q.; Wang, Y.; Zhang, Y.; Gao, D.J.; Bi, J.; Fan, G.Y. Ruthenium nanoclusters distributed on phosphorus-doped carbon derived from hypercrosslinked polymer networks for highly efficient hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2018, 43, 18253–18260. [Google Scholar] [CrossRef]
- Yao, Q.L.; Lu, Z.H.; Jia, Y.S.; Chen, X.S.; Liu, X. In situ facile synthesis of Rh nanoparticles supported on carbon nanotubes as highly active catalysts for H2 generation from NH3BH3 hydrolysis. Int. J. Hydrog. Energy 2015, 40, 2207–2215. [Google Scholar] [CrossRef]
- Hu, Y.J.; Wang, Y.Q.; Lu, Z.H.; Chen, X.S.; Xiong, L.H. Core–shell nanospheres Pt@SiO2 for catalytic hydrogen production. Appl. Surf. Sci. 2015, 341, 185–189. [Google Scholar] [CrossRef]
- Tonbul, Y.; Akbayrak, S.; Özkar, S. Palladium (0) nanoparticles supported on ceria: Highly active and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 11154–11162. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tanyıldızı, S.; Morkan, İ.; Özkar, S. Ruthenium (0) nanoparticles supported on nanotitania as highly active and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2014, 39, 9628–9637. [Google Scholar] [CrossRef]
- Garaj, S.; Hubbard, W.; Reina, A.; Kong, J.; Branton, D.; Golovchenko, J.A. Graphene as a subnanometre trans-electrode membrane. Nature 2010, 467, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Hong, J.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Hammond, P.T.; Park, H. Innovative polymer nanocomposite electrolytes: Nanoscale manipulation of ion channels by functionalized graphenes. ACS Nano 2011, 5, 5167–5174. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.J.; Sun, S.H. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef]
- Ke, D.D.; Wang, J.; Zhang, H.M.; Li, Y.; Zhang, L.; Zhao, X.; Han, S.M. Fabrication of Pt–Co NPs supported on nanoporous graphene as high-efficient catalyst for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2017, 42, 26617–26625. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Ma, Y.Y.; Zhang, H.; Gao, J.; Nie, Y.T.; Sun, X.H. Aqueous solution synthesis of Pt–M (M = Fe, Co, Ni) bimetallic nanoparticles and their catalysis for the hydrolytic dehydrogenation of ammonia borane. ACS Appl. Mater. Interfaces 2014, 6, 12429–12435. [Google Scholar] [CrossRef]
- Wang, J.M.; Ma, X.; Yang, W.R.; Sun, X.P.; Liu, J.Q. Self-supported Cu (OH)2@Co2CO3(OH)2 core–shell nanowire array as a robust catalyst for ammonia-borane hydrolysis. Nanotechnology 2016, 28, 045606. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhao, X.; Luo, W.X.; Zhang, Y.; Wang, Y.; Fan, G.Y. Bagasse-derived carbon supported Ru nanoparticles catalyst for efficient dehydrogenation of ammonia borane. ChemNanoMat 2020, 6, 1–10. [Google Scholar]
- Men, Y.N.; Su, J.; Du, X.Q.; Liang, L.J.; Cheng, G.Z.; Luo, W. CoBP nanoparticles supported on three-dimensional nitrogen-doped graphene hydrogel and their superior catalysis for hydrogen generation from hydrolysis of ammonia borane. J. Alloy. Compd. 2018, 735, 1271–1276. [Google Scholar] [CrossRef]
- Zhang, F.W.; Ma, C.; Zhang, Y.; Li, H.; Fu, D.Y.; Du, X.Q.; Zhang, X.M. N-doped mesoporous carbon embedded Co nanoparticles for highly efficient and stable H2 generation from hydrolysis of ammonia borane. J. Power Sources 2018, 399, 89–97. [Google Scholar] [CrossRef]
- Hou, C.C.; Li, Q.; Wang, C.J.; Peng, C.Y.; Chen, Q.Q.; Ye, H.F.; Fu, W.F.; Che, C.M.; López, N.; Chen, Y. Ternary Ni–Co–P nanoparticles as noble-metal-free catalysts to boost the hydrolytic dehydrogenation of ammonia-borane. Energy Environ. Sci. 2017, 10, 1770–1776. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Subrahmanyam, K.S.; Saha, S.K.; Govindaraj, A.; Krishnamurthy, H.R.; Waghmare, U.V.; Rao, C.N.R. Synthesis, structure, and properties of boron-and nitrogen-doped graphene. Adv. Mater. 2009, 21, 4726–4730. [Google Scholar] [CrossRef]
- Luo, W.L.; Zhao, X.; Cheng, W.; Zhang, Y.; Wang, Y.; Fan, G.F. A simple and straightforward strategy for synthesis of N, P co-doped porous carbon: An efficient support for Rh nanoparticles for dehydrogenation of ammonia borane and catalytic application. Nanoscale Adv. 2020, 2, 1685–1693. [Google Scholar] [CrossRef]
- Hu, M.; Ming, M.X.; Xu, C.L.; Wang, Y.; Zhang, Y.; Gao, D.J.; Bi, J.; Fan, G.Y. Towards High-Efficiency Hydrogen Production through in situ Formation of Well-Dispersed Rhodium Nanoclusters. ChemSusChem 2018, 11, 3253–3258. [Google Scholar] [CrossRef]
- Tonbul, Y.; Akbayrak, S.; Özkar, S. Group 4 oxides supported Rhodium (0) catalysts in hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2019, 44, 14164–14174. [Google Scholar] [CrossRef]
- Chen, J.Q.; Hu, M.; Ming, M.; Xu, C.L.; Wang, Y.; Zhang, Y.; Wu, J.T.; Gao, G.J.; Bi, J.; Fan, G.Y. Carbon-supported small Rh nanoparticles prepared with sodium citrate: Toward high catalytic activity for hydrogen evolution from ammonia borane hydrolysis. Int. J. Hydrog. Energy 2018, 43, 2718–2725. [Google Scholar] [CrossRef]
- Ai, W.; Zhou, W.W.; Du, Z.Z.; Chen, Y.; Sun, Z.P.; Wu, C.; Zou, C.J.; Li, C.M.; Huang, W.; Yu, T. Nitrogen and phosphorus codoped hierarchically porous carbon as an efficient sulfur host for Li-S batteries. Energy Storage Mater. 2017, 6, 112–118. [Google Scholar] [CrossRef]
- Qin, Q.; Jang, H.; Chen, L.L.; Nam, G.; Liu, X.; Cho, J. Low loading of RhxP and RuP on N, P codoped carbon as two trifunctional electrocatalysts for the oxygen and hydrogen electrode reactions. Adv. Energy Mater. 2018, 8, 1801478. [Google Scholar] [CrossRef]
- Liu, Y.; Yong, X.; Liu, Z.Y.; Chen, Z.M.; Kang, Z.H.; Lu, S.Y. Unified catalyst for efficient and stable hydrogen production by both the electrolysis of water and the hydrolysis of ammonia borane. Adv. Sustain. Syst. 2019, 3, 1800161. [Google Scholar] [CrossRef]
- Song, H.Q.; Cheng, Y.J.; Li, B.J.; Fan, Y.P.; Liu, B.Z.; Tang, Z.Y.; Lu, S.Y. Carbon dots and RuP2 nanohybrid as an efficient bifunctional catalyst for electrochemical hydrogen evolution reaction and hydrolysis of ammonia borane. ACS Sustain. Chem. Eng. 2020, 8, 3995–4002. [Google Scholar] [CrossRef]
- Lv, Y.A.; Cui, Y.H.; Xiang, Y.Z.; Wang, J.G.; Li, X.N. Modulation of bonding between noble metal monomers and CNTs by B-, N-doping. Comput. Mater. Sci. 2010, 48, 621–625. [Google Scholar] [CrossRef]
- Singh, P.; Samorì, C.; Toma, F.M.; Bussy, C.; Nunes, A.; Al-Jamal, K.T.; Menard-Moyon, C.; Kostarelos, K.; Bianco, A. Polyamine functionalized carbon nanotubes: Synthesis, characterization, cytotoxicity and siRNA binding. J. Mater. Chem. 2011, 21, 4850–4860. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ruthenium (0) nanoparticles supported on multiwalled carbon nanotube as highly active catalyst for hydrogen generation from ammonia–borane. ACS Appl. Mater. Interfaces 2012, 4, 6302–6310. [Google Scholar] [CrossRef]
- Li, S.F.; Guo, Y.H.; Sun, W.W.; Sun, D.L.; Yu, X.B. Platinum nanoparticle functionalized CNTs as nanoscaffolds and catalysts to enhance the dehydrogenation of ammonia-borane. J. Phys. Chem. C 2010, 114, 21885–21890. [Google Scholar] [CrossRef]
- Fu, W.; Han, C.; Li, D.; Chen, W.; Ji, J.; Qian, G.; Yuan, W.; Duan, X.; Zhou, X. Polyoxometalates-engineered hydrogen generation rate and durability of Pt/CNT catalysts from ammonia borane. J. Energy Chem. 2020, 41, 142–148. [Google Scholar] [CrossRef]
- Lu, Z.H.; Jiang, H.L.; Yadav, M.; Aranishi, K.; Xu, Q. Synergistic catalysis of Au-Co@SiO2 nanospheres in hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. J. Mater. Chem. 2012, 22, 5065–5071. [Google Scholar] [CrossRef]
- Metin, Ö.; Dinc, M.; Eren, Z.S.; Özkar, S. Silica embedded cobalt (0) nanoclusters: Efficient, stable and cost effective catalyst for hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2011, 36, 11528–11535. [Google Scholar] [CrossRef]
- Umegaki, T.; Yan, J.M.; Zhang, X.B.; Shioyama, H.; Kuriyama, N.; Xu, Q. Co–SiO2 nanosphere-catalyzed hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. J. Power Sources 2010, 195, 8209–8214. [Google Scholar] [CrossRef]
- Roy, B.; Manna, J.; Pal, U.; Hajari, A.; Bishnoi, A.; Sharma, P. An in situ study on the solid state decomposition of ammonia borane: Unmitigated by-product suppression by a naturally abundant layered clay mineral. Inorg. Chem. Front. 2018, 5, 301–309. [Google Scholar] [CrossRef]
- Ye, W.Y.; Ge, Y.Z.; Gao, Z.M.; Lu, R.W.; Zhang, S.F. Enhanced catalytic activity and stability of Pt nanoparticles by surface coating of nanosized graphene oxide for hydrogen production from hydrolysis of ammonia–borane. Sustain. Energy Fuels 2017, 1, 2128–2133. [Google Scholar] [CrossRef]
- Ciftci, A.; Eren, S.; Ligthart, D.M.; Hensen, E.J. Platinum-Rhenium Synergy on Reducible Oxide Supports in Aqueous-Phase Glycerol Reforming. ChemCatChem 2014, 6, 1260–1269. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Fini, D.; Ciacchi, F.T.; Munnings, C.; Kimpton, J.A.; Drennan, J. Structural and microstructural stability of ceria–gadolinia electrolyte exposed to reducing environments of high temperature fuel cells. J. Mater. Chem. A 2013, 1, 10768–10782. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S.; Bion, N.; Le Mercier, T.; Harleé, V. Reactivity of doped ceria-based mixed oxides for solar thermochemical hydrogen generation via two-step water-splitting cycles. Energy Fuels 2013, 27, 6068–6078. [Google Scholar] [CrossRef]
- Hoare, J.P. Standard Potentials in Aqueous Solution; Marcel Dekker: New York, NY, USA, 1985; p. 49. [Google Scholar]
- Lee, S.S.; Song, W.S.; Cho, M.J.; Puppala, H.L.; Nguyen, P.; Zhu, H.G.; Segatori, L.; Colvin, V.L. Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liang, B.L.; Li, L.; Yang, X.F.; Huang, Y.Q.; Wang, A.Q.; Wang, X.D.; Zhang, T. Cerium-oxide-modified nickel as a non-noble metal catalyst for selective decomposition of hydrous hydrazine to hydrogen. ACS Catal. 2015, 5, 1623–1628. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef]
- Sun, C.W.; Li, H.; Chen, L.Q. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Z.H.; Tan, H.; Chen, X.; Yao, Q. CeOx-modified RhNi nanoparticles grown on rGO as highly efficient catalysts for complete hydrogen generation from hydrazine borane and hydrazine. J. Mater. Chem. A 2015, 3, 23520–23529. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, X.L.; Peng, Z.K.; Liu, P.; Zheng, X.-C. Highly efficient hydrolysis of ammonia borane using ultrafine bimetallic RuPd nanoalloys encapsulated in porous g-C3N4. Fuel 2020, 277, 118243. [Google Scholar] [CrossRef]
- Chen, S.J.; Meng, L.; Chen, B.X.; Chen, W.Y.; Duan, X.Z.; Huang, X.; Zhang, B.S.; Fu, H.B.; Wan, Y. Poison tolerance to the selective hydrogenation of cinnamaldehyde in water over an ordered mesoporous carbonaceous composite supported Pd catalyst. ACS Catal. 2017, 7, 2074–2087. [Google Scholar] [CrossRef]
- Li, X.Y.; Song, L.H.; Gao, D.W.; Kang, B.T.; Zhao, H.Q.; Li, C.C.; Hu, X.; Chen, G.Z. Tandem of Ammonia Borane Dehydrogenation and Phenylacetylene Hydrogenation Catalyzed by CeO2 Nanotube/Pd@MIL-53 (Al). Chem. A Eur. J. 2020, 26, 4419–4424. [Google Scholar] [CrossRef]
- Ruiz, A.M.; Sakai, G.; Cornet, A.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Microstructure control of thermally stable TiO2 obtained by hydrothermal process for gas sensors. Sens. Actuators B Chem. 2004, 103, 312–317. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Kim, H.Y. Electrospun Cu-doped titania nanofibers for photocatalytic hydrolysis of ammonia borane. Appl. Catal. A Gen. 2013, 467, 98–106. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Rakap, M.; Kalu, E.E.; Özkar, S. Hydrogen generation from the hydrolysis of ammonia borane using cobalt-nickel-phosphorus (Co–Ni–P) catalyst supported on Pd-activated TiO2 by electroless deposition. Int. J. Hydrog. Energy 2011, 36, 254–261. [Google Scholar] [CrossRef]
- Shu, H.F.; Lu, L.l.; Zhu, S.F.; Liu, M.M.; Zhu, Y.; Ni, J.Q.; Ruan, Z.H.; Liu, Y. Ultra small cobalt nanoparticles supported on MCM41: One-pot synthesis and catalytic hydrogen production from alkaline borohydride. Catal. Commun. 2019, 118, 30–34. [Google Scholar] [CrossRef]
- Lu, L.L.; Zhang, H.J.; Zhang, S.W.; Li, F.L. A family of high-efficiency hydrogen-generation catalysts based on ammonium species. Angew. Chem. Int. Ed. 2015, 54, 9328–9332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.S.; Zhu, E.B.; Zheng, Y.B.; Huang, Y.; Huang, X.Q. Seedless growth of palladium nanocrystals with tunable structures: From tetrahedra to nanosheets. Nano Lett. 2015, 15, 7519–7525. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.X.; Xu, C.F.; Huang, X.Q.; Ye, J.Y.; Gu, L.; Li, G.; Tang, Z.C.; Wu, B.H.; Yang, H.Y.; Zhao, Z.P.; et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts. Nat. Mater. 2016, 15, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Tang, S.H.; Mu, X.L.; Dai, Y.; Chen, G.X.; Zhou, Z.Y.; Ruan, F.X.; Yang, Z.Y.; Zheng, N.F. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.S.; Xia, Y.N. Noble-metal nanocrystals with concave surfaces: Synthesis and applications. Angew. Chem. Int. Ed. 2012, 51, 7656–7673. [Google Scholar] [CrossRef]
- Song, J.; Gu, X.J.; Cheng, J.; Fan, N.; Zhang, H.; Su, H.Q. Remarkably boosting catalytic H2 evolution from ammonia borane through the visible-light-driven synergistic electron effect of non-plasmonic noble-metal-free nanoparticles and photoactive metal-organic frameworks. Appl. Catal. B Environ. 2018, 225, 424–432. [Google Scholar] [CrossRef]
- Huang, X.Q.; Tang, S.H.; Zhang, H.H.; Zhou, Z.Y.; Zheng, N.F. Controlled formation of concave tetrahedral/trigonal bipyramidal palladium nanocrystals. J. Am. Chem. Soc. 2009, 131, 13916–13917. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.F.; Zhang, H.; Lu, N.; Jin, M.S.; Wang, J.G.; Kim, M.J.; Xie, Z.X.; Xia, Y.N. Synthesis of rhodium concave tetrahedrons by collectively manipulating the reduction kinetics, facet-selective capping, and surface diffusion. Nano Lett. 2013, 13, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
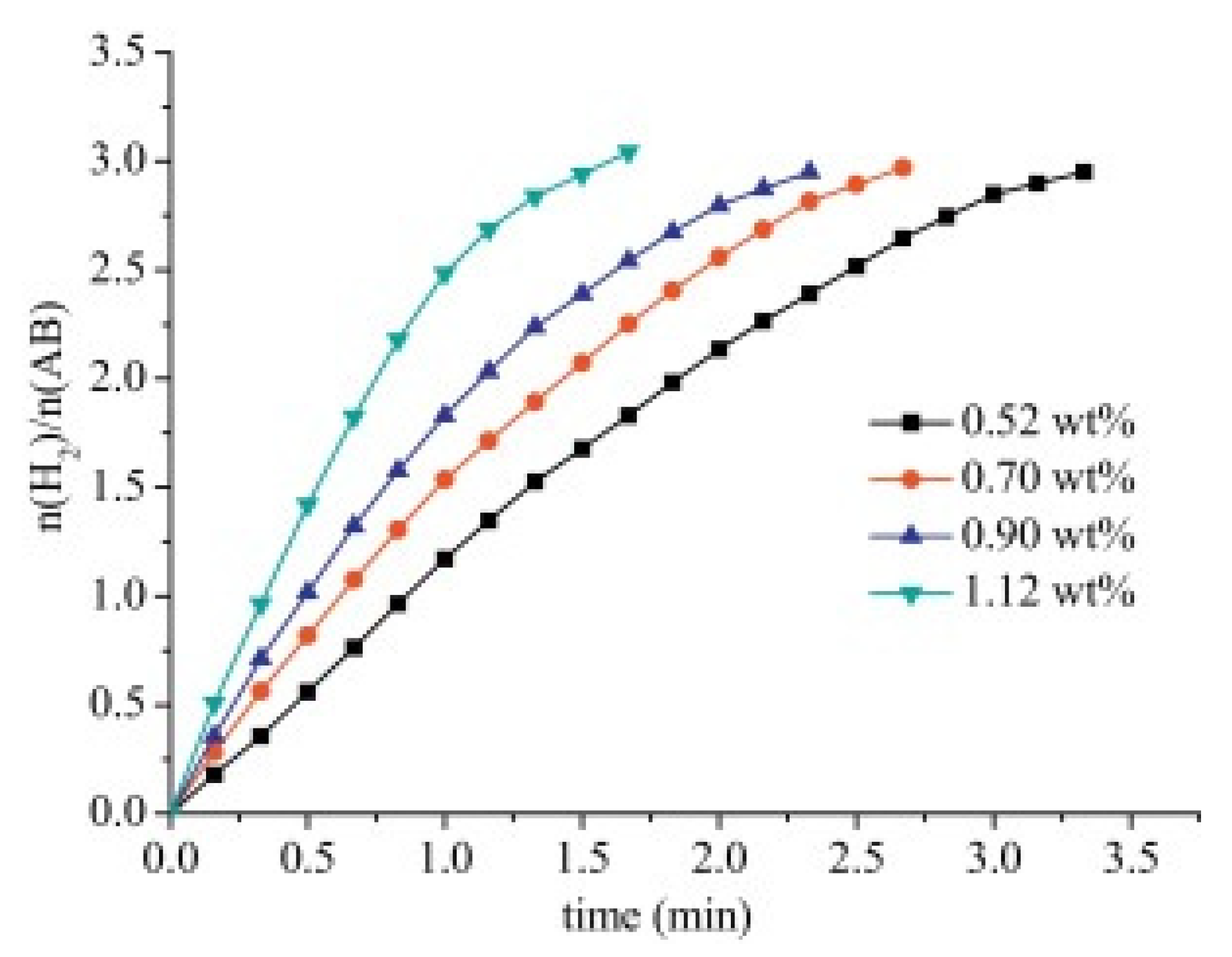
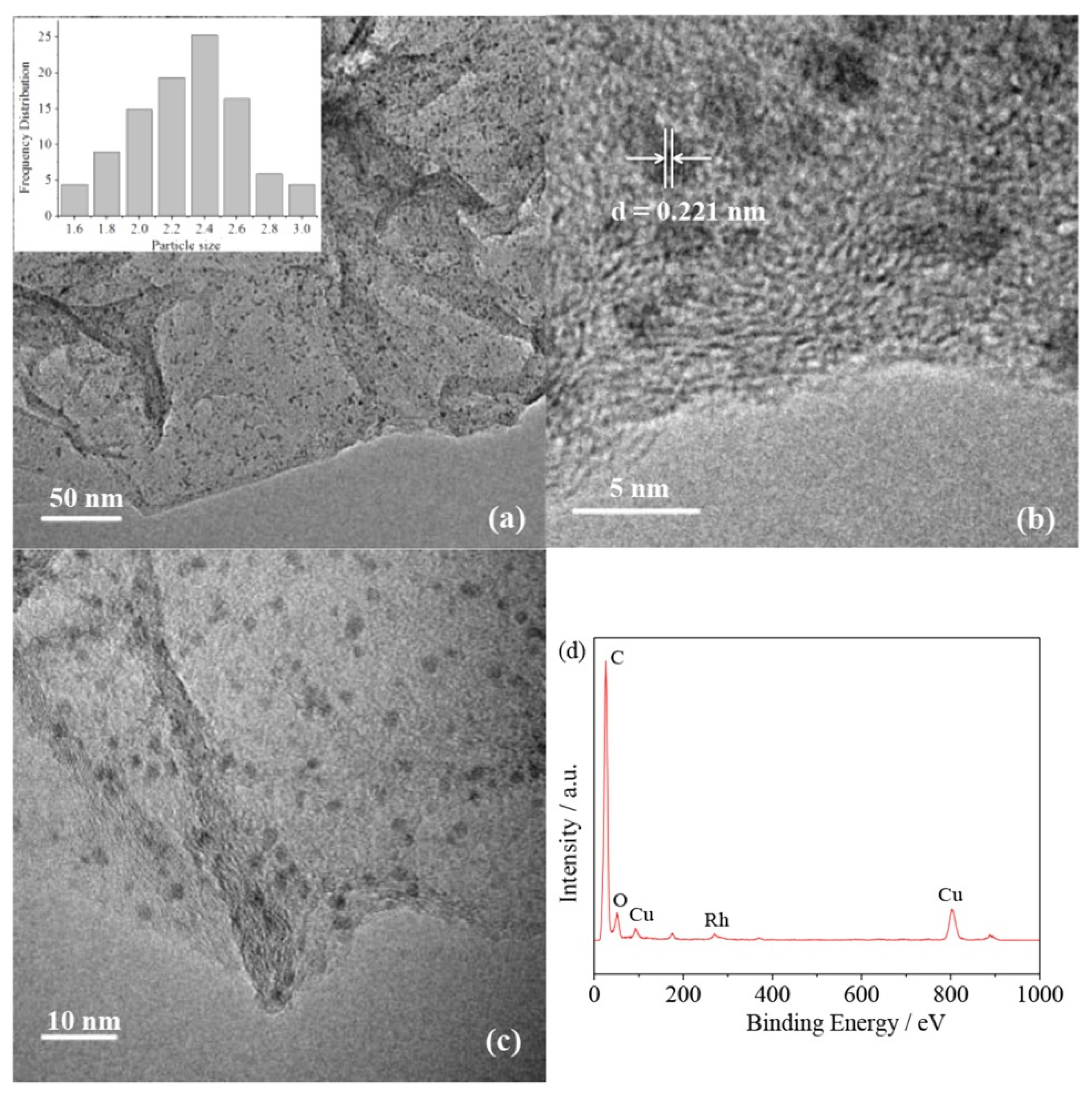
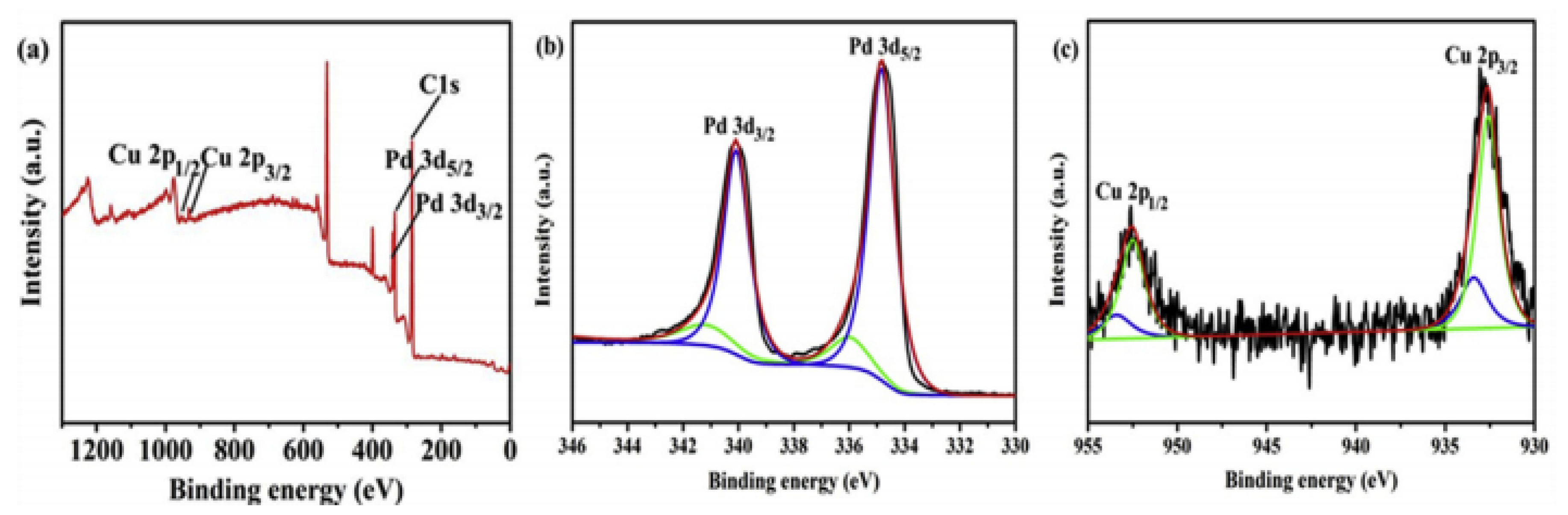
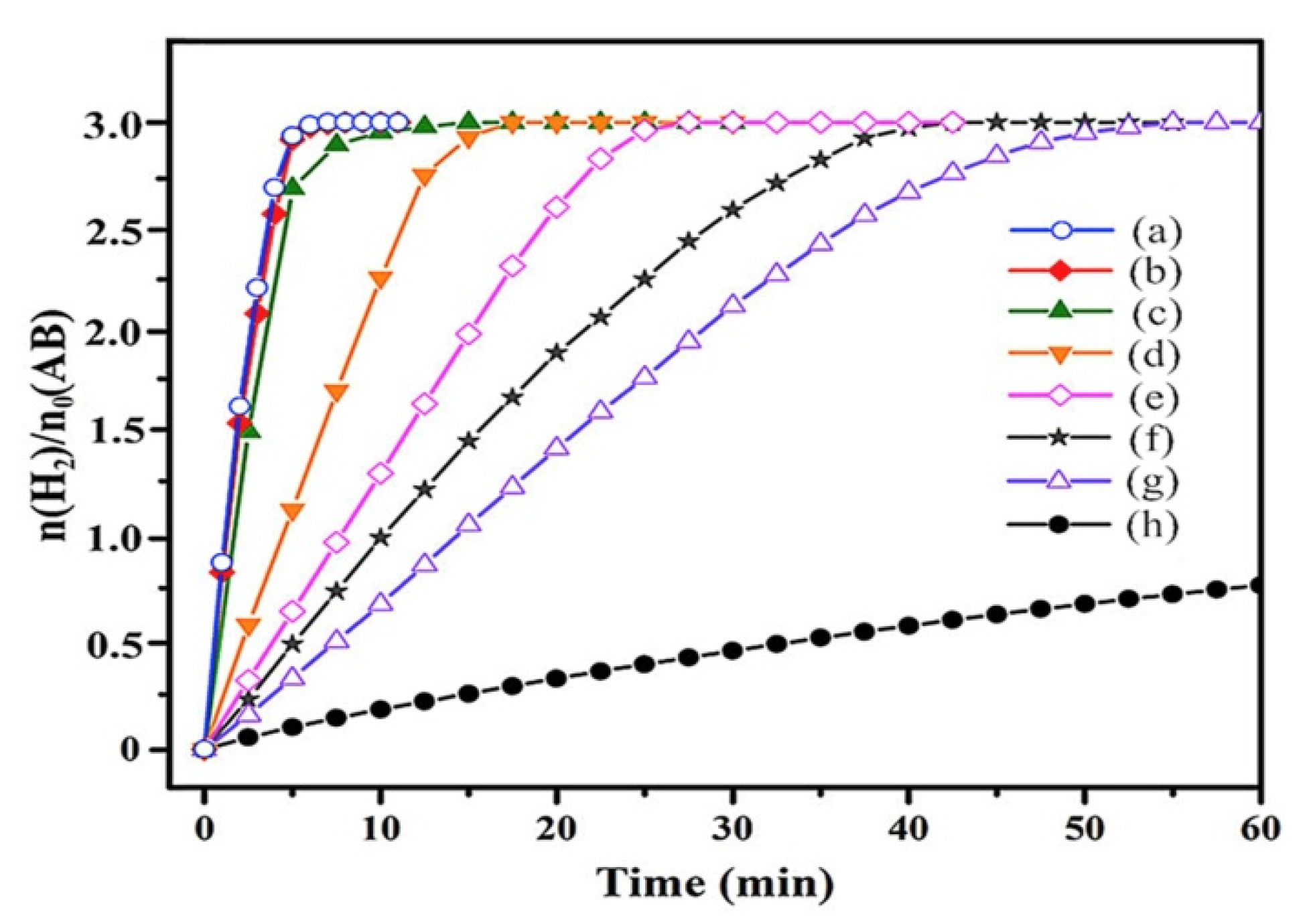
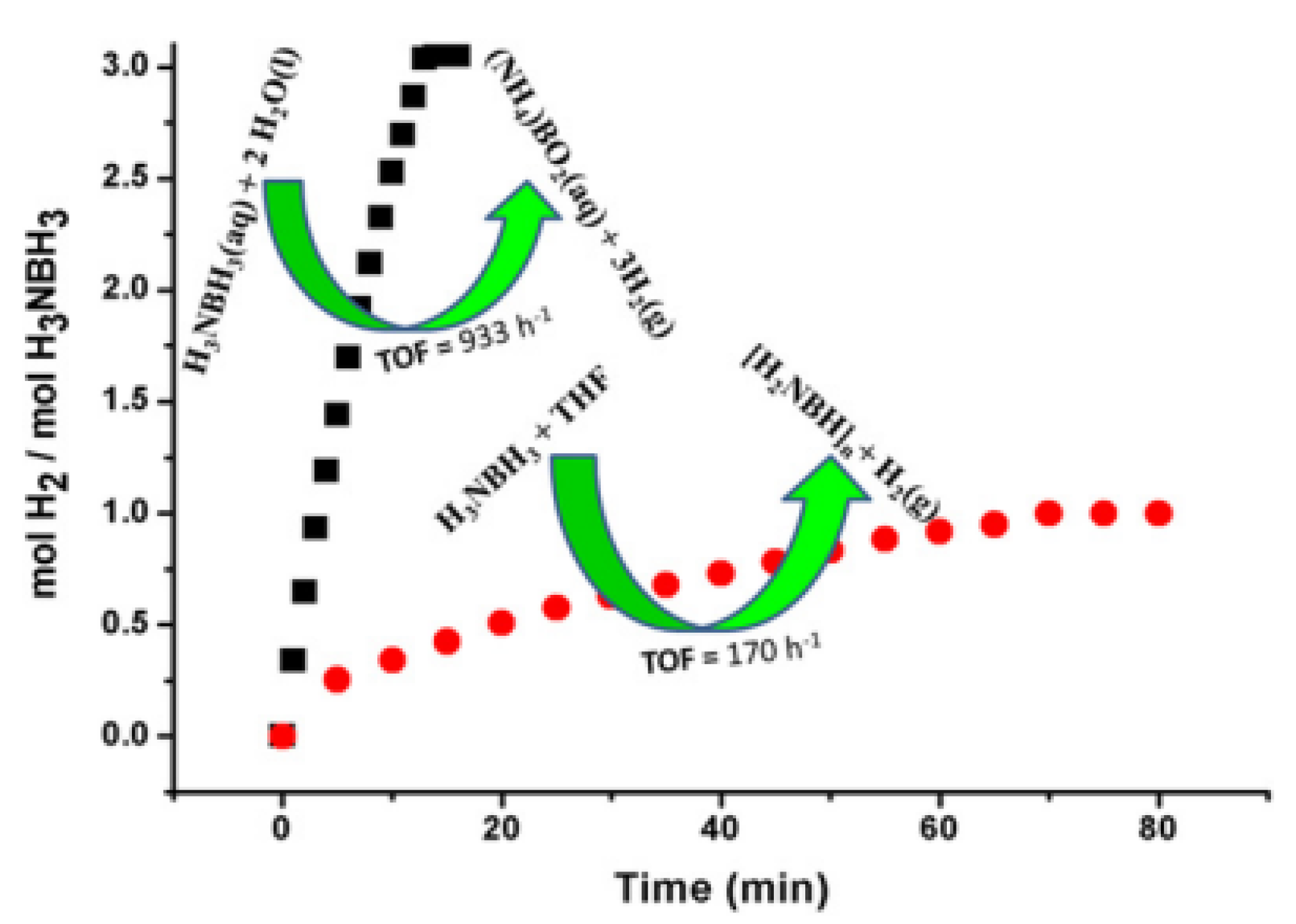
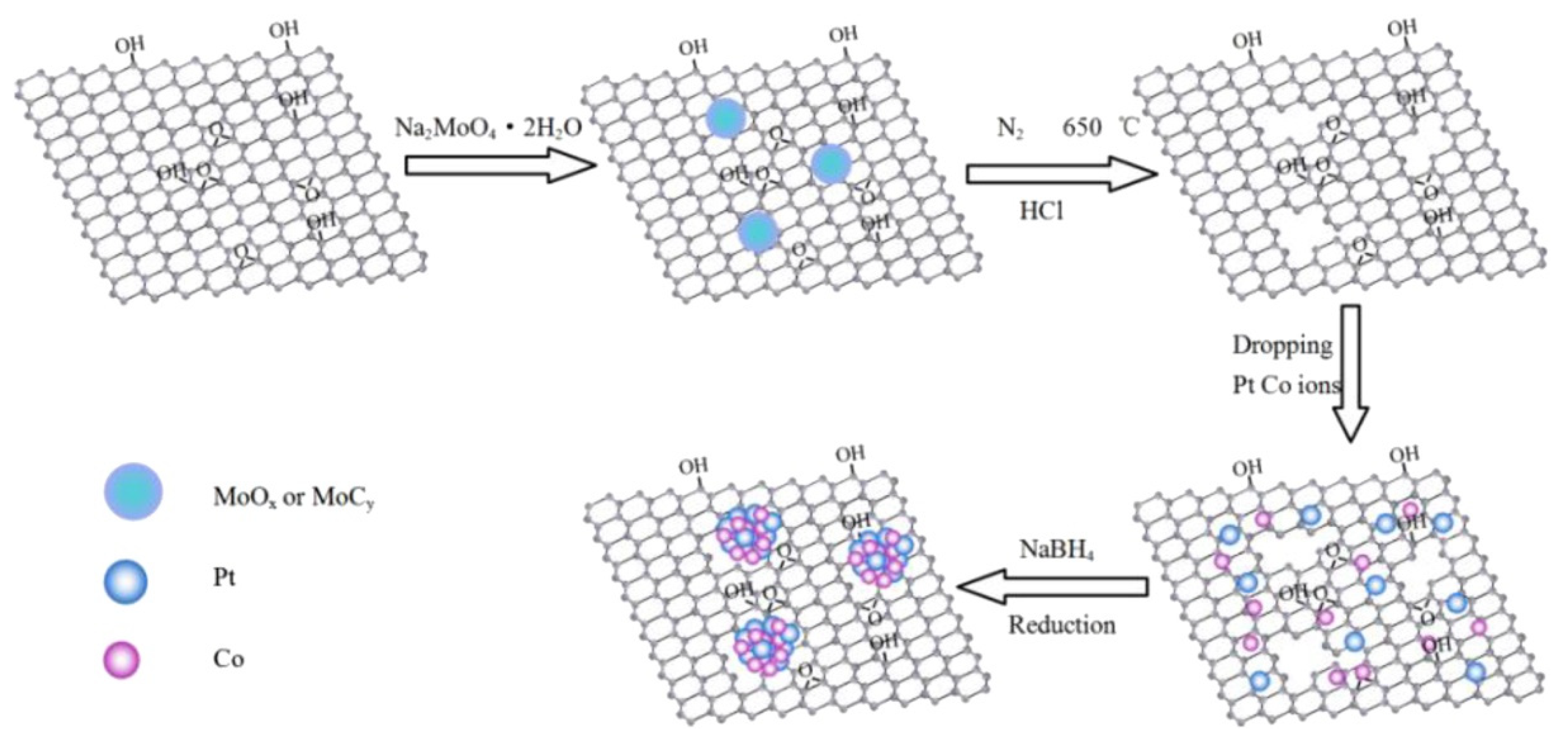
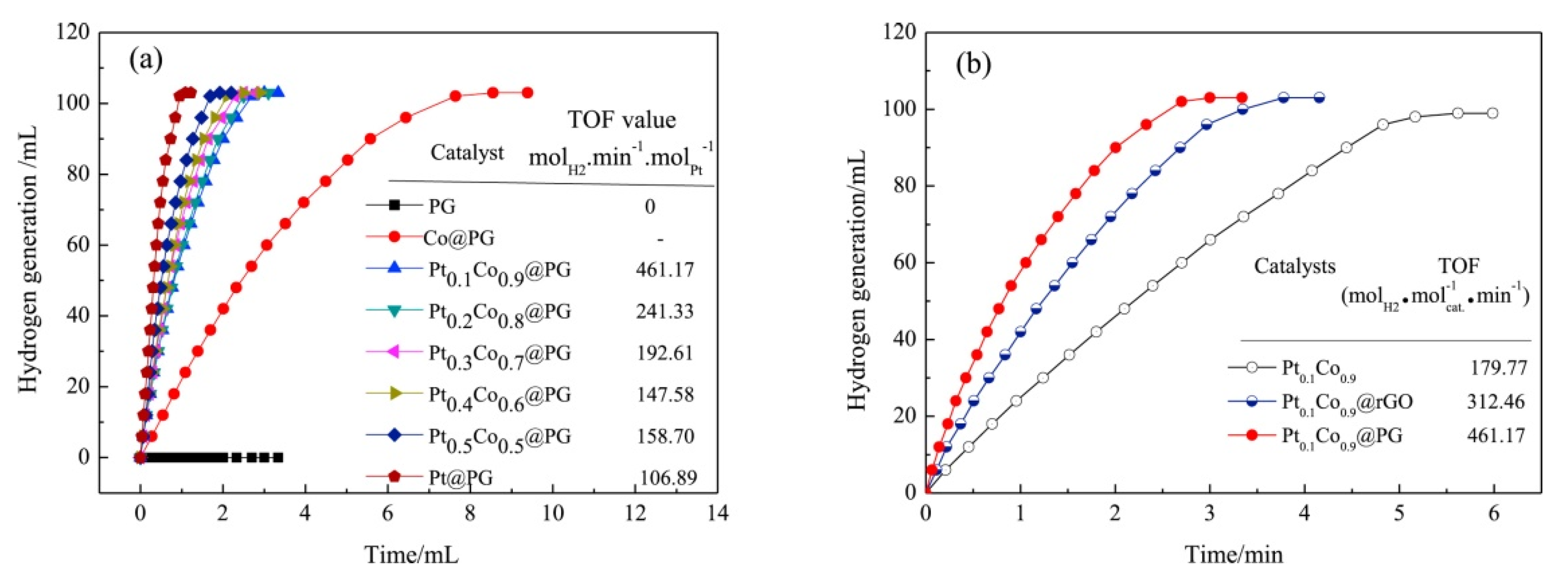
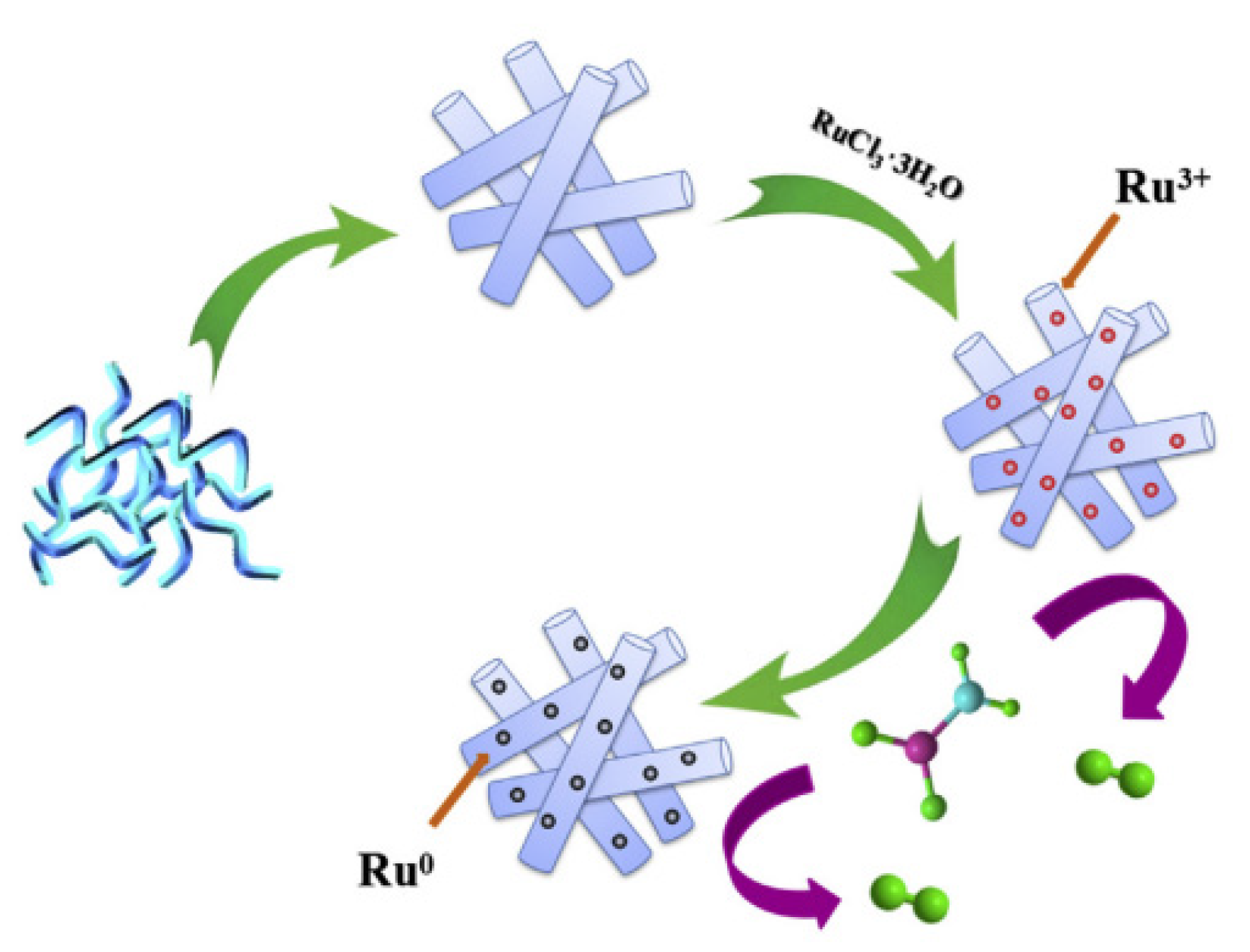



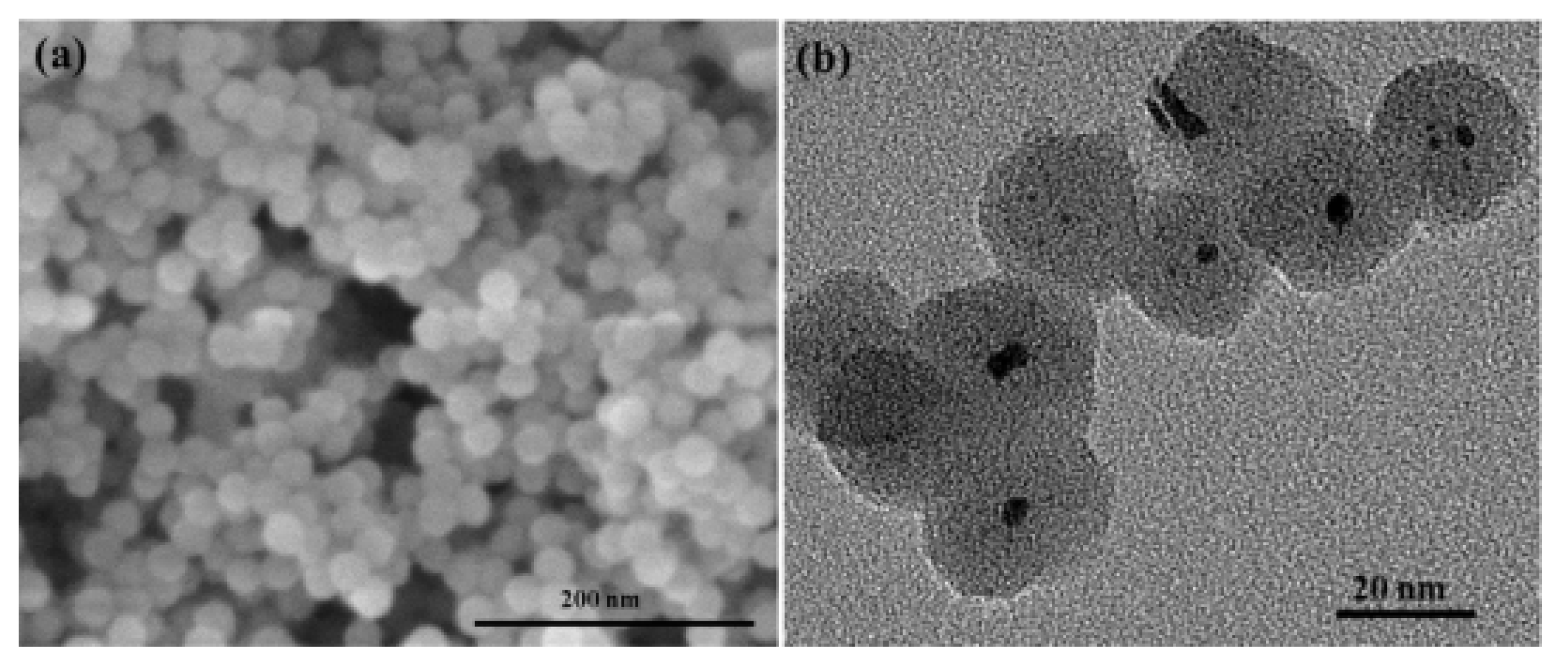
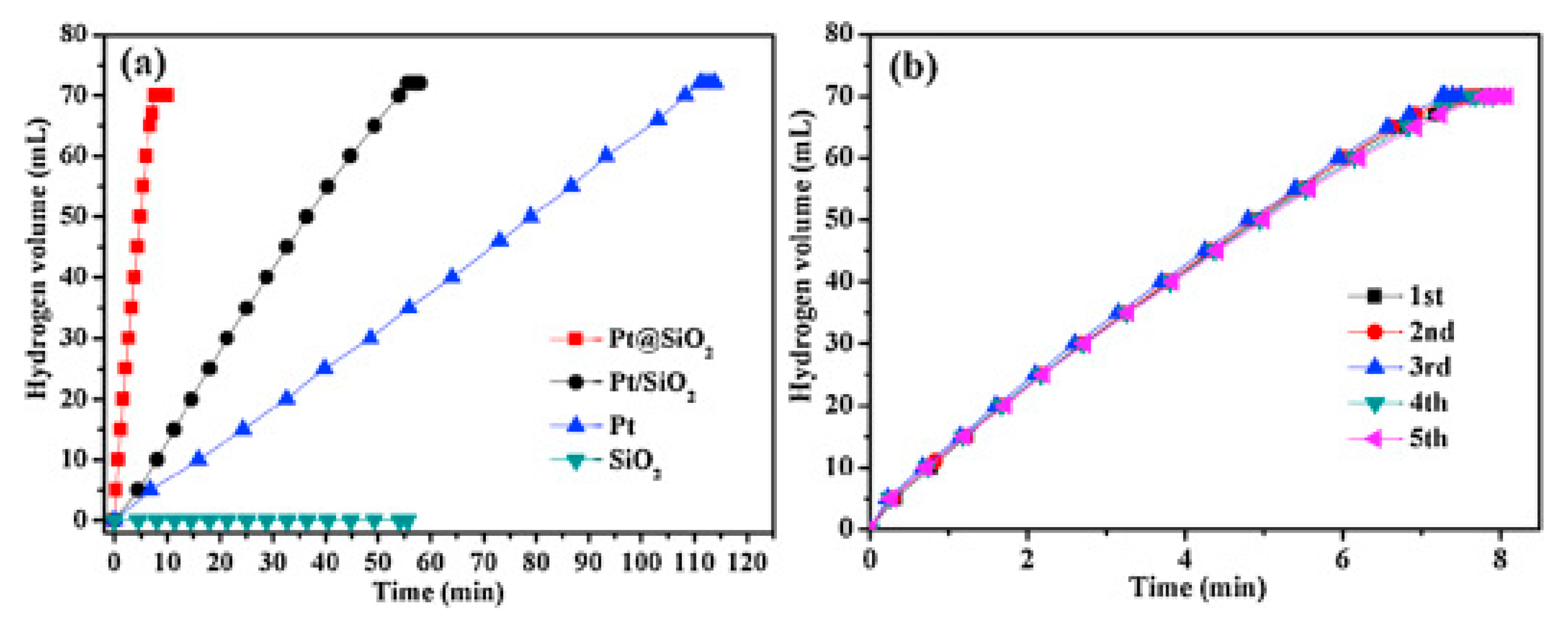
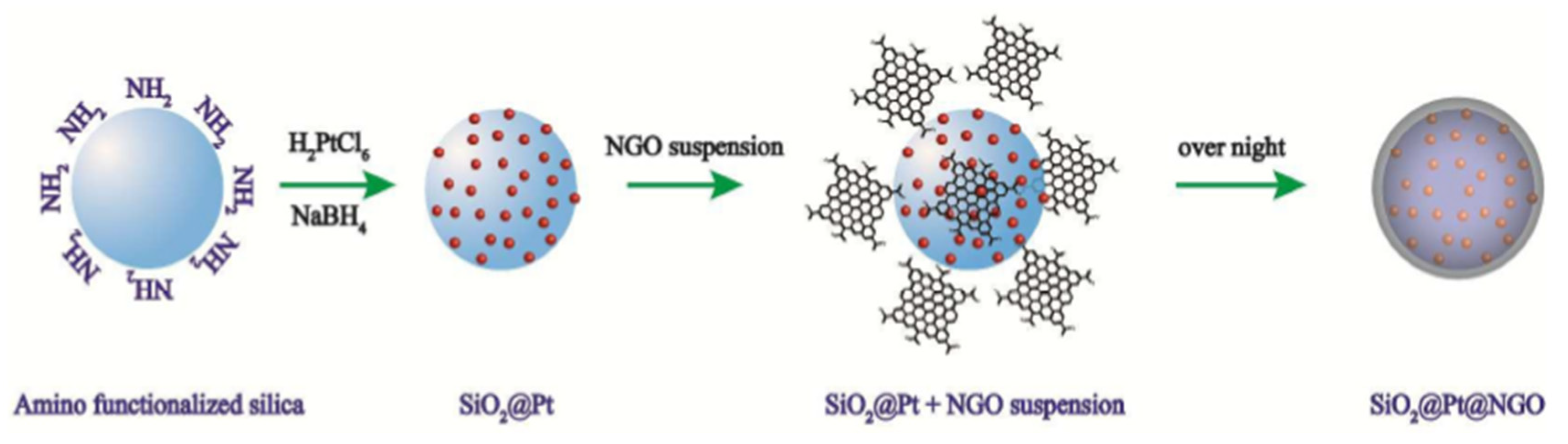

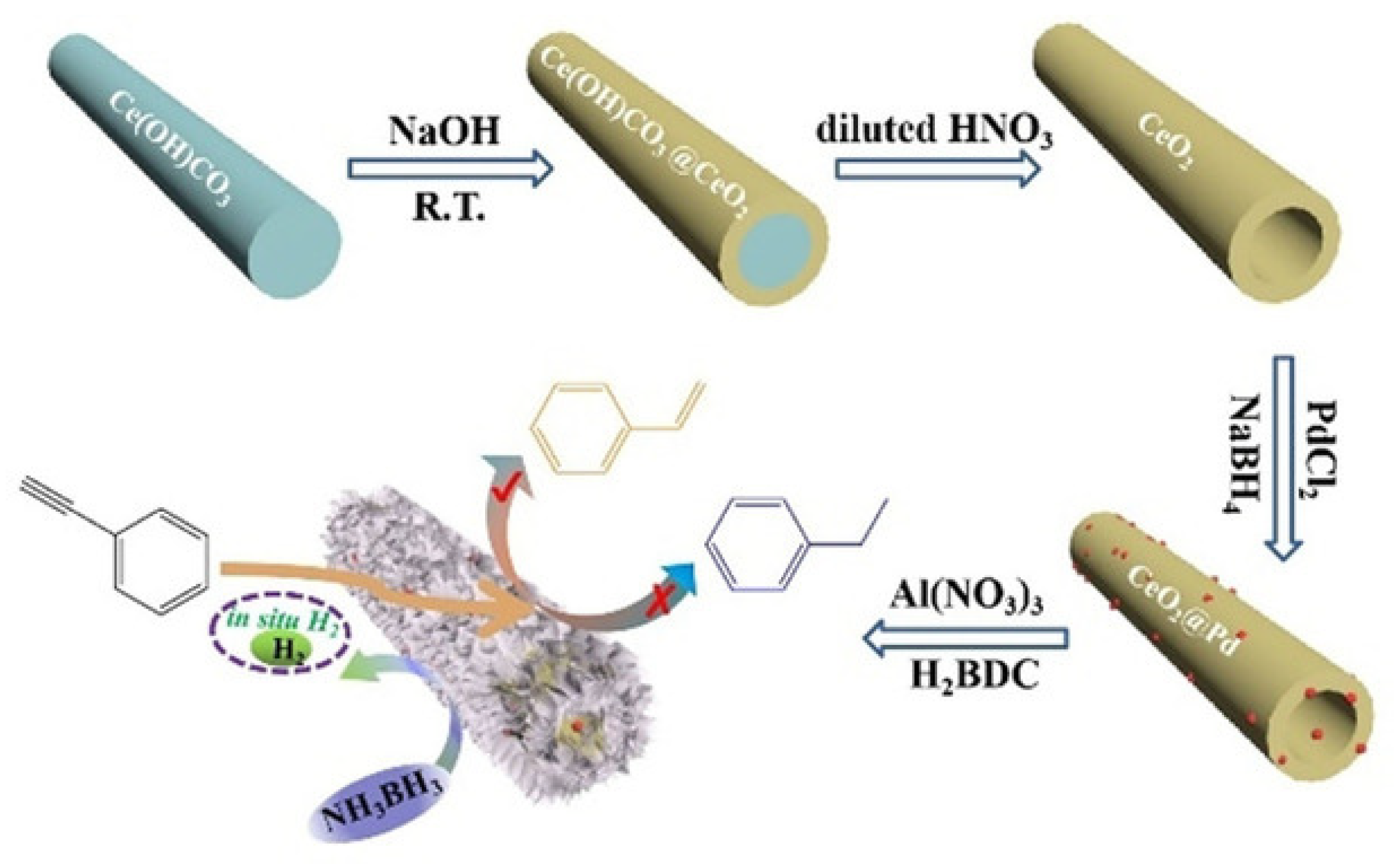
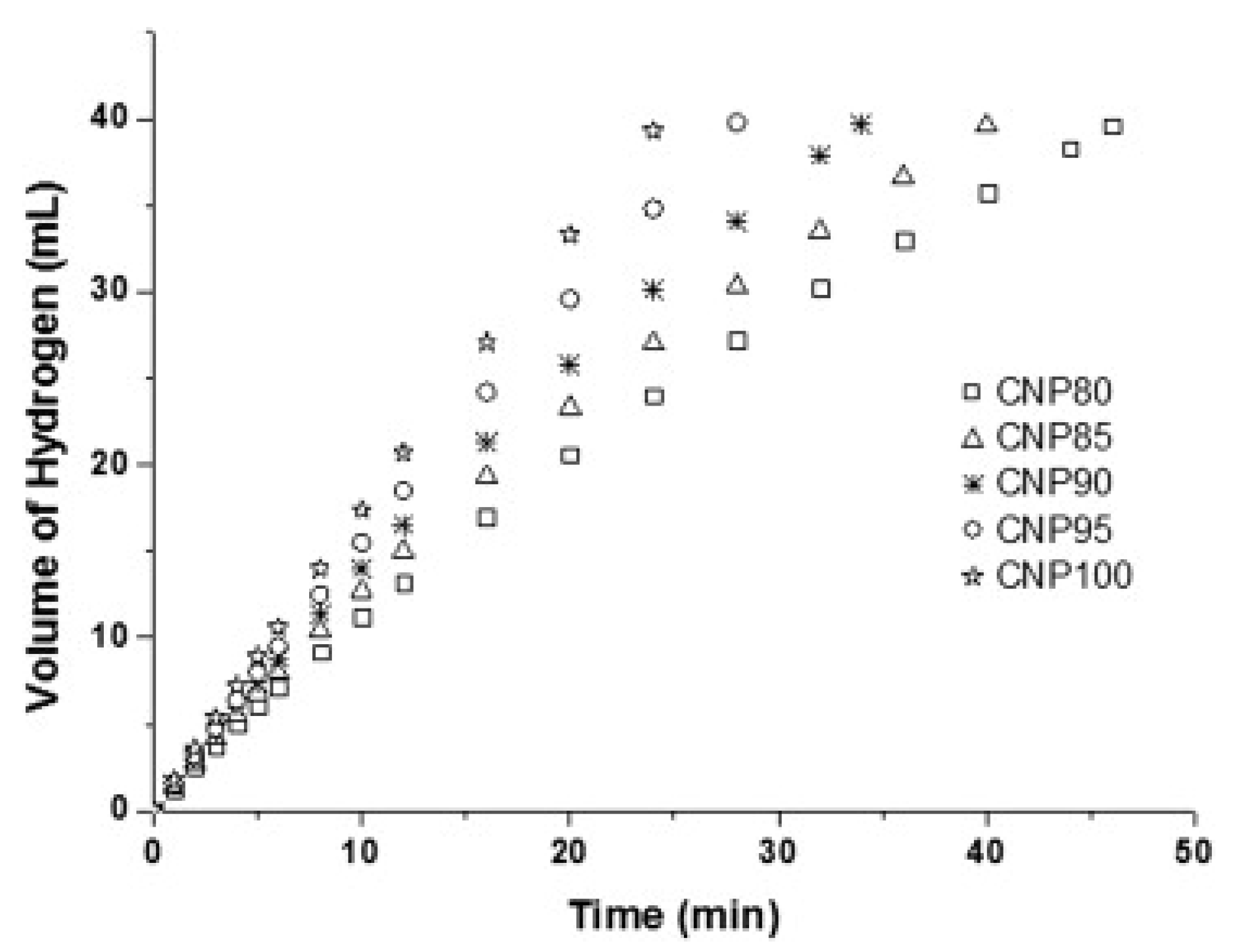
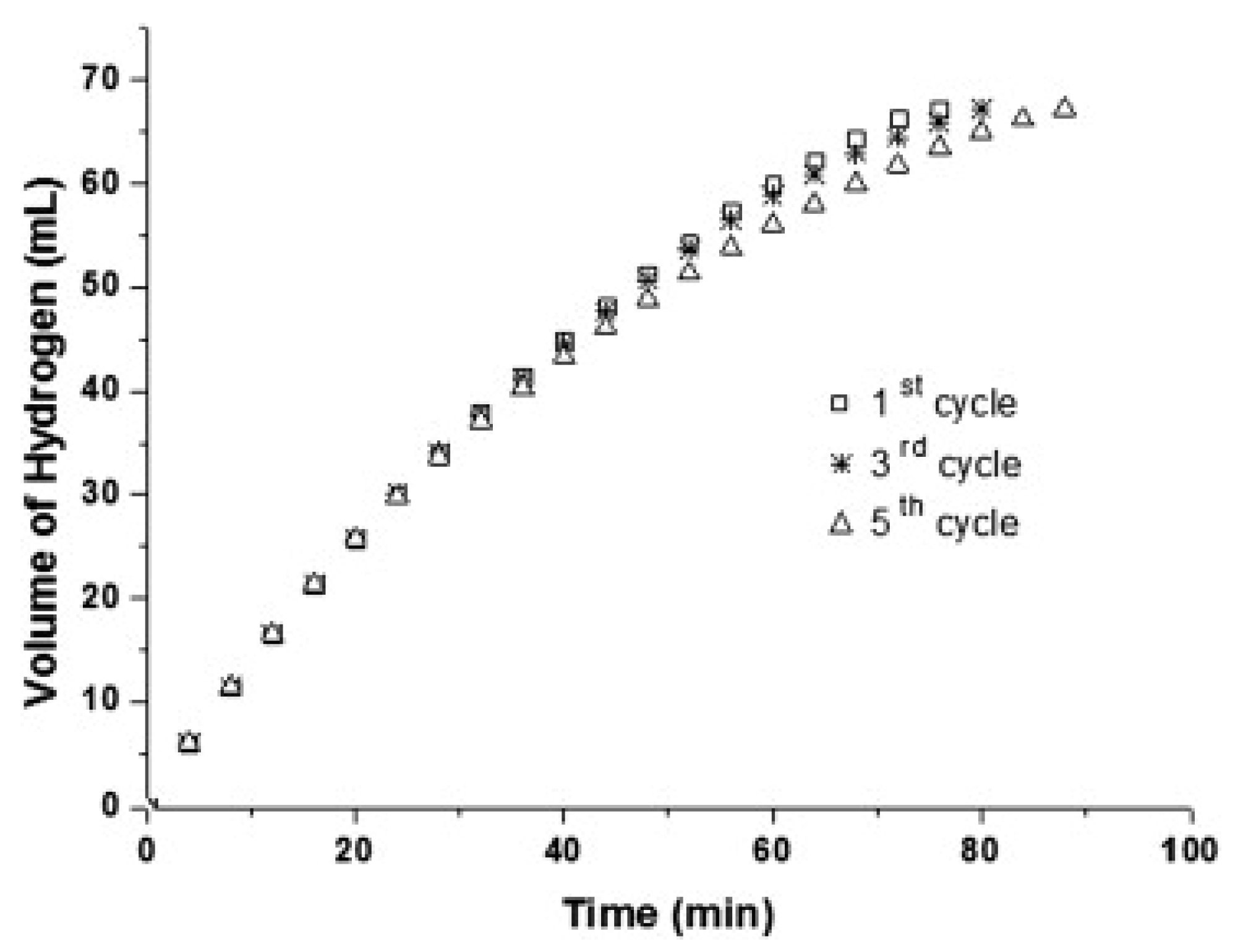
| Thermal Decomposition Step | Chemical Equation | Processes | Ref. |
|---|---|---|---|
| The first step (110 °C) | NH3BH3 → NH2BH2 + H2 | The first yield of hydrogen | [35] |
| The second step (125 °C) | nNH2BH2 → (NH2BH2)n | Intramolecular polymerization | [36] |
| The third step (150 °C) | (NH2BH2)n → (NHBH)n+ nH | The second yield of hydrogen | [37] |
| The remaining step (500 °C) | (NHBH)n → nBN+ nH2 | generation of excess hydrogen | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhou, L.; Luo, X.; Wan, C.; Xu, L. Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane. Catalysts 2020, 10, 788. https://doi.org/10.3390/catal10070788
Liu M, Zhou L, Luo X, Wan C, Xu L. Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane. Catalysts. 2020; 10(7):788. https://doi.org/10.3390/catal10070788
Chicago/Turabian StyleLiu, Mengmeng, Liu Zhou, Xianjin Luo, Chao Wan, and Lixin Xu. 2020. "Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane" Catalysts 10, no. 7: 788. https://doi.org/10.3390/catal10070788
APA StyleLiu, M., Zhou, L., Luo, X., Wan, C., & Xu, L. (2020). Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane. Catalysts, 10(7), 788. https://doi.org/10.3390/catal10070788






