Continuous Production of 2-Phenylethyl Acetate in a Solvent-Free System Using a Packed-Bed Reactor with Novozym® 435
Abstract
1. Introduction
2. Results and Discussion
2.1. Prime Experiment
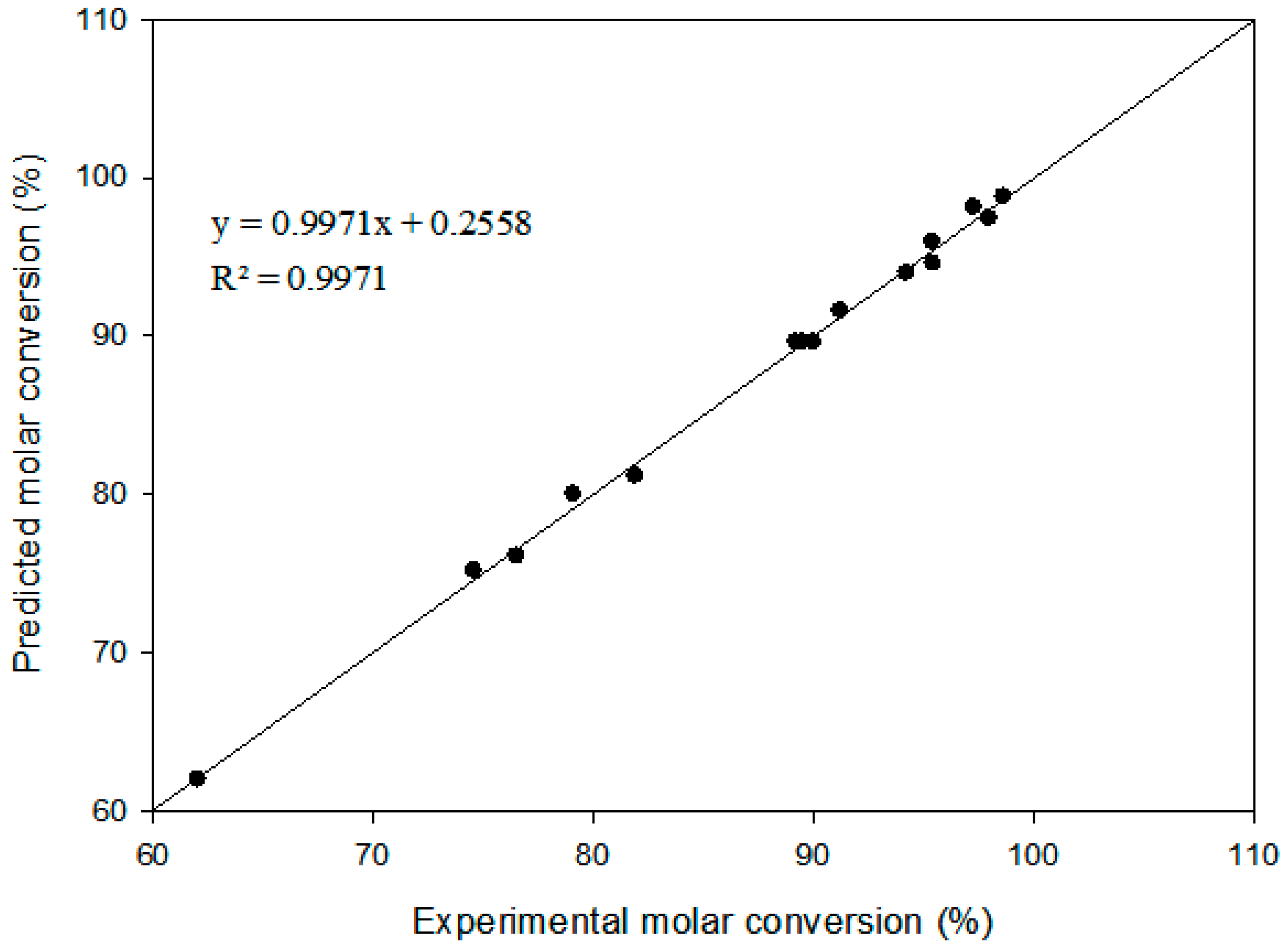
2.2. Model Fitting
+ 0.032430X2X3 − 0.0000213020X12 − 0.54497X22 − 0.00781849X32
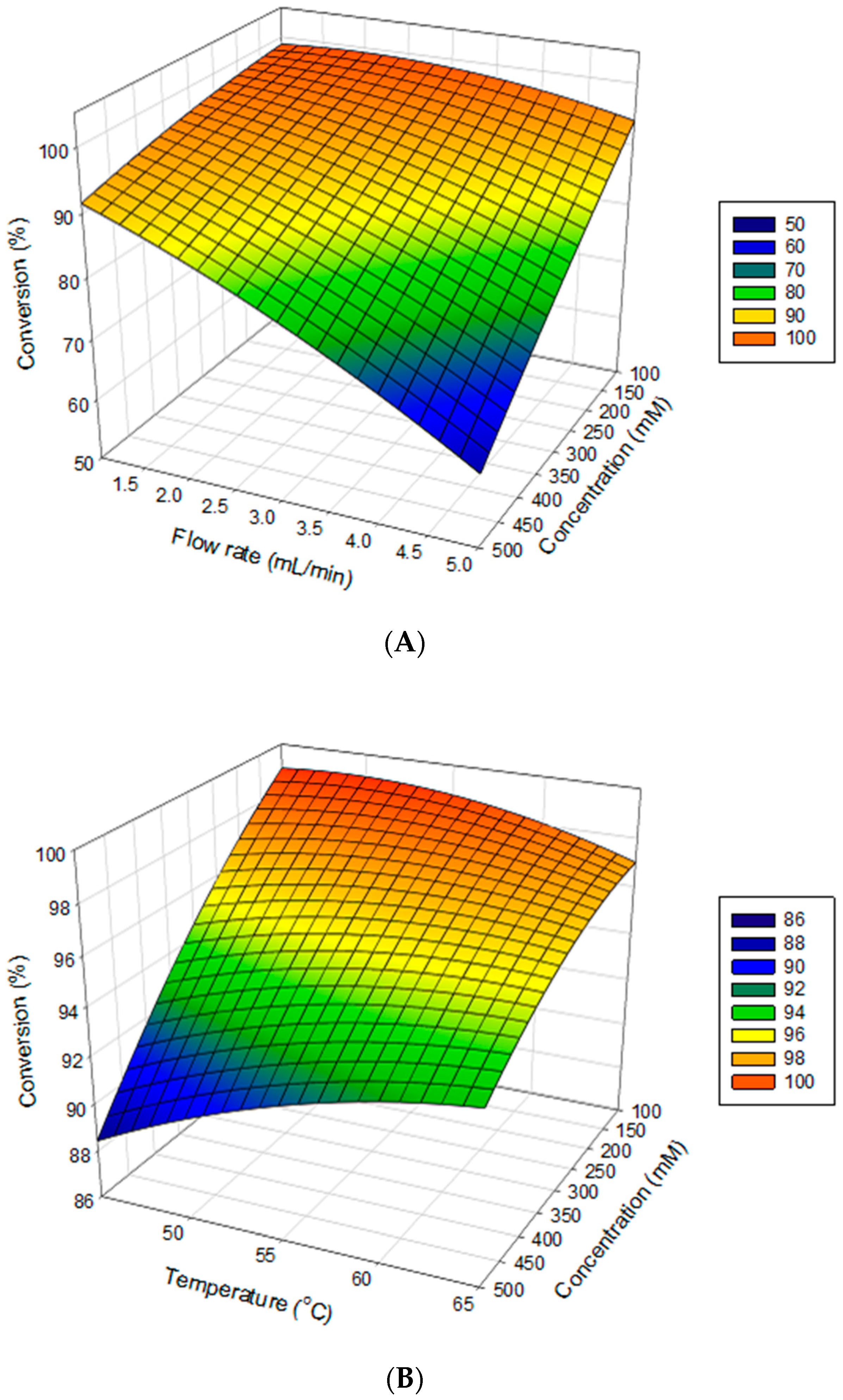
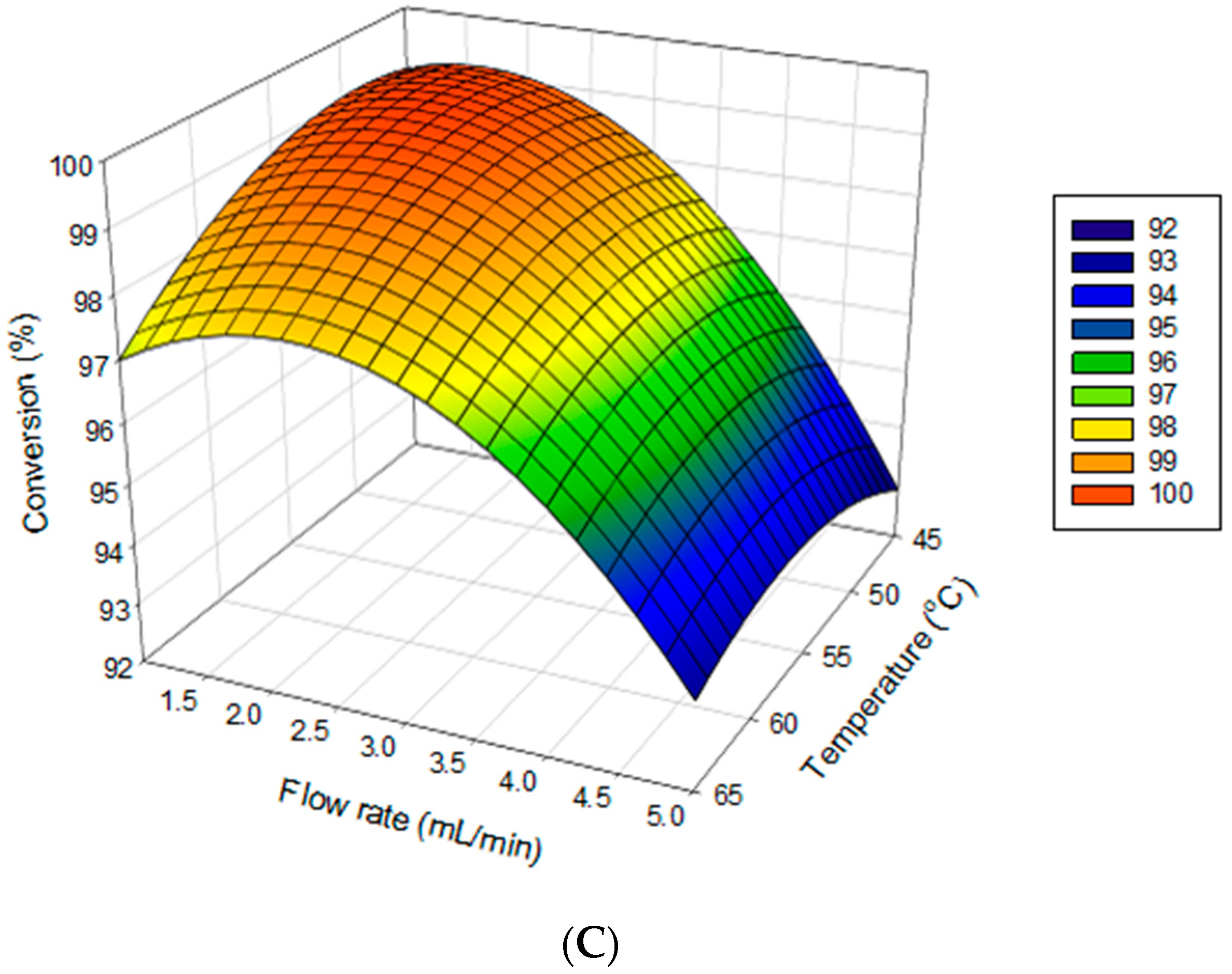
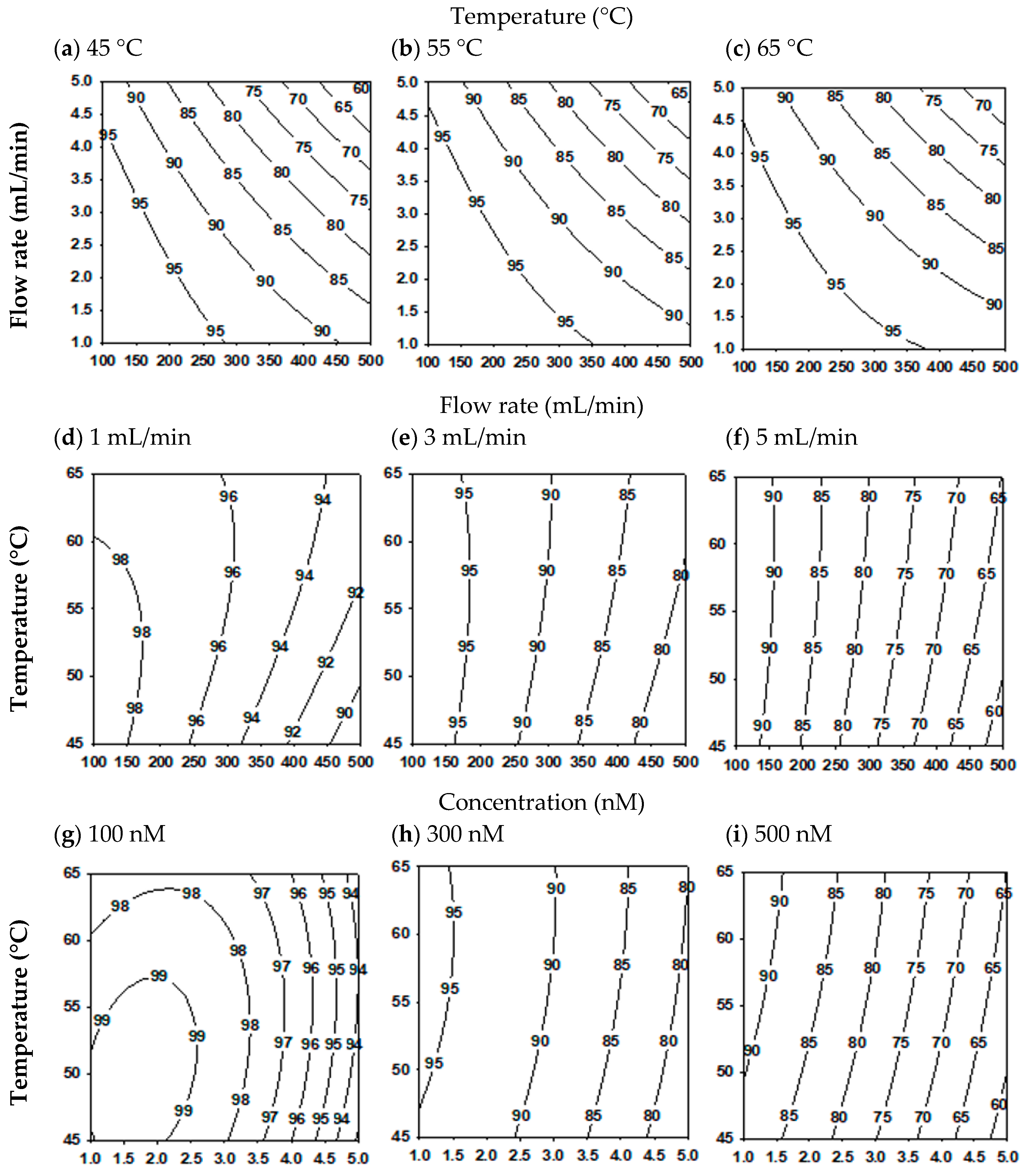
2.3. Mutual Effect of Parameters
2.4. Attaining Optimization
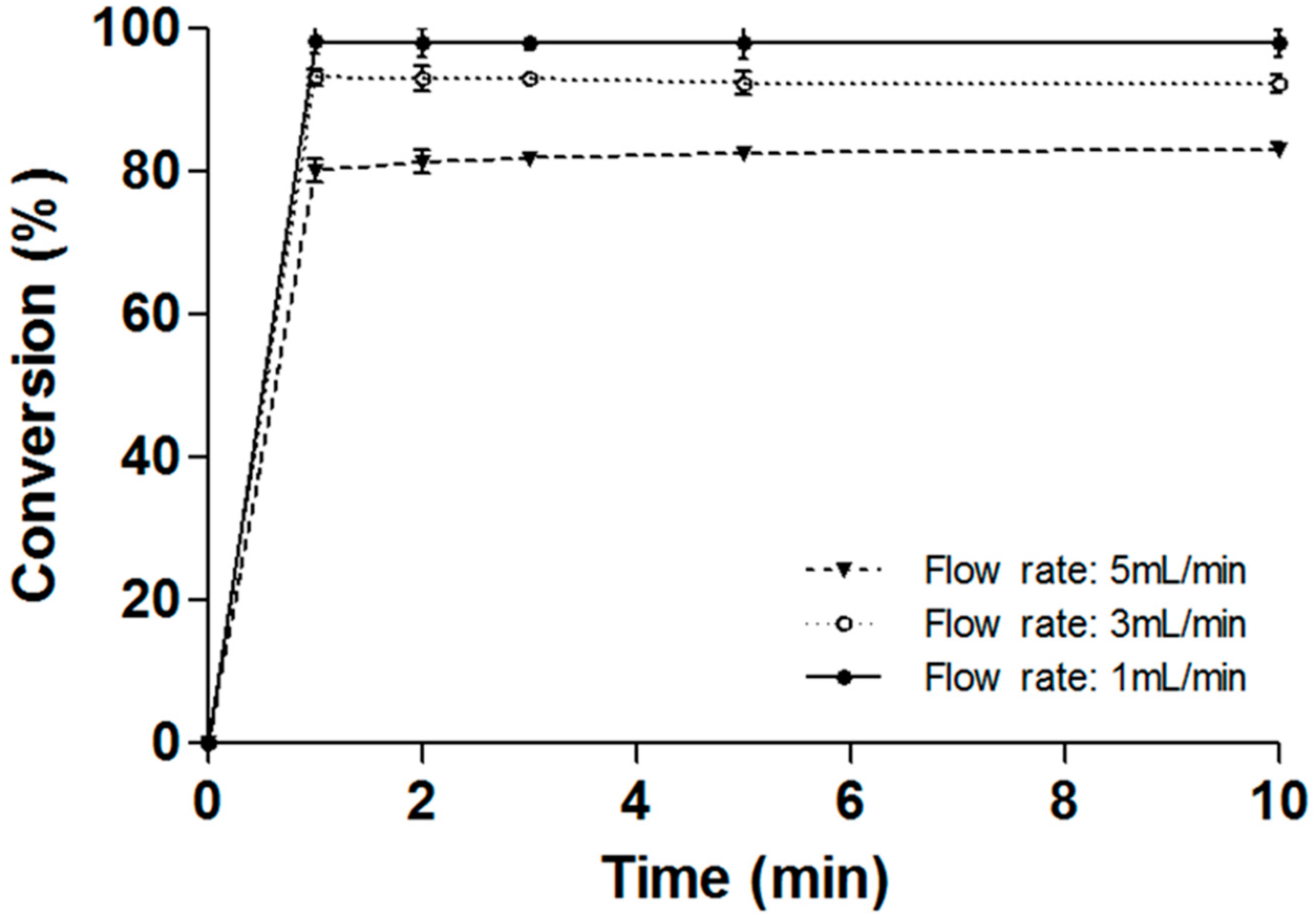
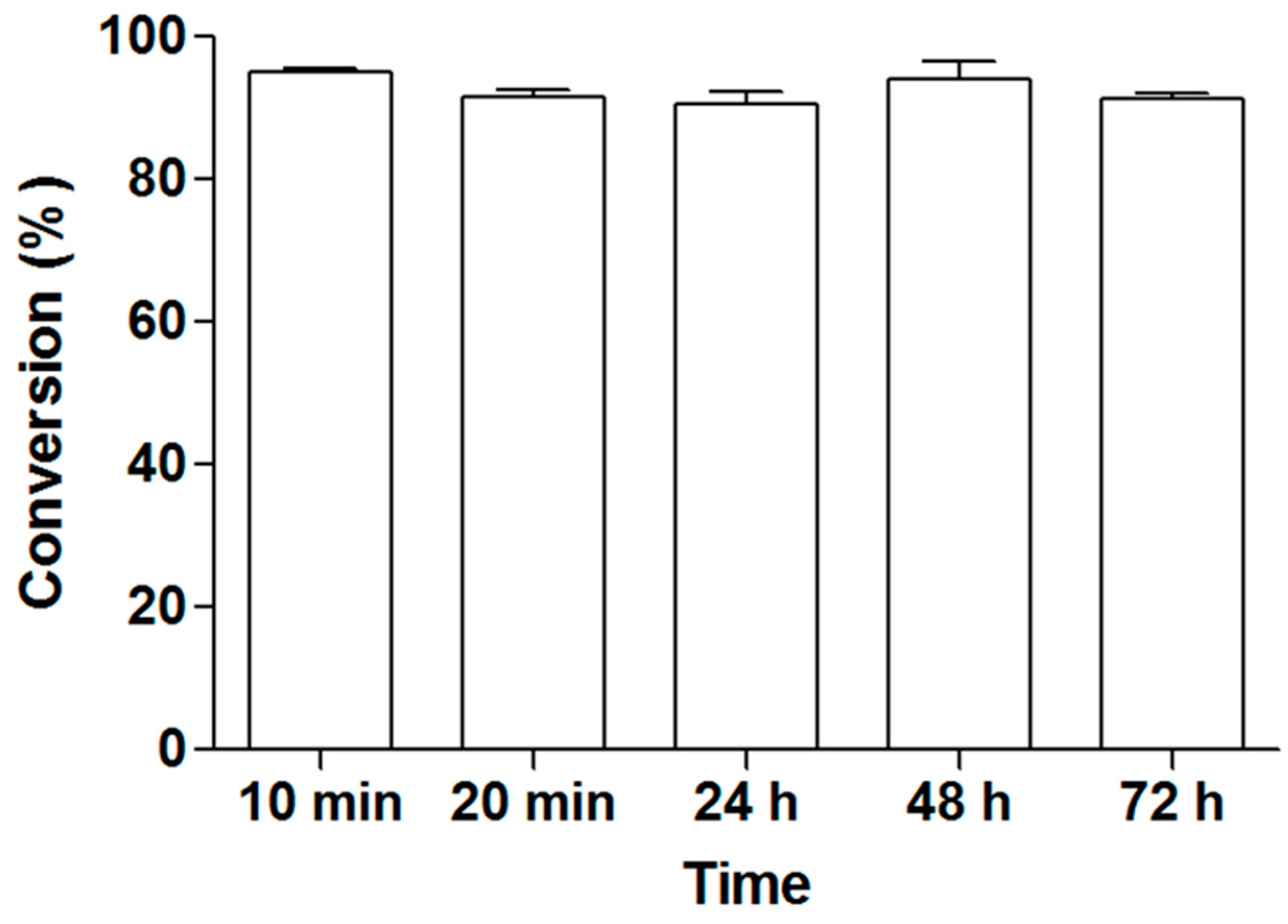
2.5. Operational Stability
3. Materials and Methods
3.1. Materials
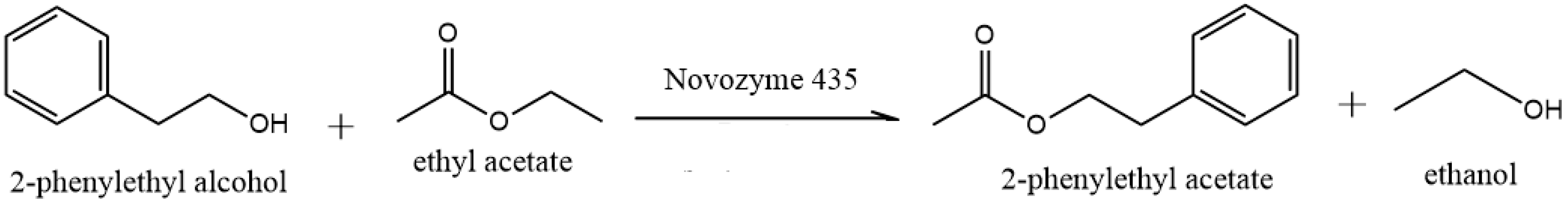
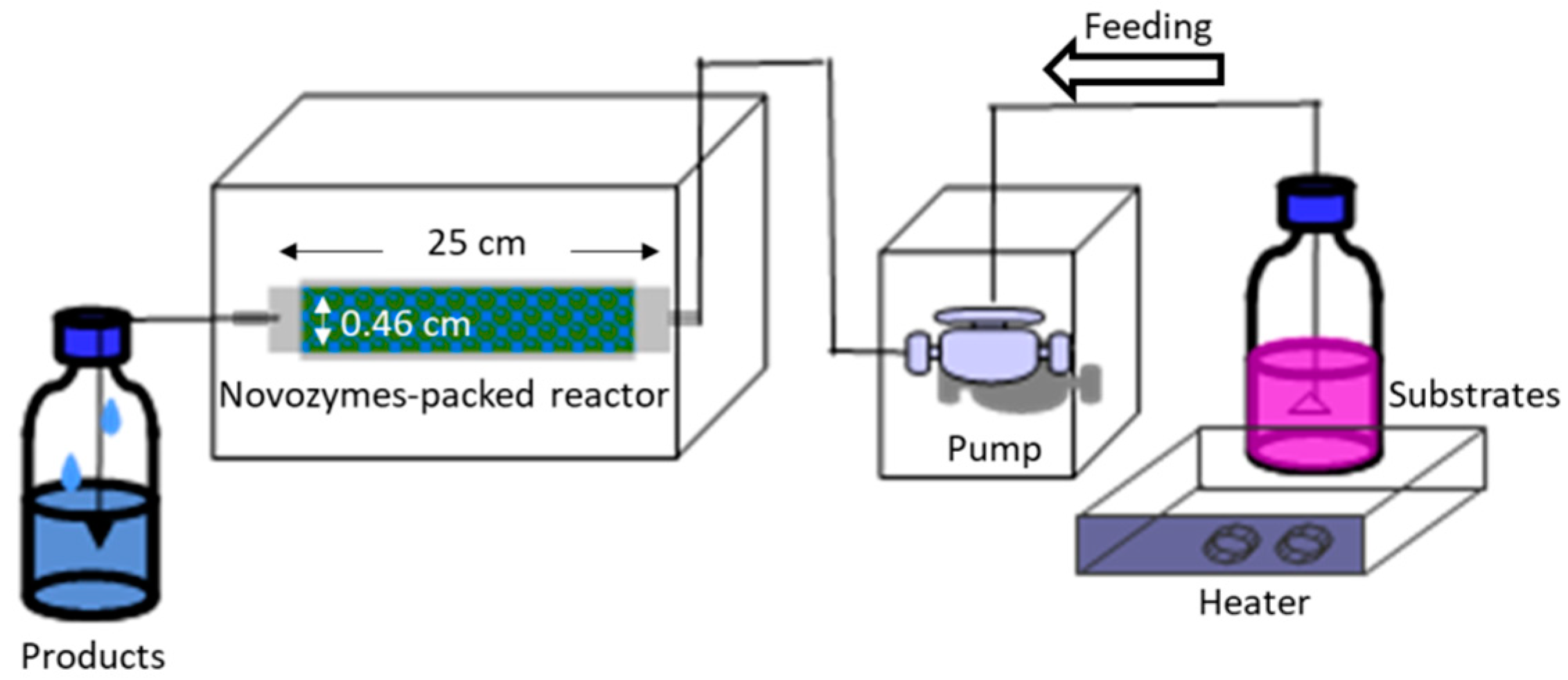
3.2. Continuous Lipase-Catalyzed Synthesis of 2-Phenylethyl Acetate (2-PEAc)
3.3. Experimental Design
3.4. Quantitation of 2-PEAc
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGinty, D.; Vitale, D.; Letizia, C.S.; Api, A.M. Fragrance material review on phenethyl acetate. Food Chem. Toxicol. 2012, 50, S491–S497. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; Meneses, A.C.D.; Araújo, P.H.H.D.; Oliveira, D.D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Bayout, I.; Bouzemi, N.; Guo, N.; Mao, X.; Serra, S.; Riva, S.; Secundo, F. Natural flavor ester synthesis catalyzed by lipases. Flavour Fragr. J. 2020, 35, 209–218. [Google Scholar] [CrossRef]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/Citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef]
- Guterman, I.; Masci, T.; Chen, X.; Negre, F.; Pichersky, E.; Dudareva, N.; Weiss, D.; Vainstein, A. Generation of phenylpropanoid pathway-derived volatiles in transgenic plants: Rose alcohol acetyltransferase produces phenylethyl acetate and benzyl acetate in petunia flowers. Plant Mol. Biol. 2006, 60, 555–563. [Google Scholar] [CrossRef]
- Hirata, H.; Ohnishi, T.; Watanabe, N. Biosynthesis of floral scent 2-phenylethanol in rose flowers. Biosci. Biotechnol. Biochem. 2016, 80, 1865–1873. [Google Scholar] [CrossRef]
- Arora, P.K. Microbial Technology for the Welfare of Society; Springer: Singapore, 2019. [Google Scholar]
- Martinez-Avila, O.; Sanchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef]
- Adler, P.; Hugen, T.; Wiewiora, M.; Kunz, B. Modeling of an integrated fermentation/membrane extraction process for the production of 2-phenylethanol and 2-phenylethylacetate. Enzyme Microb. Technol. 2011, 48, 285–292. [Google Scholar] [CrossRef]
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2014, 50, 1793–1806. [Google Scholar] [CrossRef]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.L. The impact of biochemistry vs. population membership on floral scent profiles in colour polymorphic Hesperis matronalis. Ann. Bot. 2008, 102, 911–922. [Google Scholar] [CrossRef]
- Białecka-Florjańczyk, E.; Krzyczkowska, J.; Stolarzewicz, I.; Kapturowska, A. Synthesis of 2-phenylethyl acetate in the presence of Yarrowia lipolytica KKP 379 biomass. J. Mol. Catal. B Enzym. 2012, 74, 241–245. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, L.; Pan, H.; Li, X.; Guo, D.; Zhang, L.; Pan, H.; Li, X. Metabolic engineering of Escherichia coli for production of 2-phenylethylacetate from L-phenylalanine. MicrobiologyOpen 2017, 6, e00486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Xavier Malcata, F.; Reyes, H.R.; Garcia, H.S.; Hill, C.G.; Amundson, C.H. Immobilized lipase reactors for modification of fats and oils—A review. J. Am. Oil Chem. Soc. 1990, 67, 890–910. [Google Scholar] [CrossRef]
- Fomuso, L.B.; Akoh, C.C. Lipase-catalyzed acidolysis of olive oil and caprylic acid in a bench-scale packed bed bioreactor. Food Res. Int. 2002, 35, 15–21. [Google Scholar] [CrossRef]
- Facin, B.R.; Melchiors, M.S.; Valério, A.; Oliveira, J.V.; Oliveira, D.D. Driving immobilized lipases as biocatalysts: 10 years state of the art and future prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Yang, T.; Xu, X.; Jacobsen, C. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006, 94, 53–60. [Google Scholar] [CrossRef]
- Mathpati, A.C.; Kalghatgi, S.G.; Mathpati, C.S.; Bhanage, B.M. Immobilized lipase catalyzed synthesis of n-amyl acetate: Parameter optimization, heterogeneous kinetics, continuous flow operation and reactor modeling. J. Chem. Technol. Biotechnol. 2018, 93, 2906–2916. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G.; Burton, R.; Liu, A.; Harte, F.M.; Wang, Y. Solvent-free lipase-catalyzed synthesis of technical-grade sugar esters and evaluation of their physicochemical and bioactive properties. Catalysts 2016, 6, 78. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chen, H.H.; Chen, J.H.; Liu, Y.C.; Shieh, C.J. High yield of wax ester synthesized from cetyl alcohol and octanoic acid by lipozyme RMIM and Novozym 435. Int. J. Mol. Sci. 2012, 13, 11694–11704. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gładkowski, W. Production of structured phosphatidylcholine with high content of myristic acid by lipase-catalyzed acidolysis and interesterification. Catalysts 2018, 8, 281. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. Catalysts 2018, 8, 68. [Google Scholar]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Ju, H.Y.; Yang, C.K.; Yen, Y.H.; Shieh, C.J. Continuous lipase-catalyzed synthesis of hexyl laurate in a packed-bed reactor: Optimization of the reaction conditions in a solvent-free system. J. Chem. Technol. Biotechnol. 2009, 84, 29–33. [Google Scholar] [CrossRef]
- de Meneses, A.C.; Almeida Sá, A.G.; Lerin, L.A.; Corazza, M.L.; de Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Benzyl butyrate esterification mediated by immobilized lipases: Evaluation of batch and fed-batch reactors to overcome lipase-acid deactivation. Process Biochem. 2019, 78, 50–57. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.; Dinu, C. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-Chaabouni, M.; Gargouri, Y. 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: A comparative study between n-hexane and solvent-free system. Enzyme Microb. Technol. 2004, 35, 355–363. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, P.Y.; Chen, J.H.; Kuo, C.H.; Shieh, C.J. Developing a high-temperature solvent-free system for efficient biocatalysis of octyl ferulate. Catalysts 2018, 8, 338. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, H.C.; Ju, H.Y.; Chen, J.H.; Kuo, C.H.; Chung, Y.L.; Liu, Y.C.; Shieh, C.J. Green and efficient production of octyl hydroxyphenylpropionate using an ultrasound-assisted packed-bed bioreactor. J. Ind. Microbiol. Biotechnol. 2012, 39, 655–660. [Google Scholar] [CrossRef][Green Version]
- Chen, H.C.; Kuo, C.H.; Twu, Y.K.; Chen, J.H.; Chang, C.M.J.; Liu, Y.C.; Shieh, C.J. A continuous ultrasound-assisted packed-bed bioreactor for the lipase-catalyzed synthesis of caffeic acid phenethyl ester. J. Chem. Technol. Biotechnol. 2011, 86, 1289–1294. [Google Scholar] [CrossRef]
- Karra-Châabouni, M.; Ghamgui, H.; Bezzine, S.; Rekik, A.; Gargouri, Y. Production of flavour esters by immobilized Staphylococcus simulans lipase in a solvent-free system. Process Biochem. 2006, 41, 1692–1698. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, C.Y.; Lee, C.L.; Kuo, W.C.; Hsieh, S.L.; Shieh, C.J. Synthesis of DHA/EPA ethyl esters via lipase-catalyzed acidolysis using Novozym® 435: A kinetic study. Catalysts 2020, 10, 565. [Google Scholar] [CrossRef]
- Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Santos-Moriano, P.; Ballesteros, A.O.; Plou, F.J. Continuous packed bed reactor with immobilized β-galactosidase for production of galactooligosaccharides (GOS). Catalysts 2016, 6, 189. [Google Scholar] [CrossRef]
- Neifar, S.; Cervantes, F.V.; Bouanane-Darenfed, A.; BenHlima, H.; Ballesteros, A.O.; Plou, F.J.; Bejar, S. Immobilization of the glucose isomerase from Caldicoprobacter algeriensis on Sepabeads EC-HA and its efficient application in continuous high fructose syrup production using packed bed reactor. Food Chem. 2020, 309, 125710. [Google Scholar] [CrossRef] [PubMed]
- Salvi, H.M.; Kamble, M.P.; Yadav, G.D. Synthesis of geraniol esters in a continuous-flow packed-bed reactor of immobilized lipase: Optimization of process parameters and kinetic modeling. Appl. Biochem. Biotechnol. 2018, 184, 630–643. [Google Scholar] [CrossRef]
- Sun, J.; Yu, B.; Curran, P.; Liu, S.Q. Lipase-catalysed transesterification of coconut oil with fusel alcohols in a solvent-free system. Food Chem. 2012, 134, 89–94. [Google Scholar] [CrossRef]
- Cavallaro, V.; Tonetto, G.; Ferreira, M.L. Optimization of the enzymatic synthesis of pentyl oleate with lipase immobilized onto novel structured support. Fermentation 2019, 5, 48. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; Da Fonseca, M.; Ferreira-Dias, S. Synthesis of ethyl butyrate in organic media catalyzed by Candida rugosa lipase immobilized in polyurethane foams: A kinetic study. Biochem. Eng. J. 2009, 43, 327–332. [Google Scholar] [CrossRef]
- Gawas, S.D.; Jadhav, S.V.; Rathod, V.K. Solvent free lipase catalysed synthesis of ethyl laurate: Optimization and kinetic studies. Appl. Biochem. Biotechnol. 2016, 180, 1428–1445. [Google Scholar] [CrossRef]
- Royon, D.; Daz, M.; Ellenrieder, G.; Locatelli, S. Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresour. Technol. 2007, 98, 648–653. [Google Scholar] [CrossRef]
- Halim, S.F.A.; Kamaruddin, A.H.; Fernando, W. Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: Optimization using response surface methodology (RSM) and mass transfer studies. Bioresour. Technol. 2009, 100, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, J.H.; Chieh-ming, J.C.; Wu, T.T.; Shieh, C.J. Optimization of lipase-catalyzed biodiesel by isopropanolysis in a continuous packed-bed reactor using response surface methodology. New Biotechnol. 2009, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
| Treatment No. a | Factor | Molar Conversion (%) | ||
|---|---|---|---|---|
| X1 Concentration of 2-PE (mM) | X2 Flow Rate (mL min−1) | X3 Observed Molar Conversion (%) | ||
| 1 | 0(300) | 1(5) | −1(45) | 76.53 ± 1.70 |
| 2 | 1(500) | 0(3) | −1(45) | 74.58 ± 1.62 |
| 3 | −1(100) | 0(3) | −1(45) | 97.30 ± 0.41 |
| 4 | 0(300) | −1(1) | −1(45) | 95.44 ± 0.80 |
| 5 | −1(100) | 1(5) | 0(55) | 94.26 ± 0.95 |
| 6 | 1(500) | 1(5) | 0(55) | 62.05 ± 1.01 |
| 7 | 0(300) | 0(3) | 0(55) | 90.03 ± 1.80 |
| 8 | 0(300) | 0(3) | 0(55) | 89.50 ± 1.99 |
| 9 | 0(300) | 0(3) | 0(55) | 89.23 ± 2.07 |
| 10 | −1(100) | −1(1) | 0(55) | 98.66 ± 0.01 |
| 11 | 1(500) | −1(1) | 0(55) | 91.25 ± 0.33 |
| 12 | 0(300) | 1(5) | 1(65) | 79.10 ± 1.22 |
| 13 | 1(500) | 0(3) | 1(65) | 81.93 ± 1.86 |
| 14 | −1(100) | 0(3) | 1(65) | 98.00 ± 0.32 |
| 15 | 0(300) | −1(1) | 1(65) | 95.42 ± 0.16 |
| Factors | Degree of Freedom | Sum of Squares | Prob > F |
|---|---|---|---|
| Linear | 3 | 1374.688873 | <0.0001 |
| Quadratic | 3 | 20.478243 | 0.0267 |
| Cross product | 3 | 166.589386 | 0.0002 |
| Total Model | 9 | 1561.756502 | <0.0001 |
| Lack of fit | |||
| Pure error | 3 | 4.206608 | 0.1088 |
| Total error | 2 | 0.335839 | |
| Linear | 5 | 4.542448 | |
| R2 = 0.9971 | |||
| Factors | Degree of Freedom | Sum of Squares | Prob > F |
|---|---|---|---|
| Concentration of 2-PE (X1) | 4 | 234.012890 | <0.0001 |
| Flow rate (X2) | 4 | 191.319574 | <0.0001 |
| Temperature (X3) | 4 | 7.255284 | 0.0213 |
| Coded Radius | Estimated Response (%) | Observed Response (%) | Uncoded Factor Values | ||
|---|---|---|---|---|---|
| X1 (mM) | X2 (mL/min) | X3 (°C) | |||
| 0.0 | 89.59 ± 0.55 | 89.59 ± 0.41 | 300.00 | 3.00 | 55.00 |
| 0.2 | 92.02 ± 0.54 | 91.23 ± 0.30 | 269.05 | 2.75 | 55.17 |
| 0.4 | 94.11 ± 0.53 | 92.65 ± 0.22 | 235.09 | 2.54 | 55.25 |
| 0.6 | 95.87 ± 0.51 | 95.12 ± 0.14 | 196.17 | 2.40 | 55.18 |
| 0.7 | 96.65 ± 0.51 | 95.80 ± 0.09 | 174.46 | 2.38 | 55.07 |
| 0.8 | 97.39 ± 0.52 | 97.19 ± 0.35 | 151.65 | 2.40 | 54.90 |
| 1.0 | 98.80 ± 0.59 | 98.74 ± 0.04 | 105.61 | 2.54 | 54.49 |
| 1.2 | 100.22 ± 0.73 | 99.01 ± 0.09 | 62.07 | 2.75 | 54.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Huang, H.-Y.; Chen, Y.-M.; Kuo, C.-H.; Shieh, C.-J. Continuous Production of 2-Phenylethyl Acetate in a Solvent-Free System Using a Packed-Bed Reactor with Novozym® 435. Catalysts 2020, 10, 714. https://doi.org/10.3390/catal10060714
Huang S-M, Huang H-Y, Chen Y-M, Kuo C-H, Shieh C-J. Continuous Production of 2-Phenylethyl Acetate in a Solvent-Free System Using a Packed-Bed Reactor with Novozym® 435. Catalysts. 2020; 10(6):714. https://doi.org/10.3390/catal10060714
Chicago/Turabian StyleHuang, Shang-Ming, Hsin-Yi Huang, Yu-Min Chen, Chia-Hung Kuo, and Chwen-Jen Shieh. 2020. "Continuous Production of 2-Phenylethyl Acetate in a Solvent-Free System Using a Packed-Bed Reactor with Novozym® 435" Catalysts 10, no. 6: 714. https://doi.org/10.3390/catal10060714
APA StyleHuang, S.-M., Huang, H.-Y., Chen, Y.-M., Kuo, C.-H., & Shieh, C.-J. (2020). Continuous Production of 2-Phenylethyl Acetate in a Solvent-Free System Using a Packed-Bed Reactor with Novozym® 435. Catalysts, 10(6), 714. https://doi.org/10.3390/catal10060714







