Stable Continuous Production of γ-Valerolactone from Biomass-Derived Levulinic Acid over Zr–Al-Beta Zeolite Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Zr–Al-Beta Catalyst
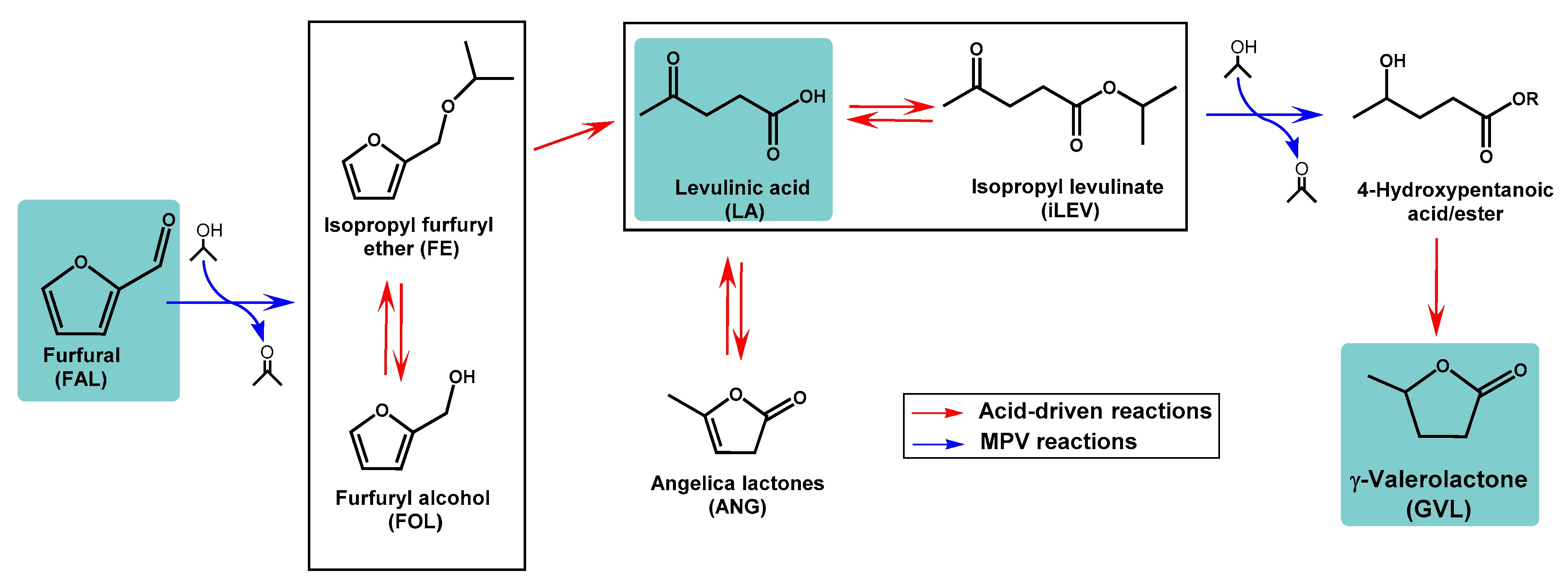
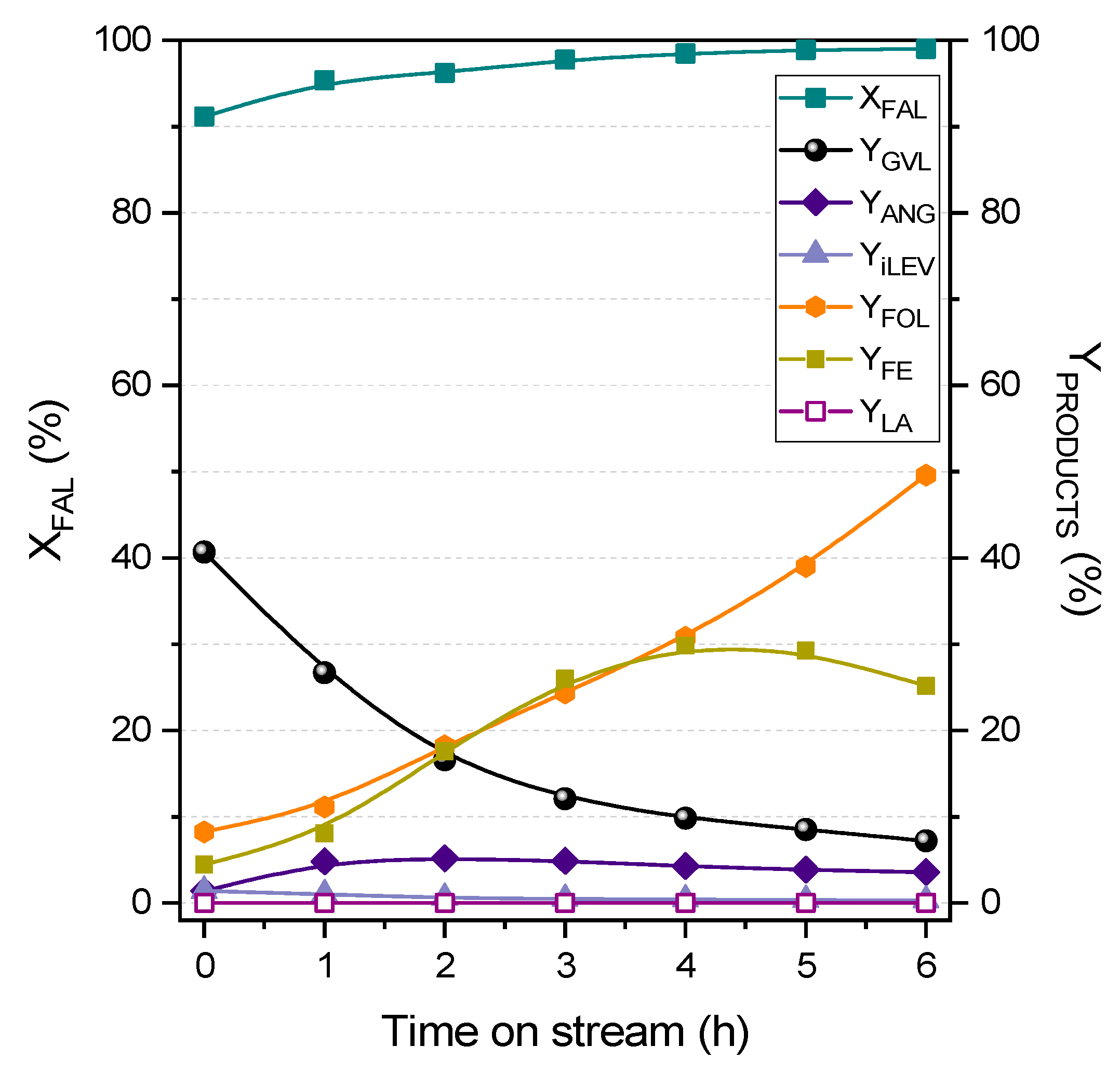
2.2. Fixed-Bed Transformation of Furfural into γ-Valerolactone
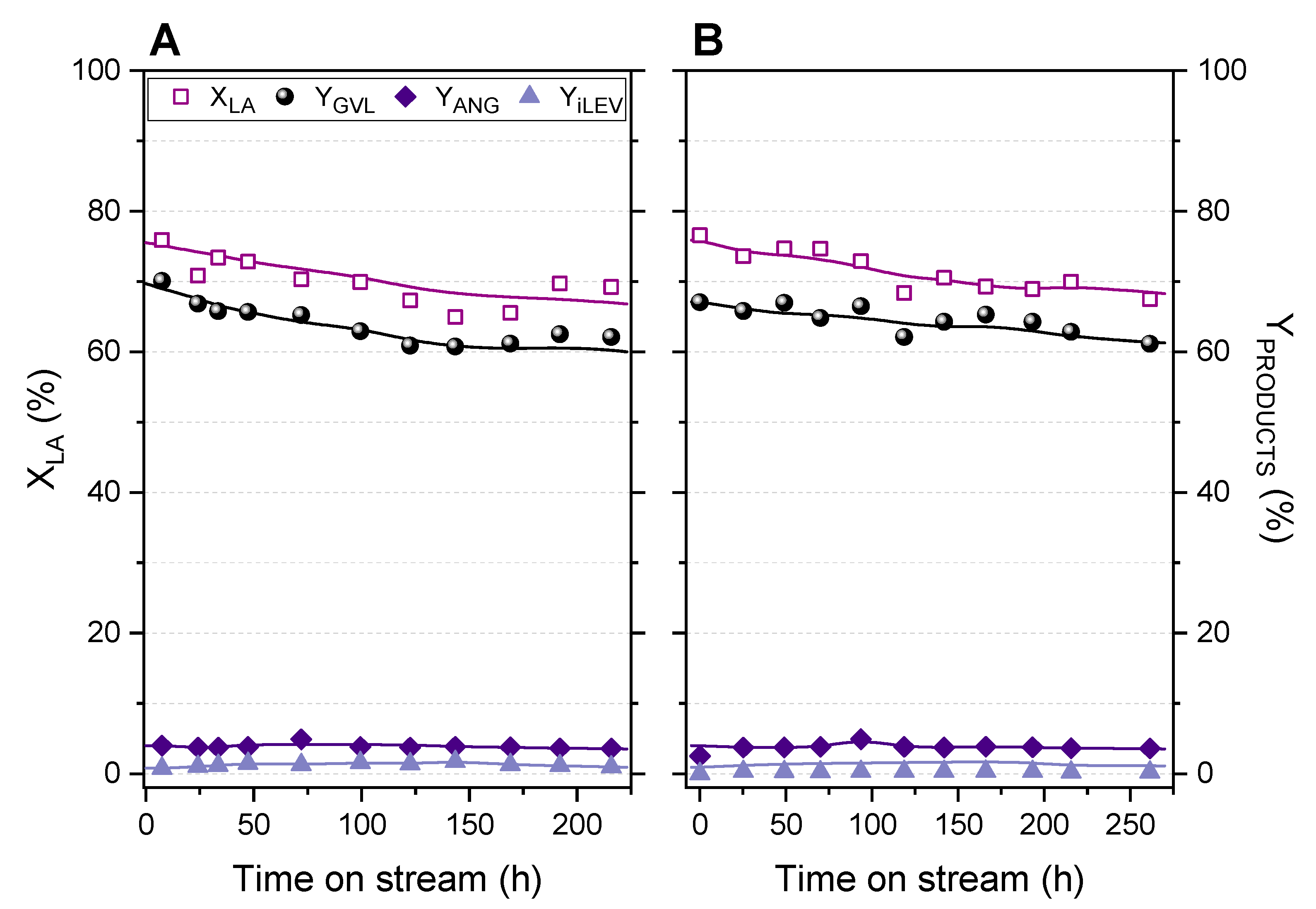
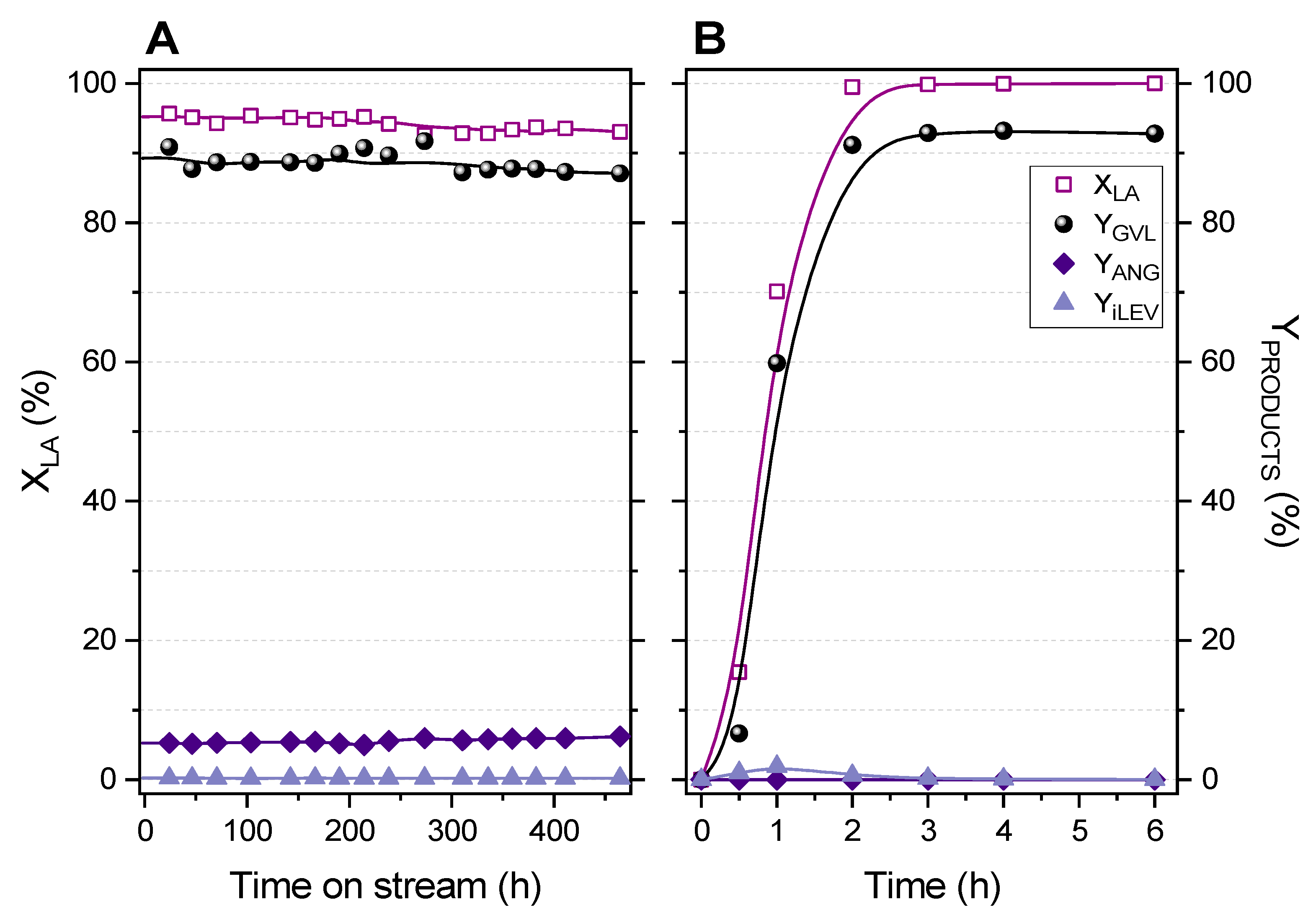
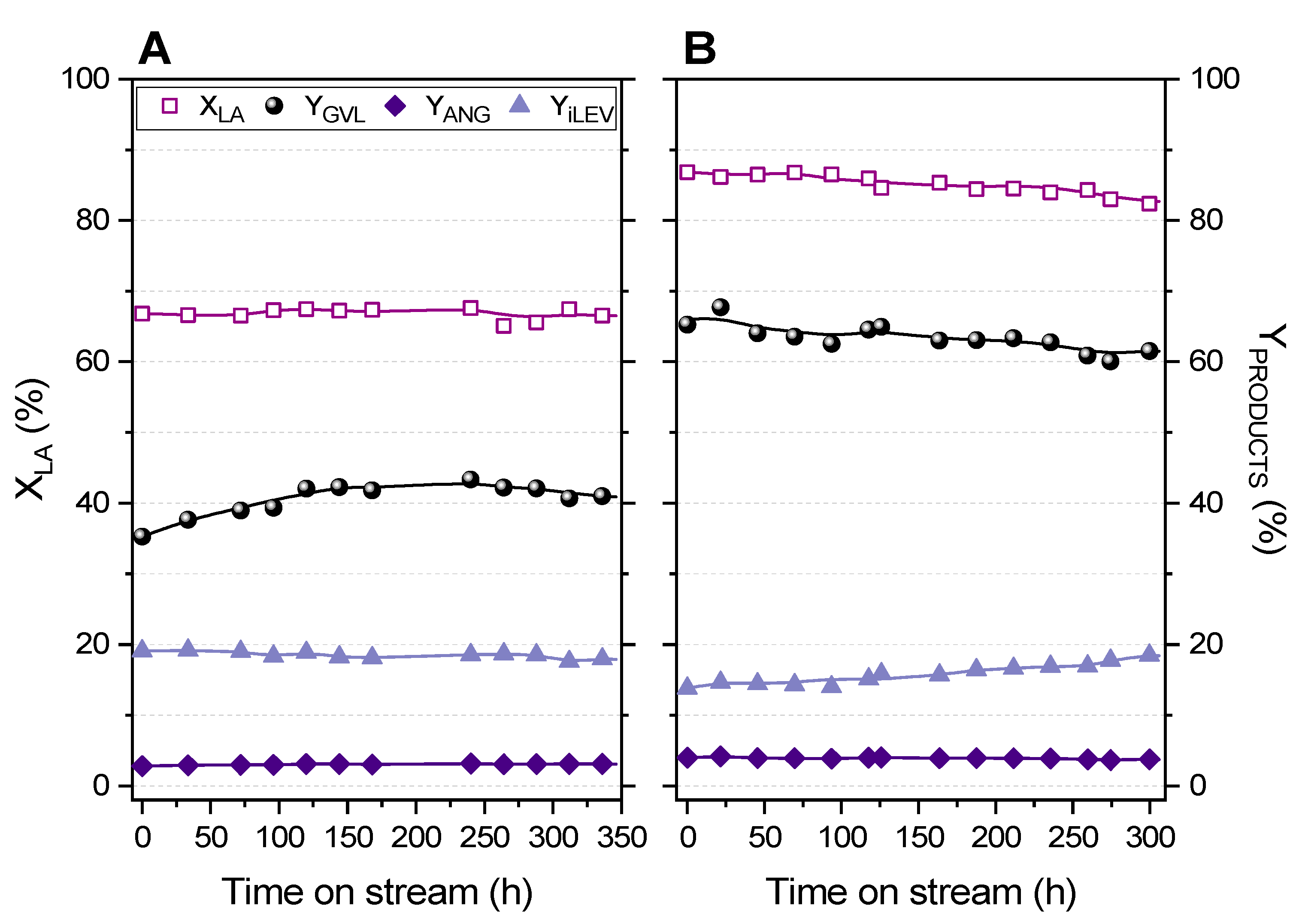
2.3. Fixed-Bed Transformation of Levulinic Acid into γ-Valerolactone
2.4. Analysis of Reaction Variables: LA Concentration, Flow Rate, Temperature
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
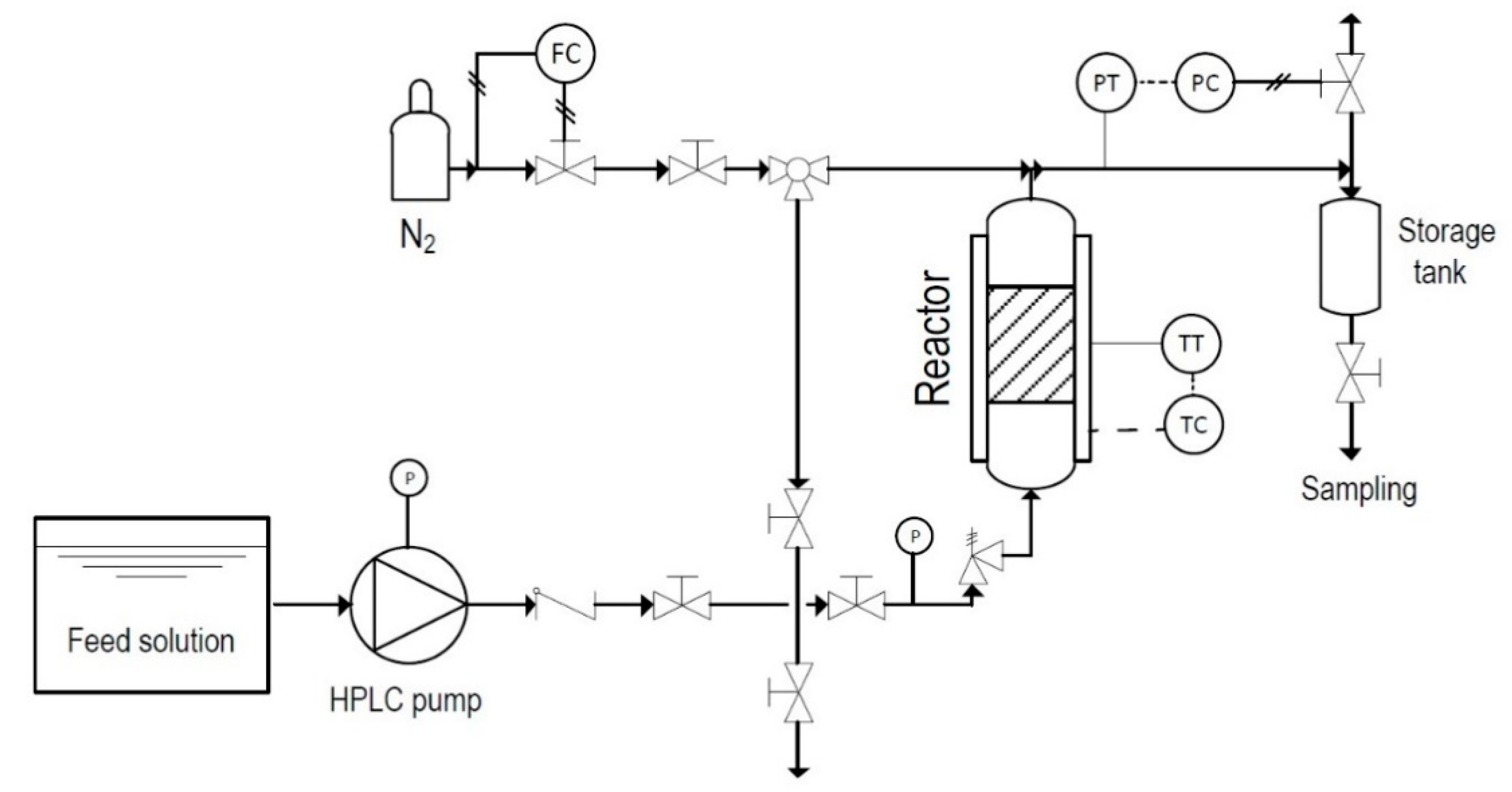
3.3. Fixed-Bed Catalytic Tests
3.4. Batch Catalytic Test
3.5. Products Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arias, P.L.; Cecilia, J.A.; Gandarias, I.; Iglesias, J.; Granados, M.L.; Mariscal, R.; Morales, G.; Moreno-Tost, R.; Maireles-Torres, P. Oxidation of lignocellulosic platform molecules to value-added chemicals using heterogeneous catalytic technologies. Catal. Sci. Technol. 2020, 10, 2721–2757. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Liu, F.; Zheng, A.; Zhang, J.; Sun, Q.; Lewis, J.P.; Zhu, L.; Meng, X.; Xiao, F.-S. Selective Catalytic Production of 5-Hydroxymethylfurfural from Glucose by Adjusting Catalyst Wettability. ChemSusChem 2014, 7, 402–406. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493. [Google Scholar] [CrossRef]
- De Clippel, F.; Dusselier, M.; van Rompaey, R.; Vanelderen, P.; Dijkmans, J.; Makshina, E.V.; Giebeler, L.; Oswald, S.; Baron, G.V.; Denayer, J.F.M.; et al. Fast and Selective Sugar Conversion to Alkyl Lactate and Lactic Acid with Bifunctional Carbon–Silica Catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef]
- Klass, D.L. Biomass for Renewable Energy, Fuels, and Chemicals; Academic Press: Cambridge, MA, USA, 1998; ISBN 9780124109506. [Google Scholar]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Sun, Z.-H.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Ennaert, T.; van Aelst, J.; Dijkmans, J.; de Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2016, 117, 6834–6880. [Google Scholar] [CrossRef]
- Serrano, D.; Melero, J.A.; Morales, G.; Iglesias, J.; Pizarro, P. Progress in the design of zeolite catalysts for biomass conversion into biofuels and bio-based chemicals. Catal. Rev. 2017, 60, 1–70. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Bosch, S.V.D.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Chang, C.; Cen, P.; Ma, X. Levulinic acid production from wheat straw. Bioresour. Technol. 2007, 98, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Fang, Z.; Smith, R.L.; Qi, X. (Eds.) Production of Platform Chemicals from Sustainable Resources; Springer: Singapore, 2017; ISBN 9789811041723. [Google Scholar]
- Mascal, M.; Dutta, S.; Wu, L. Preparation of Compounds from Levulinic Acid. U.S. Patent 16/555,710, 12 May 2020. [Google Scholar]
- Pileidis, F.D.; Titirici, M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value-Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Technical Report; Pacific Northwest National Laboratory: Oak Ridge, TN, USA; US Department of Energy: Oak Ridge, TN, USA, 2004. [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J. The Biofine Process—Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks. In Biorefineries-Industrial Processes and Products; Wiley: Hoboken, NJ, USA, 2008; pp. 139–164. [Google Scholar]
- Morales, G.; Melero, J.A.; Iglesias, J.; Paniagua, M.; López-Aguado, C. From levulinic acid biorefineries to γ-valerolactone (GVL) using a bi-functional Zr–Al-Beta catalyst. React. Chem. Eng. 2019, 4, 1834–1843. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Lee, A.; Wilson, K.; Lee, K.W.A.F. Recent advances in the production of γ-valerolactone from biomass-derived feedstocks via heterogeneous catalytic transfer hydrogenation. J. Chem. Technol. Biotechnol. 2017, 92, 1125–1135. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, N.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of -Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, G.; Zhang, X.; Li, F. Facile Fabrication of Composition-Tuned Ru–Ni Bimetallics in Ordered Mesoporous Carbon for Levulinic Acid Hydrogenation. ACS Catal. 2014, 4, 1419–1425. [Google Scholar] [CrossRef]
- Tan, J.J.; Cui, J.; Cui, X.; Deng, T.; Li, X.; Zhu, Y.; Li, Y. Graphene-Modified Ru Nanocatalyst for Low-Temperature Hydrogenation of Carbonyl Groups. ACS Catal. 2015, 5, 7379–7384. [Google Scholar] [CrossRef]
- Du, X.; Bi, Q.; Liu, Y.-M.; Cao, Y.; Fan, K. Conversion of Biomass-Derived Levulinate and Formate Esters into γ-Valerolactone over Supported Gold Catalysts. ChemSusChem 2011, 4, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.R.H.; Palkovits, R. Development of Heterogeneous Catalysts for the Conversion of Levulinic Acid to γ-Valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Lin, L.; Yan, Z. Catalytic hydrogenation, and oxidation of biomassderived levulinic acid. BioResources 2011, 6, 686–699. [Google Scholar]
- Lázaro, N.; Franco, A.; Ouyang, W.; Balu, A.M.; Romero, A.A.; Luque, R.; Pineda, A. Continuous-Flow Hydrogenation of Methyl Levulinate Promoted by Zr-Based Mesoporous Materials. Catalysts 2019, 9, 142. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, Q.; Wang, J.; Mu, T. Valorization of levulinic acid over non-noble metal catalysts: Challenges and opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Lv, J.; Rong, Z.; Wang, Y.; Xiu, J.; Wang, Y.; Qu, J. Highly efficient conversion of biomass-derived levulinic acid into γ-valerolactone over Ni/MgO catalyst. RSC Adv. 2015, 5, 72037–72045. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Dumesic, J.A. Exploring Meerwein–Ponndorf–Verley Reduction Chemistry for Biomass Catalysis Using a First-Principles Approach. ACS Catal. 2013, 3, 2694–2704. [Google Scholar] [CrossRef]
- Xu, S.; Yu, D.; Ye, T.; Tian, P. Catalytic transfer hydrogenation of levulinic acid to? Valerolactone over a bifunctional tin catalyst. RSC Adv. 2017, 7, 1026–1031. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brønsted and Lewis Acid Sites to produce γ-Valerolactone from Furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B.; Osatiashtiani, A.; Lee, A.; Wilson, K. ZrO2-SBA-15 catalysts for the one-pot cascade synthesis of GVL from furfural. Catal. Sci. Technol. 2018, 8, 4485–4493. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Y.; Li, Y.; Tan, J.J.; Deng, T.; Zheng, H. Direct conversion of carbohydrates to? Valerolactone facilitated by a solvent effect. Green Chem. 2015, 17, 3084–3089. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lewis, J.D.; Román-Leshkov, Y. Lewis Acid Zeolites for Biomass Conversion: Perspectives and Challenges on Reactivity, Synthesis, and Stability. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 663–692. [Google Scholar] [CrossRef]
- López-Aguado, C.; Paniagua, M.; Iglesias, J.; Morales, G.; García-Fierro, J.L.; Melero, J.A. Zr-USY zeolite: Efficient catalyst for the transformation of xylose into bio-products. Catal. Today 2018, 304, 80–88. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.D.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated reduction and acid-catalysed conversion of furfural in alcohol medium using Zr,Al-containing ordered micro/mesoporous silicates. Appl. Catal. B Environ. 2016, 182, 485–503. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Fernandes, A.; Neri, F.; Silva, C.M.; Rocha, S.M.; Ribeiro, M.F.; et al. One-pot conversion of furfural to useful bio-products in the presence of a Sn,Al-containing zeolite beta catalyst prepared via post-synthesis routes. J. Catal. 2015, 329, 522–537. [Google Scholar] [CrossRef]
- Antunes, M.M.; Silva, A.D.F.; Ribeiro, M.F.; Pillinger, M.; Neves, P.; Fernandes, A.; Lima, S.; Valente, A.A. Bulk and composite catalysts combining BEA topology and mesoporosity for the valorisation of furfural. Catal. Sci. Technol. 2016, 6, 7812–7829. [Google Scholar] [CrossRef]
- Song, S.; Di, L.; Wu, G.; Dai, W.; Guan, N.; Li, L. Meso-Zr–Al-beta zeolite as a robust catalyst for cascade reactions in biomass valorization. Appl. Catal. B Environ. 2017, 205, 393–403. [Google Scholar] [CrossRef]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of γ-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: Optimizing the catalyst acid properties and process conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Winoto, H.P.; Fikri, Z.A.; Ha, J.-M.; Park, Y.-K.; Lee, H.; Suh, D.J.; Jae, J. Heteropolyacid supported on Zr-Beta zeolite as an active catalyst for one-pot transformation of furfural to γ-Valerolactone. Appl. Catal. B Environ. 2019, 241, 588–597. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; López-Aguado, C.; López-Aguado, C. Rational Optimization of Reaction Conditions for the One-Pot Transformation of Furfural to γ-Valerolactone over Zr–Al-Beta Zeolite: Toward the Efficient Utilization of Biomass. Ind. Eng. Chem. Res. 2018, 57, 11592–11599. [Google Scholar] [CrossRef]
- Hernández, B.; Paniagua, M.; López-Aguado, C.; Melero, J.A.; Iglesias, J.; Morales, G.; Fierro, J.L.G.; Wolf, P.; Hermans, I. One-pot cascade transformation of xylose into? Valerolactone (GVL) over bifunctional Brønsted–Lewis Zr–Al-beta zeolite. Green Chem. 2016, 18, 5777–5781. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; López-Aguado, C.; Wilson, K.; Osatiashtiani, A. Efficient one-pot production of? Valerolactone from xylose over Zr–Al-Beta zeolite: Rational optimization of catalyst synthesis and reaction conditions. Green Chem. 2017, 19, 5114–5121. [Google Scholar] [CrossRef]
- Paniagua, M.; Morales, G.; Melero, J.A.; Iglesias, J.; López-Aguado, C.; Vidal, N.; Mariscal, R.; Granados, M.L.; Martínez-Salazar, I. Understanding the role of Al/Zr ratio in Zr–Al-Beta zeolite: Towards the one-pot production of GVL from glucose. Catal. Today 2020. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhao, D.; Wang, Y.; Balu, A.M.; Len, C.; Luque, R. Continuous Flow Conversion of Biomass-Derived Methyl Levulinate into γ-Valerolactone Using Functional Metal Organic Frameworks. ACS Sustain. Chem. Eng. 2018, 6, 6746–6752. [Google Scholar] [CrossRef]
- Molina, M.J.C.; Granados, M.L.; Gervasini, A.; Carniti, P. Exploitment of niobium oxide effective acidity for xylose dehydration to furfural. Catal. Today 2015, 254, 90–98. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Heyden, A.; Bond, J.Q. Analysis of Kinetics and Reaction Pathways in the Aqueous-Phase Hydrogenation of Levulinic Acid to Form γ-Valerolactone over Ru/C. ACS Catal. 2014, 4, 1171–1181. [Google Scholar] [CrossRef]
- Lima, C.; Monteiro, J.L.; Lima, T.; Paixão, M.W.; Correa, A. Angelica Lactones: From Biomass-Derived Platform Chemicals to Value-Added Products. ChemSusChem 2017, 11, 25–47. [Google Scholar] [CrossRef] [PubMed]

| Catalyst | Composition a | BET b (m2 g−1) | Vp c (cm3 g−1) | Acidity | |||||
|---|---|---|---|---|---|---|---|---|---|
| % Al | % Zr | Si/Al | Si/Zr | Al/Zr | (mmol H+ g−1) d | B/L Ratio e | |||
| Beta (parent) | 2.0 | 0.0 | 22 | - | - | 623 | 0.36 | 0.41 | 0.56 |
| Zr–Al-Beta | 0.3 | 4.5 | 156 | 32 | 0.20 | 685 | 0.38 | 0.29 | 0.05 |
| Experiment # | Temperature (°C) | Flow Rate (mL/min) | LA Concentr. (g/L) | YGVL b (%) | SGVL c (%) | Productivity (gGVL/h·gcat) |
|---|---|---|---|---|---|---|
| 1 | 150 | 0.050 | 6 | 60 | 88 | 2.2 |
| 2 | 170 | 0.050 | 6 | 90 | 95 | 3.4 |
| 3 | 170 | 0.025 | 60 | 75 | 88 | 14.0 |
| 4 | 170 | 0.025 | 220 | 42 | 62 | 28.7 |
| 5 | 190 | 0.025 | 220 | 65 | 76 | 44.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Aguado, C.; Paniagua, M.; Melero, J.A.; Iglesias, J.; Juárez, P.; López Granados, M.; Morales, G. Stable Continuous Production of γ-Valerolactone from Biomass-Derived Levulinic Acid over Zr–Al-Beta Zeolite Catalyst. Catalysts 2020, 10, 678. https://doi.org/10.3390/catal10060678
López-Aguado C, Paniagua M, Melero JA, Iglesias J, Juárez P, López Granados M, Morales G. Stable Continuous Production of γ-Valerolactone from Biomass-Derived Levulinic Acid over Zr–Al-Beta Zeolite Catalyst. Catalysts. 2020; 10(6):678. https://doi.org/10.3390/catal10060678
Chicago/Turabian StyleLópez-Aguado, Clara, Marta Paniagua, Juan A. Melero, Jose Iglesias, Pablo Juárez, Manuel López Granados, and Gabriel Morales. 2020. "Stable Continuous Production of γ-Valerolactone from Biomass-Derived Levulinic Acid over Zr–Al-Beta Zeolite Catalyst" Catalysts 10, no. 6: 678. https://doi.org/10.3390/catal10060678
APA StyleLópez-Aguado, C., Paniagua, M., Melero, J. A., Iglesias, J., Juárez, P., López Granados, M., & Morales, G. (2020). Stable Continuous Production of γ-Valerolactone from Biomass-Derived Levulinic Acid over Zr–Al-Beta Zeolite Catalyst. Catalysts, 10(6), 678. https://doi.org/10.3390/catal10060678










