Composite Catalyst of Palm Mill Fly Ash-Supported Calcium Oxide Obtained from Eggshells for Transesterification of Off-Grade Palm Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Off-Grade Palm Oil Characteristics
2.2. Catalyst Characterization
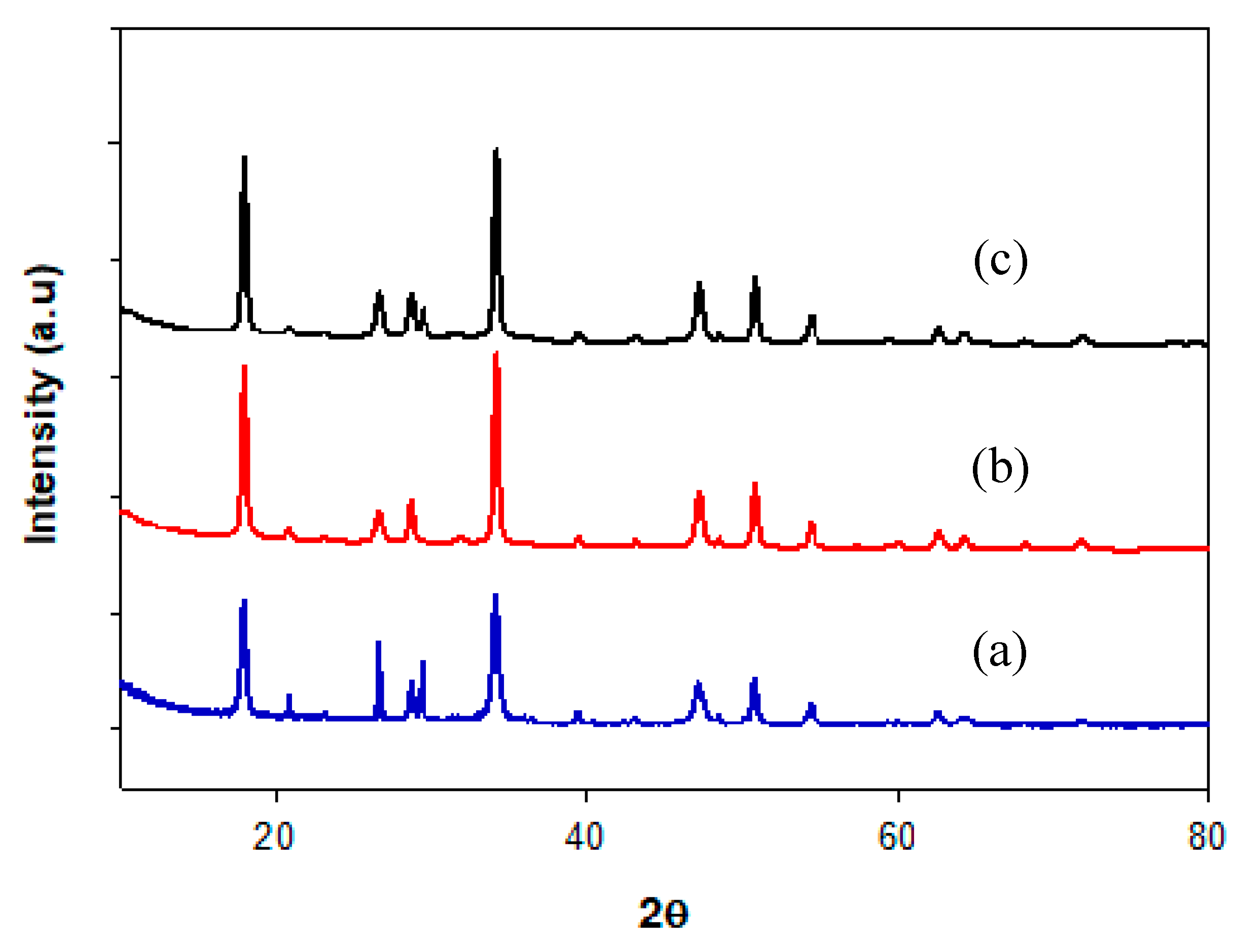
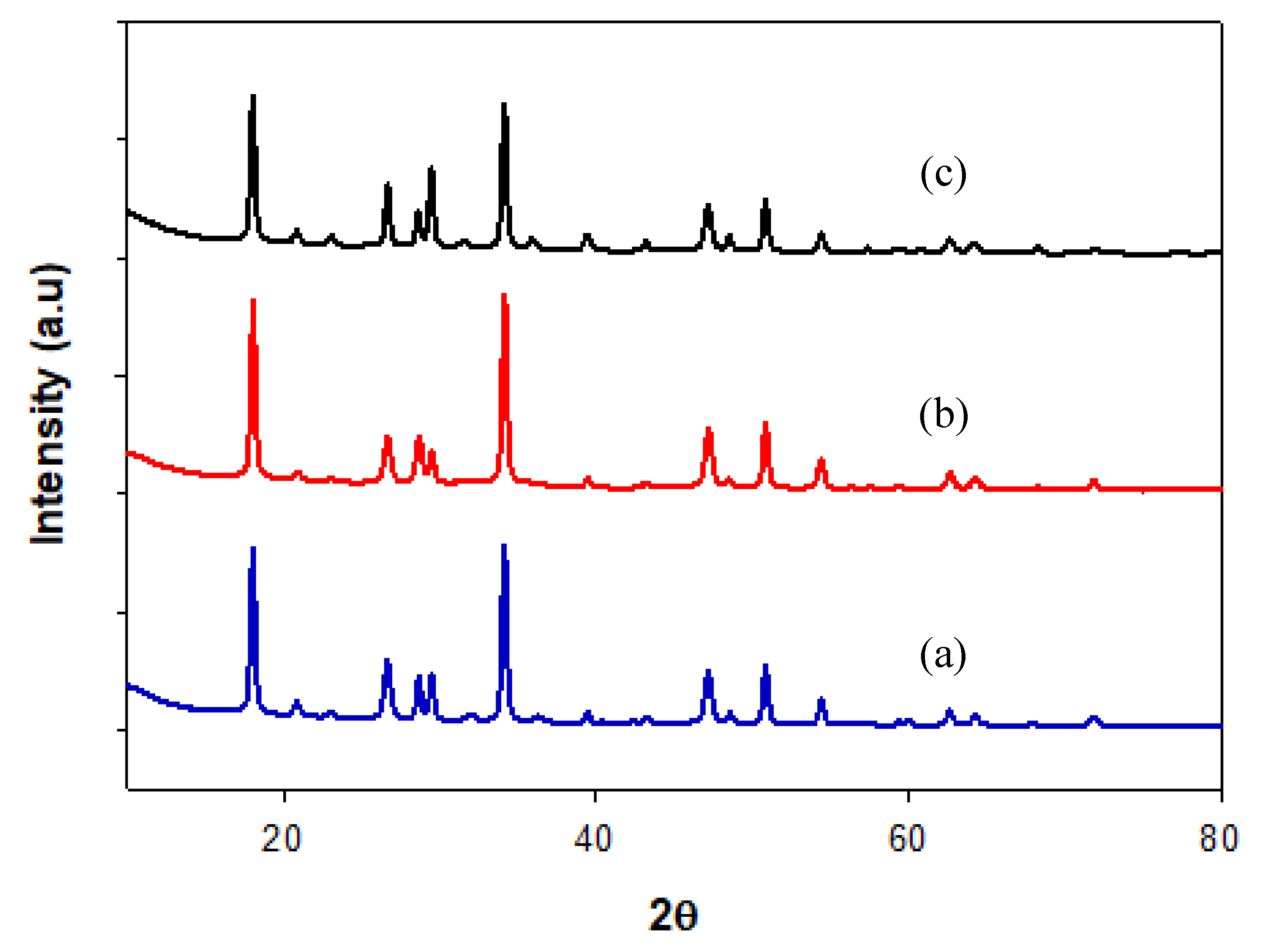
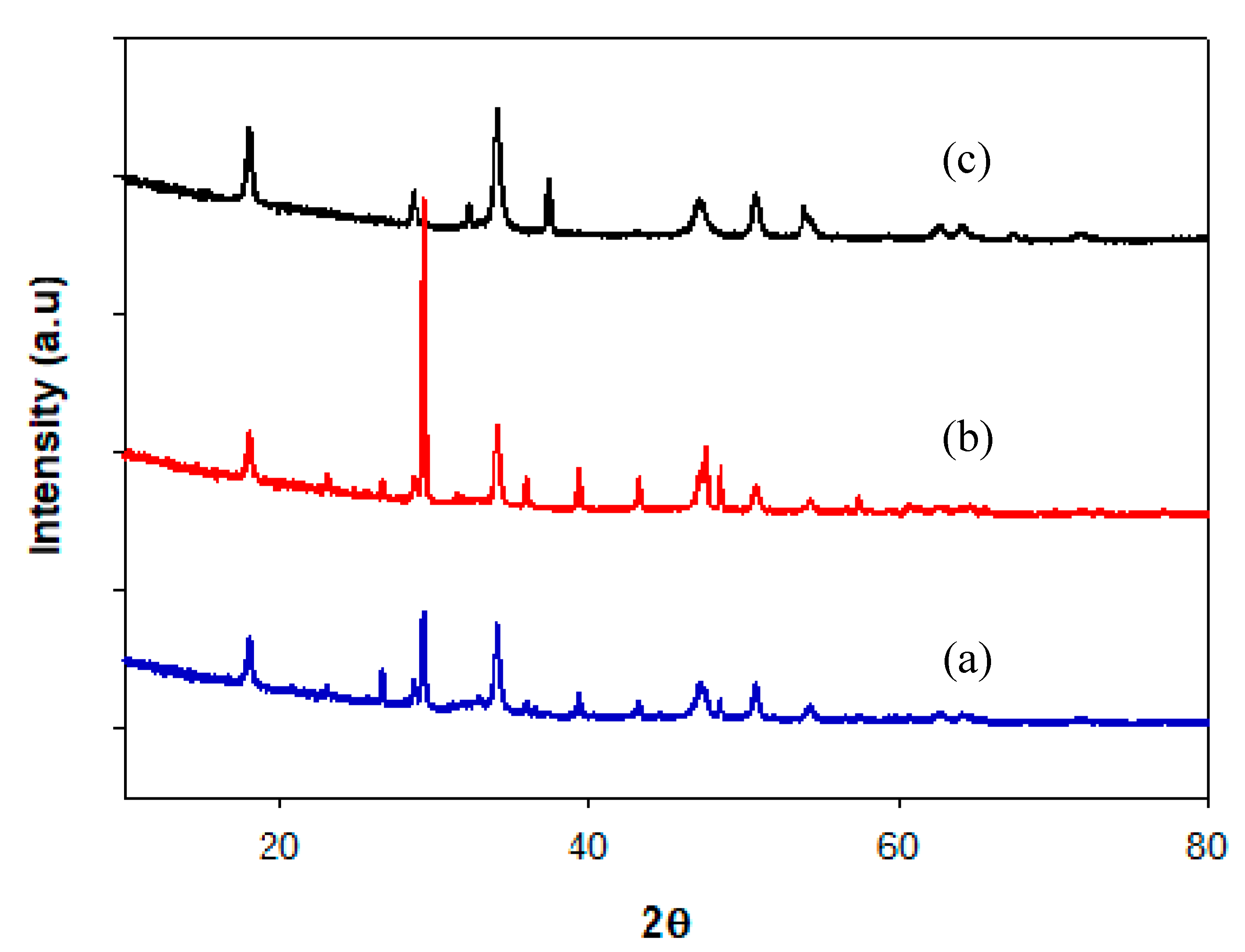
2.2.1. X-ray Diffraction
2.2.2. Brunauer–Emmett–Teller (BET) and Basic Strength
2.2.3. Hammett Indicator Titration
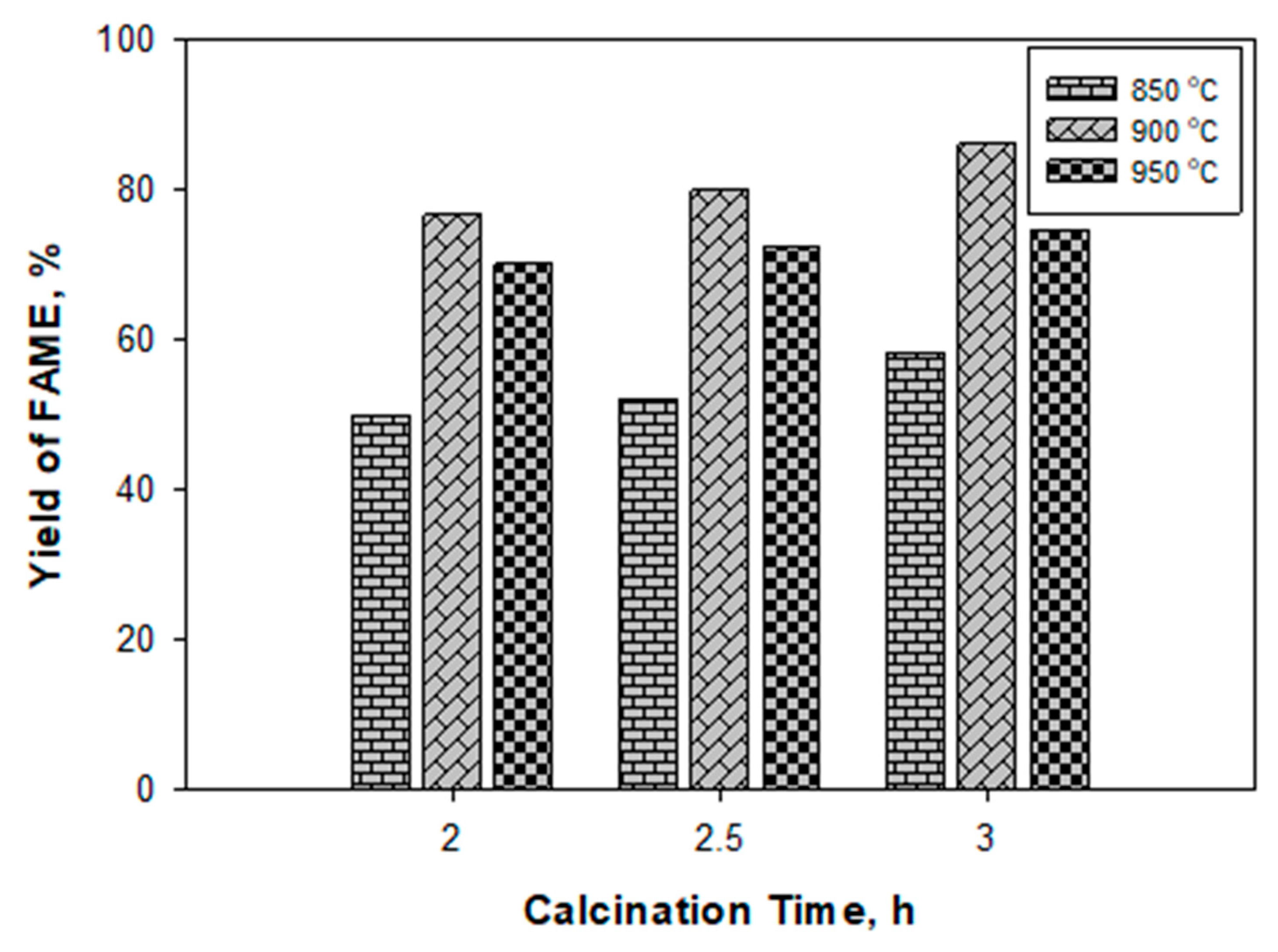
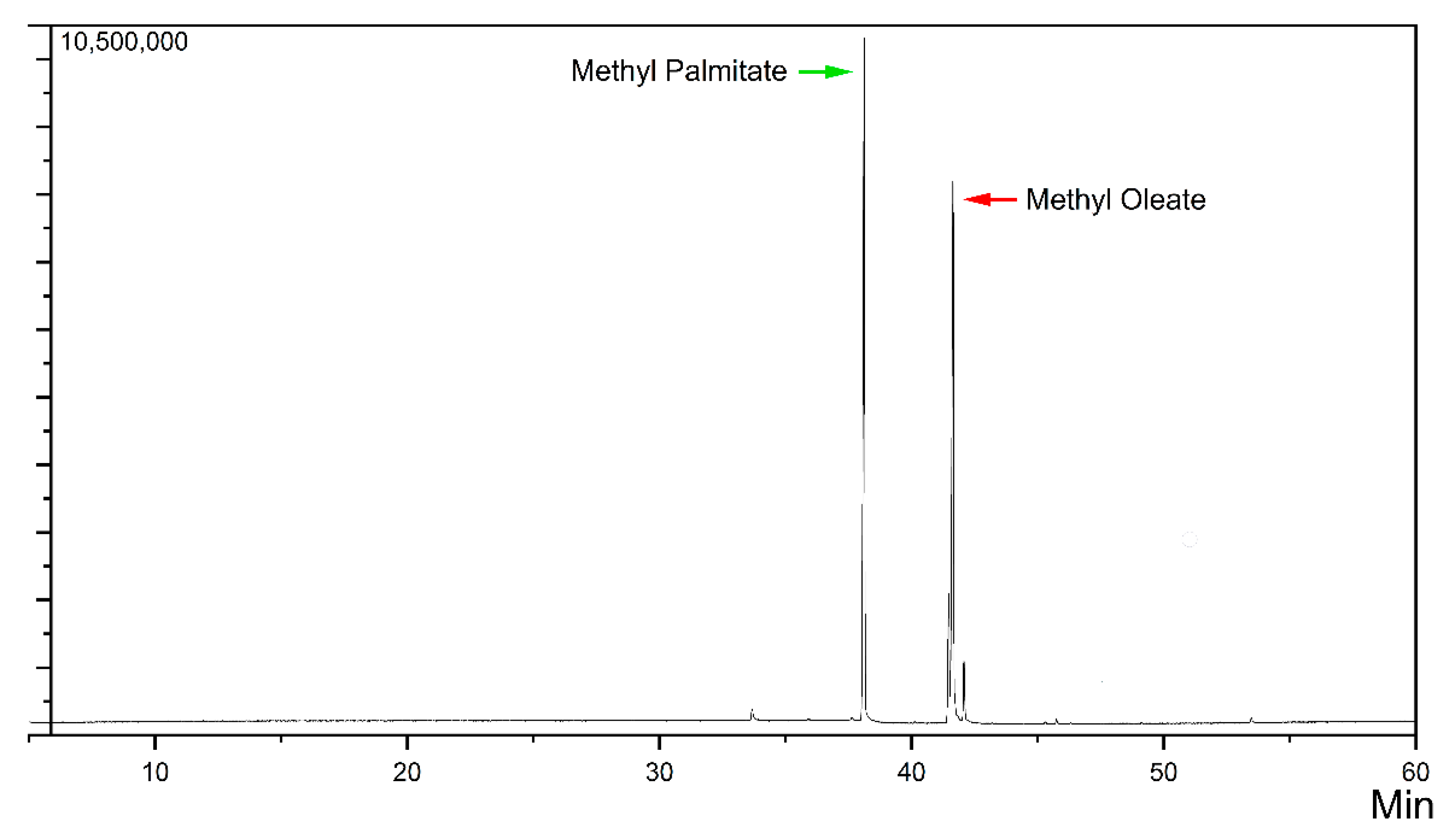
2.3. Yield and Biodiesel Characterization
2.4. Characteristics of the Biodiesel
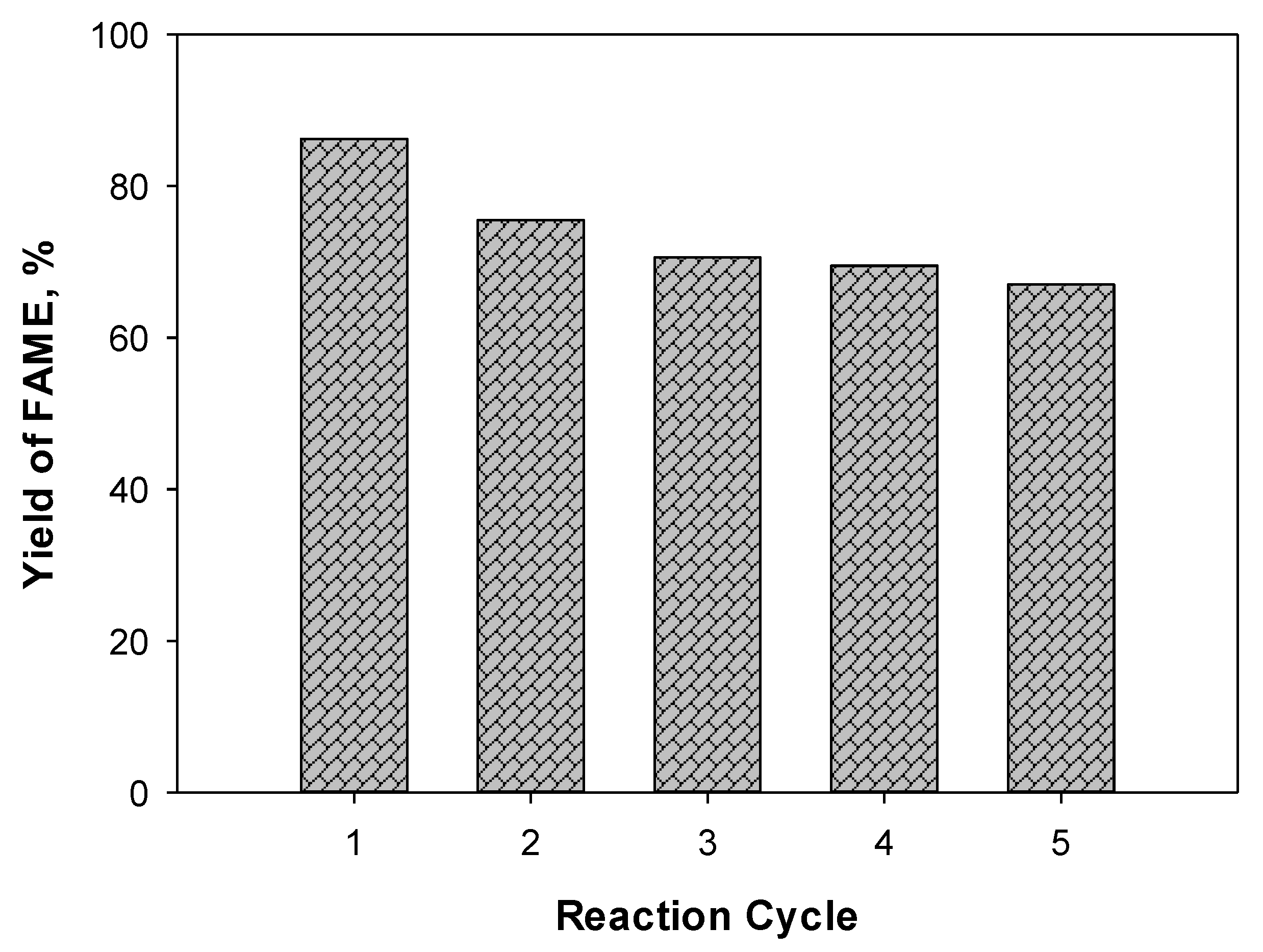
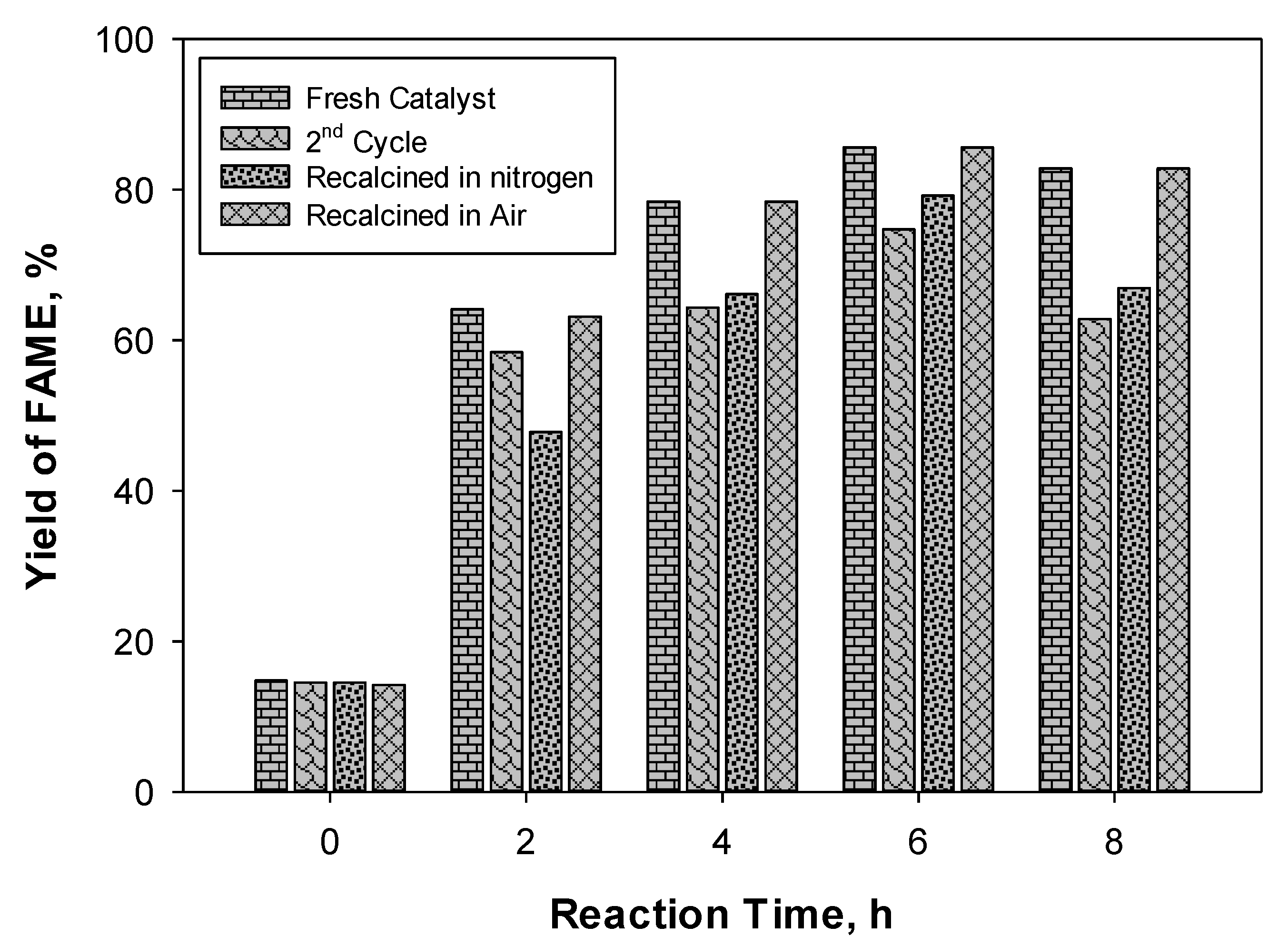
2.5. The Catalyst’s Reusability, Stability, and Regeneration
3. Experimental Procedure
3.1. Materials
3.2. Preparation of the CaO/PMFA Catalyst
3.3. Characterization
3.4. Study of the Catalytic Performance on the Transesterification Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leung, D.Y.C.; Yang, Y. Wind energy development and its environmental impact: A review. Renew. Sustain. Energy Rev. 2012, 16, 1031–1039. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Subhan; Lala, A.; Saiful, J.; Suhendra, R.; Idroes, G.M.; Marwan, M. Geochemistry of hot springs in the Ie Seu’um hydrothermal areas at Aceh Besar district, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012002. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Subhan; Lala, A.; Muhammad; Suhendra, R.; Idroes, G.M.; Marwan, M. Geochemistry of Sulphate spring in the Ie Jue geothermal areas at Aceh Besar district, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012012. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Subhan; Lala, A.; Muslem; Suhendra, R.; Idroes, G.M.; Suhendrayatna; Marwan; et al. Geochemistry of warm springs in the Ie Brôuk hydrothermal areas at Aceh Besar district. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012010. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Saiful, S.; Alatas, M.; Subhan, S.; Lala, A.; Muslem, M.; Suhendra, R.; Idroes, G.M.; Marwan, M.; et al. Geochemistry Exploration and Geothermometry Application in the North Zone of Seulawah Agam, Aceh Besar District, Indonesia. Energies 2019, 12, 4442. [Google Scholar] [CrossRef]
- Marwan, M.; Yanis, M.; Idroes, R.; Ismail, N. 2D inversion and static shift of MT and TEM data for imaging the geothermal resources of Seulawah Agam Volcano, Indonesia. Int. J. GEOMATE 2019, 17, 173–180. [Google Scholar] [CrossRef]
- Marwan, M.; Syukri, M.; Idroes, R.; Ismail, N. Deep and Shallow Structures of Geothermal Seulawah Agam Based on Electromagnetic and Magnetic Data. Int. J. GEOMATE 2019, 16, 141–147. [Google Scholar] [CrossRef]
- Putri, D.R.; Nanda, M.; Rizal, S.; Idroes, R.; Ismail, N. Interpretation of gravity satellite data to delineate structural features connected to geothermal resources at Bur Ni Geureudong geothermal field. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012003. [Google Scholar] [CrossRef]
- Joselin Herbert, G.M.; Unni Krishnan, A. Quantifying environmental performance of biomass energy. Renew. Sustain. Energy Rev. 2016, 59, 292–308. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Yüksel, I. Hydropower for sustainable water and energy development. Renew. Sustain. Energy Rev. 2010, 14, 462–469. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Earlia, N.; Muslem, M.; Suhendra, R.; Amin, M.; Prakoeswa, C.R.S.; Khairan; Idroes, R. GC/MS Analysis of Fatty Acids on Pliek U Oil and Its Pharmacological Study by Molecular Docking to Filaggrin as a Drug Candidate in Atopic Dermatitis Treatment. Sci. World J. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Earlia, N.; Rahmad, R.; Amin, M.; Prakoeswa, C.; Khairan, K.; Idroes, R. The potential effect of fatty acids from Pliek U on epidermal fatty acid binding protein: Chromatography and bioinformatic studies. Sains Malays. 2019, 48, 1019–1024. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Md Sani, N.D.; Safitri, E.; Yook Heng, L.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, U.; Setyowati, M.; Edwarsyah; Efendi, R.; Safitri, E.; Idroes, R.; Heng, L.Y.; Sani, N.D. Isolation of Pectin from coffee pulp Arabica Gayo for the development of matrices membrane. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 12014. [Google Scholar] [CrossRef]
- Hasanah, U.; Sani, N.D.M.; Heng, L.Y.; Idroes, R.; Safitri, E. Construction of a Hydrogel Pectin-Based Triglyceride Optical Biosensor with Immobilized Lipase Enzymes. Biosensors 2019, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Berawi, M.A.; Heryanto, R.; Rizalie, A. Waste to energy technology: The potential of sustainable biogas production from animal waste in Indonesia. Renew. Sustain. Energy Rev. 2019, 105, 323–331. [Google Scholar] [CrossRef]
- Muslem, M.; Kuncaka, A.; Himah, T.N.; Roto, R. Preparation of Char-Fe3O4 Composites from Polyvinyl Chloride with Hydrothermal and Hydrothermal-Pyrolysis Carbonization Methods as Co(II) Adsorbents. Indones. J. Chem. 2019, 19, 835. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Hedwig, R.; Lahna, K.; Idroes, R.; Karnadi, I.; Tanra, I.; Iqbal, J.; Kwaria, D.; Kurniawan, D.P.; Kurniawan, K.H.; Tjia, M.O.; et al. Food analysis employing high energy nanosecond laser and low pressure He ambient gas. Microchem. J. 2019, 147, 356–364. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Kim, J.; Fernando, W.J.N. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Wang, Z.-M.; Lee, J.-P.; Park, S.-C.; Lee, J.-S. Production of biodiesel from soapstock using an ion-exchange resin catalyst. Korean J. Chem. Eng. 2008, 25, 1350–1354. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel fuel production with solid superacid catalysis in fixed bed reactor under atmospheric pressure. Catal. Commun. 2004, 5, 721–723. [Google Scholar] [CrossRef]
- Peng, B.-X.; Shu, Q.; Wang, J.-F.; Wang, G.-R.; Wang, D.-Z.; Han, M.-H. Biodiesel production from waste oil feedstocks by solid acid catalysis. Process. Saf. Environ. Prot. 2008, 86, 441–447. [Google Scholar] [CrossRef]
- Abreu, F.R.; Alves, M.B.; Macêdo, C.C.S.; Zara, L.F.; Suarez, P.A.Z. New multi-phase catalytic systems based on tin compounds active for vegetable oil transesterificaton reaction. J. Mol. Catal. A Chem. 2005, 227, 263–267. [Google Scholar] [CrossRef]
- Gryglewicz, S. Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour. Technol. 1999, 70, 249–253. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Development of heterogeneous base catalysts for biodiesel production. Bioresour. Technol. 2008, 99, 3439–3443. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, S.; Wu, H.; Yang, J. Highly efficient procedure for the synthesis of biodiesel from soybean oil. Fuel Process. Technol. 2009, 90, 701–704. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Optimization of the activity of CaO/Al2O3 catalyst for biodiesel production using response surface methodology. Appl. Catal. A Gen. 2009, 366, 154–159. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef]
- Helwani, Z.; Ramli, M.; Saputra, E.; Bahruddin, B.; Yolanda, D.; Fatra, W.; Idroes, G.M.; Muslem, M.; Mahlia, T.M.I.; Idroes, R. Impregnation of CaO from Eggshell Waste with Magnetite as a Solid Catalyst (Fe3O4/CaO) for Transesterification of Palm Oil Off-Grade. Catalysts 2020, 10, 164. [Google Scholar] [CrossRef]
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Biodiesel production via transesterification of palm olein using waste mud crab (Scylla serrata) shell as a heterogeneous catalyst. Bioresour. Technol. 2009, 100, 6362–6368. [Google Scholar] [CrossRef]
- Viriya-empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef] [PubMed]
- Umdu, E.S. Methyl Ester Production from Vegetable Oils on Heterogeneous basic Catalysts. Master’s Thesis, Graduate School of Engineering and Sciences of İzmir Institute of Technology, Izmir, Turkey, July 2008. [Google Scholar]
- Albuquerque, M.C.G.; Jiménez-Urbistondo, I.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; Maireles-Torres, P. CaO supported on mesoporous silicas as basic catalysts for transesterification reactions. Appl. Catal. A Gen. 2008, 334, 35–43. [Google Scholar] [CrossRef]
- Benjapornkulaphong, S.; Ngamcharussrivichai, C.; Bunyakiat, K. Al2O3-supported alkali and alkali earth metal oxides for transesterification of palm kernel oil and coconut oil. Chem. Eng. J. 2009, 145, 468–474. [Google Scholar] [CrossRef]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Kinetics of batch alkylation of phenol with tert-butyl alcohol over a catalyst synthesized from coal fly ash. Chem. Eng. J. 2005, 112, 109–115. [Google Scholar] [CrossRef]
- Jain, D.; Khatri, C.; Rani, A. Fly ash supported calcium oxide as recyclable solid base catalyst for Knoevenagel condensation reaction. Fuel Process. Technol. 2010, 91, 1015–1021. [Google Scholar] [CrossRef]
- Arifin, J.K. Pemanfaatan Buah Sawit Sisa Sortiran Sebagai Sumber Bahan Baku Asam Lemak. Master’s Thesis, Universitas Sumatera Utara, Medan, Indonesian, 2009. [Google Scholar]
- Helwani, Z.; Fatra, W.; Saputra, E.; Maulana, R. Preparation of CaO/Fly ash as a catalyst inhibitor for transesterification process off palm oil in biodiesel production. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012077. [Google Scholar] [CrossRef]
- Subramani, V.; Gangwal, S.K. A Review of Recent Literature to Search for an Efficient Catalytic Process for the Conversion of Syngas to Ethanol. Energy Fuels 2008, 22, 814–839. [Google Scholar] [CrossRef]
- O’Brian, R.D. Fats and Oils Formulations. In Fats and Oils; CRC Press: Boca Raton, FL, USA, 2004; Chapter 4. [Google Scholar]
- Barnwal, B.K.; Sharma, M.P. Prospects of biodiesel production from vegetable oils in India. Renew. Sustain. Energy Rev. 2005, 9, 363–378. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I. Effect of calcination temperatures of CaO/Nb2O5 mixed oxides catalysts on biodiesel production. Sains Malays. 2014, 43, 783–790. [Google Scholar]
- Helwani, Z.; Aziz, N.; Kim, J.; Othman, M.R. Improving the yield of Jatropha curcas’s FAME through sol–gel derived meso-porous hydrotalcites. Renew. Energy 2016, 86, 68–74. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S.; Tan, S.H. Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energy Convers. Manag. 2014, 88, 1167–1178. [Google Scholar] [CrossRef]
- Mutreja, V.; Singh, S.; Ali, A. Potassium impregnated nanocrystalline mixed oxides of La and Mg as heterogeneous catalysts for transesterification. Renew. Energy 2014, 62, 226–233. [Google Scholar] [CrossRef]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I.; Tee, H.S. Biodiesel production via transesterification of palm oil by using CaO–CeO 2 mixed oxide catalysts. Fuel 2015, 162, 288–293. [Google Scholar] [CrossRef]
- Setiawan, A.; Widiyastuti, W.; Winardi, S.; Nugroho, A. kinetika reaksi sinthesis biomaterial hidroxyapatite dengan jenis prekursor nitrat dan asetat. Reaktor 2014, 15, 104. [Google Scholar] [CrossRef][Green Version]
- Soleimani, M.A.; Naghizadeh, R.; Mirhabibi, A.R.; Golestanifard, F. Effect of calcination temperature of the kaolin and molar Na2O/SiO2 activator ratio on physical and microstructural properties of metakaolin based geopolymers. IUST 2012, 9, 43–51. [Google Scholar]
- Kumar, D.; Ali, A. Nanocrystalline K–CaO for the transesterification of a variety of feedstocks: Structure, kinetics and catalytic properties. Biomass Bioenergy 2012, 46, 459–468. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Improving transesterification acitvity of CaO with hydration technique. Bioresour. Technol. 2010, 101, 3784–3786. [Google Scholar] [CrossRef] [PubMed]
- Kesica, Z.; Lukic, I.; Zdujic, M.; Liu, H.; Skala, D. Mechanochemically Synthesized CaO ZnO Catalyst For Biodiesel Production. Procedia Eng. 2012, 42, 1169–1178. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Development and characterisation of novel heterogeneous palm oil mill boiler ash-based catalysts for biodiesel production. Bioresour. Technol. 2012, 125, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Niju, S.; Meera, K.M.; Begum, S.; Anantharaman, N. Modification of egg shell and its application in biodiesel production. J. Saudi Chem. Soc. 2014, 18, 702–706. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Samik, E.R.; Prasetyoko, D. Review: Pengaruh Kebasaan dan Luas Permukaan Katalis Terhadap Aktivitas Katalis Basa Heterogen untuk Produksi Biodiesel. Jur. Kim. Fak. MIPA Inst. Teknol. Sepuluh Nop. 2014. [Google Scholar]
- Liu, C.; Lv, P.; Yuan, Z.; Yan, F.; Luo, W. The nanometer magnetic solid base catalyst for production of biodiesel. Renew. Energy 2010, 35, 1531–1536. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Zhang, Y.; Li, S.; Tian, S.; Wang, B. Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process. Technol. 2012, 95, 84–89. [Google Scholar] [CrossRef]

| No. | Parameter | Unit | Palm Oil Off-Grade | Palm Oil [51] |
|---|---|---|---|---|
| 1 | Density (40°) | kg/m3 | 892 | 920 |
| 2 | Viscosity (40°) | mm2/s | 29.50 | 31.45 |
| 3 | Water content | wt.% | 3.7 | 0.8 |
| 4 | FFA | mgKOHg−1 | 6.9 | 0.2 |
| 5 | Color | - | Orange | Yellow |
| Calcination Temperature (°C) | Calcination Time (h) | Surface Area (m2/g) | Basic Strength (H_) |
|---|---|---|---|
| 850–600 | 3 | 4.55 | 9.8 < H_ < 12.2 |
| 900 | 3 | 9.31 | 12.2 < H_ < 15.0 |
| 900–600 | 3 | 30.24 | 15.0 < H_ < 18.4 |
| 950–600 | 3 | 14.55 | 9.8 < H_ < 12.2 |
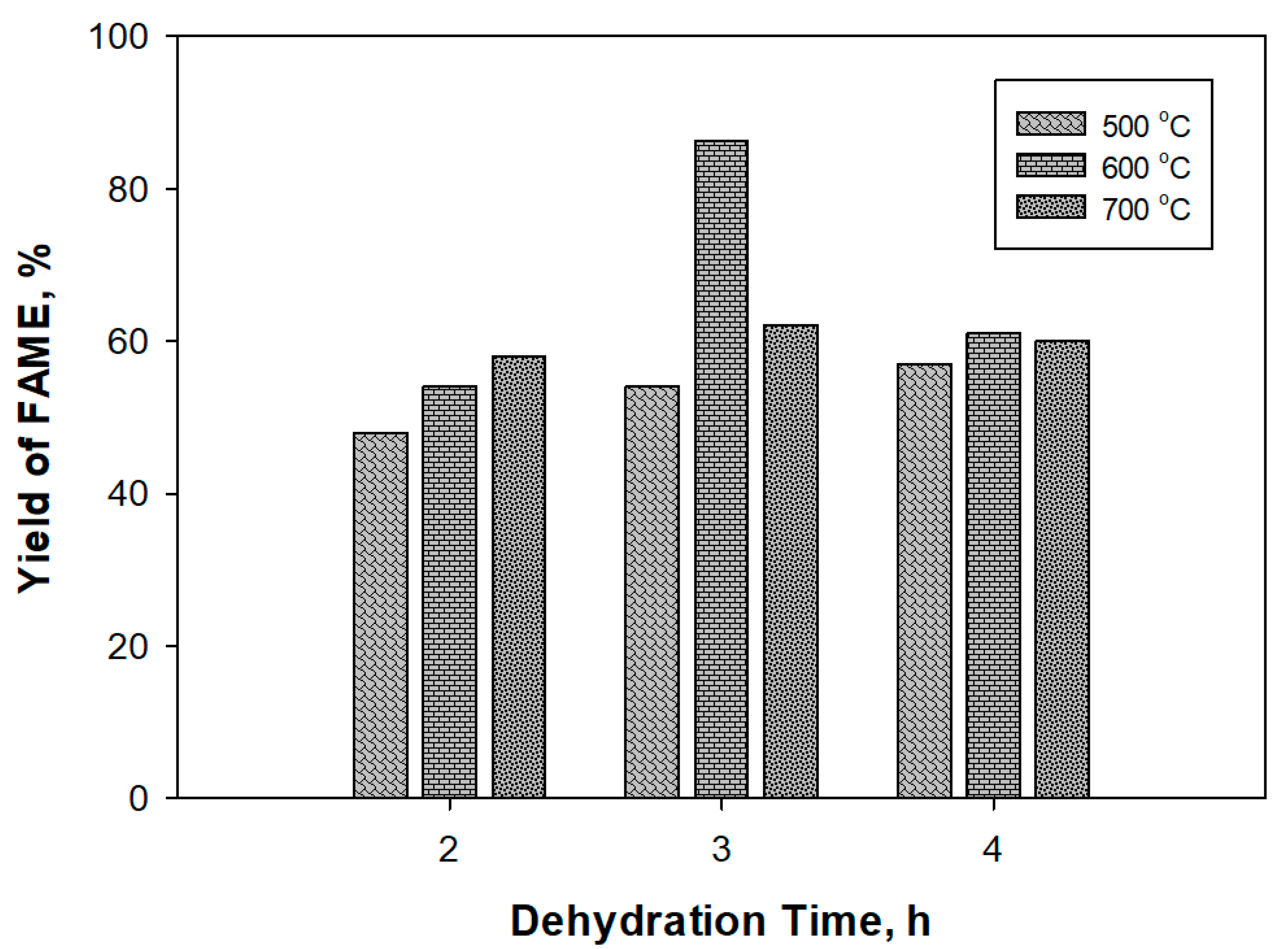
| Dehydration Temperature (°C) | Dehydration Time (h) | Surface Area (m2/g) | Basic Strength (H_) |
|---|---|---|---|
| 500 | 3 | 17.55 | 9.8 < H_ < 12.2 |
| 600 | 3 | 30.24 | 15.0 < H_ < 18.4 |
| 700 | 4 | 18.39 | 9.8 < H_ < 12.2 |
| No | Characteristics | Unit | Result | SNI 7182:2015 |
|---|---|---|---|---|
| 1 | Density | kg/m3 | 876.0 | 850–890 |
| 2 | Kinematic viscosity | mm2/s | 3.85 | 2.3–6.0 |
| 3 | Flash point | °C | 135 | Min. 100 |
| 4 5 | Acid number Cetane number | mg-KOH/g | 0.344 55 | Max. 0.5 51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helwani, Z.; Ramli, M.; Saputra, E.; Putra, Y.L.; Simbolon, D.F.; Othman, M.R.; Idroes, R. Composite Catalyst of Palm Mill Fly Ash-Supported Calcium Oxide Obtained from Eggshells for Transesterification of Off-Grade Palm Oil. Catalysts 2020, 10, 724. https://doi.org/10.3390/catal10070724
Helwani Z, Ramli M, Saputra E, Putra YL, Simbolon DF, Othman MR, Idroes R. Composite Catalyst of Palm Mill Fly Ash-Supported Calcium Oxide Obtained from Eggshells for Transesterification of Off-Grade Palm Oil. Catalysts. 2020; 10(7):724. https://doi.org/10.3390/catal10070724
Chicago/Turabian StyleHelwani, Zuchra, Muliadi Ramli, Edy Saputra, Yogi Lesmana Putra, Desly Fadila Simbolon, Mohd Roslee Othman, and Rinaldi Idroes. 2020. "Composite Catalyst of Palm Mill Fly Ash-Supported Calcium Oxide Obtained from Eggshells for Transesterification of Off-Grade Palm Oil" Catalysts 10, no. 7: 724. https://doi.org/10.3390/catal10070724
APA StyleHelwani, Z., Ramli, M., Saputra, E., Putra, Y. L., Simbolon, D. F., Othman, M. R., & Idroes, R. (2020). Composite Catalyst of Palm Mill Fly Ash-Supported Calcium Oxide Obtained from Eggshells for Transesterification of Off-Grade Palm Oil. Catalysts, 10(7), 724. https://doi.org/10.3390/catal10070724







