Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
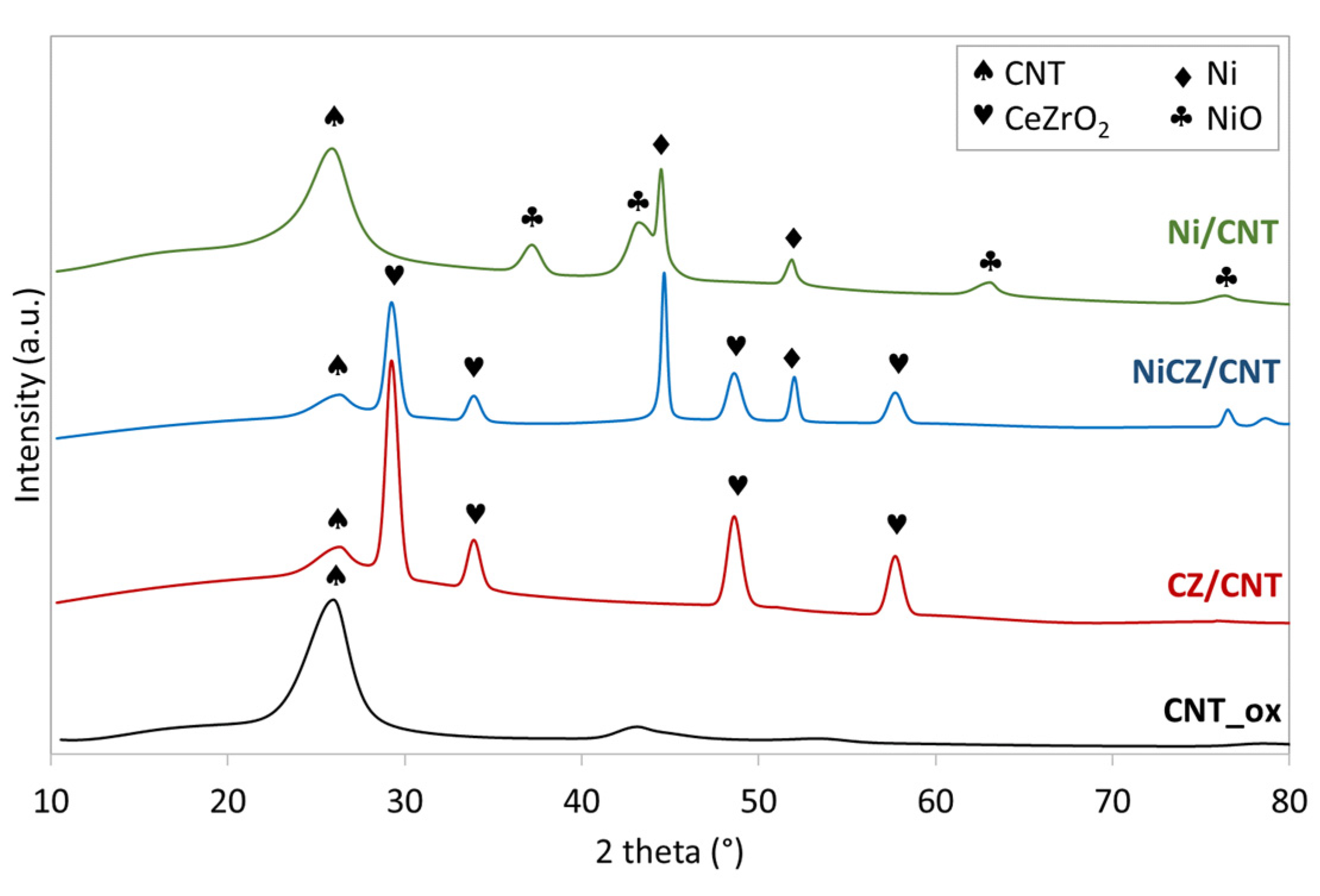
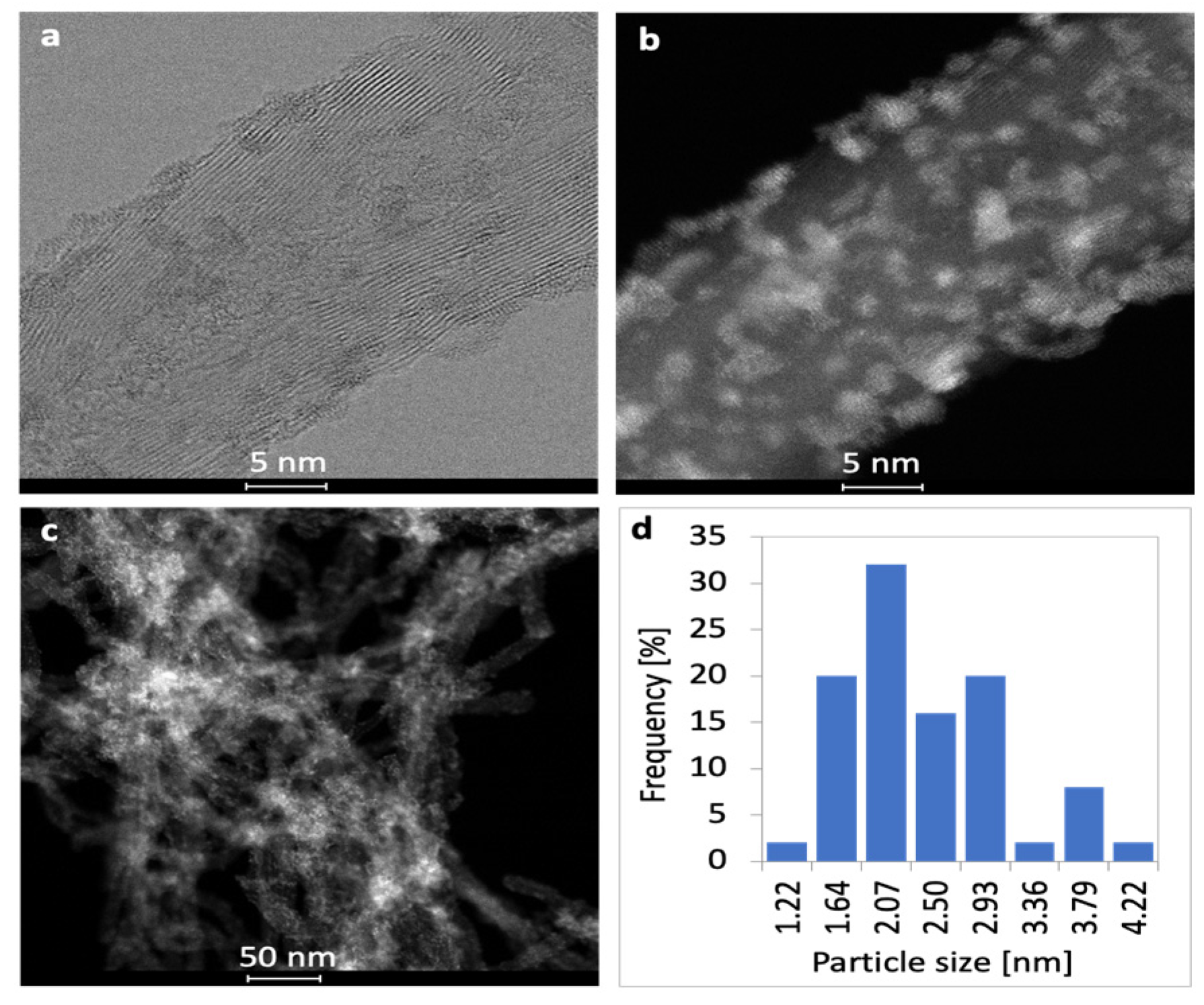
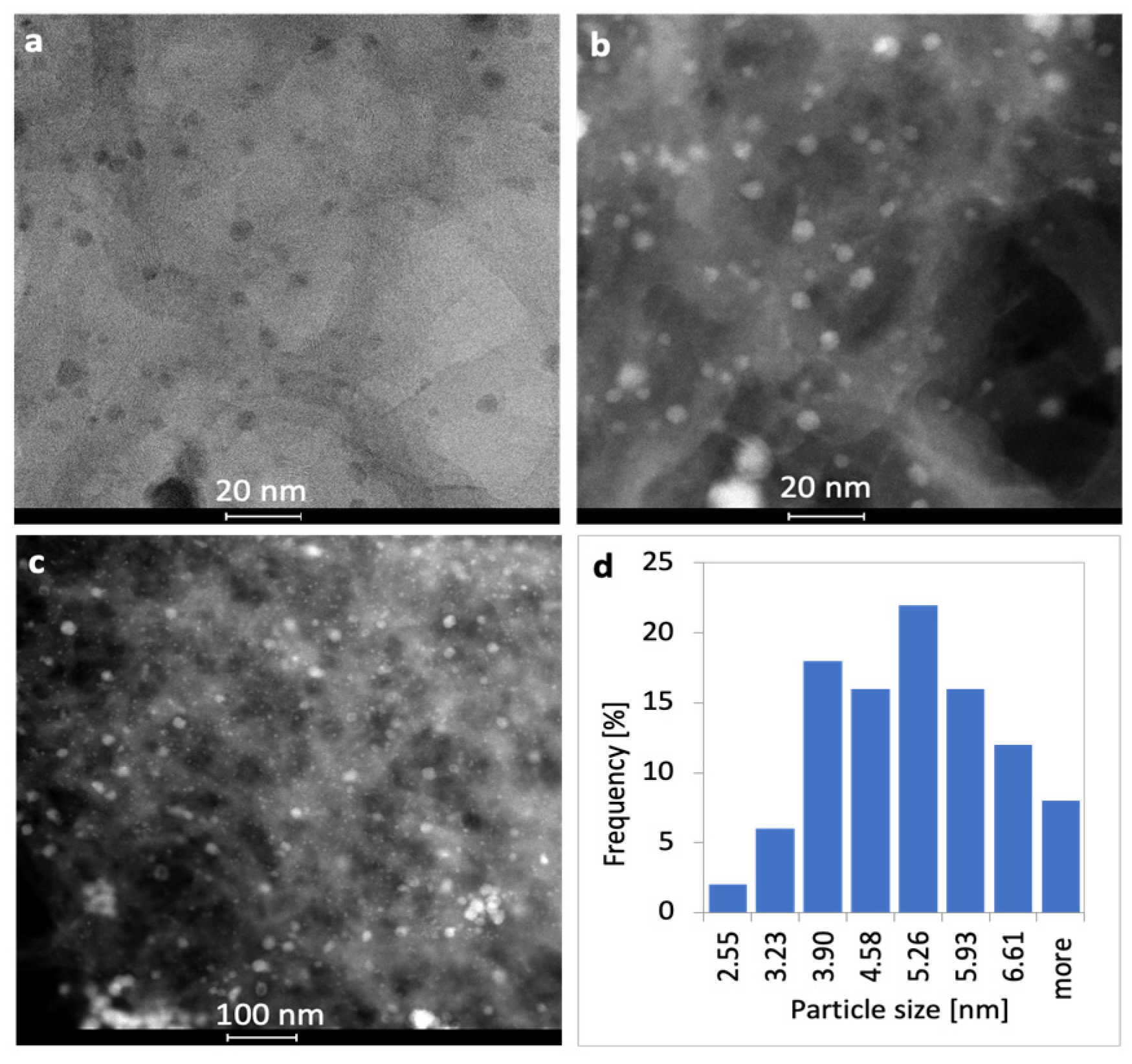
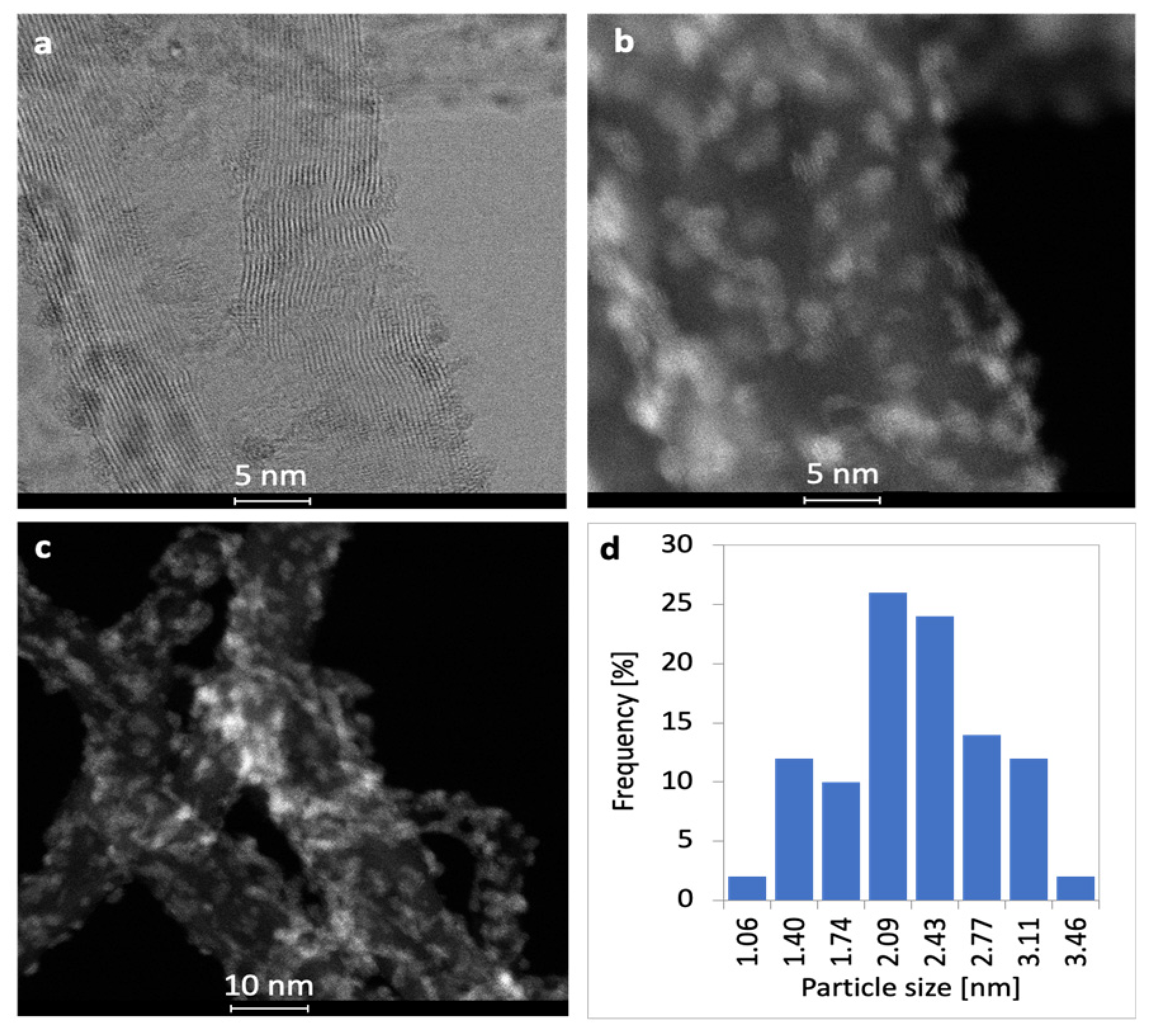
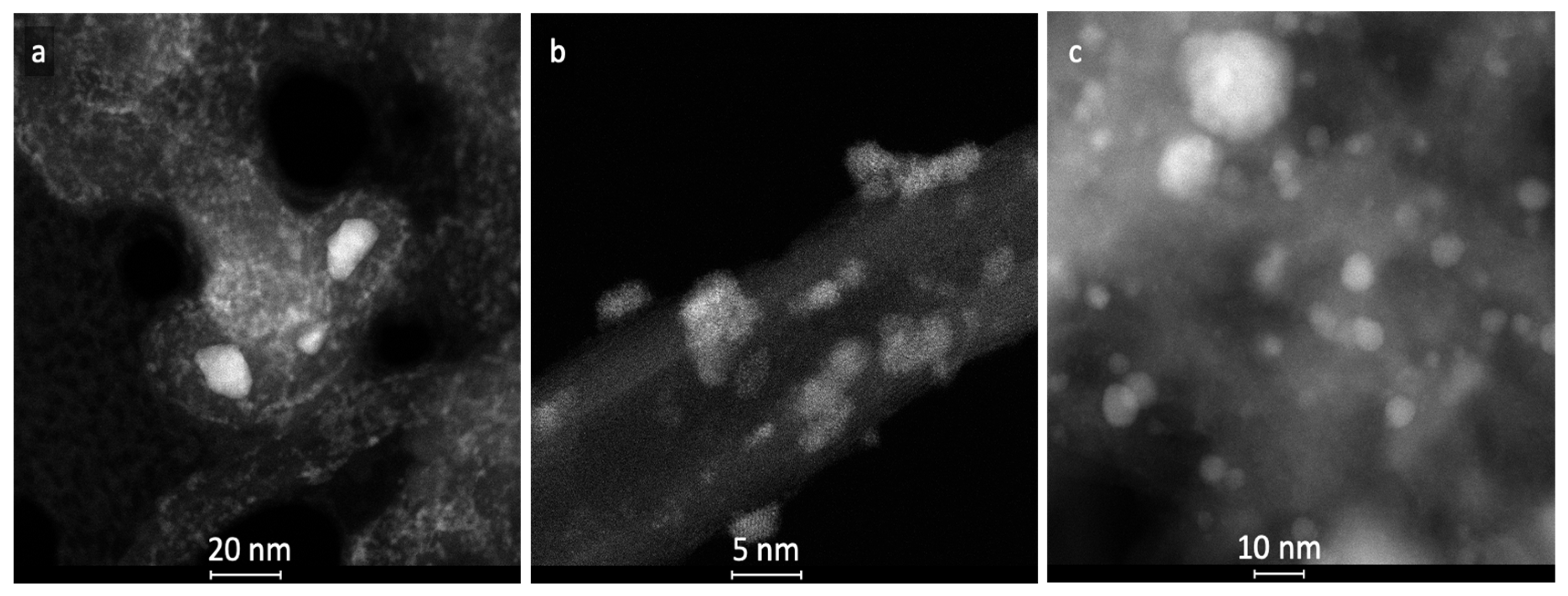
2.1. Characterization
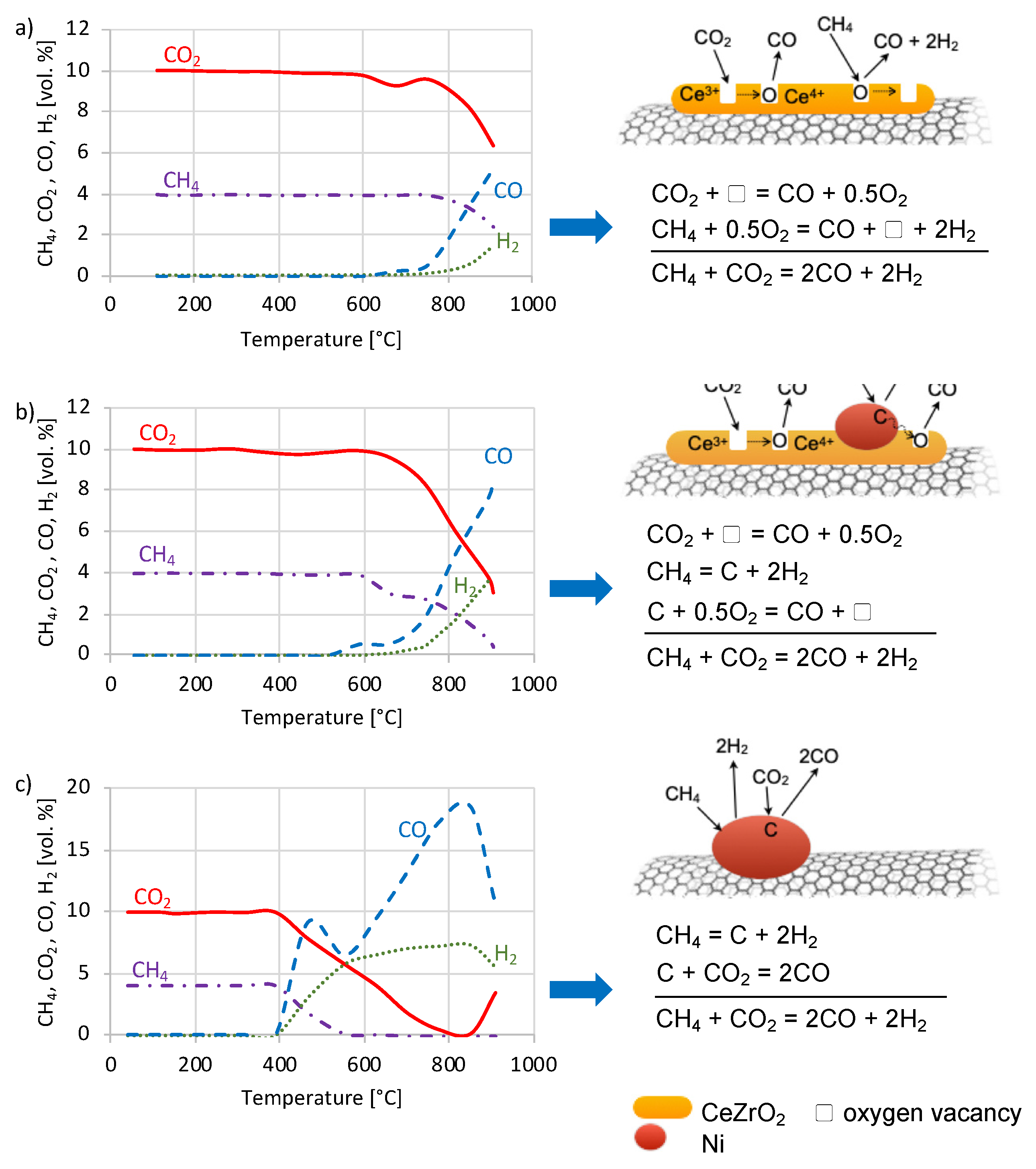
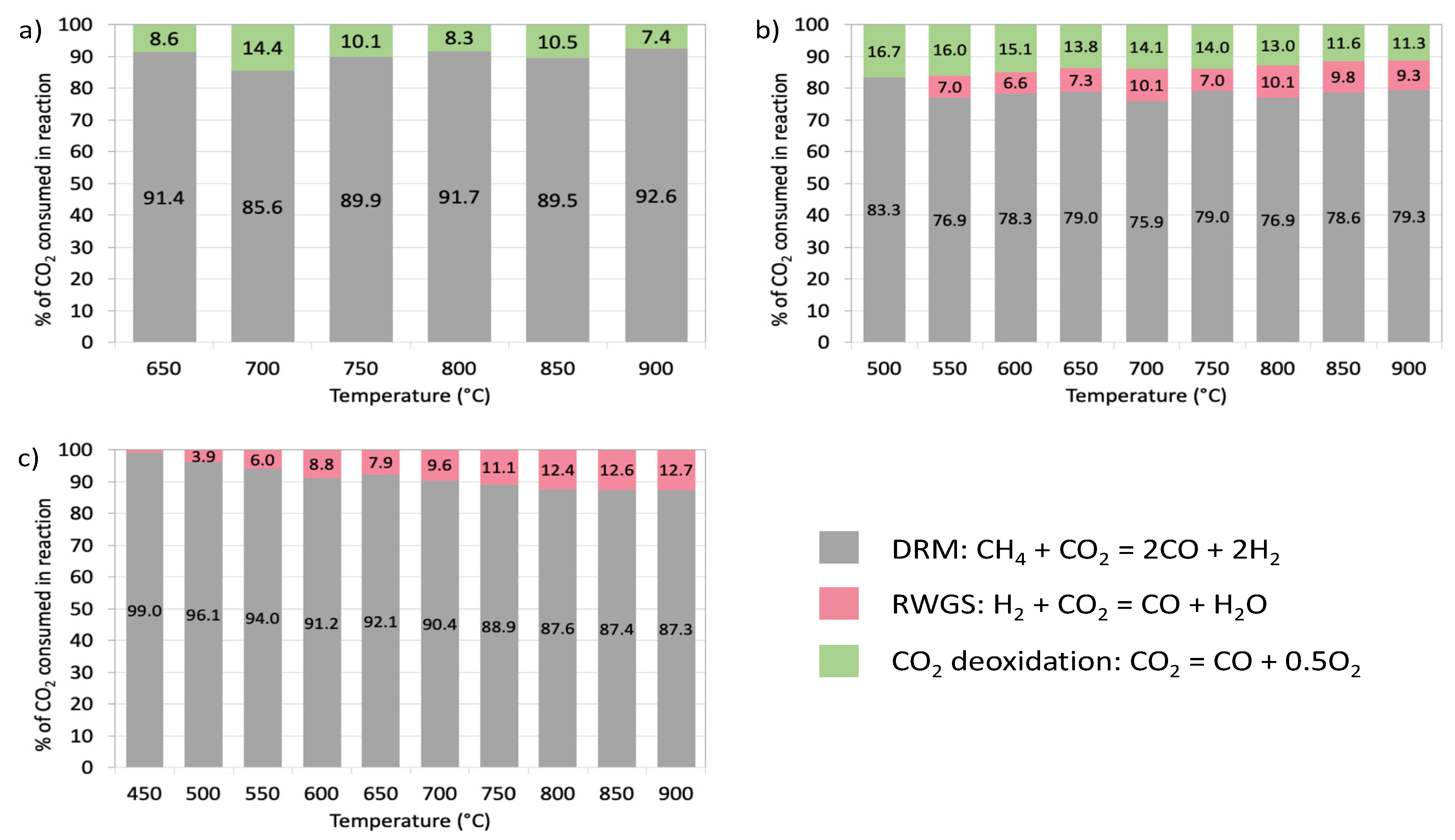
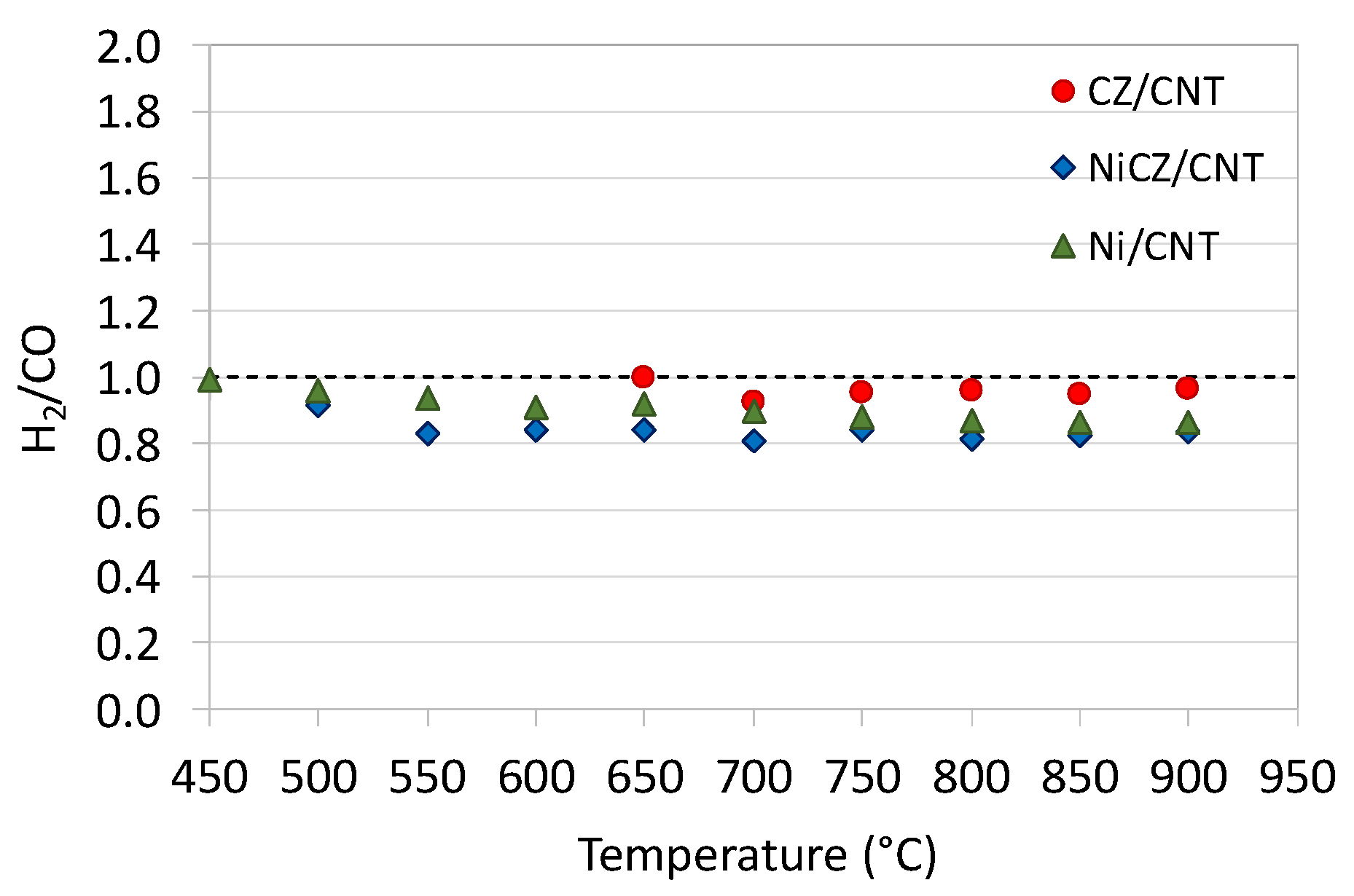
2.2. Catalytic Tests of DRM
3. Materials and Methods
3.1. Synthesis of the CNT-Supported Metal Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tuckett, R. Greenhouse Gases. In Encyclopedia of Analytical Sciences, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 362–372. [Google Scholar] [CrossRef]
- Castro Luna, A.E.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni–Al2O3 catalyst. Appl. Catal. A Gen. 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Hei, M.J.; Chen, H.B.; Yi, J.; Lin, Y.J.; Lin, Y.Z.; Wei, G.; Liao, D.W. CO2-reforming of methane on transition metal surfaces. Surf. Sci. 1998, 417, 82–96. [Google Scholar] [CrossRef]
- Cao, P.; Adegbite, S.; Wu, T. Thermodynamic equilibrium analysis of CO2 reforming of methane: Elimination of carbon deposition and adjustment of H2/CO ratio. Energy Procedia 2017, 105, 1864–1869. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Parvary, M.; Jazayeri, S.; Taeb, A.; Petit, C.; Kiennemann, A. Promotion of active nickel catalysts in methane dry reforming reaction by aluminum addition. Catal. Commun. 2001, 2, 357–362. [Google Scholar] [CrossRef]
- Schwab, G.M.; Koller, K. Combined Action of Metal and Semiconductor Catalysts. J. Am. Chem. Soc. 1968, 90, 3078–3080. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, W.H.; Doh, W.H.; Lee, S.W.; Noh, M.C.; Gallet, J.J.; Bournel, F.; Kondoh, H.; Mase, K.; Jung, Y.; et al. Adsorbate-driven reactive interfacial Pt-NiO1−x nanostructure formation on the Pt3Ni(111) alloy surface. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.J.; Tsai, M.C.; Su, W.N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.Y.; Hwang, B.J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Huang, Q.; Fang, X.; Cheng, Q.; Li, Q.; Xu, X.; Xu, L.; Liu, W.; Gao, Z.; Zhou, W.; Wang, X. Synthesis of a Highly Active and Stable Nickel-Embedded Alumina Catalyst for Methane Dry Reforming: On the Confinement Effects of Alumina Shells for Nickel Nanoparticles. ChemCatChem 2017, 9, 3563–3571. [Google Scholar] [CrossRef]
- Shin, S.A.; Noh, Y.S.; Hong, G.H.; Park, J.I.; Song, H.T.; Lee, K.Y.; Moon, D.J. Dry reforming of methane over Ni/ZrO2-Al2O3 catalysts: Effect of preparation methods. J. Taiwan Inst. Chem. Eng. 2018, 90, 25–32. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Sandupatla, A.; Deo, G.; Bal, R. Synthesis and catalytic activity of a Pd doped Ni–MgO catalyst for dry reforming of methane. J. Mater. Chem. A 2017, 5, 15688–15699. [Google Scholar] [CrossRef]
- Kroll, V.C.H.; Swaan, H.M.; Mirodatos, C. Methane Reforming Reaction with Carbon Dioxide over Ni/SiO2 Catalyst: I. Deactivation Studies. J. Catal. 1996, 161, 409–422. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Odedairo, T.; Chen, J.; Zhu, Z. Metal–support interface of a novel Ni–CeO2 catalyst for dry reforming of methane. Catal. Commun. 2013, 31, 25–31. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Vasiliades, M.A.; Djinović, P.; Pintar, A.; Kovač, J.; Efstathiou, A.M. The effect of CeO2–ZrO2 structural differences on the origin and reactivity of carbon formed during methane dry reforming over NiCo/CeO2–ZrO2 catalysts studied by transient techniques. Catal. Sci. Technol. 2017, 7, 5422–5434. [Google Scholar] [CrossRef]
- Nguyen, T.G.H.; Tran, D.L.; Sakamoto, M.; Uchida, T.; Sasaki, K.; To, T.D.; Doan, D.C.T.; Dang, M.C.; Shiratori, Y. Ni-loaded (Ce,Zr)O2-δ-dispersed paper-structured catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2018, 43, 4951–4960. [Google Scholar] [CrossRef]
- Luengnaruemitchai, A.; Kaengsilalai, A. Activity of different zeolite-supported Ni catalysts for methane reforming with carbon dioxide. Chem. Eng. J. 2008, 144, 96–102. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, D.; Wu, M.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Effect of catalytic site position: Nickel nanocatalyst selectively loaded inside or outside carbon nanotubes for methane dry reforming. Fuel 2013, 108, 430–438. [Google Scholar] [CrossRef]
- Figueira, C.E.; Moreira, P.F.; Giudici, R.; Alves, R.M.B.; Schmal, M. Nanoparticles of Ce, Sr, Co in and out the multi-walled carbon nanotubes applied for dry reforming of methane. Appl. Catal. A Gen. 2018, 550, 297–307. [Google Scholar] [CrossRef]
- Łamacz, A.; Matus, K.; Liszka, B.; Silvestre-Albero, J.; Lafjah, M.; Dintzer, T.; Janowska, I. The impact of synthesis method of CNT supported CeZrO2 and Ni-CeZrO2 on catalytic activity in WGS reaction. Catal. Today 2018, 301, 172–182. [Google Scholar] [CrossRef]
- Jardim, E.O.; Goncalves, M.; Rico-Francés, S.; Sepúlveda-Escribano, A.; Silvestre-Albero, J. Superior performance of multi-wall carbon nanotubes as support of Pt-based catalysts for the preferential CO oxidation: Effect of ceria addition. Appl. Cat. B Environ. 2012, 113, 72–78. [Google Scholar] [CrossRef]
- Tanaka, K.; Shou, M.; Zhang, H.; Yuan, Y.; Hagiwara, T.; Fukuoka, A.; Nakamura, J.; Lu, D. An Extremely Active Pt/Carbon Nano-Tube Catalyst for Selective Oxidation of CO in H2 at Room Temperature. Catal. Lett. 2008, 126, 89–95. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Donphai, W.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J. Effect of Ni-CNTs/mesocellular silica composite catalysts on carbon dioxide reforming of methane. Appl. Catal. A Gen. 2014, 475, 16–26. [Google Scholar] [CrossRef]
- Khavarian, M.; Chai, S.P.; Mohamed, A.R. The effects of process parameters on carbon dioxide reforming of methane over Co–Mo–MgO/MWCNTs nanocomposite catalysts. Fuel 2015, 158, 129–138. [Google Scholar] [CrossRef]
- Chein, R.Y.; Fung, W.Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrog. Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic Dry Reforming of Methane Over High Surface Area Ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Diaz, E.; de Rivas, B.; Lopez-Fonseca, R.; Ordóñez, S. Characterization of ceria–zirconia mixed oxides as catalysts for the combustion of volatile organic compounds using inverse gas chromatography. J. Chromatogr. A 2006, 116, 230–239. [Google Scholar] [CrossRef]
- Shah, P.R.; Kim, T.; Zhou, G.; Fornasiero, P.; Gorte, R.J. Evidence for Entropy Effects in the Reduction of Ceria−Zirconia Solutions. Chem. Mater. 2006, 18, 5363–5369. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Li, C.; Tan, P.J.; Li, X.D.; Du, Y.L.; Gao, Z.H.; Huang, W. Effect of the addition of Ce and Zr on the structure and performances of Ni-Mo/CeZr-MgAl(O) catalysts for CH4-CO2 reforming. Fuel Process. Technol. 2015, 140, 39–45. [Google Scholar] [CrossRef]
- Liu, Z.; Lustemberg, P.; Gutiérrez, R.A.; Carey, J.J.; Palomino, R.M.; Vorokhta, M.; Grinter, D.C.; Ramírez, P.J.; Matolín, V.; Nolan, M.; et al. In Situ Investigation of Methane Dry Reforming on Metal/Ceria(111) Surfaces: Metal-Support Interactions and C−H Bond Activation at Low Temperature. Angew. Chem. Int. Ed. 2017, 56, 13041–13046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.; Rui, N.; Li, X.; Lin, L.; Betancourt, L.E.; Su, D.; Xu, W.; Cen, J.; Attenkofer, K.; et al. Highly Active Ceria-Supported Ru Catalyst for the Dry Reforming of Methane: In Situ Identification of Ruδ+-Ce3+ Interactions for Enhanced Conversion. ACS Catal. 2019, 9, 3349–3359. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Bobin, A.S.; Pavlova, S.N.; Ishchenko, A.V.; Selivanova, A.V.; Kaichev, V.V.; Cherepanova, S.V.; Krieger, T.A.; Arapova, M.V.; Roger, A.C.; et al. Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids. Open Chem. 2017, 15, 412–425. [Google Scholar] [CrossRef]
- Simonov, M.N.; Rogov, V.A.; Smirnova, M.Y.; Sadykov, V.A. Pulse Microcalorimetry Study of Methane Dry Reforming Reaction on Ni/Ceria-Zirconia Catalyst. Catalysts 2017, 7, 268. [Google Scholar] [CrossRef] [Green Version]
- Mesrar, F.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Syngas production from dry reforming of methane over ni/perlite catalysts: Effect of zirconia and ceria impregnation. Int. J. Hydrog. Energy 2018, 43, 17142–17155. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High carbon-resistance Ni/CeAlO3-Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Eniviron. 2013, 136, 260–268. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno, U.R.; Zainal, Z. CeO2-SiO2 supported nickel catalysts for dry reforming of methane toward syngas production. Appl. Catal. A Gen. 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrog. Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J. Interactions of Ni Nanoparticles with Reducible CeO2(111) Thin Films. J. Phys. Chem. C 2012, 116, 9544–9549. [Google Scholar] [CrossRef]
- Zhou, Y.; Perket, J.M.; Crooks, A.B.; Zhou, J.J. Effect of Ceria Support on the Structure of Ni Nanoparticles. Phys. Chem. Lett. 2010, 1, 1447–1453. [Google Scholar] [CrossRef]
- Elizabeth, I.; Nair, A.K.; Singh, B.P.; Gopukumar, S. Multifunctional Ni-NiO-CNT Composite as High Performing Free Standing Anode for Li Ion Batteries and Advanced Electro Catalyst for Oxygen Evolution Reaction. Electrochim. Acta 2017, 230, 98–105. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.; Guan, M.; Lin, M.C.; Zhang, B.; Hu, Y.; Wang, D.Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Wang, J.; Teschner, D.; Yao, Y.; Huang, X.; Willinger, M.; Shao, L.; Schlögl, R. Fabrication of nanoscale NiO/Ni heterostructures as electrocatalysts for efficient methanol oxidation. J. Mater. Chem. 2017, 5, 9946–9951. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hui, W.; Ajay, K.D. Kinetic studies of carbon dioxide reforming of methane over Ni−Co/Al−Mg−O bimetallic catalyst. Ind. Eng. Chem. Res. 2008, 48, 677–684. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Mechanism and Site Requirements for Activation and Chemical Conversion of Methane on Supported Pt Clusters and Turnover Rate Comparisons among Noble Metals. J. Phys. Chem. B 2004, 108, 4094–4103. [Google Scholar] [CrossRef]
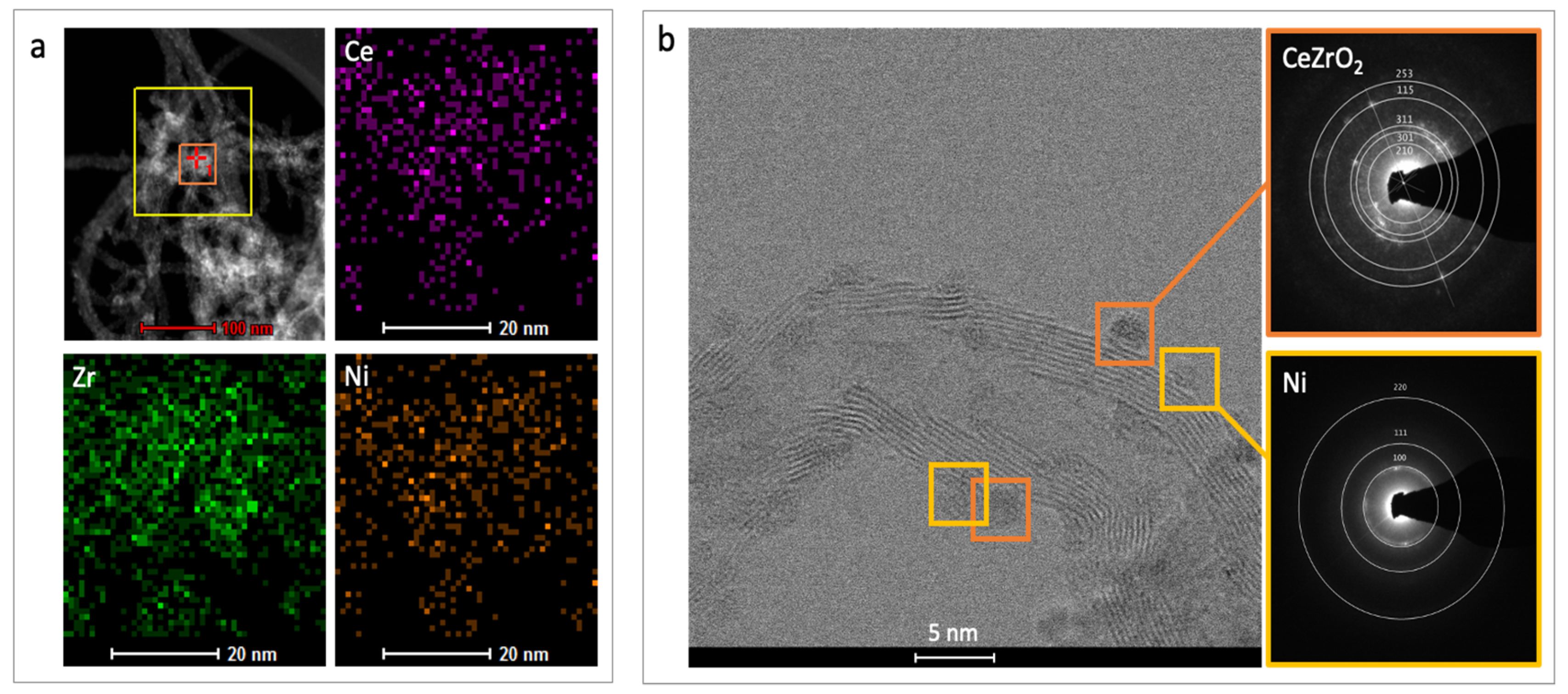
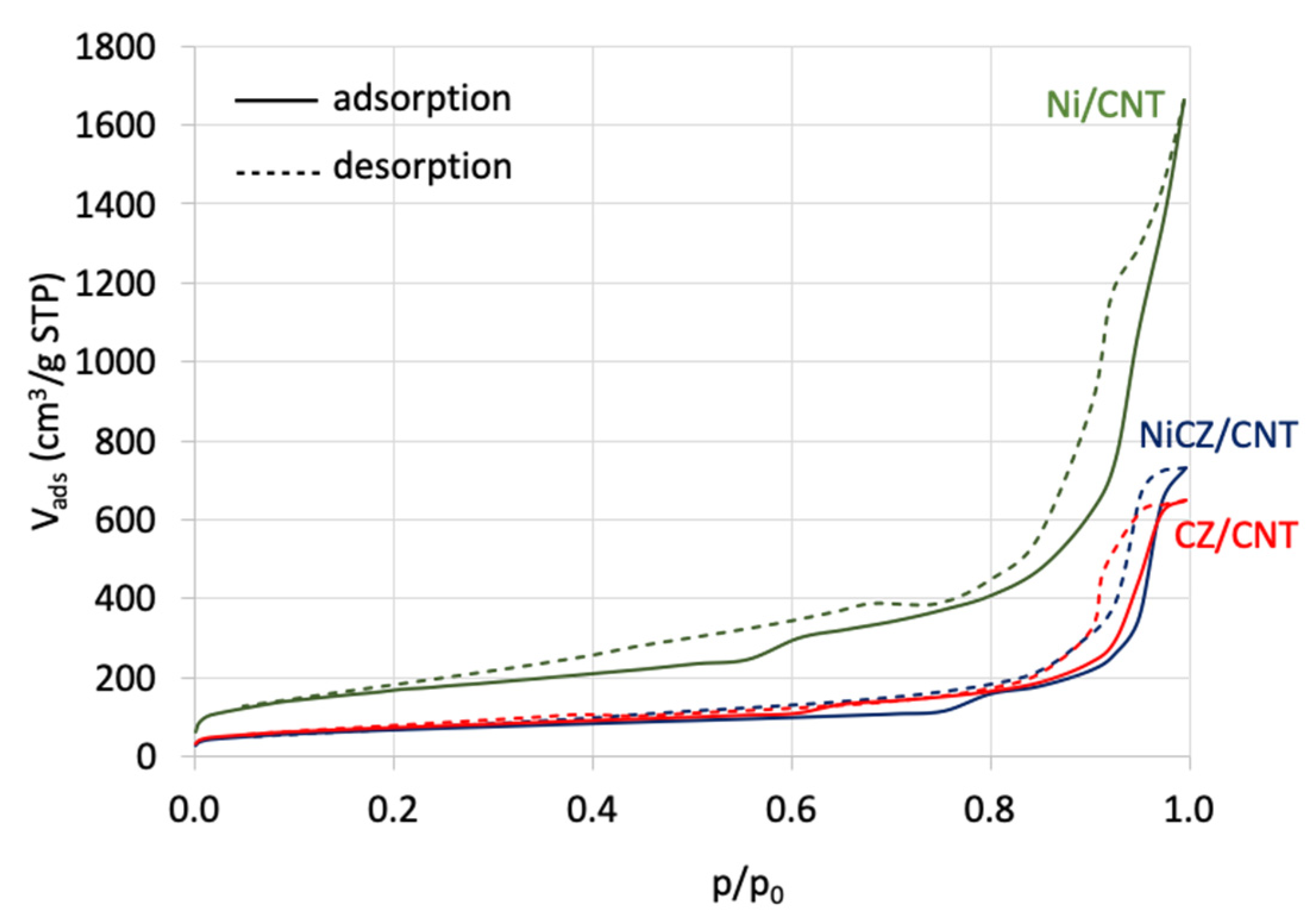


| Sample | SBET (m2/g) | Vt (cm3/g) | Vmicro (cm3/g) | Vmezo (cm3/g) | Vmacro (cm3/g) | D (nm) |
|---|---|---|---|---|---|---|
| Ni/CNT | 585 | 2.55 | 0.04 | 2.18 | 0.33 | 17.48 |
| CeZrO2/CNT | 255 | 1.00 | 0.02 | 0.97 | 0.01 | 15.70 |
| NiCeZrO2/CNT | 235 | 1.12 | 0.02 | 1.1 | 0.00 | 19.09 |
| T (°C) | Non-DRM CO2 Conversion (%) | ||
|---|---|---|---|
| CZ/CNT | NiCZ/CNT | Ni/CNT | |
| 450 | - | - | 1.0 |
| 500 | - | 20.1 | 4.0 |
| 550 | - | 30.0 | 6.3 |
| 600 | - | 27.7 | 9.7 |
| 650 | 9.4 | 26.6 | 8.6 |
| 700 | 16.8 | 31.8 | 10.6 |
| 750 | 11.2 | 26.6 | 12.5 |
| 800 | 9.0 | 30.1 | 14.2 |
| 850 | 11.7 | 27.3 | 14.5 |
| 900 | 8.0 | 26.0 | 14.5 |
| D (nm) | D (nm) | ||||
|---|---|---|---|---|---|
| Fresh Sample | Ni (111) | CeZrO2 (220) | Spent Sample | Ni (111) | CeZrO2 (220) |
| CZ/CNT | - | 9.94 | CZ/CNT_s | - | 5.03 |
| NiCZ/CNT | 6.90 | 9.88 | NiCZ/CNT_s | 9.13 | 10.40 |
| Ni/CNT | 14.13 | - | Ni/CNT_s | 16.76 | - |
| Catalyst | At% | |||
|---|---|---|---|---|
| C | Ni | Ce | Zr | |
| Ni/CNT | 97.8 | 2.2 | - | - |
| CZ/CNT | 97.2 | - | 1.9 | 0.9 |
| NiCZ/CNT | 94.6 | 2.5 | 1.9 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łamacz, A.; Jagódka, P.; Stawowy, M.; Matus, K. Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts. Catalysts 2020, 10, 741. https://doi.org/10.3390/catal10070741
Łamacz A, Jagódka P, Stawowy M, Matus K. Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts. Catalysts. 2020; 10(7):741. https://doi.org/10.3390/catal10070741
Chicago/Turabian StyleŁamacz, Agata, Paulina Jagódka, Michalina Stawowy, and Krzysztof Matus. 2020. "Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts" Catalysts 10, no. 7: 741. https://doi.org/10.3390/catal10070741
APA StyleŁamacz, A., Jagódka, P., Stawowy, M., & Matus, K. (2020). Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts. Catalysts, 10(7), 741. https://doi.org/10.3390/catal10070741







