Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting
Abstract
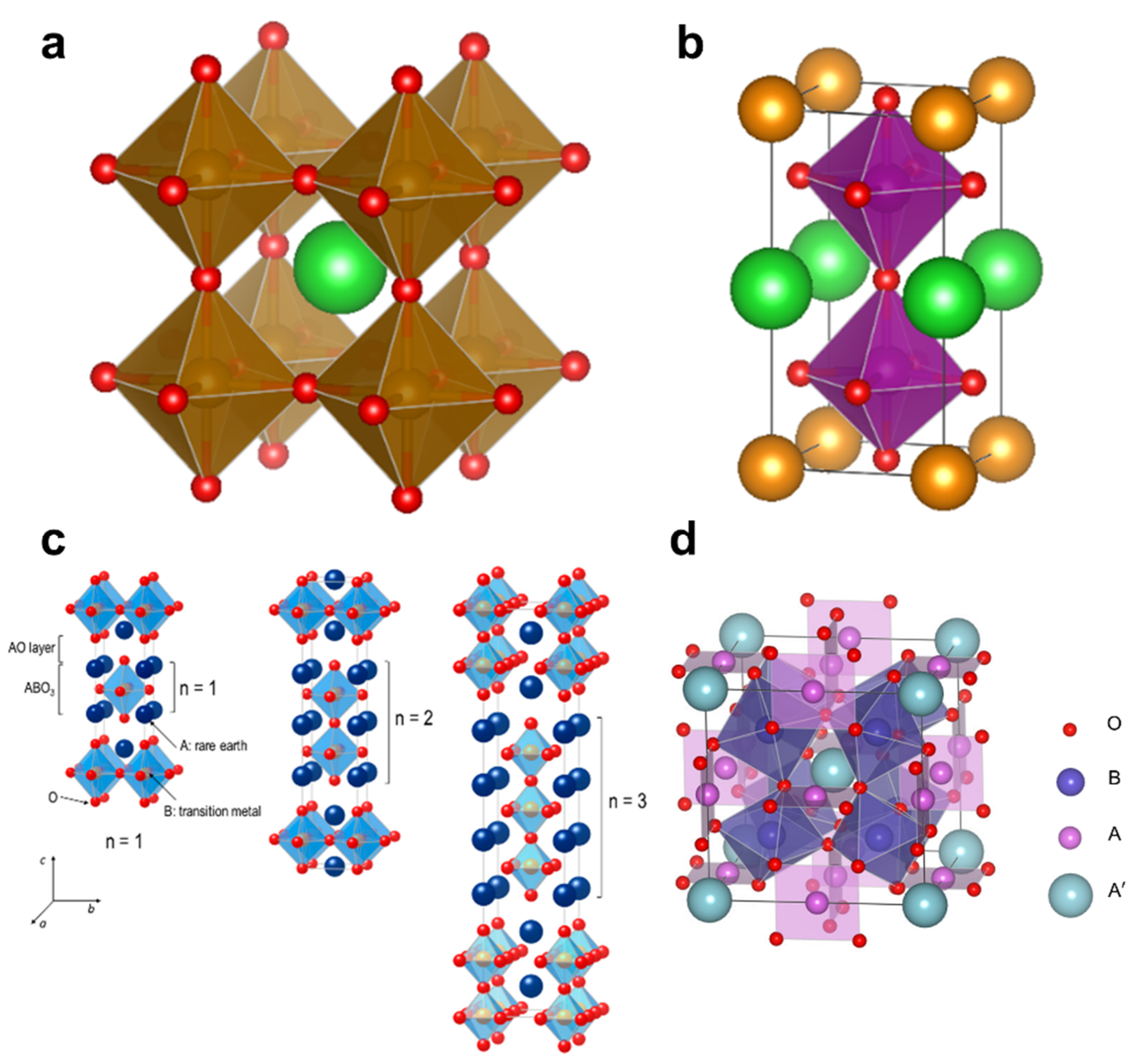
1. Introduction
2. Design Strategies
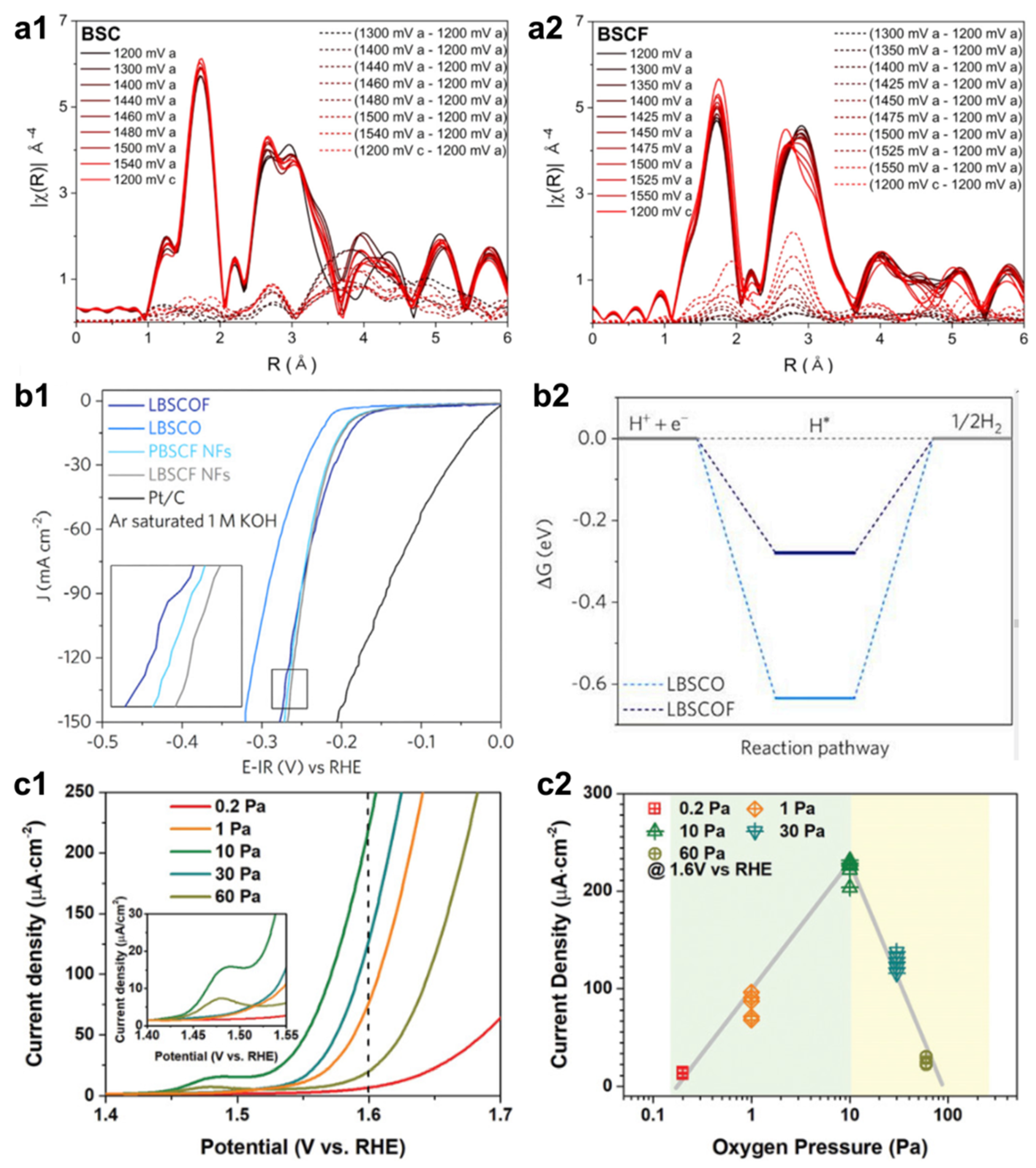
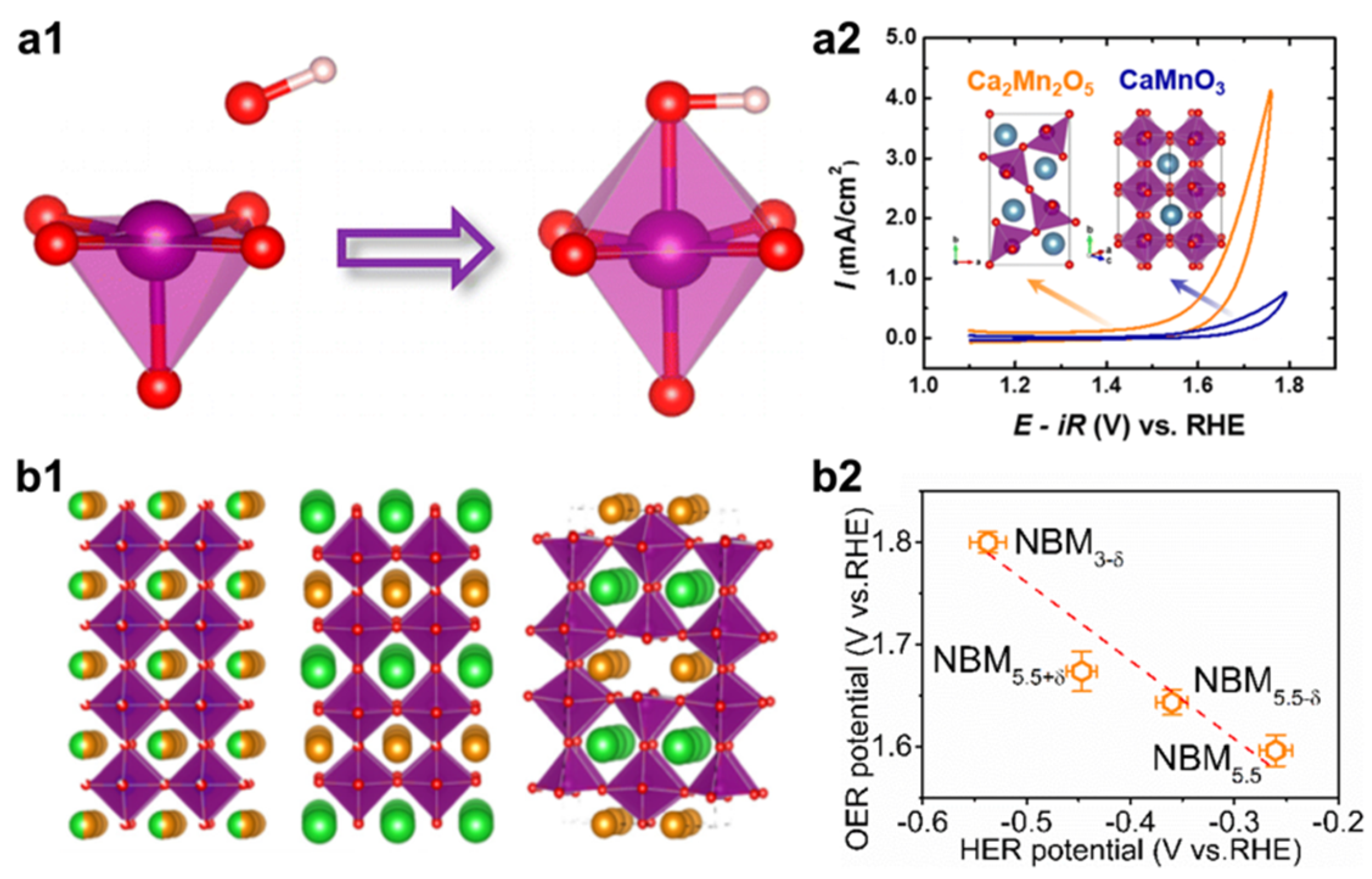
2.1. Defect Engineering
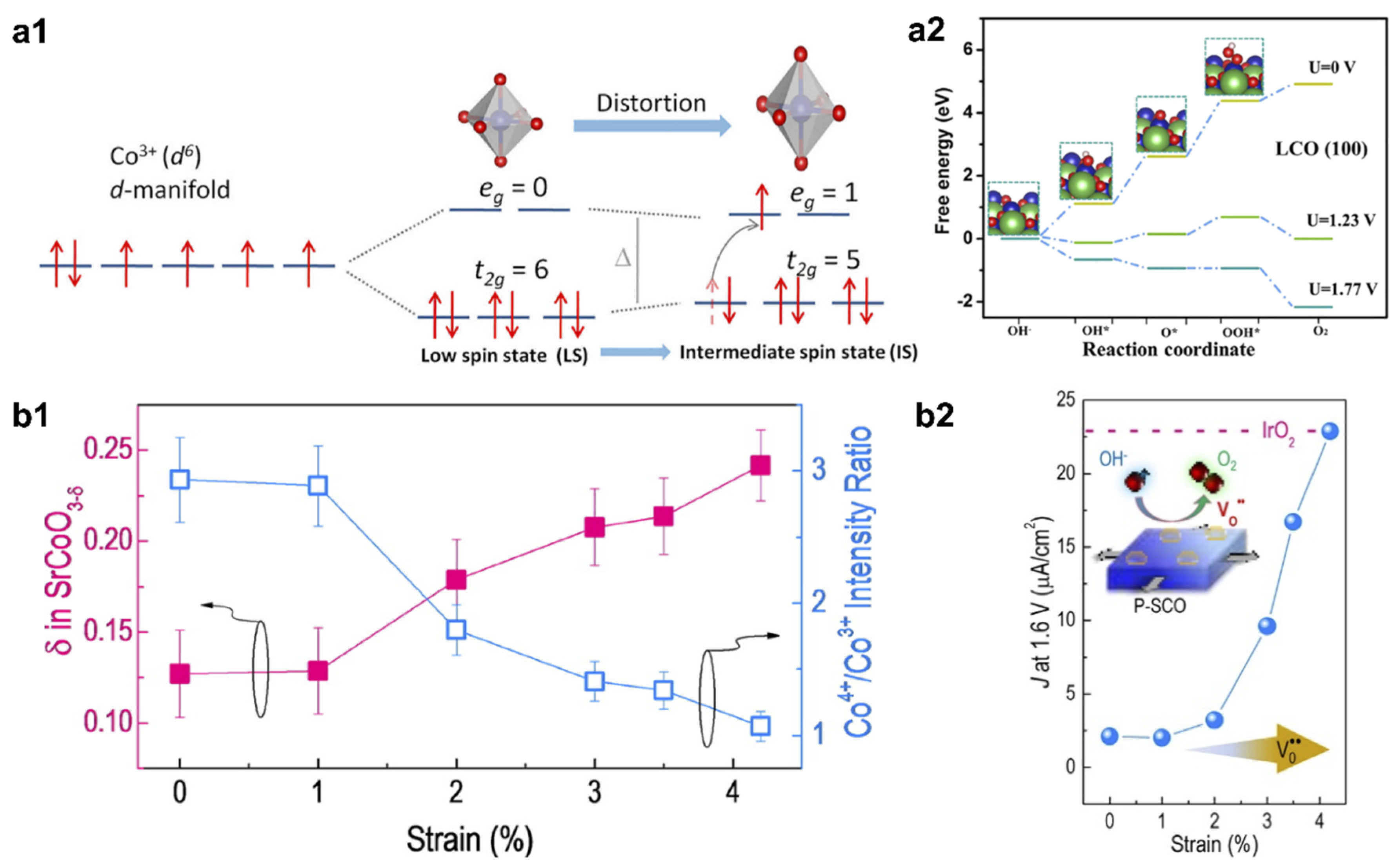
2.2. Strain Tuning
2.3. Nanostructuring
2.4. Hybridization
3. Advances of Perovskite Electrocatalysts
3.1. Co-Based
3.2. Ni-Based
3.3. Fe-Based
3.4. Mn-Based
3.5. Other First d-Block Metal-Based
4. Perspectives and Challenges
- More precise control over the perovskite composition, structure, and active sites is required. Despite the fact that strategies such as defect engineering, strain tuning, and hybridization have proven effective to modulate the electronic structure and boost the intrinsic activity, precisely modulating the specific doping/strain/interface sites and configuration still have major difficulties. In addition, we should note that many perovskite-type electrocatalysts undergo in-situ surface reconstruction during water electrolysis [37,99], which makes the identification of their active sites quite complicated. As a result, advanced in-situ/operando experimental tools are required to monitor the dynamic changes observed in perovskite oxide electrocatalysts during water electrolysis, elucidate their catalytic mechanism, and identify their active sites. DFT calculations provide effective theoretical tools to understand and predict the active species/configurations in perovskite oxide electrocatalysts. However, DFT calculations are subject to many approximations. In addition, it is also quite challenging for current theoretical modeling to precisely describe the working conditions of an electrocatalyst and rationally correlate the composition, structure, and morphology with the apparent activity. Further efforts are required to continuously optimize DFT methods to better model the real working conditions, improve the calculation accuracy, and reduce the computational cost.
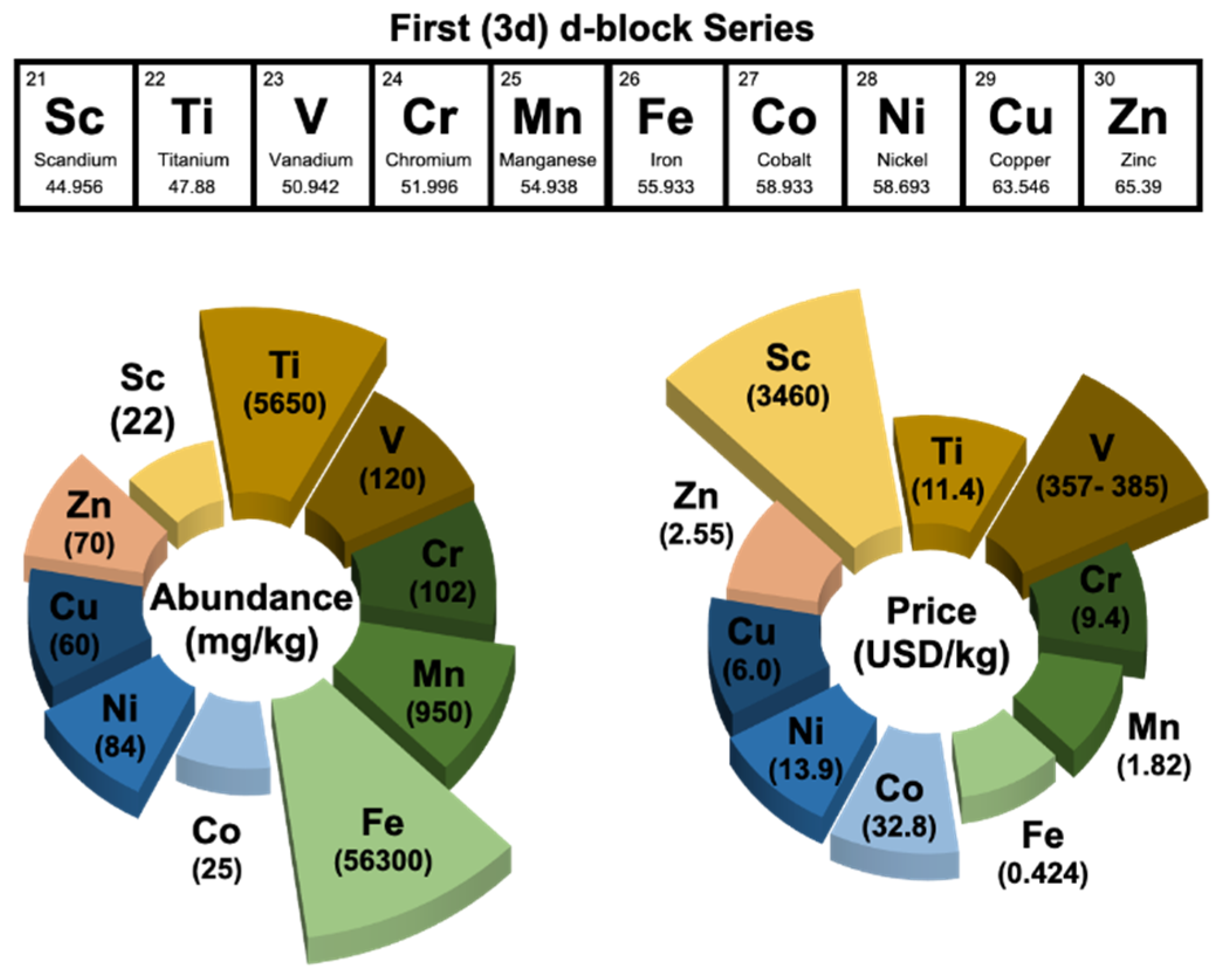
- The catalytic performance of perovskite-type water splitting electrocatalysts is far from satisfactory. Although many perovskite compositions have been reported to outperform Ru- or Ir-based electrocatalysts in catalyzing the OER, the alkaline HER activity of many perovskite oxides is still far below that of Pt metal/alloys. In addition, promising OER activity has been reported for Co-based perovskite oxides, which are relatively expensive. Developing other first d-block metal perovskite oxides (e.g., Fe, Mn, Cu) with comparable activity to Co-based perovskite oxide are favorable to save the cost, which, in turn, accelerates their practical applications. In addition to the half-cell OER/HER test, it is very crucial to build the practical water electrolyzer and exam the realistic performance where other practical and yet-unsolved issues such as the durability and catalyst loading can be clearly identified. Thus, catalytic stability is another imperative factor that should be further improved besides the activity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bockris, J.O.M. Kinetics of activation controlled consecutive electrochemical reactions: Anodic evolution of oxygen. J. Chem. Phys. 1956, 24, 817–827. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Faid, A.Y.; Oyarce Barnett, A.; Seland, F.; Sunde, S. Highly active nickel-based catalyst for hydrogen evolution in anion exchange membrane electrolysis. Catalysts 2018, 8, 614. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline water electrolysis powered by renewable energy: A review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Wang, J.; Ciucci, F. In-situ synthesis of bimetallic phosphide with carbon tubes as an active electrocatalyst for oxygen evolution reaction. Appl. Catal. B Environ. 2019, 254, 292–299. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Yang, Z.; Liang, J.; Dai, S. High-Entropy Perovskite Fluorides: A New Platform for Oxygen Evolution Catalysis. J. Am. Chem. Soc. 2020, 142, 4550–4554. [Google Scholar] [CrossRef]
- Li, B.-Q.; Tang, C.; Wang, H.-F.; Zhu, X.-L.; Zhang, Q. An aqueous preoxidation method for monolithic perovskite electrocatalysts with enhanced water oxidation performance. Sci. Adv. 2016, 2, e1600495. [Google Scholar] [CrossRef]
- Montoya, J.H.; Doyle, A.D.; Nørskov, J.K.; Vojvodic, A. Trends in adsorption of electrocatalytic water splitting intermediates on cubic ABO3 oxides. Phys. Chem. Chem. Phys. 2018, 20, 3813–3818. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Han, Z.; Yang, Y.; Li, J.; Deng, Q. Recent Advances in Oxygen Electrocatalysts Based on Perovskite Oxides. Nanomaterials 2019, 9, 1161. [Google Scholar] [CrossRef]
- Liu, S.; Luo, H.; Li, Y.; Liu, Q.; Luo, J.-L. Structure-engineered electrocatalyst enables highly active and stable oxygen evolution reaction over layered perovskite LaSr3Co1.5Fe1.5O10-δ. Nano Energy 2017, 40, 115–121. [Google Scholar] [CrossRef]
- Yagi, S.; Yamada, I.; Tsukasaki, H.; Seno, A.; Murakami, M.; Fujii, H.; Chen, H.; Umezawa, N.; Abe, H.; Nishiyama, N. Covalency-reinforced oxygen evolution reaction catalyst. Nat. Commun. 2015, 6, 8249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.N. Controlling oxygen mobility in Ruddlesden—Popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Calle-Vallejo, F.; Inoglu, N.G.; Su, H.-Y.; Martínez, J.I.; Man, I.C.; Koper, M.T.; Kitchin, J.R.; Rossmeisl, J. Number of outer electrons as descriptor for adsorption processes on transition metals and their oxides. Chem. Sci. 2013, 4, 1245–1249. [Google Scholar] [CrossRef]
- Lam, K.; Gao, Y.; Wang, J.; Ciucci, F. H2O2 Treated La0. 8Sr0. 2CoO3-δ as an Efficient Catalyst for Oxygen Evolution Reaction. Electrochim. Acta 2017, 244, 139–145. [Google Scholar] [CrossRef]
- Du, X.; Ai, H.; Chen, M.; Liu, D.; Chen, S.; Wang, X.; Lo, K.H.; Pan, H. PLD-fabricated perovskite oxide nanofilm as efficient electrocatalyst with highly enhanced water oxidation performance. Appl. Catal. B Environ. 2020, 272, 119046. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Zhang, Z.; Chen, Z.; Lin, H.; Gao, Y.; Chen, D. Double perovskite PrBaCo2O5.5: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Power Sources 2019, 427, 194–200. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhou, W.; Zhu, Z.; Su, C.; Liu, M.; Shao, Z. A perovskite electrocatalyst for efficient hydrogen evolution reaction. Adv. Mater. 2016, 28, 6442–6448. [Google Scholar] [CrossRef]
- Bu, Y.; Kim, S.; Kwon, O.; Zhong, Q.; Kim, G. A Composite Catalyst Based on Perovskites for Overall Water Splitting in Alkaline Conditions. ChemElectroChem 2019, 6, 1520–1524. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Choudhury, P.; Luo, H. Perovskite oxides as bifunctional oxygen electrocatalysts for oxygen evolution/reduction reactions—A mini review. Appl. Mater. Today 2019, 16, 56–71. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Prices of Chemical Elements. Available online: https://en.wikipedia.org/wiki/Prices_of_chemical_elements (accessed on 25 June 2020).
- Wang, J. Engineering Defective Electrode Materials for Sustainable Energy Applications. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, China, 2018. [Google Scholar]
- Zhang, Z.; Chen, Y.; Dai, Z.; Tan, S.; Chen, D. Promoting hydrogen-evolution activity and stability of perovskite oxides via effectively lattice doping of molybdenum. Electrochim. Acta 2019, 312, 128–136. [Google Scholar] [CrossRef]
- Wang, J.; Saccoccio, M.; Chen, D.; Gao, Y.; Chen, C.; Ciucci, F. The effect of A-site and B-site substitution on BaFeO3-δ: An investigation as a cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2015, 297, 511–518. [Google Scholar] [CrossRef]
- Wang, J.; Lam, K.Y.; Saccoccio, M.; Gao, Y.; Chen, D.; Ciucci, F. Ca and In co-doped BaFeO3-δ as a cobalt-free cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2016, 324, 224–232. [Google Scholar] [CrossRef]
- Li, B.-Q.; Xia, Z.-J.; Zhang, B.; Tang, C.; Wang, H.-F.; Zhang, Q. Regulating p-block metals in perovskite nanodots for efficient electrocatalytic water oxidation. Nat. Commun. 2017, 8, 934. [Google Scholar] [CrossRef] [PubMed]
- Alkan, B.; Medina, D.; Landers, J.; Heidelmann, M.; Hagemann, U.; Salamon, S.; Andronescu, C.; Wende, H.; Schulz, C.; Schuhmann, W.; et al. Spray-Flame-Prepared LaCo1-xFexO3 Perovskite Nanoparticles as Active OER Catalysts: Influence of Fe Content and Low-Temperature Heating. ChemElectroChem 2020, 7, 2564–2574. [Google Scholar] [CrossRef]
- Wang, Z.; You, Y.; Yuan, J.; Yin, Y.-X.; Li, Y.-T.; Xin, S.; Zhang, D. Nickel-Doped La0.8Sr0.2Mn1-xNixO3 Nanoparticles Containing Abundant Oxygen Vacancies as an Optimized Bifunctional Catalyst for Oxygen Cathode in Rechargeable Lithium—Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6520–6528. [Google Scholar] [CrossRef]
- Guo, Y.; Tong, Y.; Chen, P.; Xu, K.; Zhao, J.; Lin, Y.; Chu, W.; Peng, Z.; Wu, C.; Xie, Y. Engineering the Electronic State of a Perovskite Electrocatalyst for Synergistically Enhanced Oxygen Evolution Reaction. Adv. Mater. 2015, 27, 5989–5994. [Google Scholar] [CrossRef]
- Zhao, Y.; Hang, Y.; Zhang, Y.; Wang, Z.; Yao, Y.; He, X.; Zhang, C.; Zhang, D. Strontium-doped perovskite oxide La1-xSrxMnO3 (x = 0, 0.2, 0.6) as a highly efficient electrocatalyst for nonaqueous Li-O2 batteries. Electrochim. Acta 2017, 232, 296–302. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.; Wang, J.; Tan, S.; Yu, X.; Shao, Z. Highly Active and Stable Cobalt-Free Hafnium-doped SrFe0.9Hf0.1O3-δ Perovskite Cathode for Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2018, 1, 2134–2142. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Belotti, A.; Ciucci, F. P-Substituted Ba0. 95La0. 05FeO3-δ as a Cathode Material for SOFCs. ACS Appl. Energy Mater. 2019, 2, 5472–5480. [Google Scholar] [CrossRef]
- Li, Z.; Lv, L.; Wang, J.; Ao, X.; Ruan, Y.; Zha, D.; Hong, G.; Wu, Q.; Lan, Y.; Wang, C.; et al. Engineering phosphorus-doped LaFeO3-δ perovskite oxide as robust bifunctional oxygen electrocatalysts in alkaline solutions. Nano Energy 2018, 47, 199–209. [Google Scholar] [CrossRef]
- Xiong, J.; Zhong, H.; Li, J.; Zhang, X.; Shi, J.; Cai, W.; Qu, K.; Zhu, C.; Yang, Z.; Beckman, S.P.; et al. Engineering highly active oxygen sites in perovskite oxides for stable and efficient oxygen evolution. Appl. Catal. B Environ. 2019, 256, 117817. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Xi, S.; Ren, X.; Zhou, Y.; Zhang, G.; Yang, H.; Du, Y.; Xu, Z.J. Tailoring the Co 3d-O 2p Covalency in LaCoO3 by Fe Substitution To Promote Oxygen Evolution Reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Kim, B.-J.; Fabbri, E.; Abbott, D.F.; Cheng, X.; Clark, A.H.; Nachtegaal, M.; Borlaf, M.; Castelli, I.E.; Graule, T.; Schmidt, T.J. Functional Role of Fe-Doping in Co-Based Perovskite Oxide Catalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 5231–5240. [Google Scholar] [CrossRef]
- Seo, M.H.; Park, H.W.; Lee, D.U.; Park, M.G.; Chen, Z. Design of Highly Active Perovskite Oxides for Oxygen Evolution Reaction by Combining Experimental and ab Initio Studies. ACS Catal. 2015, 5, 4337–4344. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Pang, W.; Tang, W.; Zhao, S.; Jin, Z.; Zeng, Y.; Shalchi Amirkhiz, B.; Luo, J.-L. Activating p-Blocking Centers in Perovskite for Efficient Water Splitting. Chem 2018, 4, 2902–2916. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Yan, L.; Lin, Y.; Yu, X.; Xu, W.; Salas, T.; Smallidge, H.; Zhou, M.; Luo, H. La0.8Sr0.2MnO3-Based Perovskite Nanoparticles with the A-Site Deficiency as High Performance Bifunctional Oxygen Catalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2017, 9, 23820–23827. [Google Scholar] [CrossRef]
- Bian, J.; Li, Z.; Li, N.; Sun, C. Oxygen Deficient LaMn0.75Co0.25O3-δ Nanofibers as an Efficient Electrocatalyst for Oxygen Evolution Reaction and Zinc—Air Batteries. Inorg. Chem. 2019, 58, 8208–8214. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.-C.; Fang, S.; Yang, H. Ca2Mn2O5 as Oxygen-Deficient Perovskite Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Hu, C.; Wang, X.; Yao, T.; Gao, T.; Han, J.; Zhang, X.; Zhang, Y.; Xu, P.; Song, B. Enhanced Electrocatalytic Oxygen Evolution Activity by Tuning Both the Oxygen Vacancy and Orbital Occupancy of B-Site Metal Cation in NdNiO3. Adv. Funct. Mater. 2019, 29, 1902449. [Google Scholar] [CrossRef]
- Miao, X.; Wu, L.; Lin, Y.; Yuan, X.; Zhao, J.; Yan, W.; Zhou, S.; Shi, L. The role of oxygen vacancies in water oxidation for perovskite cobalt oxide electrocatalysts: Are more better? Chem. Commun. 2019, 55, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.-J.; Durst, J.; Bozza, F.; Graule, T.; Schäublin, R.; Wiles, L.; et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Jeen, H.; Barron, S.C.; Meyer, T.L.; Lee, H.N. Enhancing Perovskite Electrocatalysis through Strain Tuning of the Oxygen Deficiency. J. Am. Chem. Soc. 2016, 138, 7252–7255. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.-Y.; Eom, C.J.; Kawasaki, J.K.; Petretto, G.; Nelson, J.N.; Hautier, G.; Crumlin, E.J.; Shen, K.M.; Schlom, D.G.; Suntivich, J. Influence of Strain on the Surface—Oxygen Interaction and the Oxygen Evolution Reaction of SrIrO3. J. Phys. Chem. C 2018, 122, 4359–4364. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Choi, W.S.; Jeen, H.; Lee, H.N.; Shao-Horn, Y. Role of Strain and Conductivity in Oxygen Electrocatalysis on LaCoO3 Thin Films. J. Phys. Chem. Lett. 2015, 6, 487–492. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, Y.; Chen, P.; Liu, H.; Zhang, M.; Zhang, L.; Yan, W.; Chu, W.; Wu, C.; Xie, Y. Spin-State Regulation of Perovskite Cobaltite to Realize Enhanced Oxygen Evolution Activity. Chem 2017, 3, 812–821. [Google Scholar] [CrossRef]
- Petrie, J.R.; Mitra, C.; Jeen, H.; Choi, W.S.; Meyer, T.L.; Reboredo, F.A.; Freeland, J.W.; Eres, G.; Lee, H.N. Strain Control of Oxygen Vacancies in Epitaxial Strontium Cobaltite Films. Adv. Funct. Mater. 2016, 26, 1564–1570. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zheng, Y.; Guo, Z.; Zhu, Y.; Chen, H.; Li, F.; Liu, P.; Yu, B.; Wang, X.; et al. Uncovering the Effect of Lattice Strain and Oxygen Deficiency on Electrocatalytic Activity of Perovskite Cobaltite Thin Films. Adv. Sci. 2019, 6, 1801898. [Google Scholar] [CrossRef]
- Jung, J.-I.; Risch, M.; Park, S.; Kim, M.G.; Nam, G.; Jeong, H.-Y.; Shao-Horn, Y.; Cho, J. Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 176–183. [Google Scholar] [CrossRef]
- Zhou, S.; Miao, X.; Zhao, X.; Ma, C.; Qiu, Y.; Hu, Z.; Zhao, J.; Shi, L.; Zeng, J. Engineering electrocatalytic activity in nanosized perovskite cobaltite through surface spin-state transition. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, H.; Hao, J.; Wang, C.; Wen, Y.; Li, H.; Lu, S.; Duan, F.; Du, M. Integrating the cationic engineering and hollow structure engineering into perovskites oxides for efficient and stable electrocatalytic oxygen evolution. Electrochim. Acta 2019, 327, 135033. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.; Li, S.; Sun, C. Porous Perovskite La0.6Sr0.4Co0.8Mn0.2O3 Nanofibers Loaded with RuO2 Nanosheets as an Efficient and Durable Bifunctional Catalyst for Rechargeable Li-O2 Batteries. ACS Catal. 2017, 7, 7737–7747. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Zhang, Y.-Q.; Sun, Y.-F.; Luo, J.-L. All-In-One Perovskite Catalyst: Smart Controls of Architecture and Composition toward Enhanced Oxygen/Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700666. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A perovskite nanorod as bifunctional electrocatalyst for overall water splitting. Adv. Energy Mater. 2017, 7, 1602122. [Google Scholar] [CrossRef]
- Xu, J.-J.; Xu, D.; Wang, Z.-L.; Wang, H.-G.; Zhang, L.-L.; Zhang, X.-B. Synthesis of Perovskite-Based Porous La0.75Sr0.25MnO3 Nanotubes as a Highly Efficient Electrocatalyst for Rechargeable Lithium–Oxygen Batteries. Angew. Chem. Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, W.; Wu, S.; Yang, X.; Xia, W.; Shen, Y.; Huang, Y.; Cao, A.; Yuan, Q. Perovskite-Type LaSrMnO Electrocatalyst with Uniform Porous Structure for an Efficient Li-O2 Battery Cathode. ACS Nano 2016, 10, 1240–1248. [Google Scholar] [CrossRef]
- Jin, C.; Cao, X.; Zhang, L.; Zhang, C.; Yang, R. Preparation and electrochemical properties of urchin-like La0.8Sr0.2MnO3 perovskite oxide as a bifunctional catalyst for oxygen reduction and oxygen evolution reaction. J. Power Sources 2013, 241, 225–230. [Google Scholar] [CrossRef]
- Yu, H.; Chu, F.; Zhou, X.; Ji, J.; Liu, Y.; Bu, Y.; Kong, Y.; Tao, Y.; Li, Y.; Qin, Y. A perovskite oxide with a tunable pore-size derived from a general salt-template strategy as a highly efficient electrocatalyst for the oxygen evolution reaction. Chem. Commun. 2019, 55, 2445–2448. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.-G.; Ao, X.; Sun, H.; Wang, H.; Yuen, M.-F.; Wang, C. Conductive metal—Organic frameworks endow high-efficient oxygen evolution of La0·6Sr0·4Co0·8Fe0·2O3 perovskite oxide nanofibers. Electrochim. Acta 2020, 334, 135638. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Jin, C.; Li, C.; Li, X.; Li, Y.; Yang, R.; Liu, M. Enhanced overall water electrolysis on a bifunctional perovskite oxide through interfacial engineering. Electrochim. Acta 2019, 318, 120–129. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Lyu, Y.-Q.; Lam, K.; Ciucci, F. In situ growth of Pt 3 Ni nanoparticles on an A-site deficient perovskite with enhanced activity for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 6399–6404. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Z.; You, T.L.; Wang, J.; Xie, L.; He, J.; Ciucci, F. Energetics of Nanoparticle Exsolution from Perovskite Oxides. J. Phys. Chem. Lett. 2018, 9, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Lindenthal, L.; Rameshan, R.; Summerer, H.; Ruh, T.; Popovic, J.; Nenning, A.; Löffler, S.; Opitz, A.K.; Blaha, P.; Rameshan, C. Modifying the Surface Structure of Perovskite-Based Catalysts by Nanoparticle Exsolution. Catalysts 2020, 10, 268. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Ji, D.; Cai, S.; Paudel, T.R.; Sun, H.; Zhang, C.; Han, L.; Wei, Y.; Zang, Y.; Gu, M.; Zhang, Y.; et al. Freestanding crystalline oxide perovskites down to the monolayer limit. Nature 2019, 570, 87–90. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, C.; Chen, D.; Saccoccio, M.; Wang, J.; Gao, Y.; Wan, T.H.; Ciucci, F. Ba0.95La0.05FeO3-δ-multi-layer graphene as a low-cost and synergistic catalyst for oxygen evolution reaction. Carbon 2015, 90, 122–129. [Google Scholar] [CrossRef]
- Xu, Y.; Tsou, A.; Fu, Y.; Wang, J.; Tian, J.-H.; Yang, R. Carbon-Coated Perovskite BaMnO3 Porous Nanorods with Enhanced Electrocatalytic Perporites for Oxygen Reduction and Oxygen Evolution. Electrochim. Acta 2015, 174, 551–556. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Gao, Y.; Chen, D.; Chen, C.; Saccoccio, M.; Ciucci, F. Ba0.5Sr0.5Co0.8Fe0.2O3-δ on N-doped mesoporous carbon derived from organic waste as a bi-functional oxygen catalyst. Int. J. Hydrog. Energy 2016, 41, 10744–10754. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Ciucci, F. Mechanochemical Coupling of MoS2 and Perovskites for Hydrogen Generation. ACS Appl. Energy Mater. 2018, 1, 6409–6416. [Google Scholar] [CrossRef]
- Oh, N.K.; Kim, C.; Lee, J.; Kwon, O.; Choi, Y.; Jung, G.Y.; Lim, H.Y.; Kwak, S.K.; Kim, G.; Park, H. In-situ local phase-transitioned MoSe2 in La0.5Sr0.5CoO3-δ heterostructure and stable overall water electrolysis over 1000 hours. Nat. Commun. 2019, 10, 1723. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Cheng, X.; Schmidt, T.J. Superior Bifunctional Electrocatalytic Activity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ/Carbon Composite Electrodes: Insight into the Local Electronic Structure. Adv. Energy Mater. 2015, 5, 1402033. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Chen, Y.; Tan, S.; Shao, Z.; Chen, D. In situ formation of a 3D core-shell and triple-conducting oxygen reduction reaction electrode for proton-conducting SOFCs. J. Power Sources 2018, 385, 76–83. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Teraoka, Y.; Yamazoe, N. Preparation of perovskite-type oxides with large surface area by citrate process. Chem. Lett. 1987, 16, 665–668. [Google Scholar] [CrossRef]
- Bu, Y.; Jang, H.; Gwon, O.; Kim, S.H.; Joo, S.H.; Nam, G.; Kim, S.; Qin, Y.; Zhong, Q.; Kwak, S.K. Synergistic interaction of perovskite oxides and N-doped graphene in versatile electrocatalyst. J. Mater. Chem. A 2019, 7, 2048–2054. [Google Scholar] [CrossRef]
- Guan, D.; Zhou, J.; Huang, Y.-C.; Dong, C.-L.; Wang, J.-Q.; Zhou, W.; Shao, Z. Screening highly active perovskites for hydrogen-evolving reaction via unifying ionic electronegativity descriptor. Nat. Commun. 2019, 10, 3755. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Zhong, Y.; Miao, J.; Lin, B.; Zhou, W.; Shao, Z. Enabling High and Stable Electrocatalytic Activity of Iron-Based Perovskite Oxides for Water Splitting by Combined Bulk Doping and Morphology Designing. Adv. Mater. Interfaces 2019, 6, 1801317. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Remarkable Oxygen-Evolution Activity of a Perovskite Oxide from the Ca2-xSrxFe2O6-δ Series. Angew. Chem. Int. Ed. 2019, 58, 2060–2063. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhen, D.; Yoo, S.; Ding, Y.; Chen, D.; Chen, Y.; Zhang, Q.; Doyle, B.; Xiong, X.; et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat. Commun. 2017, 8, 14586. [Google Scholar] [CrossRef]
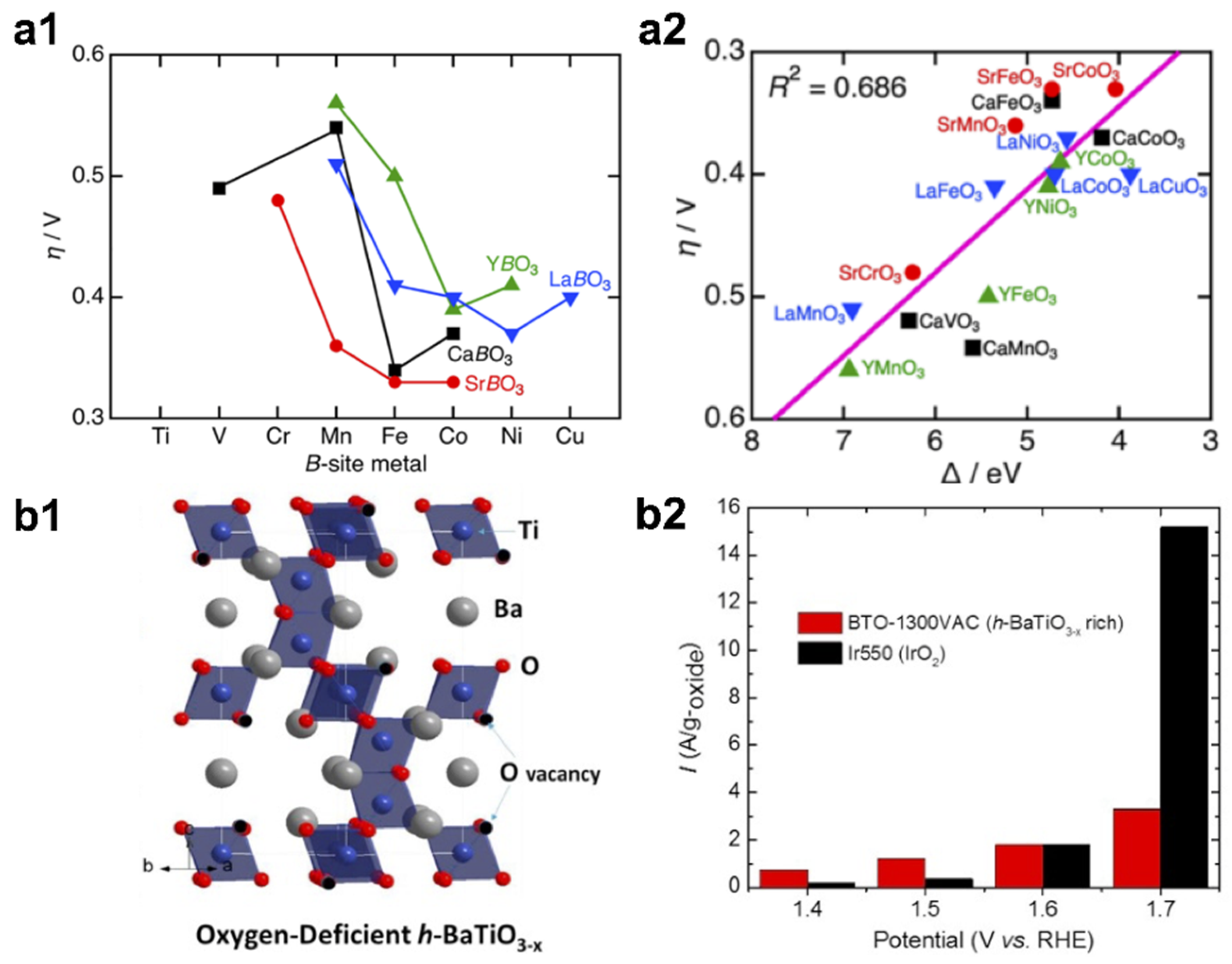
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270. [Google Scholar] [CrossRef]
- Jung, J.-I.; Park, S.; Kim, M.-G.; Cho, J. Tunable Internal and Surface Structures of the Bifunctional Oxygen Perovskite Catalysts. Adv. Energy Mater. 2015, 5, 1501560. [Google Scholar] [CrossRef]
- Ou, G.; Yang, C.; Liang, Y.; Hussain, N.; Ge, B.; Huang, K.; Xu, Y.; Wei, H.; Zhang, R.; Wu, H. Surface Engineering of Perovskite Oxide for Bifunctional Oxygen Electrocatalysis. Small Methods 2019, 3, 1800279. [Google Scholar] [CrossRef]
- Zhang, C.; Berlinguette, C.P.; Trudel, S. Water oxidation catalysis: An amorphous quaternary Ba-Sr-Co-Fe oxide as a promising electrocatalyst for the oxygen-evolution reaction. Chem. Commun. 2016, 52, 1513–1516. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Pi, Y.; Shao, Q.; Tan, Y.; Huang, X. Double Perovskite LaFexNi1-xO3 nanorods enable efficient oxygen evolution electrocatalysis. Angew. Chem. 2019, 131, 2338–2342. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Du, Z.; Wang, L.; Li, Y.; Goodenough, J.B. Active LaNi1-xFexO3 bifunctional catalysts for air cathodes in alkaline media. J. Mater. Chem. A 2015, 3, 9421–9426. [Google Scholar] [CrossRef]
- Höfer, H.E.; Schmidberger, R. Electronic Conductivity in the La(Cr, Ni)O3 Perovskite System. J. Electrochem. Soc. 1994, 141, 782–786. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Otagawa, T. The electrocatalysis of oxygen evolution on perovskites. J. Electrochem. Soc. 1984, 131, 290. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef]
- Wang, J.; Ciucci, F. Boosting Bifunctional Oxygen Electrolysis for N-Doped Carbon via Bimetal Addition. Small 2017, 13, 1604103. [Google Scholar] [CrossRef] [PubMed]
- Forslund, R.P.; Hardin, W.G.; Rong, X.; Abakumov, A.M.; Filimonov, D.; Alexander, C.T.; Mefford, J.T.; Iyer, H.; Kolpak, A.M.; Johnston, K.P.; et al. Exceptional electrocatalytic oxygen evolution via tunable charge transfer interactions in La0.5Sr1.5Ni1-xFexO4±δ Ruddlesden-Popper oxides. Nat. Commun. 2018, 9, 3150. [Google Scholar] [CrossRef]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Retuerto, M.; Pereira, A.G.; Pérez-Alonso, F.J.; Peña, M.A.; Fierro, J.L.G.; Alonso, J.A.; Fernández-Díaz, M.T.; Pascual, L.; Rojas, S. Structural effects of LaNiO3 as electrocatalyst for the oxygen reduction reaction. Appl. Catal. B Environ. 2017, 203, 363–371. [Google Scholar] [CrossRef]
- Bak, J.; Bin Bae, H.; Chung, S.-Y. Atomic-scale perturbation of oxygen octahedra via surface ion exchange in perovskite nickelates boosts water oxidation. Nat. Commun. 2019, 10, 2713. [Google Scholar] [CrossRef]
- Lee, J.G.; Hwang, J.; Hwang, H.J.; Jeon, O.S.; Jang, J.; Kwon, O.; Lee, Y.; Han, B.; Shul, Y.-G. A new family of perovskite catalysts for oxygen-evolution reaction in alkaline media: BaNiO3 and BaNi0.83O2.5. J. Am. Chem. Soc. 2016, 138, 3541–3547. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, Y.; Jia, L.; He, B.; Zhang, K.; Zhao, L. Engineering anion defect in LaFeO2.85Cl0.15 perovskite for boosting oxygen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 24077–24085. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhou, W.; Zhong, Y.; Guan, D.; Shao, Z. Earth-Abundant Silicon for Facilitating Water Oxidation over Iron-Based Perovskite Electrocatalyst. Adv. Mater. Interfaces 2018, 5, 1701693. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, H.; Li, X.; Li, Q.; Wang, J.; Zhu, X.; Yang, W. Oxygen evolution reaction over Fe site of BaZrxFe1-xO3-δ perovskite oxides. Electrochim. Acta 2017, 241, 433–439. [Google Scholar] [CrossRef]
- Shen, Z.; Zhuang, Y.; Li, W.; Huang, X.; Oropeza, F.E.; Hensen, E.J.M.; Hofmann, J.P.; Cui, M.; Tadich, A.; Qi, D.; et al. Increased activity in the oxygen evolution reaction by Fe4+-induced hole states in perovskite La1-xSrxFeO3. J. Mater. Chem. A 2020, 8, 4407–4415. [Google Scholar] [CrossRef]
- Najafpour, M.M. Calcium-manganese oxides as structural and functional models for active site in oxygen evolving complex in photosystem II: Lessons from simple models. J. Photochem. Photobiol. B Biol. 2011, 104, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gagrani, A.; Alsultan, M.; Swiegers, G.F.; Tsuzuki, T. Comparative evaluation of the structural and other features governing photo-electrochemical oxygen evolution by Ca/Mn oxides. Catal. Sci. Technol. 2020, 10, 2152–2164. [Google Scholar] [CrossRef]
- Yamada, I.; Fujii, H.; Takamatsu, A.; Ikeno, H.; Wada, K.; Tsukasaki, H.; Kawaguchi, S.; Mori, S.; Yagi, S. Bifunctional Oxygen Reaction Catalysis of Quadruple Manganese Perovskites. Adv. Mater. 2017, 29, 1603004. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Boosting oxygen reduction/evolution reaction activities with layered perovskite catalysts. Chem. Commun. 2016, 52, 10739–10742. [Google Scholar] [CrossRef]
- Miao, H.; Wang, Z.; Wang, Q.; Sun, S.; Xue, Y.; Wang, F.; Zhao, J.; Liu, Z.; Yuan, J. A new family of Mn-based perovskite (La1-xYxMnO3) with improved oxygen electrocatalytic activity for metal-air batteries. Energy 2018, 154, 561–570. [Google Scholar] [CrossRef]
- Yamada, I.; Takamatsu, A.; Asai, K.; Ohzuku, H.; Shirakawa, T.; Uchimura, T.; Kawaguchi, S.; Tsukasaki, H.; Mori, S.; Wada, K.; et al. Synergistically Enhanced Oxygen Evolution Reaction Catalysis for Multielement Transition-Metal Oxides. ACS Appl. Energy Mater. 2018, 1, 3711–3721. [Google Scholar] [CrossRef]
- Yoo, J.S.; Rong, X.; Liu, Y.; Kolpak, A.M. Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites. ACS Catal. 2018, 8, 4628–4636. [Google Scholar] [CrossRef]
- Zhang, L.; Raman, A.S.; Vojvodic, A. Reviving Inert Oxides for Electrochemical Water Splitting by Subsurface Engineering. Chem. Mater. 2020. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; Halder, A.; Thomas, P.; Vaish, R. CaCu3Ti4O12: A Bifunctional Perovskite Electrocatalyst for Oxygen Evolution and Reduction Reaction in Alkaline Medium. Electrochim. Acta 2017, 252, 532–540. [Google Scholar] [CrossRef]
- Yamada, I.; Takamatsu, A.; Asai, K.; Shirakawa, T.; Ohzuku, H.; Seno, A.; Uchimura, T.; Fujii, H.; Kawaguchi, S.; Wada, K.; et al. Systematic Study of Descriptors for Oxygen Evolution Reaction Catalysis in Perovskite Oxides. J. Phys. Chem. C 2018, 122, 27885–27892. [Google Scholar] [CrossRef]
- Chen, C.-F.; King, G.; Dickerson, R.M.; Papin, P.A.; Gupta, S.; Kellogg, W.R.; Wu, G. Oxygen-deficient BaTiO3-x perovskite as an efficient bifunctional oxygen electrocatalyst. Nano Energy 2015, 13, 423–432. [Google Scholar] [CrossRef]
- Tymińska, N.; Wu, G.; Dupuis, M. Water oxidation on oxygen-deficient barium titanate: A first-principles study. J. Phys. Chem. C 2017, 121, 8378–8389. [Google Scholar] [CrossRef]
- Artrith, N.; Sailuam, W.; Limpijumnong, S.; Kolpak, A.M. Reduced overpotentials for electrocatalytic water splitting over Fe- and Ni-modified BaTiO3. Phys. Chem. Chem. Phys. 2016, 18, 29561–29570. [Google Scholar] [CrossRef] [PubMed]
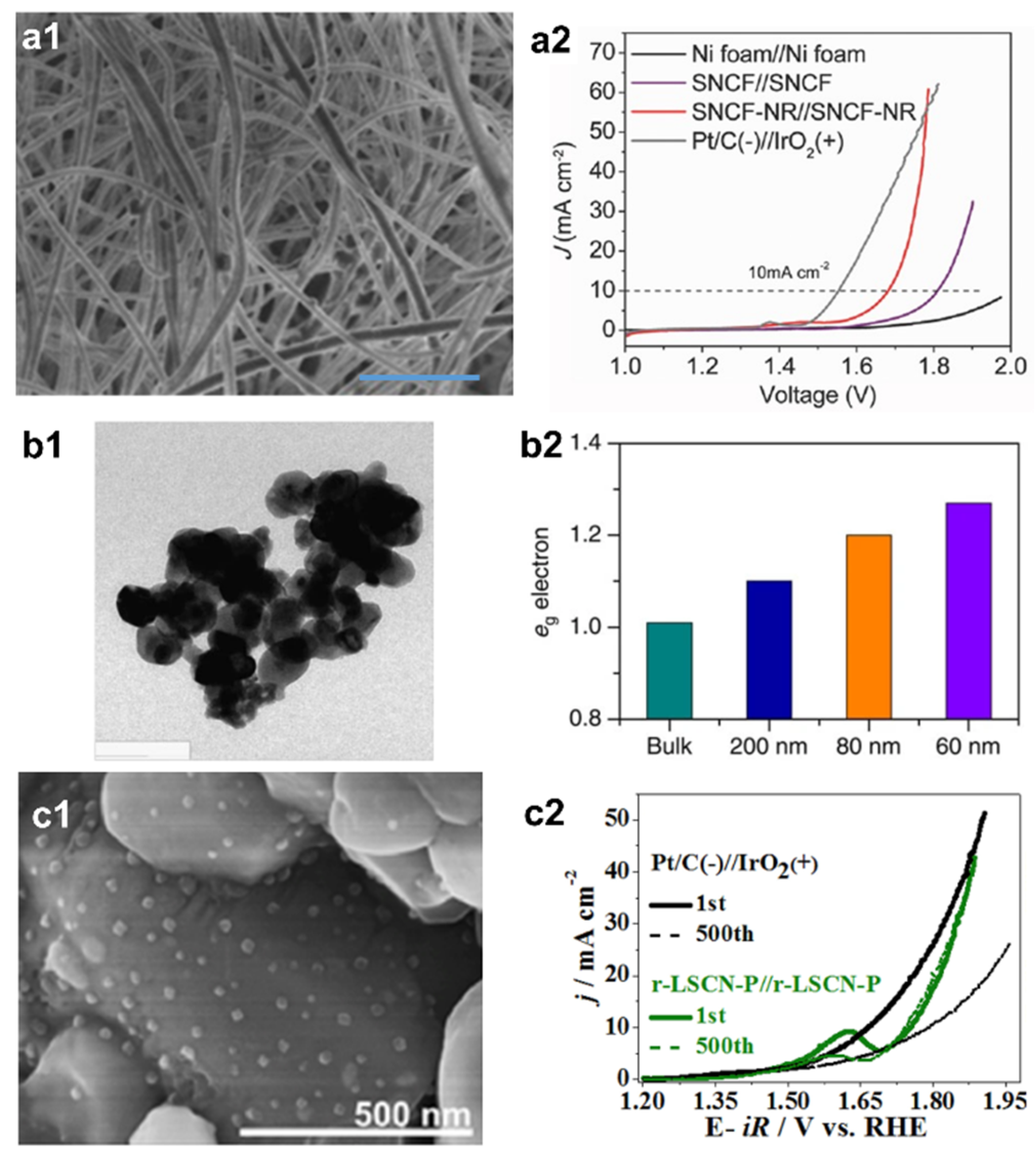
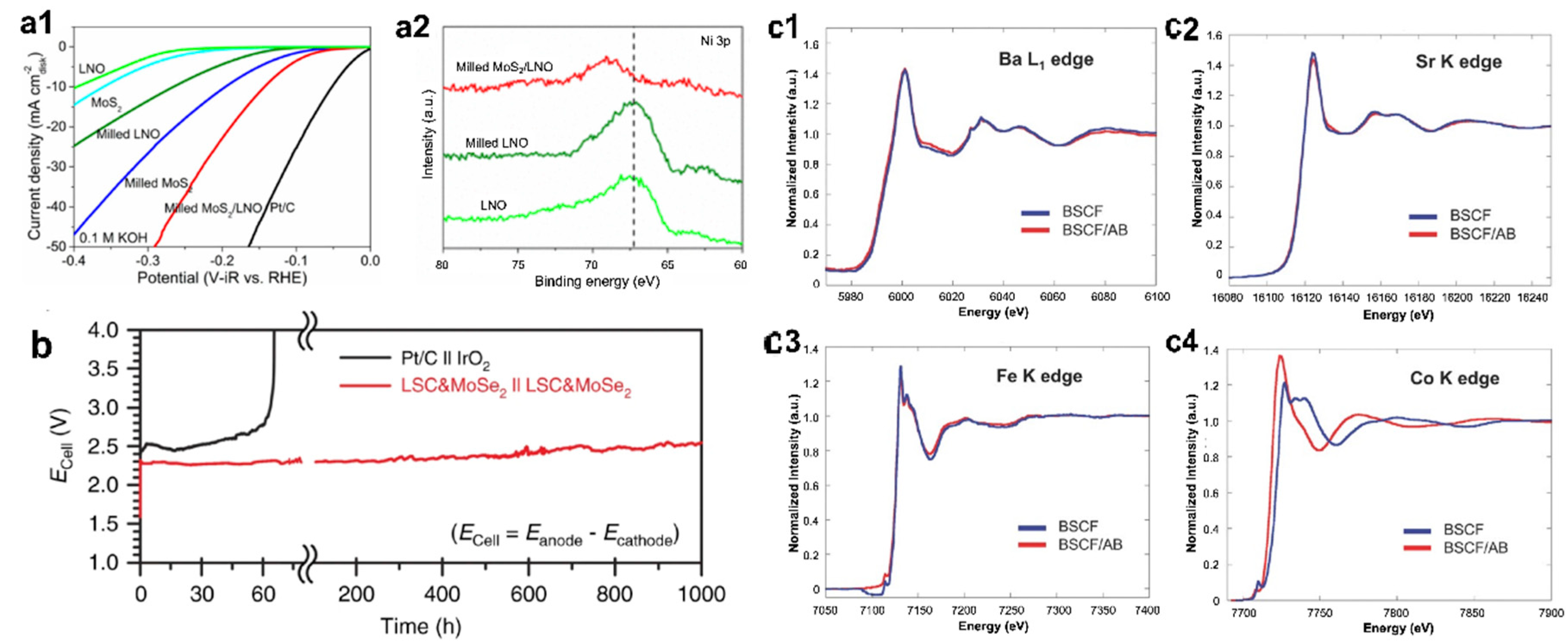
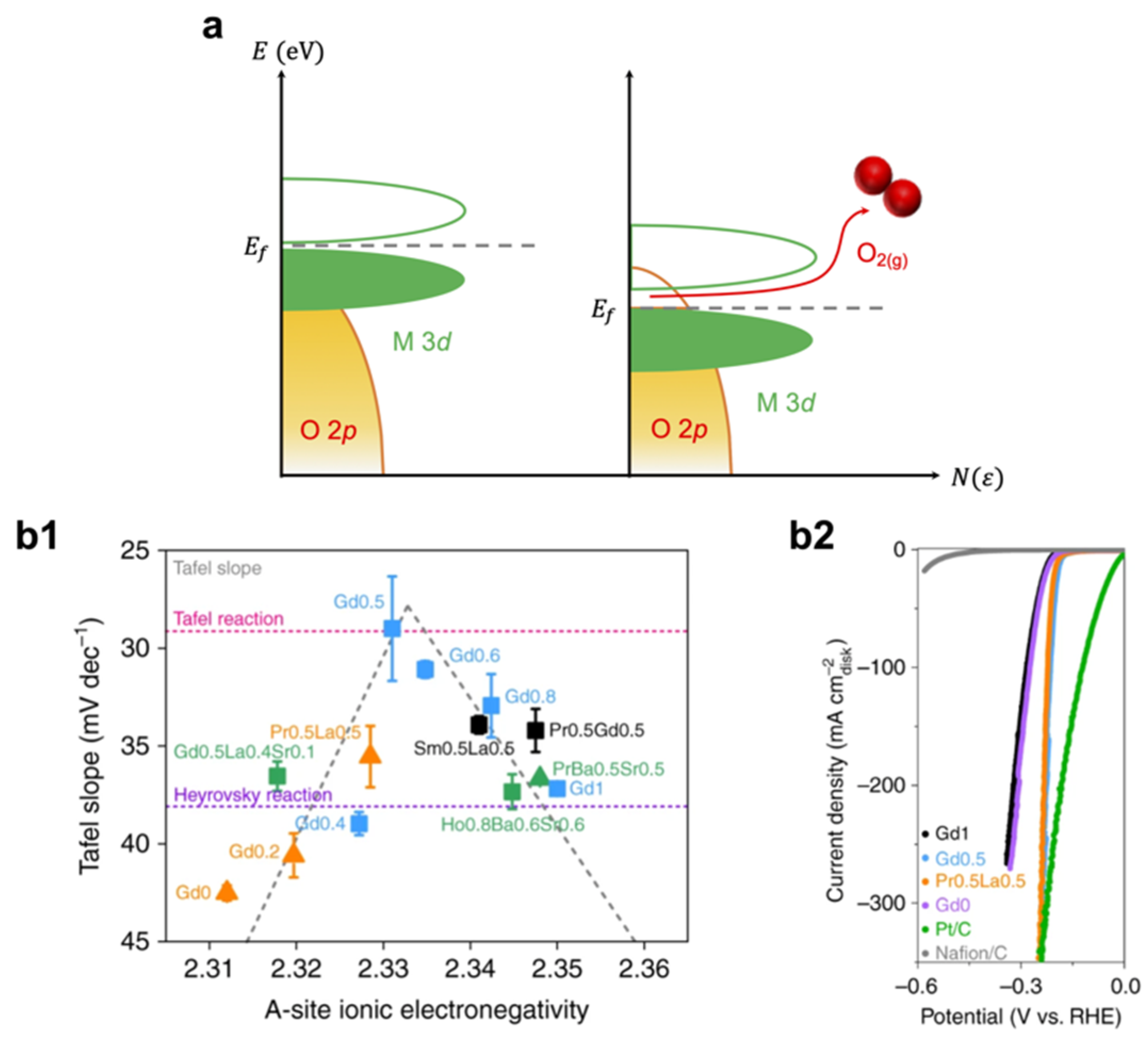

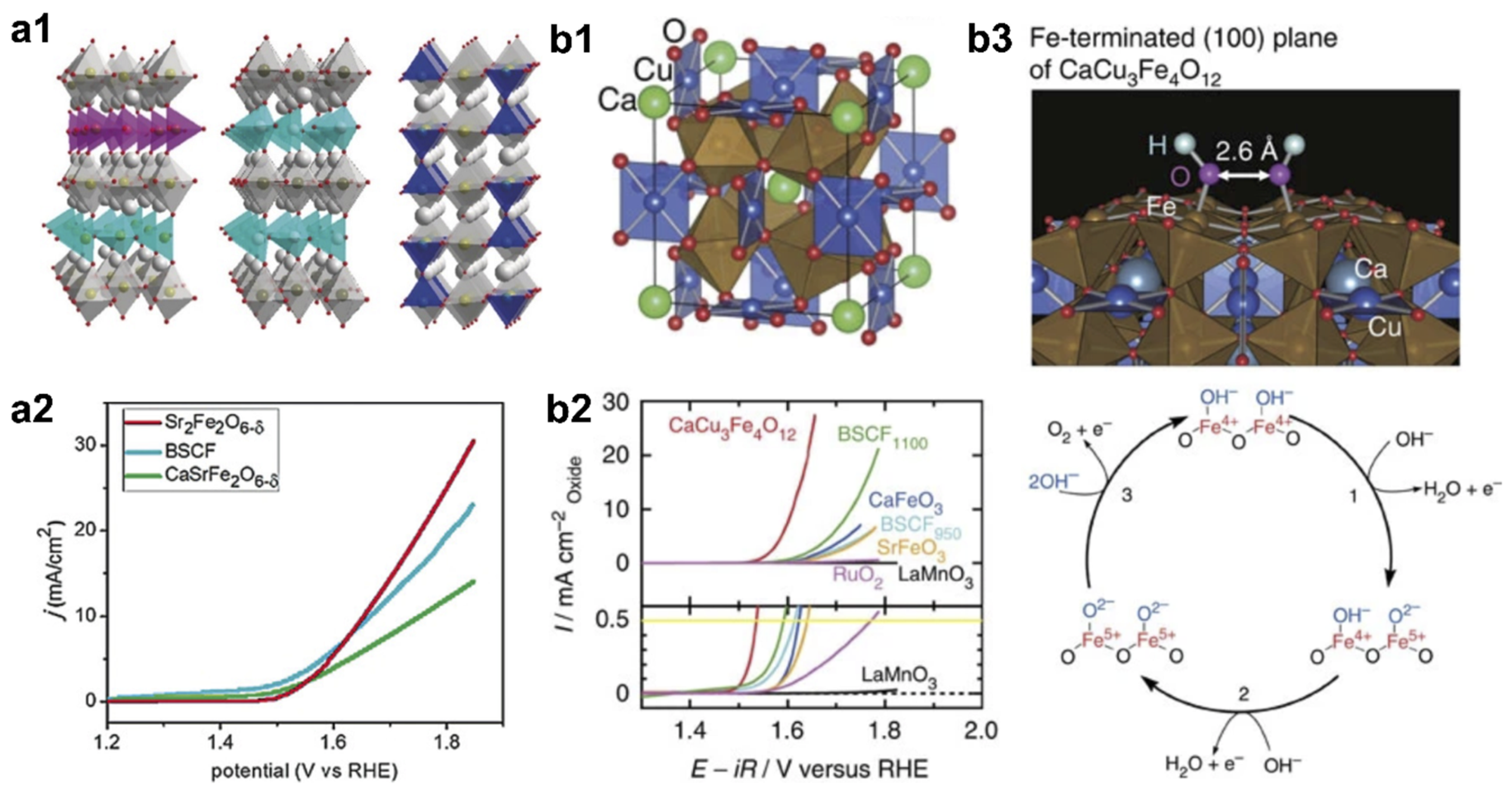
| Catalyst | Synthesis Method | OER Potential (V vs. RHE) at 10 mA cm−2 | HER Potential (V vs. RHE) at −10 mA cm−2 | Reference |
|---|---|---|---|---|
| NBM5.5 | Reductive annealing | 1.62 | −0.29 | [12] |
| PBC-1100 | Sol-gel | - | −0.245 | [17] |
| Pr0.5BSCF | Sol-gel | - | ~ −0.24 | [18] |
| C-NSCFNb | Exsolution | 1.65 | −0.47 | [19] |
| LBSCF | Solid-state reaction | ~1.5 | ~−0.02 | [39] |
| C-NSCFNb | - | - | - | - |
| SNCF-NR | Electrospinning | 1.62 | ~−0.24 | [58] |
| LSCN-P | Exsolution & phosphatization | 1.63 | −0.339 | [64] |
| MoS2/LNO | Ball milling | - | ~ −0.15 | [73] |
| LSC/MoSe2 | Ball milling | ~1.6 | ~−0.24 | [74] |
| Gd0.5 | Sol-gel | - | ~−0.21 | [79] |
| 3DOM-LF | colloidal template | 1.64 | ~−0.4 | [80] |
| Sr2Fe2O6−δ | Sol-gel | 1.71 | / | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Choi, S.; Kim, J.; Cha, S.W.; Lim, J. Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting. Catalysts 2020, 10, 770. https://doi.org/10.3390/catal10070770
Wang J, Choi S, Kim J, Cha SW, Lim J. Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting. Catalysts. 2020; 10(7):770. https://doi.org/10.3390/catal10070770
Chicago/Turabian StyleWang, Jian, Subin Choi, Juwon Kim, Suk Won Cha, and Jongwoo Lim. 2020. "Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting" Catalysts 10, no. 7: 770. https://doi.org/10.3390/catal10070770
APA StyleWang, J., Choi, S., Kim, J., Cha, S. W., & Lim, J. (2020). Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting. Catalysts, 10(7), 770. https://doi.org/10.3390/catal10070770





