Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature
Abstract
1. Introduction
2. Results and Discussion
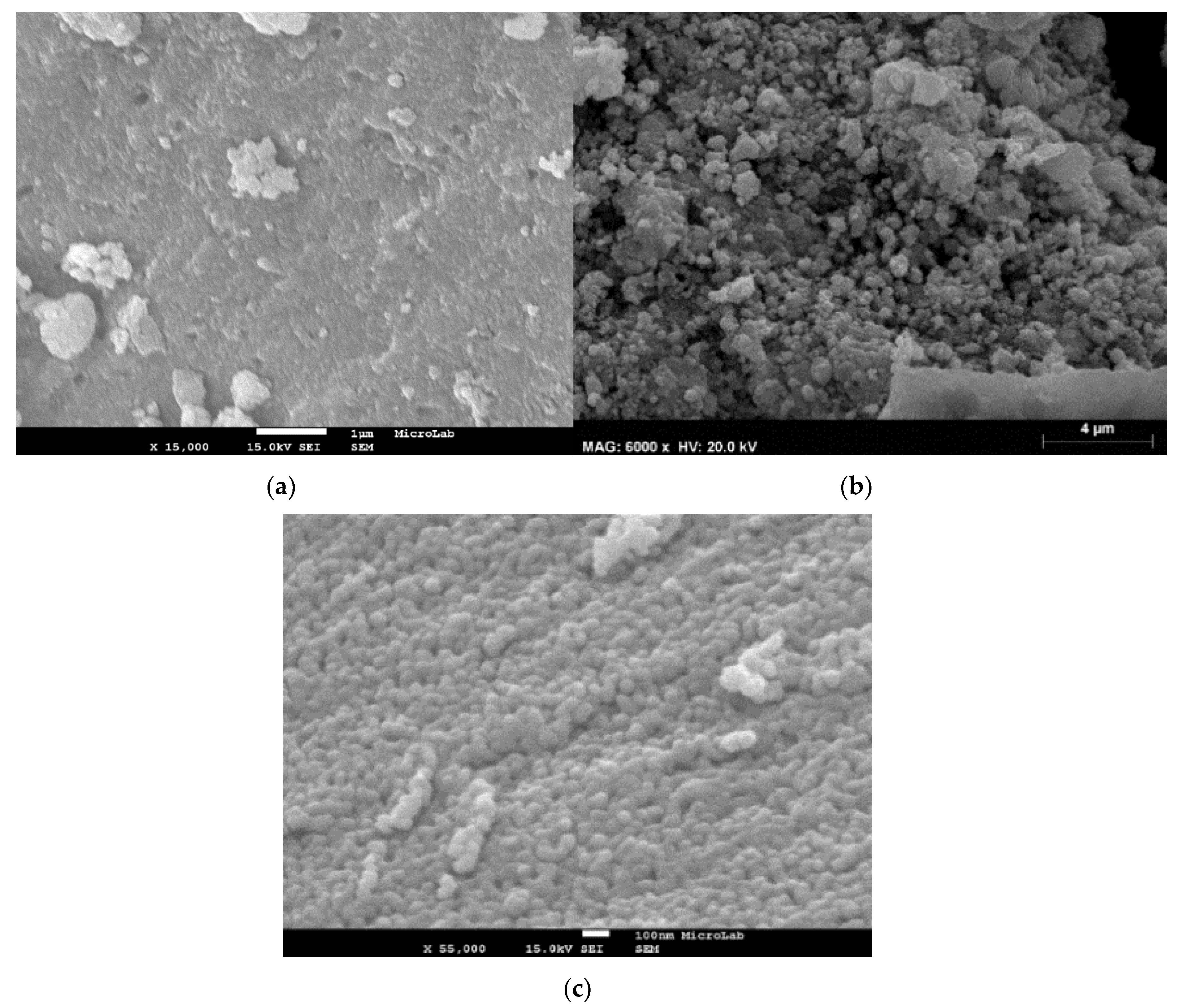
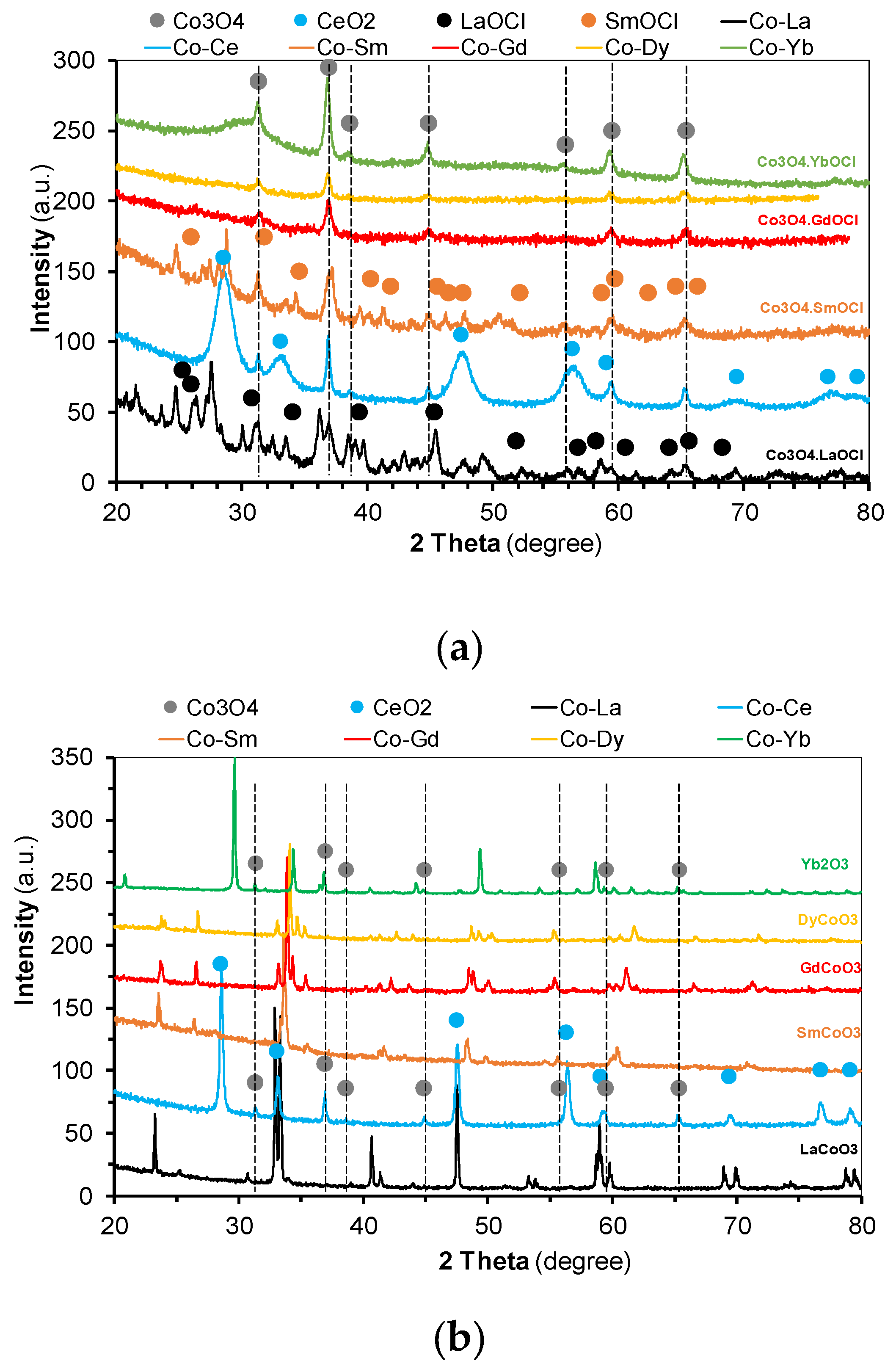
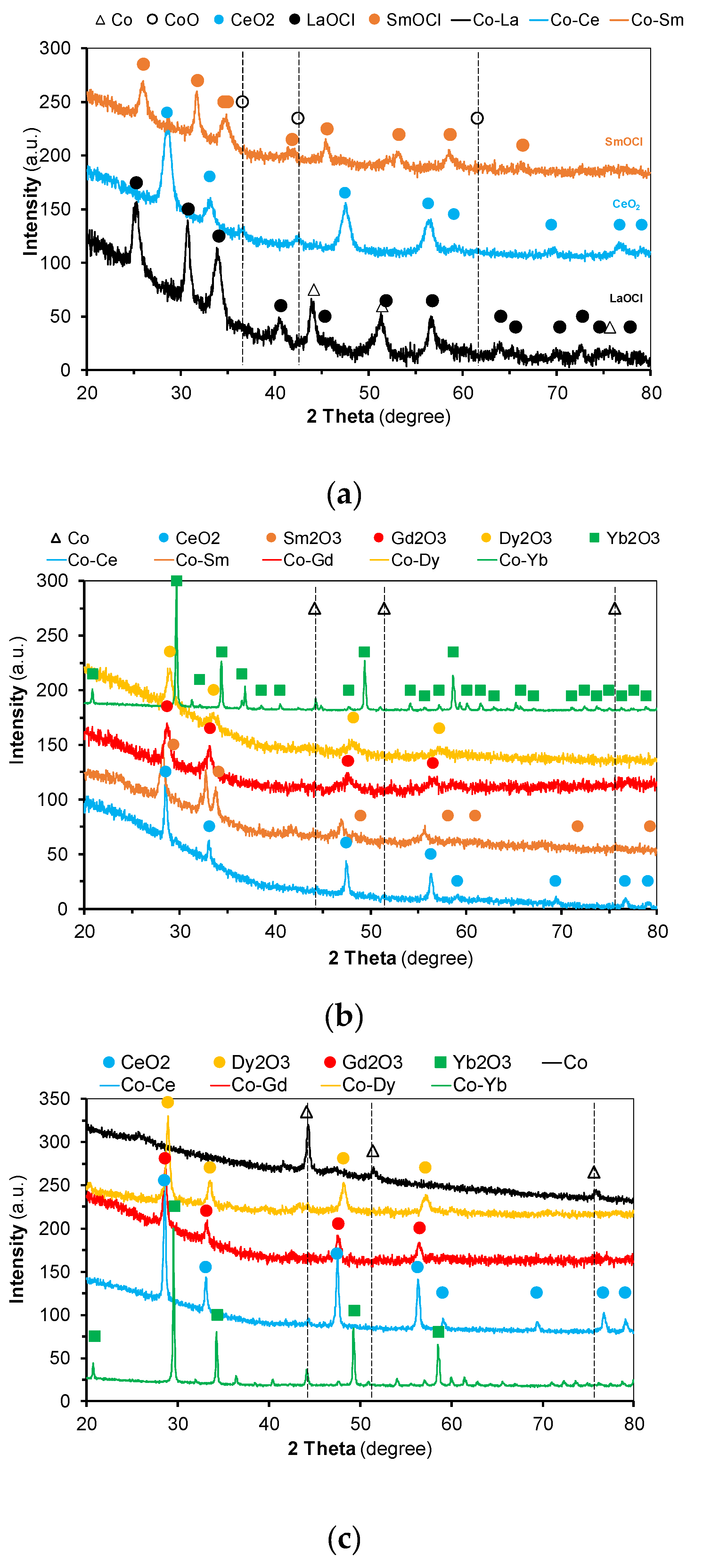
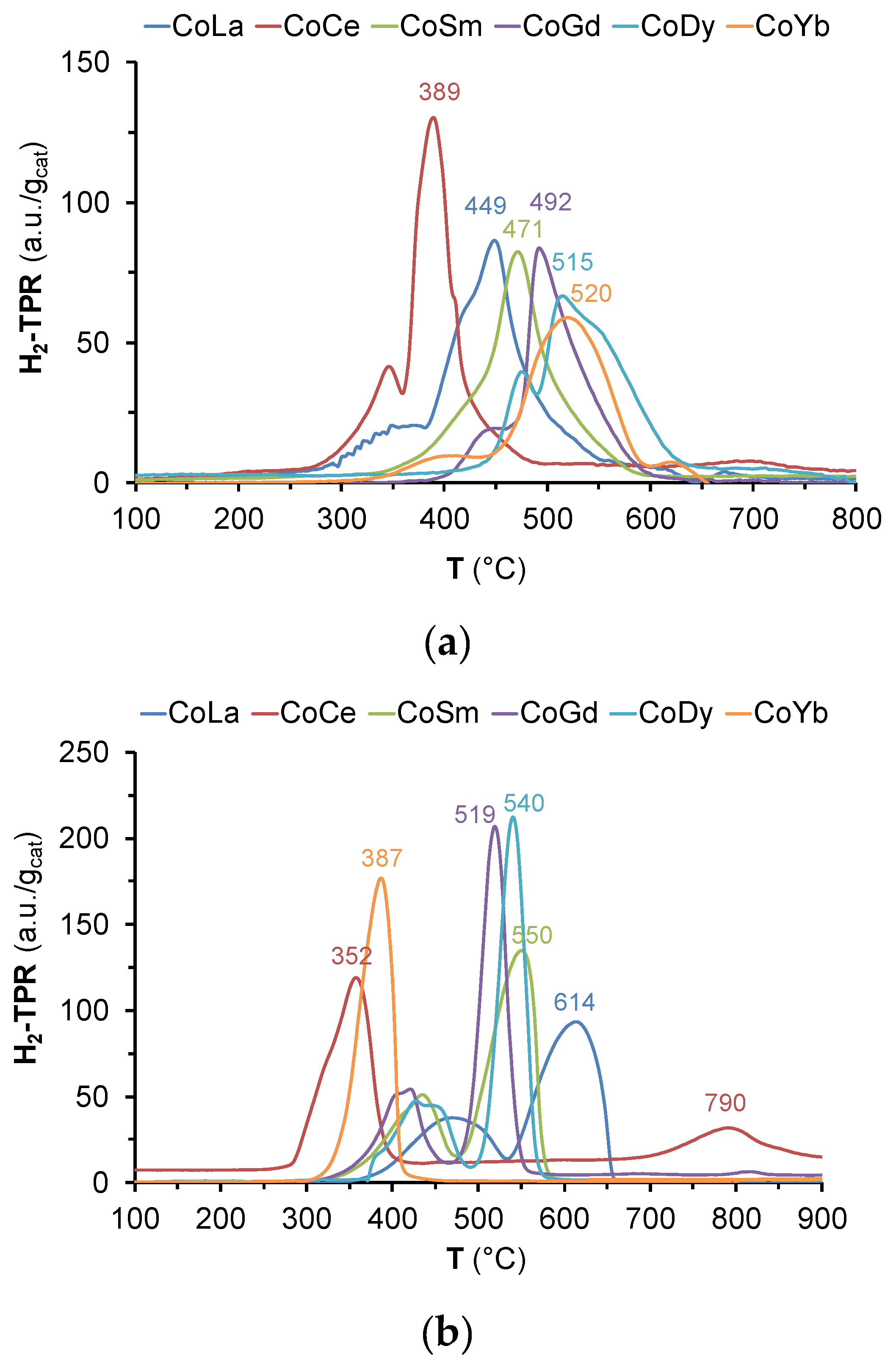
2.1. Catalysts Characterization
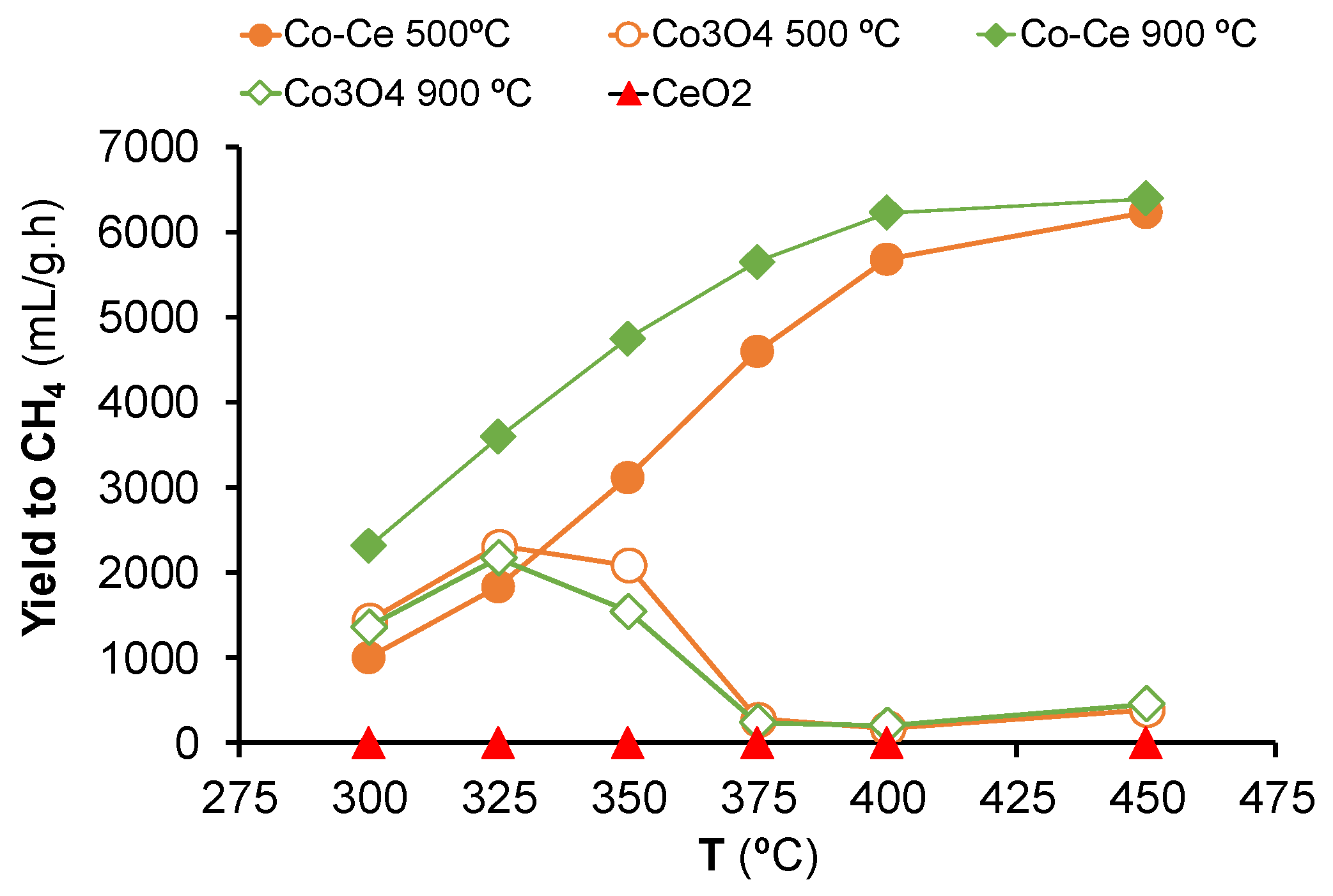
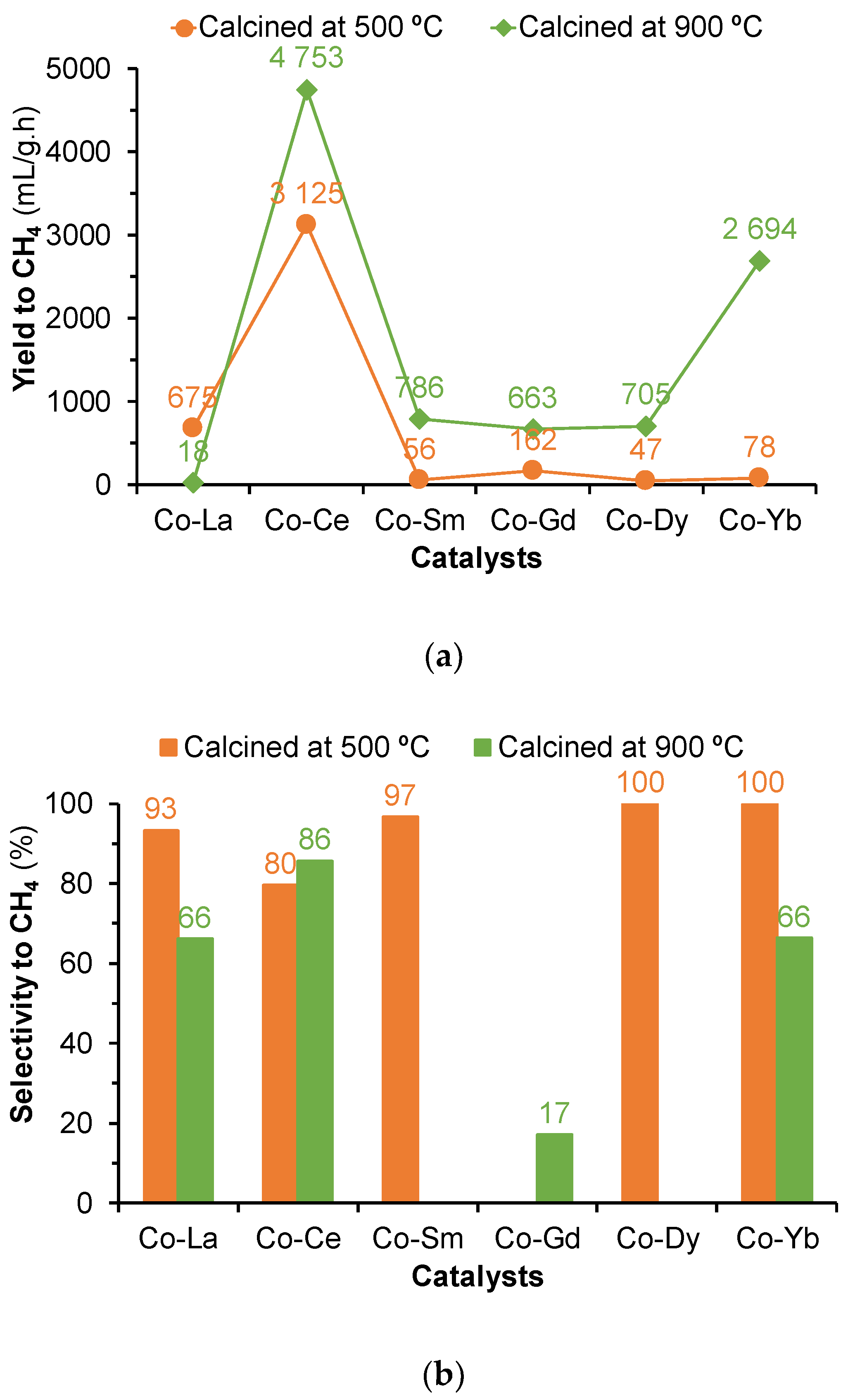
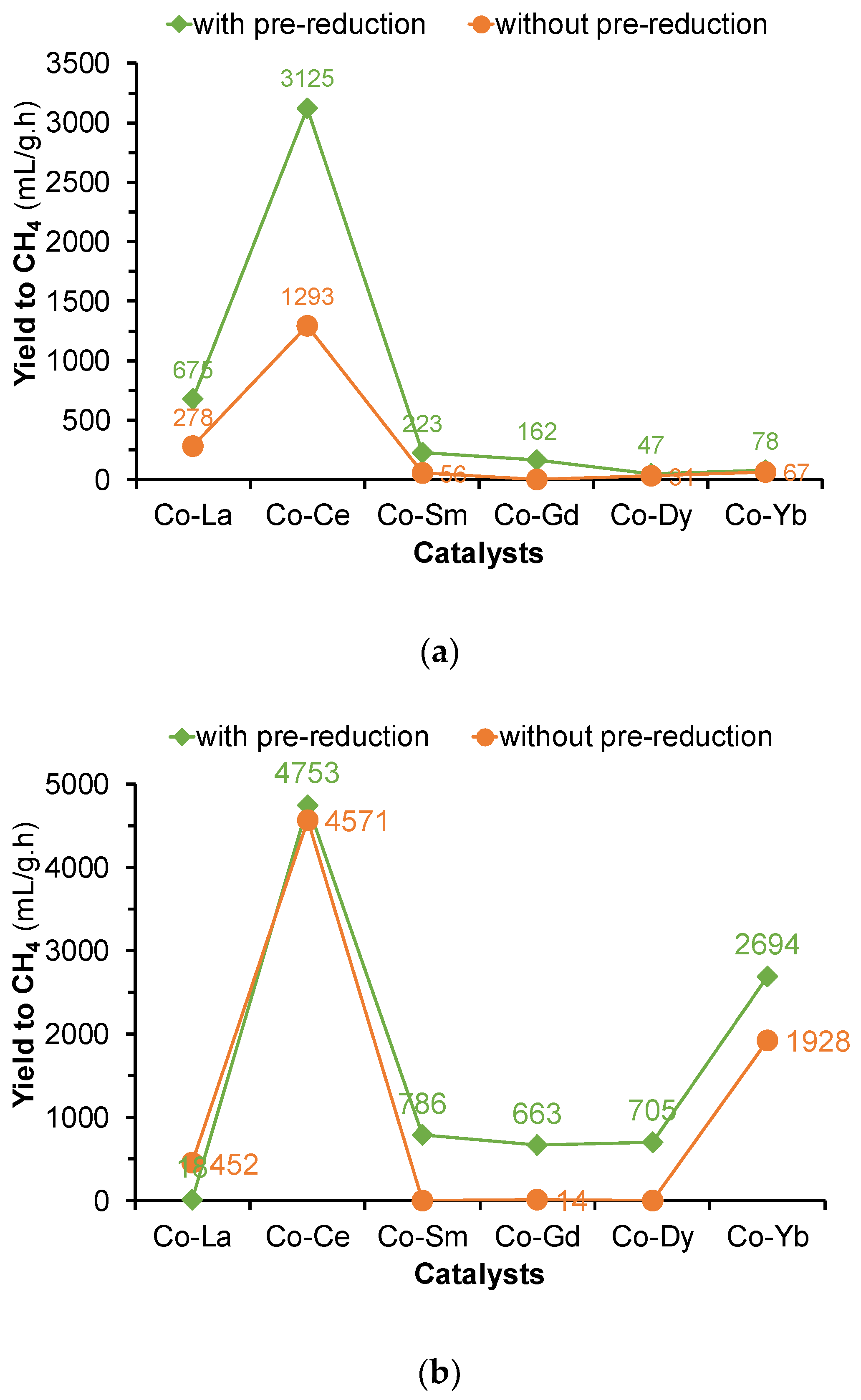
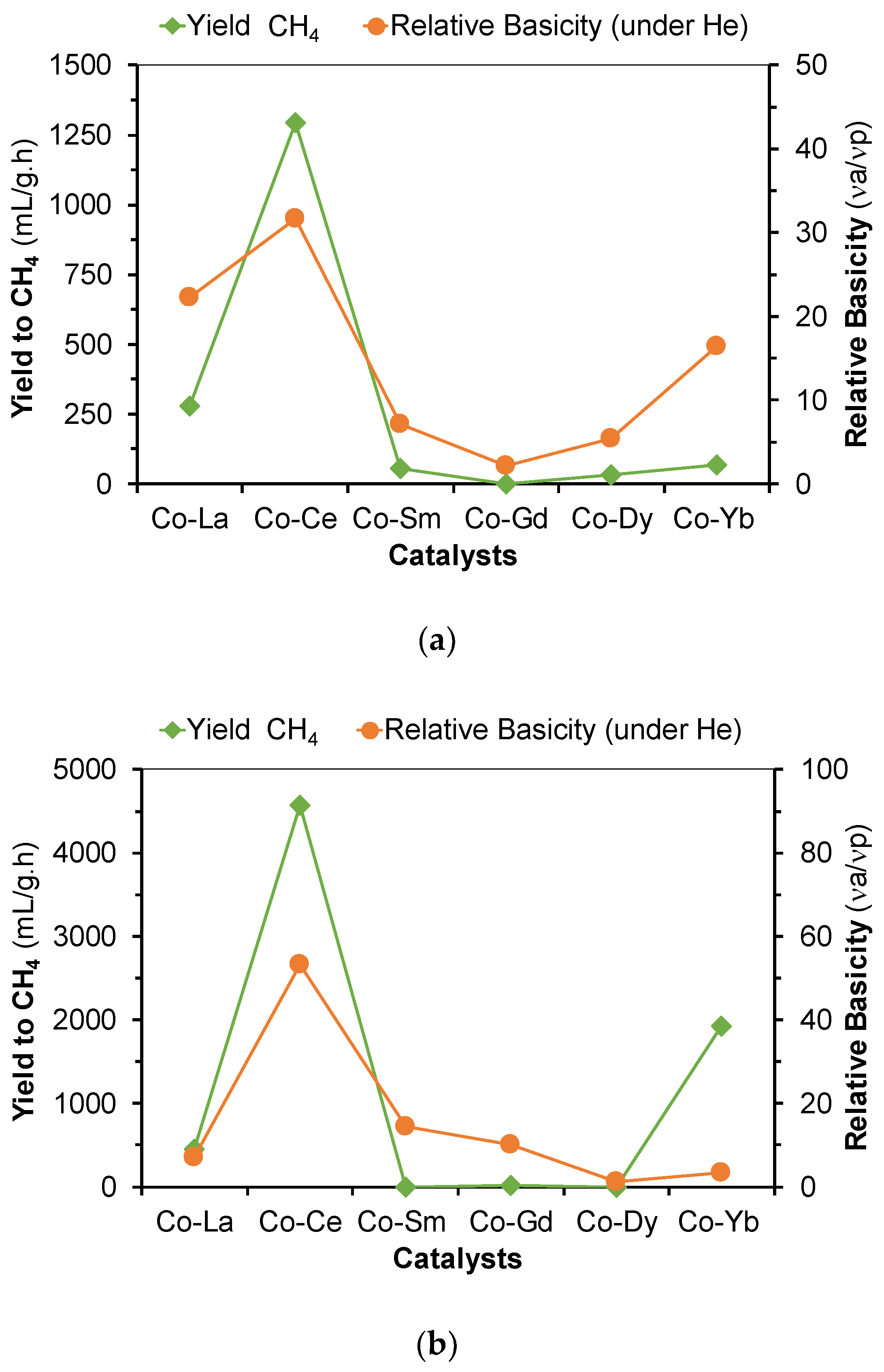
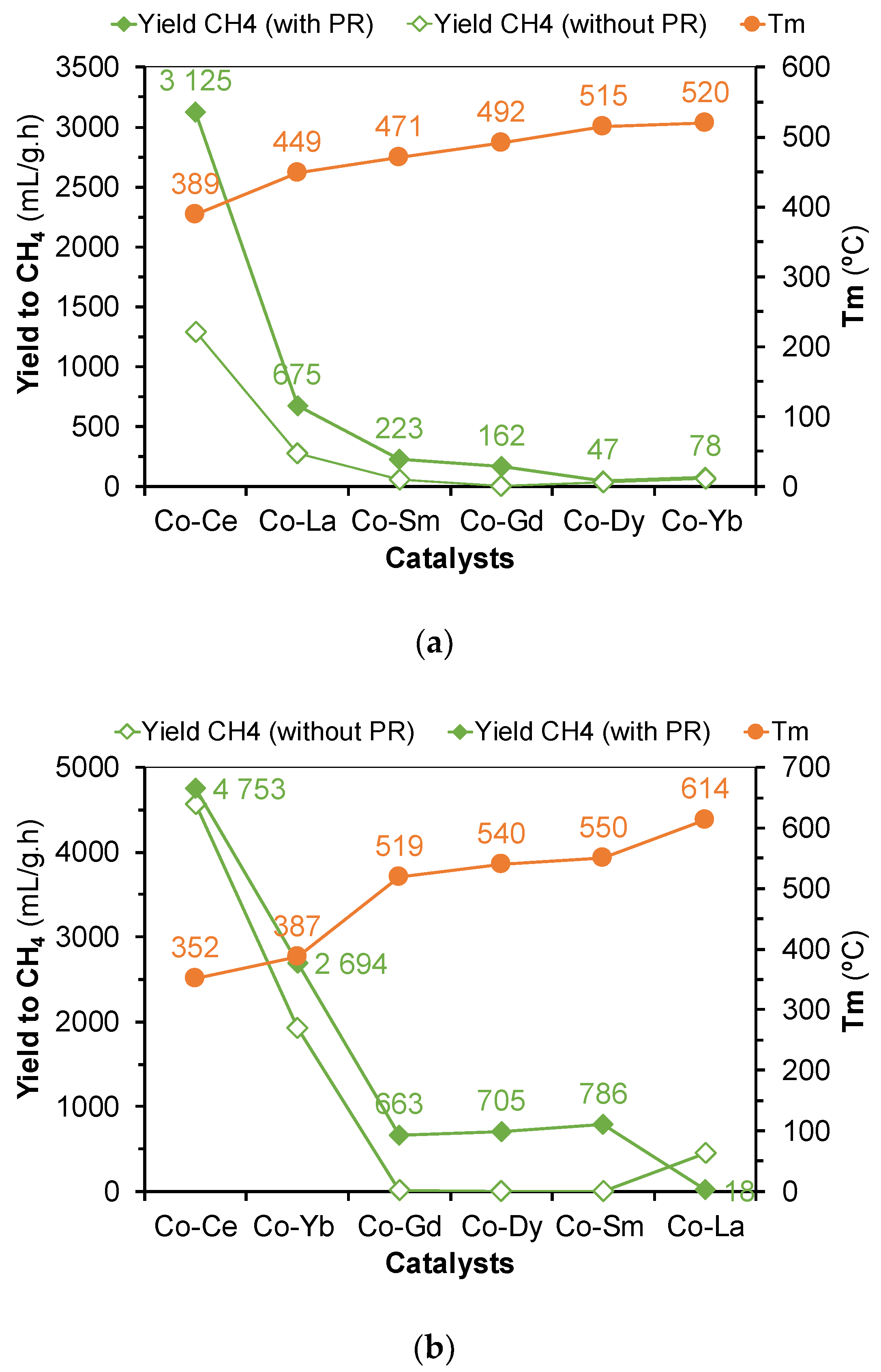
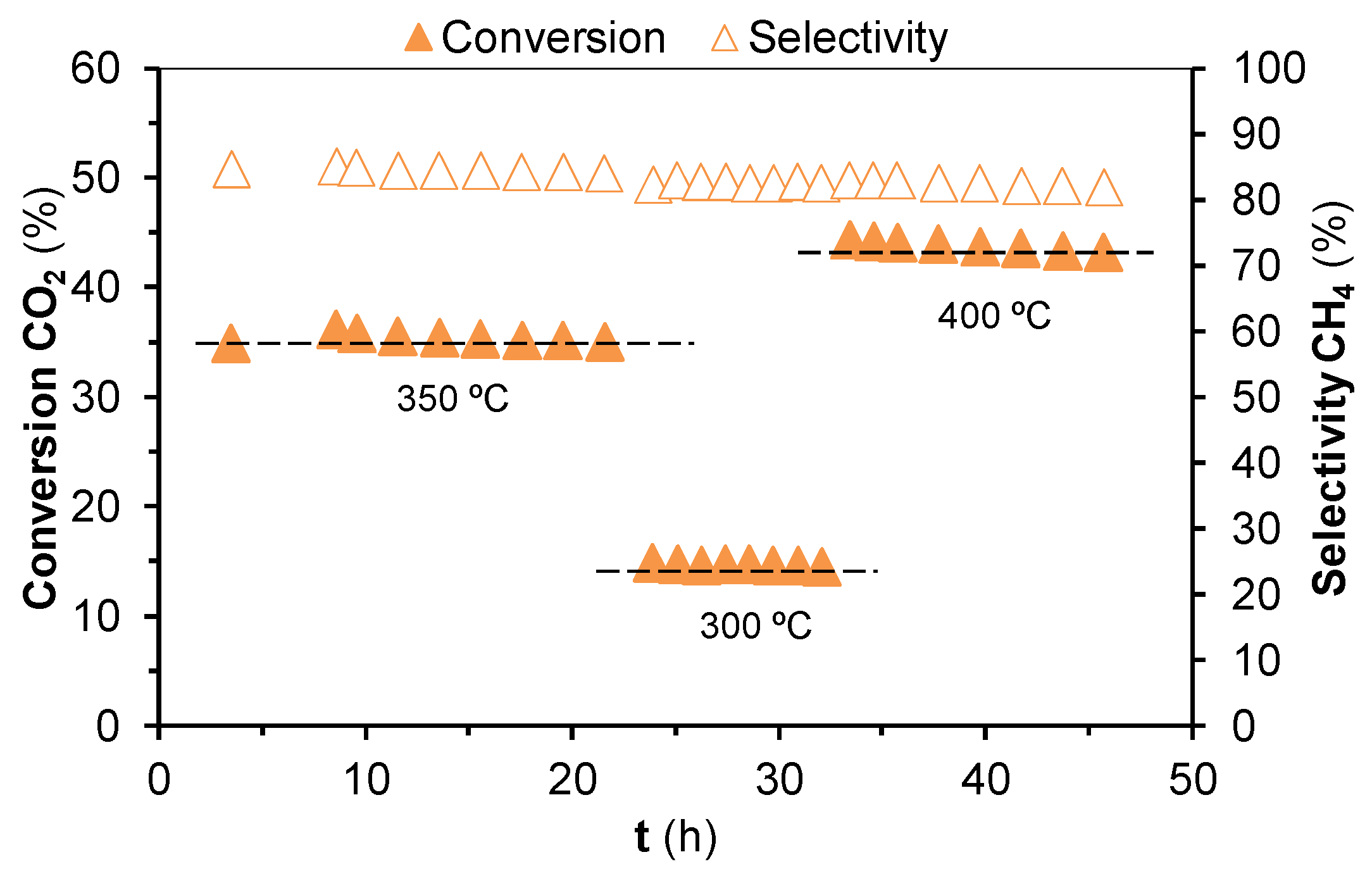
2.2. Hydrogenation of CO2
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.H.; Wang, H.Z.; Jiang, X.; Zhu, J.; Liu, Z.M.; Guo, X.W.; Song, C.S. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Sanz-Perez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Ronsch, S.; Schneider, J.; Matthischke, S.; Schluter, M.; Gotz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.Y.; Chu, W.; Zhou, Y.L.; Kohler, K. CO2 methanation over supported Ru/Al2O3 catalysts: Mechanistic studies by in situ infrared spectroscopy. Chemistryselect 2016, 1, 3197–3203. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.S.; Jun, K.W.; Choi, M.J.; Kishan, G.; Lee, K.W. Comparative study of Fischer-Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe- and Co-based catalysts. Appl. Catal. A Gen. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Davis, B.H.; Willauer, H.D. Influence of Gas Feed Composition and Pressure on the Catalytic Conversion of CO2 to Hydrocarbons using a traditional cobalt-based fischer-tropsch catalyst. Energy Fuels 2009, 23, 4190–4195. [Google Scholar] [CrossRef]
- Zhang, J.L.; Su, X.J.; Wang, X.; Ma, Q.X.; Fan, S.B.; Zhao, T.S. Promotion effects of Ce added Fe-Zr-K on CO2 hydrogenation to light olefins. React. Kinet. Mech. Cat. 2018, 124, 575–585. [Google Scholar] [CrossRef]
- Zeng, S.H.; Du, Y.; Su, H.Q.; Zhang, Y.L. Promotion effect of single or mixed rare earths on cobalt-based catalysts for Fischer-Tropsch synthesis. Catal. Commun. 2011, 13, 6–9. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Park, E.D. CO and CO2 methanation over supported cobalt catalysts. Top. Catal. 2017, 60, 714–720. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Jiang, Y.X.; Qin, Z.Z.; Xie, Q.R.; Ji, H.B. Influence of Zr, Ce, and La on Co3O4 catalyst for CO2 methanation at low temperature. Chin. J. Chem. Eng. 2018, 26, 768–774. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ferraria, A.M.; do Rego, A.M.B.; Goncalves, A.P.; Correia, M.R.; Gasche, T.A.; Branco, J.B. Partial oxidation of methane over bimetallic nickel-lanthanide oxides. J. Alloys Compd. 2010, 489, 316–323. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ferraria, A.M.; do Rego, A.M.B.; Goncalves, A.P.; Girao, A.V.; Correia, R.; Gasche, T.A.; Branco, J.B. Partial oxidation of methane over bimetallic copper-cerium oxide catalysts. J. Mol. Catal. A Chem. 2010, 320, 47–55. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Goncalves, A.P.; Gasche, T.A.; Ferraria, A.M.; do Rego, A.M.B.; Correia, M.R.; Bola, A.M.; Branco, J.B. Partial oxidation of methane over bimetallic copper- and nickel-actinide oxides (Th, U). J. Alloys Compd. 2010, 497, 249–258. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C.; Gasche, T.A.; Pimenta, G.; Leal, J.P. Low temperature partial oxidation of methane over bimetallic nickel-f block element oxide nanocatalysts. Adv. Synth. Catal. 2014, 356, 3048–3058. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C.; Leal, J.P. Light hydrocarbons production over bimetallic calcium-actinide oxide catalysts using N2O as oxidant. J. Mol. Catal. A Chem. 2014, 390, 45–51. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Gasche, T.A.; Leal, J.P.; Branco, J.B. Methane activation with nitrous oxide over bimetallic oxide Ca-lanthanide nanocatalysts. Mol. Catal. 2017, 443, 155–164. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C. Methanation of CO2 over bimetallic Ni-5f block element (Th, U) oxides. Eur. J. Inorg. Chem. 2019, 1039–1045. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Branco, J.B. Methanation of CO2 over nanostructured nickel-4f block element bimetallic oxides. Int. J. Hydrog. Energy 2019, 44, 6505–6513. [Google Scholar] [CrossRef]
- Zhou, G.L.; Liu, H.R.; Cui, K.K.; Xie, H.M.; Jiao, Z.J.; Zhang, G.Z.; Xiong, K.; Zheng, X.X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrog. Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Clapsaddle, B.J.; Neumann, B.; Wittstock, A.; Sprehn, D.W.; Gash, A.E.; Satcher, J.H.; Simpson, R.L.; Baumer, M. A sol-gel methodology for the preparation of lanthanide-oxide aerogels: Preparation and characterization. J. Solgel Sci. Technol. 2012, 64, 381–389. [Google Scholar] [CrossRef]
- Cho, Y.J.; Yang, H.C.; Eun, H.C.; Kim, E.H.; Kim, I.T. Characteristics of oxidation reaction of rare-earth chlorides for precipitation in LiCl-KCl molten salt by oxygen sparging. J. Nucl. Sci. Technol. 2006, 43, 1280–1286. [Google Scholar] [CrossRef]
- Ben Farhat, L.; Ben Hassen, R.; Dammak, L. Rietveld refinement of YbCoO3 prepared from aqueous solution-gel precursor. Powder Diffr. 2007, 22, 35–39. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhao, M.; Song, Z.X.; Zhao, H.; Liu, W.; Zhao, J.G.; Ma, Z.A.; Xing, Y. The effect of different metal oxides on the catalytic activity of a Co3O4 catalyst for toluene combustion: Importance of the structure-property relationship and surface active species. New J. Chem. 2019, 43, 10868–10877. [Google Scholar] [CrossRef]
- Lago, R.; Bini, G.; Pena, M.A.; Fierro, J.L.G. Partial oxidation of methane to synthesis gas using LnCoO3 perovskites as catalyst precursors. J. Catal. 1997, 167, 198–209. [Google Scholar] [CrossRef]
- Arakawa, T.; Ohara, N.; Shiokawa, J. Reduction of Perovskite Oxide La-EuCoO3 in a Hydrogen Atmosphere. J. Mater. Sci. 1986, 21, 1824–1827. [Google Scholar] [CrossRef]
- Hattori, H. Heterogeneous Basic Catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. New Acids and Bases-Their catalytic Properties; Studies in Surface Science and Catalysis; Delmon, B., Yates, J.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 51, pp. 200–211. [Google Scholar]
- Williams, J.; Jones, R.H.; Thomas, J.M.; Kent, J. A comparison of the catalytic performance of the layered oxychlorides of bismuth, lanthanum and samarium in the conversion of methane to ethylene. Catal. Lett. 1989, 3, 247–255. [Google Scholar] [CrossRef]
- Ueda, W.; Sakyu, F.; Isozaki, T.; Morikawa, Y.; Thomas, J.M. Catalytic oxidative dimerization of methane to form C2-Compounds over Arppe phase oxychlorides of Bi, La and Sm. Catal. Lett. 1991, 10, 83–90. [Google Scholar] [CrossRef]
- Chanaud, P.; Julbe, A.; Larbot, A.; Guizard, C.; Cot, L.; Borges, H.; Fendler, A.G.; Mirodatos, C. Catalytic membrane reactor for oxidative coupling of methane. 1. preparation and characterization of LaOCl membranes. Catal. Today 1995, 25, 225–230. [Google Scholar] [CrossRef]
- Kienneman, A.; Kieffer, R.; Kaddouri, A.; Poix, P.; Rehspringer, J.L. Oxidative coupling of methane over LnLiO2, LnNaO2 and LnOX catalysts (Ln = Sm, Nd, La; X = Cl, Br). Promoting effect of MgO, CaO and SrO. Catal. Today 1990, 6, 409–416. [Google Scholar] [CrossRef]
- Molander, G.A. Application of lanthanide reagents in organic-synthesis. Chem. Rev. 1992, 92, 29–68. [Google Scholar] [CrossRef]
- Yasuda, H.; He, L.N.; Sakakura, T. Cyclic carbonate synthesis from supercritical carbon dioxide and epoxide over lanthanide oxychloride. J. Catal. 2002, 209, 547–550. [Google Scholar] [CrossRef]
- Xu, J.H.; Su, X.; Duan, H.M.; Hou, B.L.; Lin, Q.Q.; Liu, X.Y.; Pan, X.L.; Pei, G.X.; Geng, H.R.; Huang, Y.Q.; et al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar] [CrossRef]
- Tada, S.; Kikuchi, R.; Urasaki, K.; Satokawa, S. Effect of reduction pretreatment and support materials on selective CO methanation over supported Ru catalysts. Appl. Catal. A Gen. 2011, 404, 149–154. [Google Scholar] [CrossRef]
- Ahn, C.I.; Koo, H.M.; Jin, M.; Kim, J.M.; Kim, T.; Suh, Y.W.; Yoon, K.J.; Bae, J.W. Catalyst deactivation by carbon formation during CO hydrogenation to hydrocarbons on mesoporous Co3O4. Microporous Mesoporous Mater. 2014, 188, 196–202. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chang, K.H.; Lu, S.Y.; Hu, C.C. Cobalt oxide aerogels of ideal supercapacitive properties prepared with an epoxide synthetic route. Chem. Mater. 2009, 21, 3228–3233. [Google Scholar] [CrossRef]
- Ren, L.L.; Cui, S.M.; Cao, F.C.; Guo, Q.H. An easy way to prepare monolithic inorganic oxide aerogels. Angew. Chem. Int. Ed. 2014, 53, 10147–10149. [Google Scholar] [CrossRef]
- JCPDS. The Powder Diffraction File; JCPDS: Swarthmore, PA, USA, 1981. [Google Scholar]
- Dekker, F.H.M.; Bliek, A.; Kapteijn, F.; Moulijn, J.A. Analyisis of mass and heat transfer in trasient experiments over heterogeneous catalysts. Chem. Eng. Sci. 1995, 50, 3573–3580. [Google Scholar] [CrossRef]
- Oyama, S.T.; Zhang, X.; Lu, J.; Gu, Y.; Fujitani, T. Epoxidation of propylene with H2 and O2 in the explosive regime in a packed-bed catalytic membrane reactor. J. Catal. 2008, 257, 1–4. [Google Scholar] [CrossRef]
- Froment, G.F.; Bischoff, K.B.; De Wilde, J. Chemical Reactor Analysis and Design; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]

| Catalyst | Conversion CO2 (%) | Yield CH4 (%) | Selectivity CH4 (%) |
|---|---|---|---|
| Co-Ce a,b | 37.0 | 31.7 | 85.7 |
| NiO/CeO2 ref1 a,c | 35.0 | 33.7 | 96.3 |
| 5%Rh/Al2O3 ref2 a,d | 35.6 | 35.5 | 99.8 |
| Co-Ce e | 26.1 | 24.9 | 95.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, J.B.; da Silva, R.P.; Ferreira, A.C. Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature. Catalysts 2020, 10, 704. https://doi.org/10.3390/catal10060704
Branco JB, da Silva RP, Ferreira AC. Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature. Catalysts. 2020; 10(6):704. https://doi.org/10.3390/catal10060704
Chicago/Turabian StyleBranco, Joaquim Badalo, Ricardo Pinto da Silva, and Ana Cristina Ferreira. 2020. "Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature" Catalysts 10, no. 6: 704. https://doi.org/10.3390/catal10060704
APA StyleBranco, J. B., da Silva, R. P., & Ferreira, A. C. (2020). Methanation of CO2 over Cobalt-Lanthanide Aerogels: Effect of Calcination Temperature. Catalysts, 10(6), 704. https://doi.org/10.3390/catal10060704