Glycerol Dehydration to Acrolein over Supported Vanadyl Orthophosphates Catalysts
Abstract
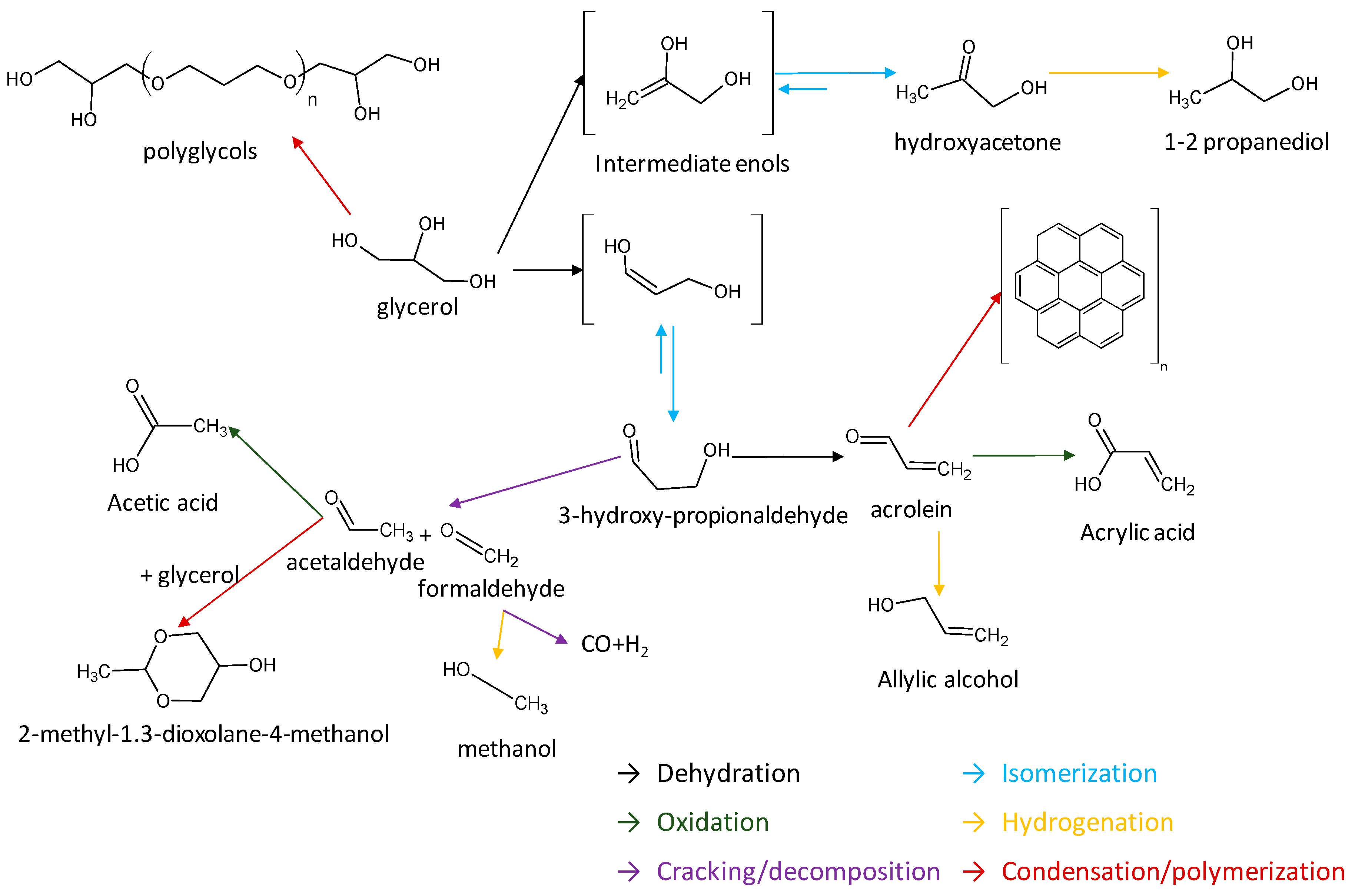
1. Introduction
2. Results
2.1. Catalysts Characterization
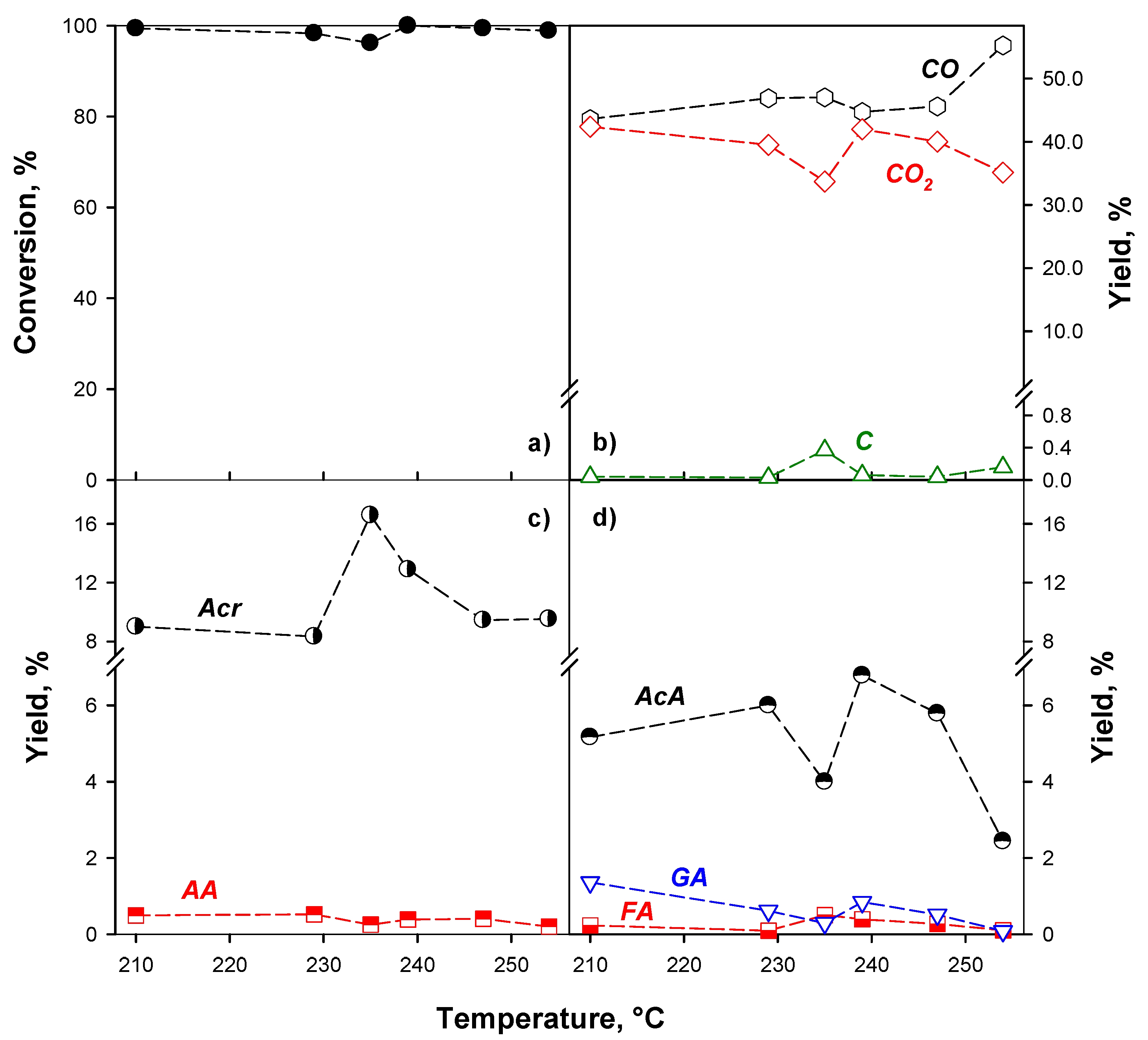
2.2. Catalytic Activity
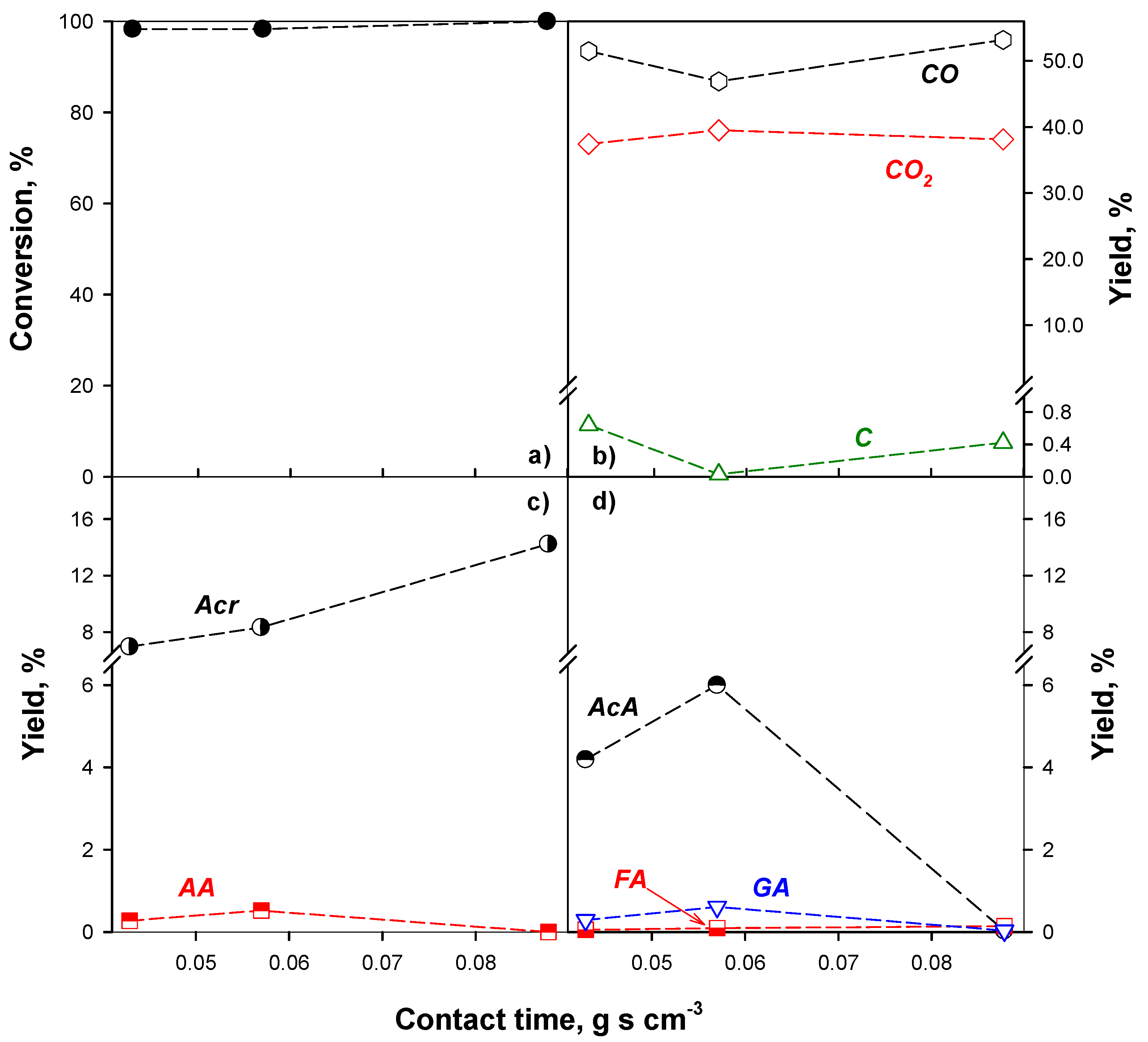
2.2.1. Effect of the Reaction Conditions
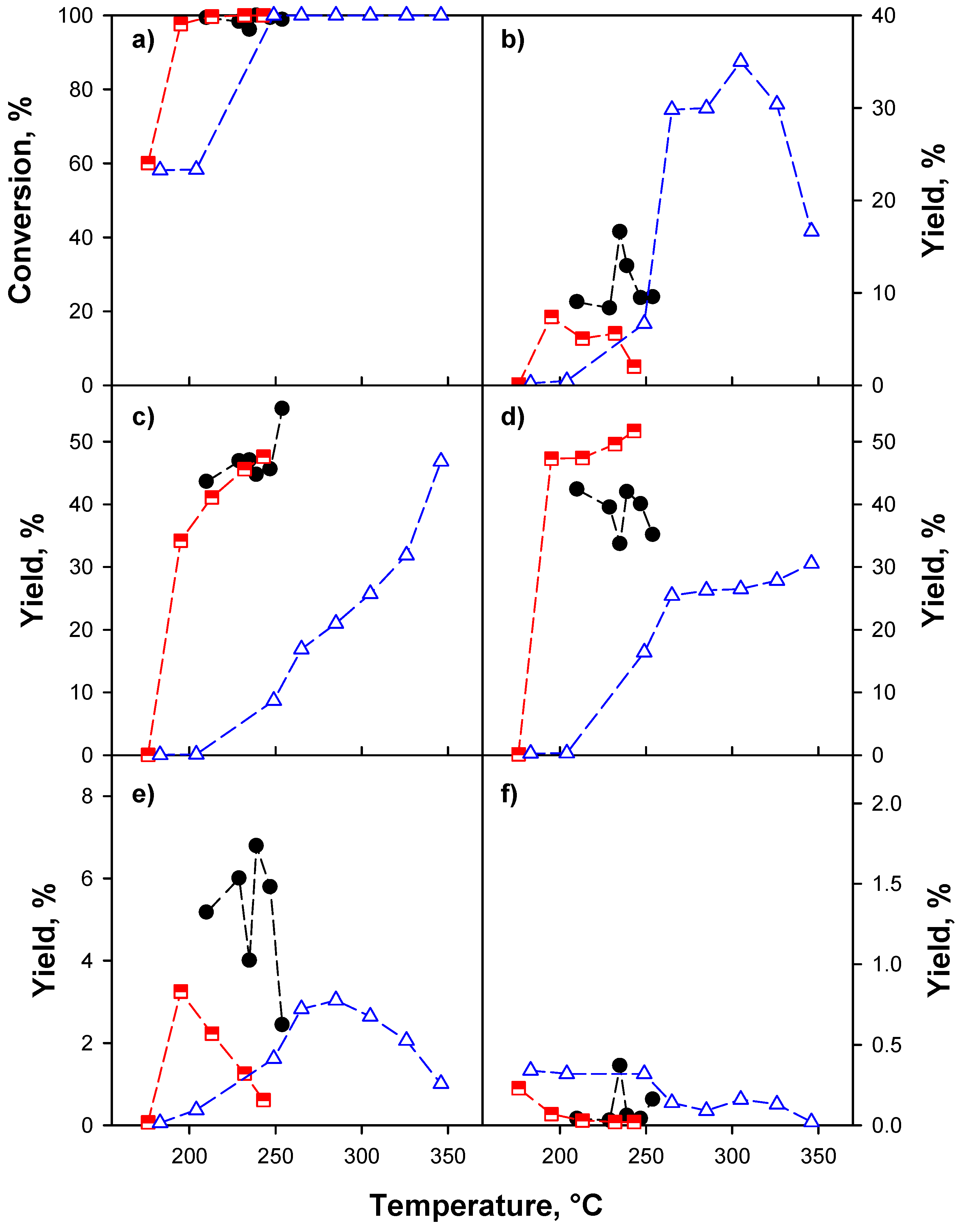
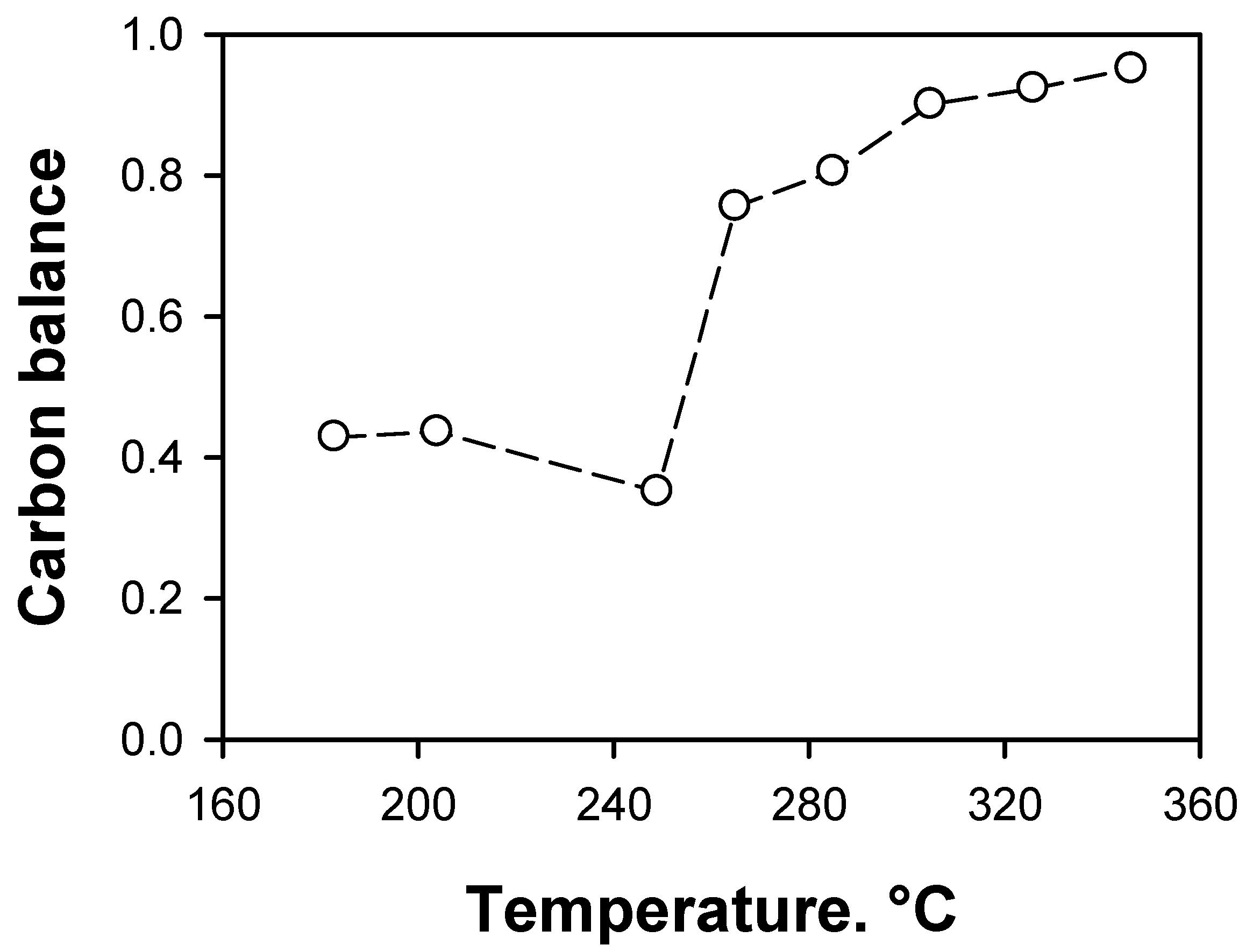
2.2.2. Effect of the Support
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Physico-Chemical Characterizations
4.3. Catalytic Tests
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass-A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Dulf, F.V.; Pop, O.L.; Socaciu, C. L (+)-lactic acid production by pellet-form Rhizopus oryzae NRRL 395 on biodiesel crude glycerol. Microb. Cell Factories 2013, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.; Dulf, F.V.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Factories 2018, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, F.; Sudhakar, M.; Wang, N.; Dai, J.; Liu, L. Gas-phase dehydration of glycerol to acrolein catalyzed by hybrid acid sites derived from transition metal hydrogen phosphate and meso-HZSM-5. Catal. Today 2019, 332, 20–27. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C. Della Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Dharmadi, Y.; Murarka, A.; Gonzalez, R. Anaerobic fermentation of glycerol byEscherichia coli: A new platform for metabolic engineering. Biotechnol. Bioeng. 2006, 94, 821–829. [Google Scholar] [CrossRef]
- Deckwer, W.-D. Microbial conversion of glycerol to 1,3-propanediol. FEMS Microbiol. Rev. 1995, 16, 143–149. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Stelmachowski, M. Utilization of glycerol, a by-product of the transestrification process of vegetable oils: A review. Ecol. Chem. Eng. S 2011, 18, 9–30. [Google Scholar]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Li, C.; He, B.; Ling, Y.; Tsang, C.-W.; Liang, C. Glycerol hydrogenolysis to n-propanol over Zr-Al composite oxide-supported Pt catalysts. Chin. J. Catal. 2018, 39, 1121–1128. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.-M.; Morgan, P.J.; Jackson, R.E.; Liu, X.; Saunders, G.C.; Lorenzini, F.; Marr, A.C. Designing effective homogeneous catalysis for glycerol valorisation: Selective synthesis of a value-added aldehyde from 1,3-propanediol via hydrogen transfer catalysed by a highly recyclable, fluorinated Cp*Ir(NHC) catalyst. Catal. Today 2018, 307, 248–259. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Perspective on catalyst development for glycerol reduction to C3 chemicals with molecular hydrogen. Res. Chem. Intermed. 2018, 44, 3879–3903. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, M.; Yao, Y.; Wang, J.; Wang, D.; Zhang, M.; Li, Y. One-pot catalytic production of 1,3-propanediol and γ-valerolactone from glycerol and levulinic acid. Catal. Today 2018, 302, 217–226. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Ipsakis, D.; Lemonidou, A.A. Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. React. Chem. Eng. 2018, 3, 559–571. [Google Scholar] [CrossRef]
- De Abreu, T.H.; Meyer, C.I.; Padró, C.; Martins, L. Acidic V-MCM-41 catalysts for the liquid-phase ketalization of glycerol with acetone. Microporous Mesoporous Mater. 2019, 273, 219–225. [Google Scholar] [CrossRef]
- Basile, F.; Cavani, F.; Chieregato, A.; Concepción, P.; Liosi, G.; López Nieto, J.M.; Soriano, M.D.; Trevisanut, C. Glycerol oxidehydration into acrolein and acrylic acid over W/V/Nb bronzes with hexagonal structure. DGMK Tag. 2012, 2012, 189–193. [Google Scholar]
- Bozga, E.; Plesu, V.; Bozga, G. Conversion of Glycerol to Propanediol and Acrolein by Heterogeneous Catalysis. Rev. Chim. Bucharest 2011, 62, 646–654. [Google Scholar]
- Célerier, S.; Morisset, S.; Batonneau-Gener, I.; Belin, T.; Younes, K.; Batiot-Dupeyrat, C. Glycerol dehydration to hydroxyacetone in gas phase over copper supported on magnesium oxide (hydroxide) fluoride catalysts. Appl. Catal. A Gen. 2018, 557, 135–144. [Google Scholar] [CrossRef]
- Christy, S.; Noschese, A.; Lomelí-Rodriguez, M.; Greeves, N.; Lopez-Sanchez, J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Deleplanque, J.; Dubois, J.L.; Devaux, J.F.; Ueda, W. Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts. Catal. Today 2010, 157, 351–358. [Google Scholar] [CrossRef]
- Gabrysch, T.; Peng, B.; Bunea, S.; Dyker, G.; Muhler, M. The Role of Metallic Copper in the Selective Hydrodeoxygenation of Glycerol to 1,2-Propanediol over Cu/ZrO2. ChemCatChem 2018, 10, 1344–1350. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P.; Bozell, J.J. A Comparative Review of Petroleum-Based and Bio-Based Acrolein Production. ChemSusChem 2012, 5, 1162–1180. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, X.; Huang, H.; Huang, J.; Sudhakar, M.; Liu, L. High-performance phosphate supported on HZSM-5 catalyst for dehydration of glycerol to acrolein. Chin. J. Chem. Eng. 2018, 26, 1031–1040. [Google Scholar] [CrossRef]
- Ding, J.; Ma, T.; Shao, R.; Xu, W.; Wang, P.; Song, X.; Guan, R.; Yeung, K.; Han, W. Gas phase dehydration of glycerol to acrolein on an amino siloxane-functionalized MCM-41 supported Wells–Dawson type H6P2W18O62 catalyst. New. J. Chem. 2018, 42, 14271–14280. [Google Scholar] [CrossRef]
- Possato, L.G.; Chaves, T.F.; Cassinelli, W.H.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. The multiple benefits of glycerol conversion to acrolein and acrylic acid catalyzed by vanadium oxides supported on micro-mesoporous MFI zeolites. Catal. Today 2017, 289, 20–28. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, J.M.; Santamaría González, J.; Moreno-Tost, R.; Maireles-Torres, P. WO3 supported on Zr doped mesoporous SBA-15 silica for glycerol dehydration to acrolein. Appl. Catal. A Gen. 2016, 516, 30–40. [Google Scholar] [CrossRef]
- Diallo, M.M.; Laforge, S.; Pouilloux, Y.; Mijoin, J. Highly Efficient Dehydration of Glycerol to Acrolein Over Isomorphously Substituted Fe-MFI Zeolites. Catal. Lett. 2018, 148, 2283–2303. [Google Scholar] [CrossRef]
- Beerthuis, R.; Huang, L.; Shiju, N.R.; Rothenberg, G.; Shen, W.; Xu, H. Facile Synthesis of a Novel Hierarchical ZSM-5 Zeolite: A Stable Acid Catalyst for Dehydrating Glycerol to Acrolein. ChemCatChem 2018, 10, 211–221. [Google Scholar] [CrossRef]
- Han, Q.; Ge, J.; Yang, Y.; Liu, B. Nickel substituted tungstophosphoric acid supported on Y-ASA composites as catalysts for the dehydration of gas-phase glycerol to acrolein. React. Kinet. Mech. Catal. 2019, 127, 331–343. [Google Scholar] [CrossRef]
- Ding, J.; Yan, C.; Ma, T.; Shao, R.; Xu, W.; Wang, P. Gas phase dehydration of glycerol to acrolein over NaHSO4@Zr-MCM-41 catalyst. Can. J. Chem. Eng. 2019, 97, 1152–1159. [Google Scholar] [CrossRef]
- Kraleva, E.; Atia, H. Keggin-type heteropolyacids supported on sol–gel oxides as catalysts for the dehydration of glycerol to acrolein. React. Kinet. Mech. Catal. 2019, 126, 103–117. [Google Scholar] [CrossRef]
- Shan, J.; Li, Z.; Zhu, S.; Liu, H.; Li, J.; Wang, J.; Fan, W. Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts 2019, 9, 121. [Google Scholar] [CrossRef]
- Lauriol-Garbey, P.; Millet, J.M.M.; Loridant, S.; Bellire-Baca, V.; Rey, P. New efficient and long-life catalyst for gas-phase glycerol dehydration to acrolein. J. Catal. 2011, 281, 362–370. [Google Scholar] [CrossRef]
- Ding, J.; Ma, T.; Cui, M.; Shao, R.; Guan, R.; Wang, P. Gas phase dehydration of glycerol to acrolein over Cs2.5H0.5PW12O40/Zr-MCM-41 catalysts prepared by supercritical impregnation. Mol. Catal. 2018, 461, 1–9. [Google Scholar] [CrossRef]
- Srinivasa Rao, G.; Hussain, S.; Chary, K.V.R. Porous zirconium phosphate solid acid catalysts with variable Zr/P ratio for gas phase glycerol dehydration to acrolein. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25773–25781. [Google Scholar]
- Ding, J.; Ma, T.; Yun, Z.; Shao, R. Heteropolyacid (H3PW12O40) supported MCM-41: An effective solid acid catalyst for the dehydration of glycerol to acrolein. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 1511–1516. [Google Scholar] [CrossRef]
- Wang, F.; Dubois, J.L.; Ueda, W. Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen. J. Catal. 2009, 268, 260–267. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Zakaria, Z.Y. Gas phase selective conversion of glycerol to acrolein over supported silicotungstic acid catalyst. J. Ind. Eng. Chem. 2016, 34, 300–312. [Google Scholar] [CrossRef]
- Huang, L.; Qin, F.; Huang, Z.; Zhuang, Y.; Ma, J.; Xu, H.; Shen, W. Metal–Organic Framework Mediated Synthesis of Small-Sized γ-Alumina as a Highly Active Catalyst for the Dehydration of Glycerol to Acrolein. ChemCatChem 2018, 10, 381–386. [Google Scholar] [CrossRef]
- Ma, T.; Yun, Z.; Xu, W.; Chen, L.; Li, L.; Ding, J.; Shao, R. Pd-H3PW12O40/Zr-MCM-41: An Efficient Catalyst for the Sustainable Dehydration of Glycerol to Acrolein; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nadji, L.; Massó, A.; Delgado, D.; Issaadi, R.; Rodriguez-Aguado, E.; Rodriguez-Castellón, E.; Nieto, J.M.L. Gas phase dehydration of glycerol to acrolein over WO3-based catalysts prepared by non-hydrolytic sol–gel synthesis. RSC Adv. 2018, 8, 13344–13352. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Yun, Z. Catalytic conversion of glycerol to acrolein over MCM-41 by the grafting of phosphorus species. Can. J. Chem. Eng. 2016, 94, 924–930. [Google Scholar] [CrossRef]
- Lauriol-Garbey, P.; Loridant, S.; Bellière-Baca, V.; Rey, P.; Millet, J.M.M. Gas phase dehydration of glycerol to acrolein over WO3/ZrO2 catalysts: Improvement of selectivity and stability by doping with SiO2. Catal. Commun. 2011, 16, 170–174. [Google Scholar] [CrossRef]
- Znaiguia, R.; Brandhorst, L.; Christin, N.; Bellière Baca, V.; Rey, P.; Millet, J.M.M.; Loridant, S. Toward longer life catalysts for dehydration of glycerol to acrolein. Microporous Mesoporous Mater. 2014, 196, 97–103. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Capron, M.; Dumeignil, F. Towards the sustainable production of acrolein by glycerol dehydration. ChemSusChem 2009, 2, 719–730. [Google Scholar] [CrossRef]
- Jiang, X.C.; Zhou, C.H.; Tesser, R.; Di Serio, M.; Tong, D.S.; Zhang, J.R. Coking of Catalysts in Catalytic Glycerol Dehydration to Acrolein. Ind. Eng. Chem. Res. 2018, 57, 10736–10753. [Google Scholar] [CrossRef]
- Dos Santos, M.B.; Andrade, H.M.C.; Mascarenhas, A.J.S. Reduced coke formation during the gas phase oxidative dehydration of glycerol over ferrierite zeolites synthesized in fluoride medium. Microporous Mesoporous Mater. 2016, 223, 105–113. [Google Scholar] [CrossRef]
- Park, H.; Yun, Y.S.; Kim, T.Y.; Lee, K.R.; Baek, J.; Yi, J. Kinetics of the dehydration of glycerol over acid catalysts with an investigation of deactivation mechanism by coke. Appl. Catal. B Environ. 2015, 176–177, 1–10. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S. Coke-tolerant SiW20-Al/Zr10 catalyst for glycerol dehydration to acrolein. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 1697–1710. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Lisi, L.; Mattogno, G.; Patrono, P.; Ruoppolo, G.; Russo, G. Oxidative dehydrogenation of ethane on γ-Al2O3 supported vanadyl and iron vanadyl phosphates: Physico-chemical characterisation and catalytic activity. Appl. Catal. A Gen. 2002, 226, 41–48. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Landi, G.; Lisi, L.; Patrono, P.; Pinzari, F. Effect of the support on the catalytic properties of vanadyl phosphate in the oxidative dehydrogenation of propane. J. Mol. Catal. A Chem. 2010, 329, 50–56. [Google Scholar] [CrossRef]
- Cavani, F.; Luciani, S.; Esposti, E.D.; Cortelli, C.; Leanza, R. Surface Dynamics of A Vanadyl Pyrophosphate Catalyst for n-Butane Oxidation to Maleic Anhydride: An In Situ Raman and Reactivity Study of the Effect of the P/V Atomic Ratio. Chem. A Eur. J. 2010, 16, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, A.; Cavani, F.; Folco, F.; Luciani, S.; Cortelli, C.; Leanza, R. The design of a new ZrO2-supported V/P/O catalyst for n-butane oxidation to maleic anhydride: The build-up of the active phase during thermal treatment. In Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2010; pp. 204–210. [Google Scholar]
- Chieregato, A.; Bandinelli, C.; Concepción, P.; Soriano, M.D.; Puzzo, F.; Basile, F.; Cavani, F.; Nieto, J.M.L. Structure-Reactivity Correlations in Vanadium-Containing Catalysts for One-Pot Glycerol Oxidehydration to Acrylic Acid. ChemSusChem 2017, 10, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Lisi, L.; Volta, J.C. Oxidation of propane to acrylic acid over vanadyl pyrophosphate: Modifications of the structural and acid properties during the precursor activation and their relationship with catalytic performances. J. Mol. Catal. A Chem. 2004, 222, 175–181. [Google Scholar] [CrossRef]
- Pethan Rajan, N.; Rao, G.S.; Pavankumar, V.; Chary, K.V.R. Vapour phase dehydration of glycerol over VPO catalyst supported on zirconium phosphate. Catal. Sci. Technol. 2014, 4, 81–92. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Lisi, L.; Mattogno, G.; Patrono, P.; Ruoppolo, G. Effect of the support on the surface composition of vanadium phosphate catalysts in the oxidative dehydrogenation of ethane. In Surface and Interface Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 737–740. [Google Scholar]
- García-Sancho, C.; Cecilia, J.A.; Mérida-Robles, J.M.; Santamaría González, J.; Moreno-Tost, R.; Infantes-Molina, A.; Maireles-Torres, P. Effect of the treatment with H3PO4on the catalytic activity of Nb2O5supported on Zr-doped mesoporous silica catalyst. Case study: Glycerol dehydration. Appl. Catal. B Environ. 2018, 221, 158–168. [Google Scholar] [CrossRef]
- Vieira, L.H.; Carvalho, K.T.G.; Urquieta-González, E.A.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. Effects of crystal size, acidity, and synthesis procedure on the catalytic performance of gallium and aluminum MFI zeolites in glycerol dehydration. J. Mol. Catal. A Chem. 2016, 422, 148–157. [Google Scholar] [CrossRef]
- Wang, F.; Dubois, J.L.; Ueda, W. Catalytic performance of vanadium pyrophosphate oxides (VPO) in the oxidative dehydration of glycerol. Appl. Catal. A Gen. 2010, 376, 25–32. [Google Scholar] [CrossRef]
- Xie, Q.; Li, S.; Gong, R.; Zheng, G.; Wang, Y.; Xu, P.; Duan, Y.; Yu, S.; Lu, M.; Ji, W.; et al. Microwave-assisted catalytic dehydration of glycerol for sustainable production of acrolein over a microwave absorbing catalyst. Appl. Catal. B Environ. 2019, 243, 455–462. [Google Scholar] [CrossRef]
- Katryniok, B.; Meléndez, R.; Bellière-Baca, V.; Rey, P.; Dumeignil, F.; Fatah, N.; Paul, S. Catalytic Dehydration of Glycerol to Acrolein in a Two-Zone Fluidized Bed Reactor. Front. Chem. 2019, 7, 127. [Google Scholar] [CrossRef]
- Lauriol-Garbey, P.; Postole, G.; Loridant, S.; Auroux, A.; Belliere-Baca, V.; Rey, P.; Millet, J.M.M. Acid-base properties of niobium-zirconium mixed oxide catalysts for glycerol dehydration by calorimetric and catalytic investigation. Appl. Catal. B Environ. 2011, 106, 94–102. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, R.; Xia, G. Sequential Dehydration and Oxidation of Biodiesel-derived Crude Glycerol into Acrylic Acid. Russ. J. Appl. Chem. 2018, 91, 235–244. [Google Scholar] [CrossRef]

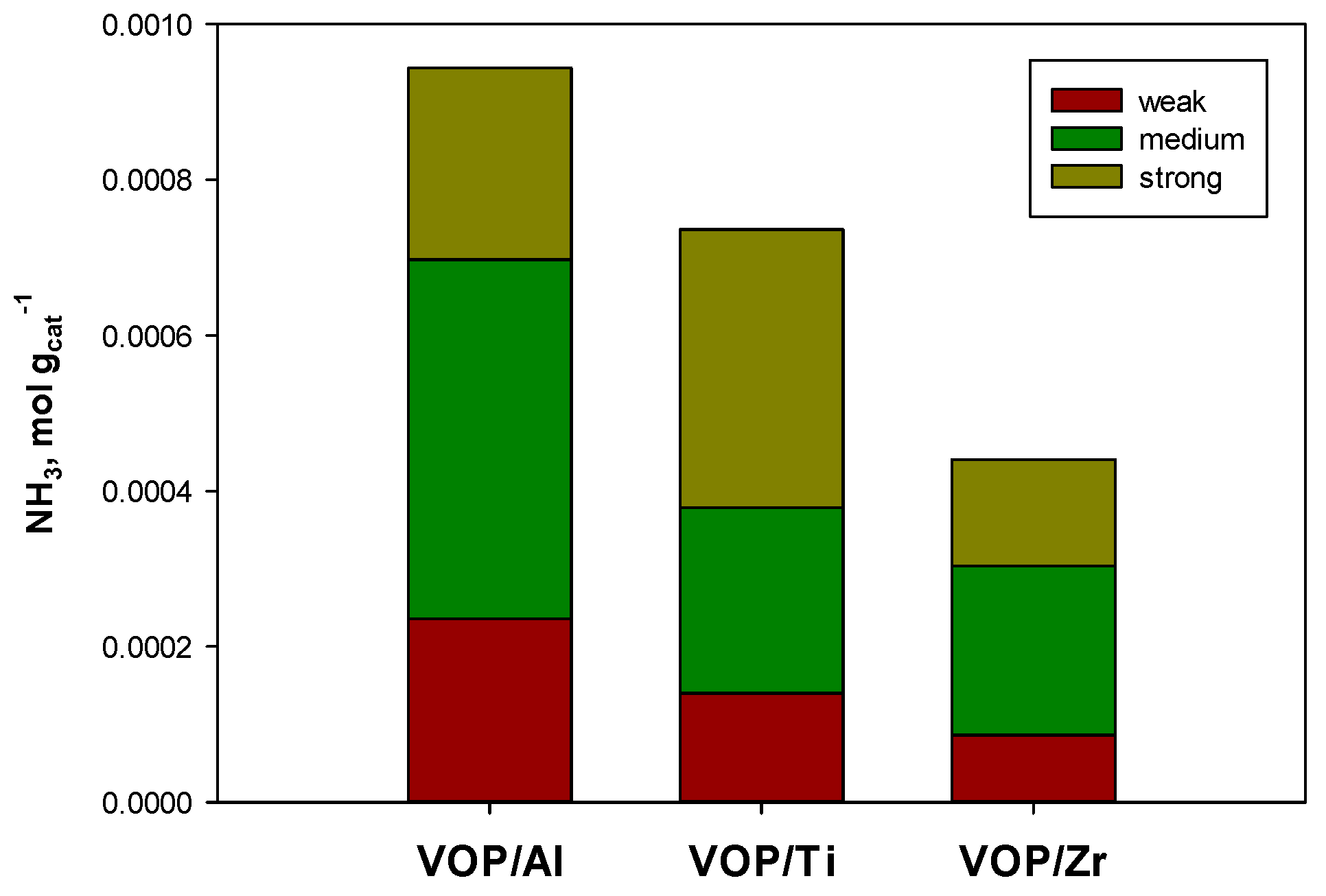


| Catalyst | Nominal VOP Content | Support Surface Area | Catalyst Surface Area | H2/V a |
|---|---|---|---|---|
| VOP/Ti | 9.6 | 125 | 125 | 0.71 |
| VOP/Al | 14.0 | 190 | 183 | 0.67 |
| VOP/Zr | 3.6 | 49 | 43 | 1.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruoppolo, G.; Landi, G.; Di Benedetto, A. Glycerol Dehydration to Acrolein over Supported Vanadyl Orthophosphates Catalysts. Catalysts 2020, 10, 673. https://doi.org/10.3390/catal10060673
Ruoppolo G, Landi G, Di Benedetto A. Glycerol Dehydration to Acrolein over Supported Vanadyl Orthophosphates Catalysts. Catalysts. 2020; 10(6):673. https://doi.org/10.3390/catal10060673
Chicago/Turabian StyleRuoppolo, Giovanna, Gianluca Landi, and Almerinda Di Benedetto. 2020. "Glycerol Dehydration to Acrolein over Supported Vanadyl Orthophosphates Catalysts" Catalysts 10, no. 6: 673. https://doi.org/10.3390/catal10060673
APA StyleRuoppolo, G., Landi, G., & Di Benedetto, A. (2020). Glycerol Dehydration to Acrolein over Supported Vanadyl Orthophosphates Catalysts. Catalysts, 10(6), 673. https://doi.org/10.3390/catal10060673