Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis
Abstract
1. Introduction
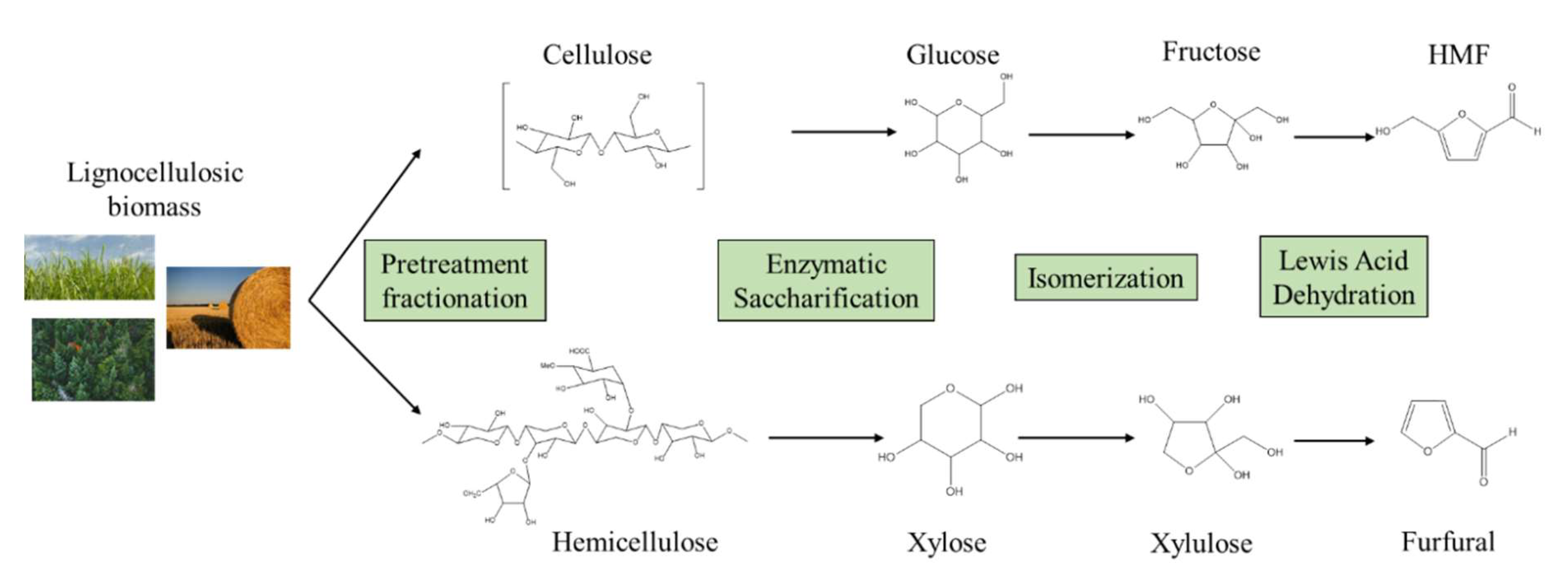
2. Conversion of Lignocellulosic Feedstocks to Monomeric Sugars
2.1. Plant Cell Wall Composition
2.2. Fractionation Technologies
2.3. Enzymatic Hydrolysis of Cellulose and Hemicellulose
Inhibition of Enzymatic Hydrolysis
2.4. Isomerisation of Hexoses and Pentoses for the Production of Furan Derivatives
3. Enzymatic Conversion of Furans to Building Blocks for Polymer Synthesis
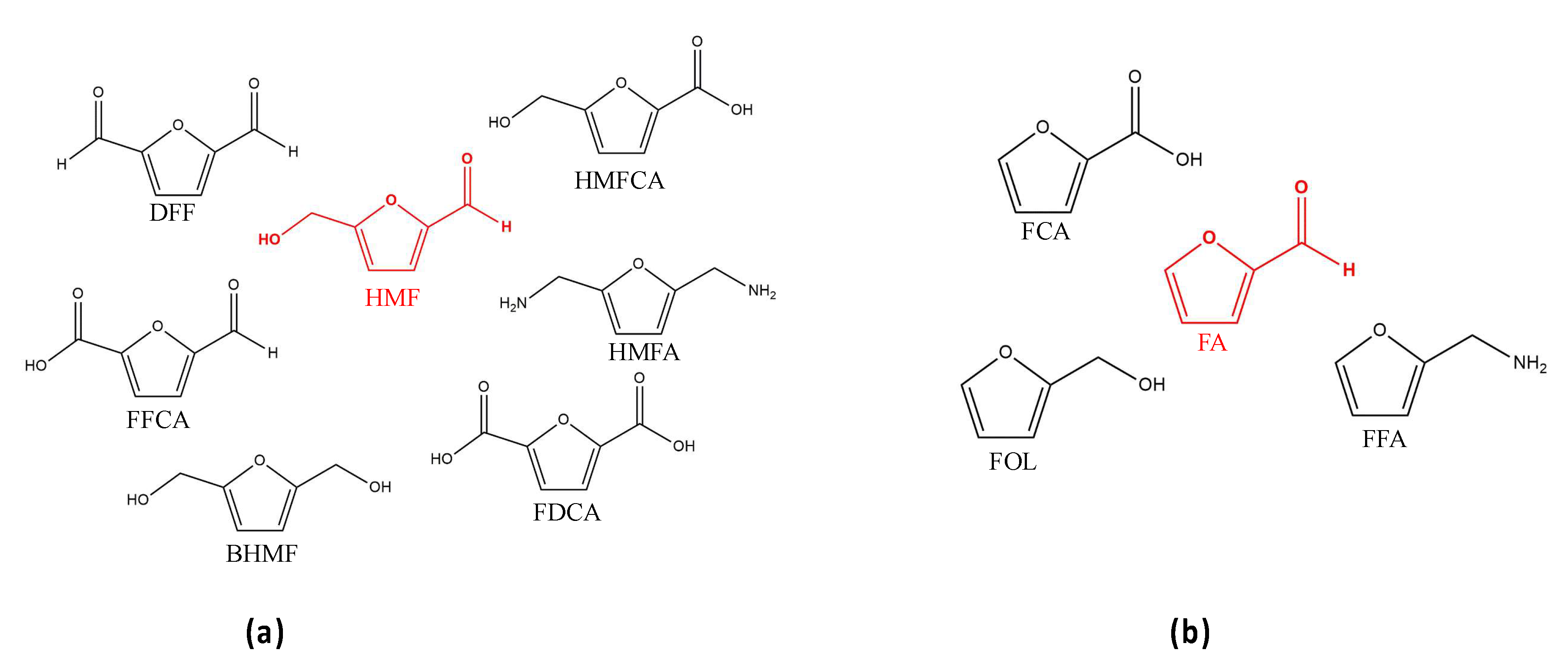
3.1. Oxidation Routes of Furans
3.1.1. Oxidative Reactions of HMF
Enzymes of the Recently Revisited AA5 Family
3.1.2. Oxidative Reactions for FA
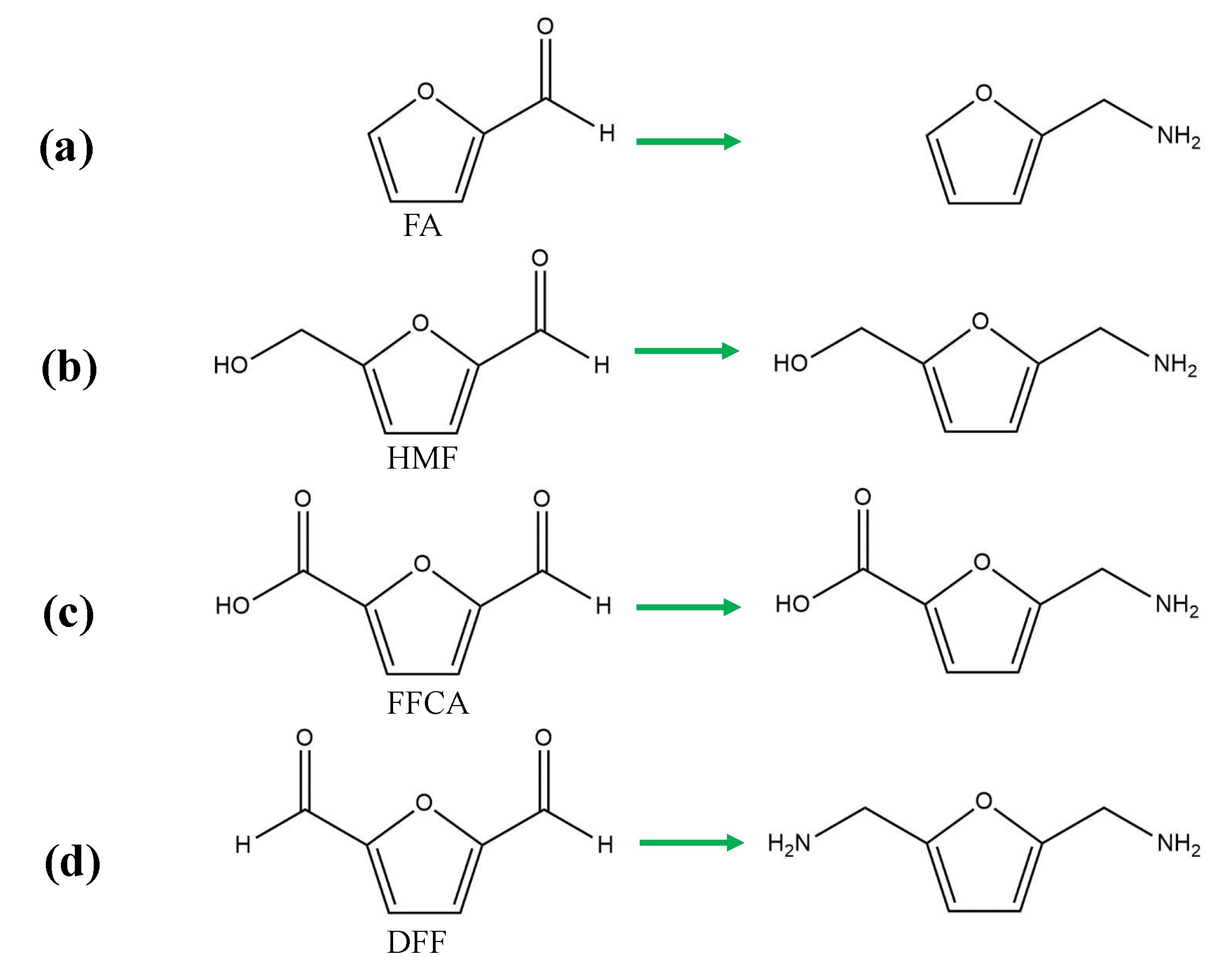
3.2. Reductive Amination
3.3. Reduction to Furan Alcohols
3.4. Other Enzymatic Activities and Future Perspectives
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
References
- Zhang, J.; Li, J.; Tang, Y.; Lin, L.; Long, M. Advances in catalytic production of bio-based polyester monomer 2,5-furandicarboxylic acid derived from lignocellulosic biomass. Carbohydr. Polym. 2015, 130, 420–428. [Google Scholar] [CrossRef] [PubMed]
- de Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Kudakasseril Kurian, J.; Raveendran Nair, G.; Hussain, A.; Vijaya Raghavan, G.S. Feedstocks, logistics and pre-treatment processes for sustainable lignocellulosic biorefineries: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 25, 205–219. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.; Matos, M.; Freire, C.S. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Χu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane production from lignocellulose: Biomass recalcitrance and its impacts on anaerobic digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Liu, R.; Liang, L.; Li, F.; Wu, M.; Chen, K.; Ma, J.; Jiang, M.; Wei, P.; Ouyang, P. Efficient succinic acid production from lignocellulosic biomass by simultaneous utilization of glucose and xylose in engineered Escherichia coli. Bioresour. Technol. 2013, 149, 84–91. [Google Scholar] [CrossRef]
- Karnaouri, A.; Chalima, A.; Kalogiannis, K.G.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of lignocellulosic biomass towards the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Bioresour. Technol. 2020, 303, 122899. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 2016, 6, 46834–46852. [Google Scholar] [CrossRef]
- Huang, R.; Qi, W.; Su, R.; He, Z. Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem. Commun. (Camb.) 2010, 46, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Van Lewis, D.; Chen, W.H.; Huang, H.W.; ALOthman, Z.A.; Yamauchi, Y.; Wu, K.C.W. Combined treatments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass. Catal. Today 2016, 278, 344–349. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value-Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. 2004. Available online: http://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 3 July 2020).
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.; Taarning, E.; Pedersen, C.M.; Bols, M. Conversion of d-glucose into 5-hydroxymethylfurfural (HMF) using zeolite in [Bmim]Cl or tetrabutylammonium chloride (TBAC)/CrCl 2. Tetrahedron Lett. 2012, 53, 983–985. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Sørensen, M.G.; Riisager, A. Direct conversion of glucose to 5-(hydroxymethyl)furfural in ionic liquids with lanthanide catalysts. Green Chem. 2010, 12, 321–325. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Du, J.; Liu, K.; Wang, T.; Liu, L. Biocatalytic production of 2,5-furandicarboxylic acid: Recent advances and future perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 527–543. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuel Bioprod. Biore-fining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Ks, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Nhuchhen, D.; Basu, P.; Acharya, B.A. Comprehensive review on biomass torrefaction. Int. J. Renew. Energ. Biofuels 2014, 1–56. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Arsène, M.A.; Bilba, K.; Junior, H.S.; Ghavami, K. Treatments of non-wood plant fibres used as reinforcement in composite materials. Mater. Res. 2013, 16, 903–923. [Google Scholar] [CrossRef]
- Smit, A.; Huijgen, W. Effective fractionation of lignocellulose in herbaceous biomass and hardwood using a mild acetone organosolv process. Green Chem. 2017, 19, 5505–5514. [Google Scholar] [CrossRef]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Karnaouri, A.; Matsakas, L.; Krikigianni, E.; Christakopoulos, P.; Rova, U. Valorization of waste forest biomass towards the production of cello-oligosaccharides with prebiotic potential by utilizing customized enzyme cocktails. Biotechnol. Biofuels 2020, 12, 285. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) production from real biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed]
- Timell, T.E. Recent progress in the chemistry of wood hemicelluloses. Wood Sci. Technol. 1967, 1, 45–70. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Dedes, G.; Bistis, P.; Kekos, D.; Kalogiannis, K.G.; Topakas, E. Acetone/water oxidation of corn stover for the production of bioethanol and prebiotic oligosaccharides. Bioresour. Technol. 2018, 270, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Sticklen, M.B. Plant genetic engineering for biofuel production: Towards affordable cellulosic ethanol. Nat. Rev. Genet. 2008, 9, 433. [Google Scholar] [CrossRef]
- Palonen, H.; Thomsen, A.B.; Tenkanen, M.; Schmidt, A.S.; Viikari, L. Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Appl. Biochem. Biotechnol. 2004, 117, 1–17. [Google Scholar] [CrossRef]
- Sewalt, V.J.H.; Ni, W.; Jung, H.G.; Dixon, R.A. Lignin impact on fiber degradation: Increased enzymatic digestibility of genetically engineered tobacco (Nicotiana tabacum) stems reduced in lignin content. J. Agric. Food Chem. 1997, 45, 1977–1983. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnol. Bioeng. 2006, 94, 611–617. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Cheng, K.; Liu, D. Enhancement of the enzymatic digestibility of sugarcane bagasse by alkali-peracetic acid pretreatment. Enzyme Microb. Technol. 2009, 44, 17–23. [Google Scholar] [CrossRef]
- Kienberger, M. Potential Applications of Lignin. In Economics of Bioresources; Krozer, Y., Narodoslawsky, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 183–193. [Google Scholar]
- Klinke, H.B.; Ahring, B.K.; Schmidt, A.S.; Thomsen, A.B. Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour. Technol. 2002, 82, 15–26. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Arora, A.; Martin, E.M.; Pelkki, W.H.; Carrier, D.J. Effect of formic acid and furfural on the enzymatic hydrolysis of cellulose powder and dilute acid-pretreated poplar hydrolysates. ACS Sustain. Chem. Eng. 2013, 1, 23–28. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Kalogiannis, K.G.; Kalogianni, A.; Lappas, A.A. Production of high concentrated cellulosic ethanol by acetone/water oxidized pretreated beech wood. Biotechnol. Biofuels 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K.; Julson, J.L. Influence of high shear bioreactor parameters on carbohydrate release from different biomasses. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, ASABE, Providence, RI, USA, 29 June–2 July 2008; Volume 6, pp. 3562–3577. [Google Scholar]
- Carvalheiro, F.; Duarte, L.C.; Girio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. India 2008, 67, 849–864. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Mosier, N.; Hendrickson, R.; Ho, N.; Sedlak, M.; Ladisch, M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef]
- Kootstra, A.M.J.; Beeftink, H.H.; Scott, E.L.; Sanders, J.P.M. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem. Eng. J. 2009, 46, 126–131. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Pielhop, T.; Amgarten, J.; von Rohr, P.R.; Studer, M.H. Steam explosion pretreatment of softwood: The effect of the explosive decompression on enzymatic digestibility. Biotechnol. Biofuels 2016, 9, 152. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef]
- Teymouri, F.; Laureano-Perez, L.; Alizadeh, H.; Dale, B.E. Optimization of the Ammonia Fiber Explosion (AFEX) Treatment Parameters for Enzymatic Hydrolysis of Corn Stover; Cambridge University Press: Cambridge, UK, 2014; pp. 1–30. [Google Scholar] [CrossRef]
- Martín, C.; Thomsen, M.H.; Hauggaard-Nielsen, H.; BelindaThomsen, A. Wet oxidation pretreatment, enzymatic hydrolysis and simultaneous saccharification and fermentation of clover-ryegrass mixtures. Bioresour. Technol. 2008, 99, 8777–8782. [Google Scholar] [CrossRef]
- Kumar, R.; Wyman, C.E. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Prog. 2009, 25, 302–314. [Google Scholar] [CrossRef]
- Itoh, H.; Wada, M.; Honda, Y.; Kuwahara, M.; Watanabe, T. Bioorganosolve pretreatments for simultaneous saccharification and fermentation of beech wood by ethanolysis and white rot fungi. J. Biotechnol. 2003, 103, 273–280. [Google Scholar] [CrossRef]
- Eriksson, T.; Karlsson, J.; Tjerneld, F. A model explaining declining rate in hydrolysis of lignocellulose substrates with cellobiohydrolase I (Cel7A) and endoglucanase I (Cel7B) of Trichoderma reesei. Appl. Biochem. Biotechnol. 2002, 101, 41–60. [Google Scholar] [CrossRef]
- Väljamäe, P.; Kipper, K.; Pettersson, G.; Johansson, G. Synergistic cellulose hydrolysis can be described in terms of fractal-like kinetics. Biotechnol. Bioeng. 2003, 84, 254–257. [Google Scholar] [CrossRef]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant expression of thermostable processive MtEG5 endoglucanase and its synergism with MtLPMO from Myceliophthora thermophila during the hydrolysis of lignocellulosic substrates. Biotechnol. Biofuels 2017, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Haven, M.Ø.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and Cellic® CTec2: Saccharification/fermentation of wheat straw, solid-liquid partition and potential of enzyme recycling by alkaline washing. Enzyme Microb. Technol. 2015, 79–80, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.C.; Topakas, E.; Christakopoulos, P. Cloning, expression, and characterization of a thermostable GH7 endoglucanase from Myceliophthora thermophila capable of high-consistency enzymatic liquefaction. Appl. Microbiol. Biotechnol. 2014, 98, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Topakas, E.; Matsakas, L.; Rova, U.; Christakopoulos, P. Fine-tuned enzymatic hydrolysis of organosolv pretreated forest materials for the efficient production of cellobiose. Front. Chem. 2018, 6, 128. [Google Scholar] [CrossRef]
- Karnaouri, A.; Topakas, E.; Paschos, T.; Taouki, I.; Christakopoulos, P. Cloning, expression and characterization of an ethanol tolerant GH3 β-glucosidase from Myceliophthora thermophila. PeerJ. 2013, 1, e46. [Google Scholar] [CrossRef] [PubMed]
- Katsimpouras, C.; Dimarogona, M.; Petropoulos, P.; Christakopoulos, P.; Topakas, E. A thermostable GH26 endo-β-mannanase from Myceliophthora thermophila capable of enhancing lignocellulose degradation. Appl. Microbiol. Biotechnol. 2016, 100, 8385–8397. [Google Scholar] [CrossRef]
- Kumar, G.P.; Pushpa, A.; Prabha, H. A Review on Xylooligosaccharides. IRJP 2012, 3, 71–74. [Google Scholar]
- Katsimpouras, C.; Dedes, G.; Thomaidis, N.S.; Topakas, E. A novel fungal GH30 xylanase with xylobiohydrolase auxiliary activity. Biotechnol. Biofuels 2019, 12, 120. [Google Scholar] [CrossRef]
- Moukouli, M.; Topakas, E.; Christakopoulos, P. Cloning and optimized expression of a GH-11 xylanase from Fusarium oxysporum in Pichia pastoris. New Biotechnol. 2011, 28, 369–374. [Google Scholar] [CrossRef]
- Várnai, A.; Mäkelä, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-binding modules of fungal cellulases: Occurrence in nature, function, and relevance in industrial biomass conversion. Adv. Appl. Microbiol. 2014, 88, 103–165. [Google Scholar] [CrossRef]
- Decker, C.H.; Visser, J.; Schreier, P. β-Glucosidases from five black Aspergillus species: Study of their physico-chemical and biocatalytic properties. J. Agric. Food Chem. 2000, 48, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Holtzapple, M.; Cognata, M.; Hendrickson, C. Inhibition of Trichoderma reesei celIulase by sugars and Solvents. Biotechnol. Bioeng. 1990, 36, 275–287. [Google Scholar] [CrossRef]
- Panagiotou, G.; Olsson, L. Effect of compounds released during pretreatment of wheat straw on microbial growth and enzymatic hydrolysis rates. Biotechnol. Bioeng. 2007, 96, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Danon, B.; Gianluca Marcotullio, G.; de Jong, W. Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem. 2014, 16, 39–54. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose isomerization to xylulose and its dehydration to furfural in aqueous media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Seyhan Tükel, S.; Alagöz, D. Catalytic efficiency of immobilized glucose isomerase in isomerization of glucose to fructose. Food Chem. 2008, 111, 658–662. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Tehrany, M.S.; Safari, I. Kinetics of glucose isomerization to fructose by immobilized glucose isomerase (Sweetzyme IT). Ind. Eng. Chem. Res. 2009, 48, 3271–3278. [Google Scholar] [CrossRef]
- Takasaki, Y. Studies on sugar-isomerizing enzymes effect of borate on glucose-fructose isomerization catalyzed by glucose isomerase. Agric. Biol. Chem. 1971, 35, 1371–1375. [Google Scholar] [CrossRef][Green Version]
- an den Berg, R.; Peters, J.A.; van Bekkum, H. The structure and (local) stability constants of borate esters of mono- and di-saccharides as studied by 11B and 13C NMR spectroscopy. Carbohydr. Res. 1994, 253, 1–12. [Google Scholar] [CrossRef]
- Wang, W.; Mittal, A.; Pilath, H.; Chen, X.; Tucker, M.P.; Johnson, D.K. Simultaneous upgrading of biomass-derived sugars to HMF/furfural via enzymatically isomerized ketose intermediates. Biotechnol. Biofuels 2019, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Ohara, M.; Nishimura, S.; Ebitani, K. One-pot formation of furfural from xylose via isomerization and successive dehydration reactions over heterogeneous acid and base catalysts. Chem. Lett. 2010, 39, 838–840. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.W.; Abu-Omar, M.M. Synthesis of furfural from xylose, xylan, and biomass using AlCl 3·6H2O in biphasic media via xylose isomerization to xylulose. ChemSusChem 2012, 5, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wierckx, N.; Koopman, F.; Ruijssenaars, H.J.; de Winde, J.H. Microbial degradation of furanic compounds: Biochemistry, genetics, and impact. Appl. Microbiol. Biotechnol. 2011, 92, 1095–1105. [Google Scholar] [CrossRef]
- Krystof, M.; Pérez-Sánchez, M.; de María, P.D. Lipase-mediated selective oxidation of furfural and 5-hydroxymethylfurfural. ChemSusChem 2013, 6, 826–830. [Google Scholar] [CrossRef]
- Carro, J.; Ferreira, P.; Rodríguez, L.; Prieto, A.; Serrano, A.; Balcells, B.; Ardá, A.; Jiménez-Barbero, J.; Gutiérrez, A.; Ullrich, R.; et al. 5-Hydroxymethylfurfural conversion by fungal aryl-alcohol oxidase and unspecific peroxygenase. FEBS J. 2015, 282, 3218–3229. [Google Scholar] [CrossRef]
- Serrano, A.; Calviño, E.; Carro, J.; Sánchez-Ruiz, M.I.; Cañada, J.F.; Martínez, A.T. Complete oxidation of hydroxymethylfurfural to furandicarboxylic acid by aryl-alcohol oxidase. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-catalyzed oxidation of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid. Angew. Chem. 2014, 126, 6633–6636. [Google Scholar] [CrossRef]
- Koopman, F.; Wierckx, N.; De Winde, J.H.; Ruijssenaars, H.J. Identification and characterization of the furfural and 5-(hydroxymethyl)furfural degradation pathways of Cupriavidus basilensis HMF14. Proc. Natl. Acad. Sci. USA 2010, 107, 4919–4924. [Google Scholar] [CrossRef]
- Viña-Gonzalez, J.; Martinez, A.T.; Guallar, V.; Alcalde, M. Sequential oxidation of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid by an evolved aryl-alcohol oxidase. Biochim. Biophys. Acta 2020, 1868, 140293. [Google Scholar] [CrossRef]
- McKenna, S.M.; Leimkühler, S.; Herter, S.; Turner, N.J.; Carnell, A.J. Enzyme cascade reactions: Synthesis of furandicarboxylic acid (FDCA) and carboxylic acids using oxidases in tandem. Green Chem. 2015, 17, 3271–3275. [Google Scholar] [CrossRef]
- McKenna, S.M.; Mines, P.; Law, P.; Kovacs-Schreiner, K.; Birmingham, W.R.; Turner, N.J.; Leimkühler, S.; Carnell, A.J. The continuous oxidation of HMF to FDCA and the immobilisation and stabilisation of periplasmic aldehyde oxidase (PaoABC). Green Chem. 2017, 19, 4660–4665. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Li, Y.M.; Zong, M.H.; Wu, H.; Li, N. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- Karich, A.; Kleeberg, S.B.; Ullrich, R.; Hofrichter, M. Enzymatic preparation of 2,5-furandicarboxylic acid (FDCA)—A substitute of terephthalic acid—By the joined action of three fungal enzymes. Microorganisms 2018, 6, 5. [Google Scholar] [CrossRef]
- Daou, M.; Yassine, B.; Wikee, S.; Record, E.; Duprat, F.; Bertrand, E.; Faulds, C.B. Pycnoporus cinnabarinus glyoxal oxidases display differential catalytic efficiencies on 5-hydroxymethylfurfural and its oxidized derivatives. Fungal Biol. Biotechnol. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Mathieu, Y.; Offen, W.A.; Forget, S.M.; Ciano, L.; Viborg, A.H.; Bloagova, E.; Henrissat, B.; Walton, P.H.; Davies, G.J.; Brumer, H. Discovery of a fungal copper radical oxidase with high catalytic efficiency toward 5-hydroxymethylfurfural and benzyl alcohols for bioprocessing. ACS Catal. 2020, 10, 3042–3058. [Google Scholar] [CrossRef]
- Dong, J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. 2018, 57, 9238–9261. [Google Scholar] [CrossRef] [PubMed]
- Pérez, H.I.; Manjarrez, N.; Solís, A.; Luna, H.; Ramírez, M.A.; Cassani, J. Microbial biocatalytic preparation of 2-furoic acid by oxidation of 2-furfuryl alcohol and 2-furanaldehyde with Nocardia corallina. Afr. J. Biotechnol. 2009, 8, 2279–2282. [Google Scholar]
- Ran, H.; Zhang, J.; Gao, Q.; Lin, Z.; Bao, J. Analysis of biodegradation performance of furfural and 5- hydroxymethylfurfural by Amorphotheca resinae ZN1. Biotechnol. Biofuels 2014, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Fraaije, M.W. Conversion of furans by Baeyer-Villiger monooxygenases. Catalysts 2017, 7, 179. [Google Scholar] [CrossRef]
- Lankenaua, A.W.; Kanan, M.W. Polyamide monomers via carbonate-promoted C–H carboxylation of furfurylamine. Chem. Sci. 2020, 11, 248–252. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; He, X.; Feng, T.; Ramdani, N.; Luan, M.; Liu, W.; Xu, X. Synthesis of novel furan-containing tetrafunctional fluorene-based benzoxazine monomer and its high performance thermoset. RSC Adv. 2014, 4, 64798–64801. [Google Scholar] [CrossRef]
- Haas, T.; Pfeffer, J.C.; Faber, K.; Fuchs, M. (Evonik Degussa Gmbh) Enzymatic Amination. U.S. Patent WO2012171666, 20 December 2012. [Google Scholar]
- Schaub, T.; Buschhaus, B.; Brinks, M.K.; Schelwies, M.; Paciello, R.; Melder, J.-P.; Merger, M. (BASF SE) Process for the Preparation of Primary Amines by Homogenously Catalysed Alcohol Amination. U.S. Patent 8785693, 22 July 2014. [Google Scholar]
- Meng, J.; Zeng, Y.; Zhu, G.; Zhang, J.; Chen, P.; Cheng, Y.; Fang, Z.; Guo, K. Sustainable bio-based furan epoxy resin with flame retardancy. Polym. Chem. 2019, 10, 2370–2375. [Google Scholar] [CrossRef]
- Wei, H.; Yao, K.; Chu, H.; Li, Z.C.; Zhu, J.; Zhao, Z.X.; Feng, Y.L. Click synthesis of the thermo- and pH-sensitive hydrogels containing β-cyclodextrins. J. Mater. Sci. 2012, 47, 332–340. [Google Scholar] [CrossRef]
- Roylance, J.J.; Choi, K.S. Electrochemical reductive amination of furfural-based biomass intermediates. Green Chem. 2016, 18, 5412–5417. [Google Scholar] [CrossRef]
- Dunbabin, A.; Subrizi, F.; Ward, J.M.; Sheppard, T.D.; Hailes, H.C. Furfurylamines from biomass: Transaminase catalysed upgrading of furfurals. Green Chem. 2017, 19, 397–404. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Moody, T.S.; Gilmore, B.F. Biocatalysis in seawater: Investigating a halotolerant ω-transaminase capable of converting furfural in a seawater reaction medium. Eng. Life Sci. 2019, 19, 721–725. [Google Scholar] [CrossRef]
- Deska, J.; Blume, F.; Albeiruty, M. Alkylative amination of biogenic furans through imine-to-azaallyl anion umpolung. Synthesis 2015, 47, 2093–2099. [Google Scholar] [CrossRef]
- Neto, W.; Schürmann, M.; Panella, L.; Vogel, A.; Woodley, J.M. Immobilisation of ω-transaminase for industrial application: Screening and characterisation of commercial ready to use enzyme carriers. J. Mol. Catal. B Enzym. 2015, 117, 54–61. [Google Scholar] [CrossRef]
- Petri, A.; Masia, G.; Piccolo, O. Biocatalytic conversion of 5- hydroxymethylfurfural: Synthesis of 2,5-bis(hydroxymethyl)furan and 5-(hydroxymethyl)furfurylamine. Catal. Commun. 2018, 114, 15–18. [Google Scholar] [CrossRef]
- Meng, Q.; Capra, N.; Palacio, C.M.; Lanfranchi, E.; Otzen, M.; van Schie, L.Z.; Rozeboom, H.J.; Thunnissen, A.-M.W.H.; Wijma, H.J.; Janssen, D.B. Robust ω-transaminases by computational stabilization of the subunit interface. ACS Catalysis 2020, 10, 2915–2928. [Google Scholar] [CrossRef]
- Höhne, M.; Bornscheuer, U.T. Biocatalytic routes to optically active amines. ChemCatChem 2009, 1, 42–51. [Google Scholar] [CrossRef]
- Knaus, T.; Böhmer, W.; Mutti, F.G. Amine dehydrogenases: Efficient biocatalysts for the reductive amination of carbonyl compounds. Green Chem. 2017, 9, 453–463. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; van Alberda Ekenstein, G.O.R.; Petrović, D.M.; Loos, K. Enzymatic synthesis of biobased polyesters using 2,5-bis(hydroxymethyl)furan as the building block. Biomacromolecules 2014, 15, 2482–2493. [Google Scholar] [CrossRef]
- Baraldi, S.; Fantin, G.; Di Carmine, G.; Ragno, D.; Brandolese, A.; Massi, A.; Bortolini, O.; Marchetti, N.; Giovannini, P.P. Enzymatic synthesis of biobased aliphatic–aromatic oligoesters using 5,5′-bis(hydroxymethyl)furoin as a building block. RSC Adv. 2019, 9, 29044–29050. [Google Scholar] [CrossRef]
- Bradshaw, C.W.; Hummel, W.; Wong, C.H. Lactobacillus kefir alcohol dehydrogenase: A useful catalyst for synthesis. Org. Chem. 1992, 57, 1532–1536. [Google Scholar] [CrossRef]
- Oberleitner, N.; Peters, C.; Rudroff, F.; Bornscheuer, U.T.; Mihovilovic, M.D. In vitro characterization of an enzymatic redox cascade composed of an alcohol dehydrogenase, an enoate reductases and a Baeyer-Villiger monooxygenase. J. Biotechnol. 2014, 192, 393–399. [Google Scholar] [CrossRef]
- Kaswurm, V.; Van Hecke, W.; Kulbe, K.D.; Ludwig, R. Engineering of a bi-enzymatic reaction for efficient production of the ascorbic acid precursor 2-keto-L-gulonic acid. Adv. Synth. Catal. 2013, 355, 1709–1714. [Google Scholar] [CrossRef]
- Feldman, D.; Kowbel, D.J.; Glass, N.L.; Yarden, O.; Hadar, Y. Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol. Biofuels 2015, 8, 63. [Google Scholar] [CrossRef]
- Dominguez, P.D.M.; Guajardo, N.V. Biocatalytic valorization of furans: Opportunities for inherently unstable substrates. Chemsuschem 2017, 10, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bu, C.; He, Q.; Zheng, Z.; Ouyang, J. Efficient bioconversion of furfural to furfuryl alcohol by Bacillus coagulans NL01. RSC Adv. 2018, 8, 26720–26727. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Ingram, L.O.; Preston, J.F. Purification and characterization of a furfural reductase (FFR) from Escherichia coli strain LYO1—An enzyme important in the detoxification of furfural during ethanol production. J. Biotechnol. 2006, 121, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Metthew Lam, L.K.; Xun, L. Cupriavidus necator JMP134 rapidly reduces furfural with a Zn-dependent alcohol dehydrogenase. Biodegradation 2011, 22, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Metthew Lam, L.K.; Xun, L. Biochemical characterization of ethanol-dependent reduction of furfural by alcohol dehydrogenases. Biodegradation 2011, 22, 1227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Qiao, H.; Zheng, Z.; Chu, Q.; Li, X.; Qiang, Y.; Jia, O. Lactic acid production from pretreated hydrolysates of corn stover by a newly developed Βacillus coagulans strain. PLoS ONE 2016, 11, e0149101. [Google Scholar] [CrossRef]
- Laadan, B.; Almeida, J.R.; Radstrom, P.; Hahn-Hagerdal, B.; Gorwa-Grauslund, M. Identification of an NADH-dependent 5-hydroxymethylfurfural-reducing alcohol dehydrogenase in Saccharomyces cerevisiae. Yeast 2008, 25, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhang, X.Y.; Li, N.; Xu, P.; Lou, W.Y.; Zong, M.H. Biocatalytic reduction of HMF to 2,5-bis(hydroxymethyl)furan by HMF-tolerant whole cells. ChemSusChem 2017, 10, 372–378. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Ma, C.; Di, J.; Jiang, C.; Li, A. One-pot conversion of biomass-derived xylose to furfuralcohol by a chemo-enzymatic sequential acid-catalyzed dehydration and bioreduction. Green Chem. 2017, 19, 3844–3850. [Google Scholar] [CrossRef]
- Xia, Z.-H.; Zong, M.-H.; Li, N. Catalytic synthesis of 2,5-bis(hydroxymethyl)furan from 5-hydroxymethylfurfual by recombinant Saccharomyces cerevisiae. Enzyme Microb. Technol. 2019, 134, 109491. [Google Scholar] [CrossRef]
- Gong, X.M.; Qin, Z.; Li, F.L.; Zeng, B.B.; Zheng, G.W.; Xu, J.H. Development of an engineered ketoreductase with simultaneously improved thermostability and activity for making a bulky atorvastatin precursor. ACS Catal. 2019, 9, 147–153. [Google Scholar] [CrossRef]
- Zheng, G.-W.; Liu, Y.-Y.; Chen, Q.; Huang, L.; Yu, H.-L.; Lou, W.-Y.; Li, C.-X.; Bai, Y.-P.; Li, A.; Xu, J.-H. Preparation of structurally diverse chiral alcohols by engineering ketoreductase CgKR1. ACS Catal. 2017, 7, 7174–7181. [Google Scholar] [CrossRef]
- Jiang, Y.; Loos, K. Enzymatic synthesis of biobased polyesters and polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Marotta, A.; Ambrogi, V.; Cerruti, P.; Mija, A. Green approaches in the synthesis of furan-based diepoxy monomers. RSC Adv. 2018, 8, 16330. [Google Scholar] [CrossRef]
- Peterson, L.A.; Cummings, M.E.; Vu, C.C.; Matter, B.A. Glutathione trapping to measure microsomal oxidation of furan to cis-2-butene-1,4-dial. Drug Metab Dispos. 2005, 33, 1453–1458. [Google Scholar] [CrossRef]
- Thibodeaux, C.J.; Chang, W.C.; Liu, H.W. Enzymatic chemistry of cyclopropane, epoxide, and aziridine biosynthesis. Chem Rev. 2012, 112, 1681–1709. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 oxidations in the generation of reactive electrophiles: Epoxidation and related reactions. Arch. Biochem. Biophys. 2003, 409, 59–71. [Google Scholar] [CrossRef]
- Peterson, L.A. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem Res Toxicol. 2013, 26, 6–25. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, D.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Fagúndez, N.; Agirrezabal-Telleria, I.; Aria, P.L.; Fierro, J.L.G.; Mariscal, R.; López Granados, M. Aqueous-phase catalytic oxidation of furfural with H2O2: High yield of maleic acid by using titanium silicalite-1. RSC Adv. 2014, 4, 54960–54972. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedes, G.; Karnaouri, A.; Topakas, E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts 2020, 10, 743. https://doi.org/10.3390/catal10070743
Dedes G, Karnaouri A, Topakas E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts. 2020; 10(7):743. https://doi.org/10.3390/catal10070743
Chicago/Turabian StyleDedes, Grigorios, Anthi Karnaouri, and Evangelos Topakas. 2020. "Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis" Catalysts 10, no. 7: 743. https://doi.org/10.3390/catal10070743
APA StyleDedes, G., Karnaouri, A., & Topakas, E. (2020). Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts, 10(7), 743. https://doi.org/10.3390/catal10070743






