1. Introduction
β-Glucuronidases (β-GIc) is a lysosomal acid hydrolase and member of the glucosidase family that catalyzes the breakdown of complex carbohydrates. It is ubiquitous being found in mammalian tissues and body fluids and also in plants, fishes, insects, and mollusks [
1,
2]. It elicits different physiological responses as a consequence of the principal function to catalytically cleave the glycosidic bond of glycans. Moreover, β-GIc is regularly used for in vitro drug metabolism studies, as well as in routine drug testing applications [
3]. Glucuronidation (conjugation with glucuronic acid) by the uracil-diphosphate-glucuronosyltransferase (UGT) family of enzymes plays an important role in the metabolic fate of many drugs and other xenobiotics. This biosynthesis reaction also has a role in the conjugation and excretion of endogenous substrates, such as steroids, bilirubin, and bile acids. The glucuronides formed are more polar (water soluble) than the main organic substrate and in general excreted through the kidney. Glucuronide tags complicates the direct and quantitative analysis of controlled substances and therefore sample processing necessitates the deconjugation of β-d-glucuronides from target molecules. β-GIc cleavage of glucuronide tags is commonly performed in analytical labs as the chemical approach is known to cause the formation of multiple by-products for a given conjugate [
4].
Aside from this in vitro application, β-GIc have an increasing number of biocatalytic utilization in pharmaceutical and food industry [
5,
6,
7]. For instance, hydrolyzation of one of the glucuronic acid moieties of Glycyrrhizin (GL), a typical triterpenoid saponin efficacious against inflammation, allergy, tumor, and asthma can overcome its low target organ bioavailability. A successful β-GIc mediated biotransformation of GL not only rendered the target pharmaceutical intermediate but also a functional sweetener that is 940-fold sweeter than sucrose [
5]. β-GIc has been also used as a tool in enzyme prodrug therapy (EPT) to enable localized conversion of inert inactive prodrugs. Glucuronide prodrugs, including prodrugs of effective antineoplastic activity, do not readily enter cells due to their charged carboxy group, minimizing interactions with endogenous β-GIc located inside lysosomes. Prodrug activation selectively occurs at target sites where exogenous β-GIc is applied.
For all the above mentioned applications, integration of β-GIc to different materials has provided a number of advantages that circumvented the problems associated with the use of the enzyme in its soluble form. In general, enzyme immobilization has proved essential to achieve better specific activities, robustness, and reusability of biocatalysts. However, obtention of active and stable immobilized preparations of enzymes remains a challenge as one is still unable to predict the behavior of an enzyme when cross talking with a particular material. What it is known is that the nature of the carriers as well as the chemistry used to associate them to the biocatalysts may affect the physico-chemical properties of the enzymes and consequently, impact their functionality [
8,
9,
10,
11]. Given the multiple applications of β-GIc, the pursuit for better immobilization strategies could still provide better immobilized biocatalysts.
A recent trend in the field of enzyme immobilization is the use of combined or hybrid materials [
12,
13]. In this approach, different materials are involved with a specific aim in aiding the catalysis, the stability of the biocatalysts, or providing an added advantageous property. Such is the case of a recent composite that we have developed in our laboratory that combined biomimetic silica with magnetic nanoparticles (MNPs) and a horseradish peroxidase. The biomimetic synthesis of silica nanoparticles has various advantages, such as it is a mild and fast reaction, wherein the formation of the nanoparticles takes place within minutes and under neutral pH and ambient temperatures. Due to its rigid structure it provides stability and enables the reuse of the enzyme. Any material contained in the synthetic mixture may become entrapped within the biomimetic Si nanoparticles [
14,
15,
16,
17]. The mild synthetic approach is compatible with a range of enzymes for which the strategy has also resulted in stabilization. The synthesis necessitates an aminated molecule to catalyze de deposition of the nanoparticles. Various synthetic polymers (polypeptides and polyamines) as well as biopolymers (proteins and polysaccharides) have been used as inducers for the synthesis. As they become integrated in the support matrix, they provide opportunities to tailor the support for better specific activity and stability of the catalysts. Moreover, integration of MNPs in this particular type of silica supports would allow a facile magnetic separation of the biocatalysts for its reuse or even a remote activation of a thermophilic enzyme upon application of an alternate magnetic field.
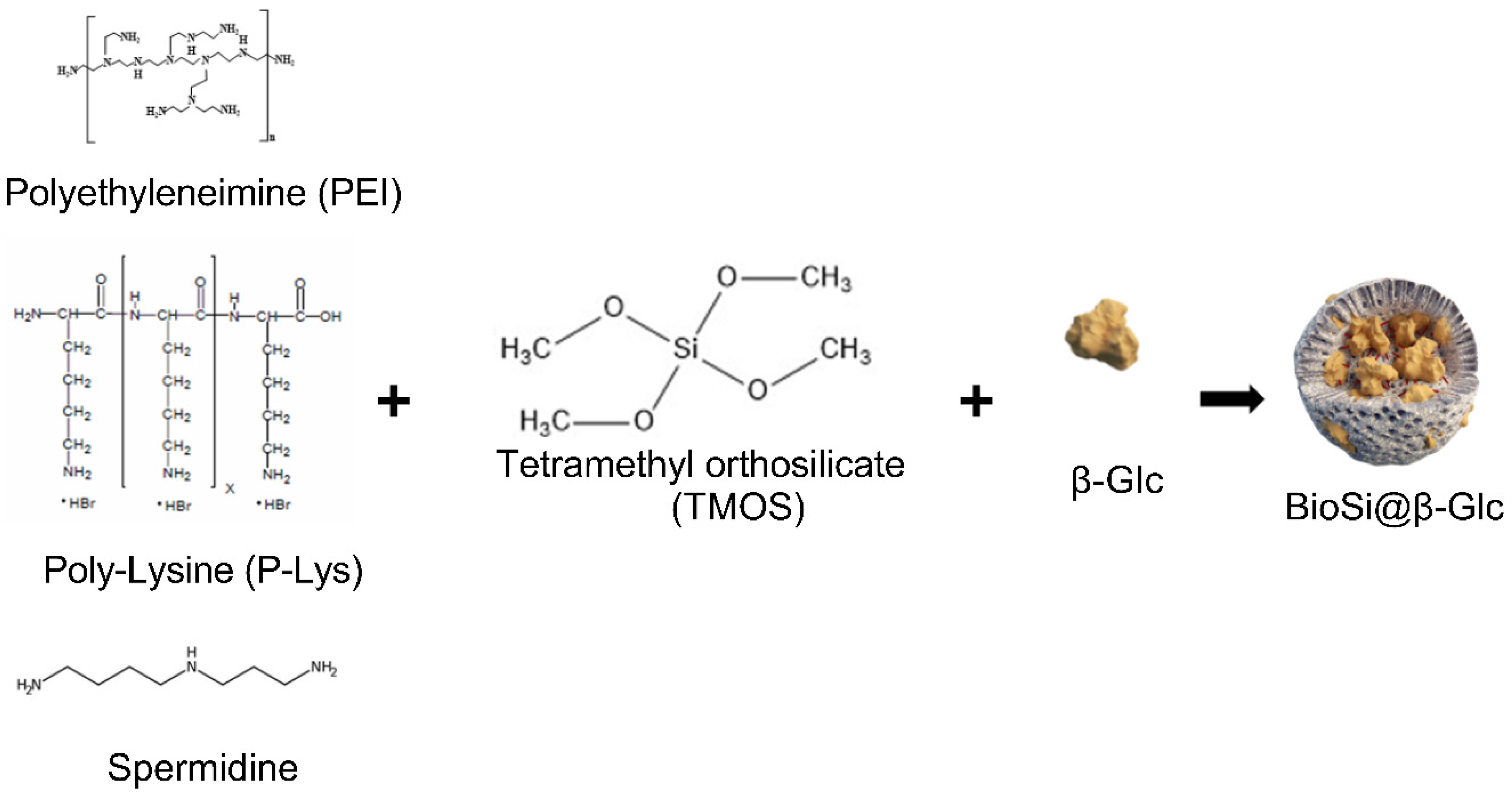
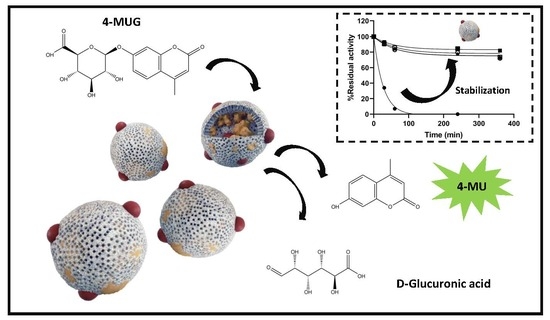
In this work, the immobilization of β-GIc from Patella vulgata was carried out following different strategies under the hypothesis that a tailored combination of materials could produce an active and stable immobilized biocatalyst. Entrapment in biomimetic silica nanoparticles in the presence of three polyaminated molecules: polyethyleneimine, spermidine, and poly-lysine, was first tested followed by immobilization on magnetic nanoparticles (MNPs) via covalent binding and ionic adsorption strategies. Combined silica and magnetic materials were tested proving that tailoring the immobilization strategy resulted essential in the final properties of the immobilized biocatalysts.
2. Results and Discussion
Although immobilization studies are commonly based on trial and error approaches, knowledge of as many properties as possible of the enzyme in advance could dictate the selection of immobilized conditions and help explain the results obtained. β-GIc from
Patella vulgata has shown before to have superior hydrolytic activity on drug-glucuronides [
18]. Despite being a commercial enzyme, there is scarce information on its biochemical properties. Its structure has not been elucidated, however, considering the deposited structures of glucuronidases in the Protein Data Bank (PDB) from the Research Collaboratory for Structural Bioinformatics (RSCD)and data compiled in BRENDA (Braunschweig Enzyme Database) it is likely that it is organized as a multimer. An analysis in our laboratory by gel filtration of the commercial preparation using fast protein liquid chromatography (FPLC), showed a main peak of 396 kDa that concentrated all the glucuronidase activity (data not shown). This molecular weight is similar to several tetrameric β-GIc from other sources [
19].
The properties of immobilized biocatalysts on hybrid materials are partially determined by the individual effect of each material on the enzyme upon immobilization [
13]. Therefore, as a first step in the integration of β-GIc in a material containing silica (Si) and MNPs, we studied the entrapment of the enzyme solely in biomimetic Si.
The classical rapid and green one-pot procedure was carried out in the presence of a polyaminated catalyst and derivatives of silicic acid in mild reaction conditions [
14]. Silica entrapped preparations were designated as Si@. Because there is a distinct dependence of the amount, size, and form of the silica precipitates on the molecular structure of the polyamine [
20,
21], we explored the immobilization using polyethyleneimine (PEI), poli Lysine (PL) and spermidine (Spr) (
Scheme 1). Upon entrapment using PEI, the immobilization was unsuccessful resulting in Si particles that contained the totality of the offered enzyme with no activity (
Table 1).
Further studies demonstrated that the amine catalyst was responsible for the inactivation of the enzyme as incubation with different PEIs (not branched MW 1300, 2000, 60,000. and branched 25,000) inactivated the enzyme after 15 min at 25 °C (data not shown). Although PEI has demonstrated in the past to be very useful to stabilize and immobilize enzymes [
9,
22], it has also been shown that interaction of the polycation with internal pockets of the protein can lead to enzyme inactivation [
23].
When using poly-lysine (P-Lys) (MW 70,000–150,000) as amine catalysts, the entrapment resulted in a yield of 49 ± 2% and expressed activity of 55 ± 6% (
Table 1). Similar results were obtained for Spr providing two novel immobilized preparations of β-GIc with this rapid and mild procedure.
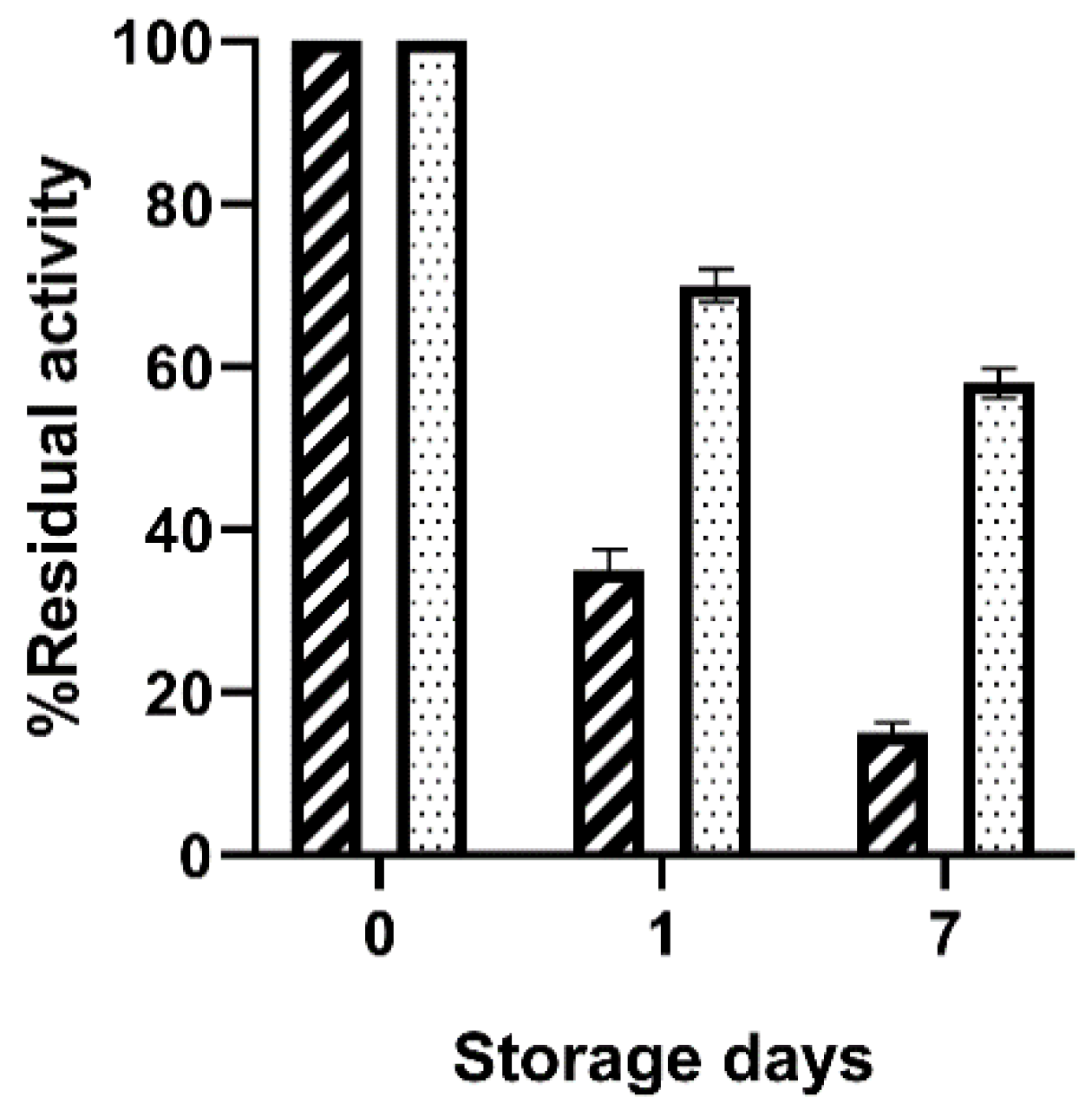
The activity of the immobilized preparations were measured after storage at 4 °C demonstrating that BioSi@β-Glc_PL retained 15 ± 1% of the incubated initial activity whereas 58 ± 1.8 was preserved in the immobilized preparation using Spr after 7 days (
Figure 1).
The analysis of the supernatant in this experiment showed no enzyme activity indicating negligible leakage from neither of the immobilized preparations. Though the entrapment using PL was successful, the immobilized biocatalysts obtained using Spr showed better potential for further use due to a superior storage stability. The latter was therefore selected for the deposition of silica for subsequent experiments in this work.
The chemistry used for the attachment of an enzyme to a support significantly determines the properties of an immobilized biocatalysts. Different orientations of the biological molecule on the support or number of enzyme-support interactions, can be dictated by the availability of exposed groups on the surface of the enzyme, degree of support functionalization, or conditions that promote enzyme–support reactivity. Both oriented immobilization and intensity of interaction in immobilized enzymes have demonstrated in the past dramatic impacts in the activity, selectivity, and stability of heterogeneous biocatalysts [
11,
24,
25,
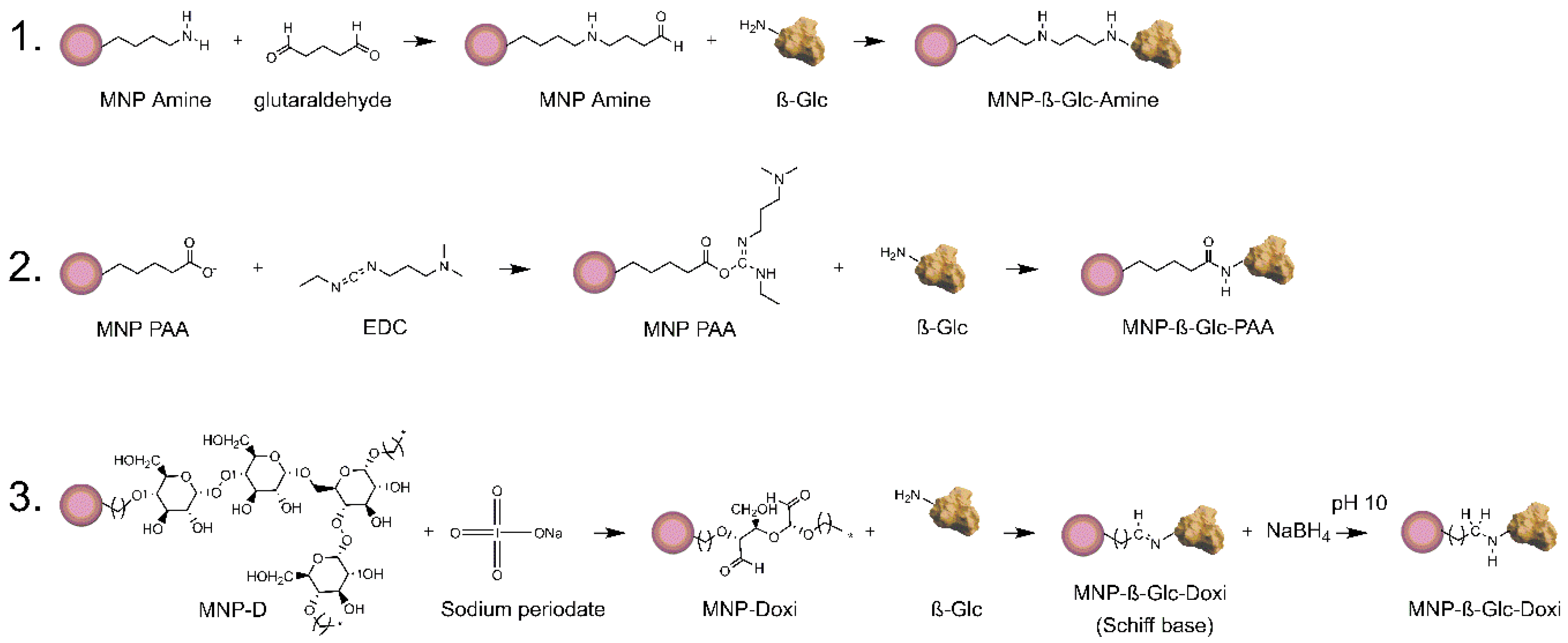
26]. We, therefore, studied the immobilization of β-GIc onto differently functionalized MNPs (
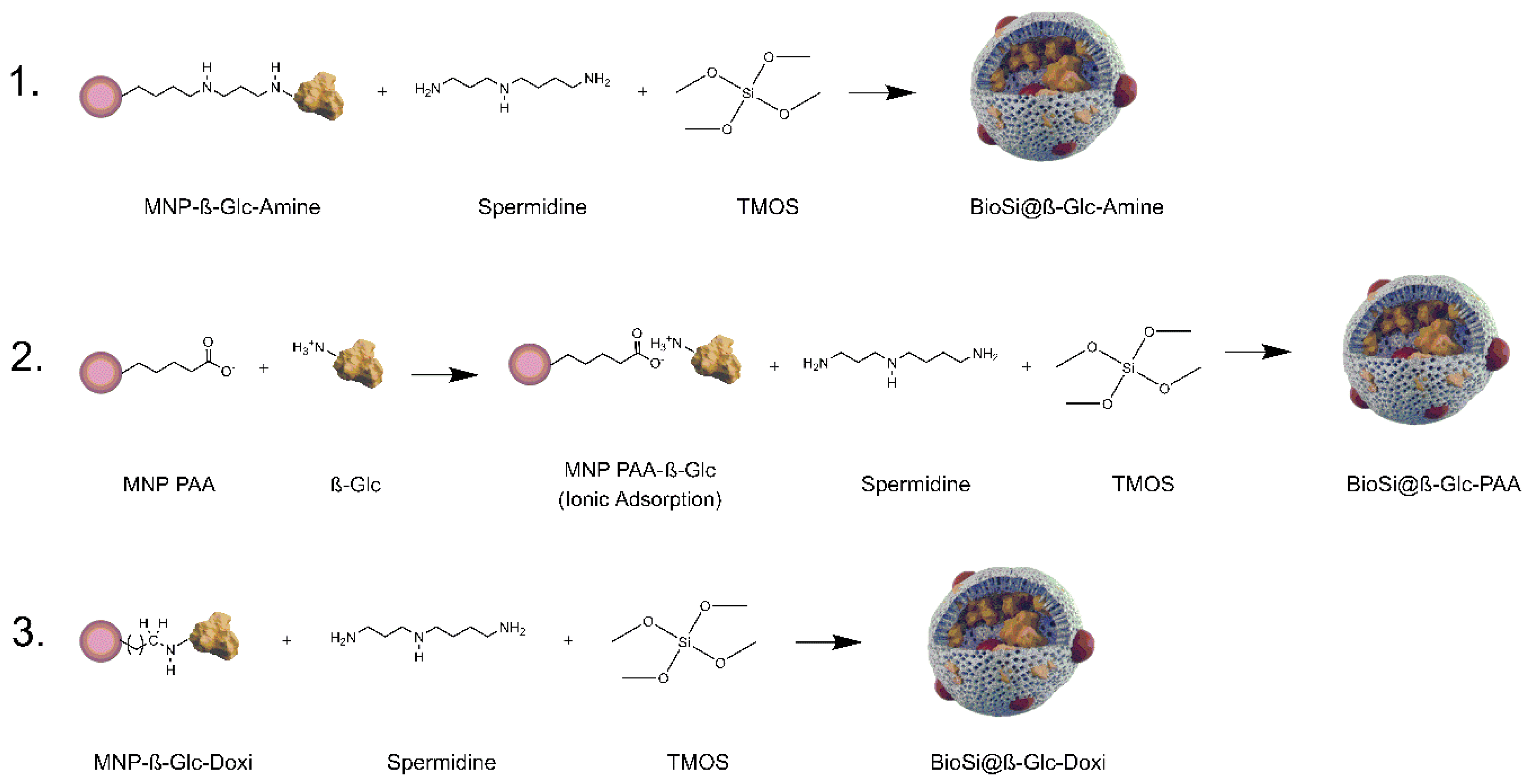
Scheme 2) namely MNP PAA for poly aspartic acid activated nanoparticles, MNP Amine for amino silane activated nanoparticles and MNP D for dextran activated nanoparticles. Further chemical modification with sodium periodate of the latter MNPs allowed us to obtain dextran oxidized nanoparticles MNP DOxi.
Following the well-established 1-Ethyl-3-[3-dimethylami-nopropyl]-carbodiimide hydrochloride (EDC) and N-hydroxy succinimide (NHS) chemistry [
27], the enzyme was covalently attached via amide bonds on carboxylated MNPs (
Scheme 2). Upon immobilization, 4 ± 1% of the offered enzyme was attached to the MNPs retaining 87 ± 3% of its activity (
Table 1). Alternatively, aminated MNPs were functionalized with glutaraldehyde for a preferential immobilization via primary amines of the enzyme. In this case, the yield was 44 ± 5% and the expressed activity was 100%. Finally, the dextran activated MNPs were mildly oxidized with sodium periodate to create Schiff’s bases to which the amines of the enzyme could covalently attach [
28]. On further reduction with sodium borohyride the once reversible Schiff’s bases are converted into irreversible bonds. Using this approach, the yield obtained was 75 ± 2% and a preparation containing 0.73 IU/g was obtained.
Practical applications of enzymes are often hindered by the instability albeit their capacity to catalyze a wide variety of chemical reactions. Stabilization of biocatalysts is therefore of paramount importance for applied purposes. Thermal stability of the MNPs immobilized enzyme was tested at 75 °C demonstrating an improved resistance to T of the immobilized preparations regardless of the chemistry used (
Figure 2). MNP PAA preparations were not included in this experiment as they had very poor specific activity. The covalent immobilization of the enzyme on the MNPs provided a significant stabilization to the enzyme. While the soluble enzyme lost 66% of its initial activity in the first 30 min of the experiments, both immobilized preparations kept 52.5 ± 8% and 38 ± 3% after 240 min for the MNP DOxi and MNP Amine, respectively. It is worth noting that lengthening the incubations at 75 °C caused precipitation of the soluble preparations and colloidal instability of the particles. Below 75 °C, the immobilized preparations showed remarkable stability which make it unpractical to test their inactivation kinetics. Therefore, in order to consider both the activity of the immobilized biocatalysts and their stability, the lumped parameter catalytic potential (CP) was used. Comparison of the CP of the preparations showed a three-fold stabilization factor of the immobilized preparations compared to the soluble enzyme as CP were 31.19, 96.75, and 108.1 µmol of hydrolyzed 4-methyl-umbelliferyl-β-d-glucuronide (4-MUG)/g catalyst at 250 min for the soluble, MNPs Amine and MNPs DOxi respectively. To the best of our knowledge, there is only one previous report of β-GIc immobilized on a magnetic support [
29]. In this case a glucuronidase from
P. purpurogenum was immobilized on a graphene/Fe
2O
3 hybrid aerogel. However, no investigation on the thermal stability of the immobilized preparation was conducted.
MNPs Amine and MNPs DOxi covalent preparations of β-GIc were included as part of the mixture in a Si synthesis aiming to integrate both the MNPs and covalently attached enzyme to the Si matrix (
Scheme 3). For both preparations it was possible to immobilize more than 70% of the initial offered activity to the hybrid and approximately 40% of it remained after the Si entrapment process (
Table 1).
Given the poor results obtained following the EDC-NHS chemistry, MNPs PAA were integrated in Si matrix along with the enzyme without previous covalent attachment in further experiments. Previous testing of ionic adsorption of the enzyme on non-modified MNPs PAA revealed a rapid adsorption that produced an immobilized preparation of a similar specific activity of those on MNP Doxi and MNP Amine (0.6 ± 0.04). Integration in the Si was achieved by following the one-pot synthesis for the co-entrapment and adding MNPs-PAA and the enzyme in the reaction mixture. Similar results than those obtained with the covalently attached enzyme were found upon entrapment of this preparation (
Table 1).
Particle size of immobilized preparations could condition their technological application and affect the design of bioprocesses as it can directly impact intrinsic properties such us catalytic potential or robustness [
30,
31]. The hydrodynamic size of the hybrid immobilized preparations prepared herein was evaluated using dynamic light scattering (DLS) (
Table 2).
All the preparations were in the micro-sized range with BioSi@ β-Glc _Spr showing the largest hydrodynamic diameter. A reduction in the diameter was observed when the MNPs were integrated in the hybrid. The difference was particularly significant when 100 nm MNPs (BioSi@β-Glc _Amine) were used instead of 200 nm MNPs (BioSi@β-Glc _PAA and BioSi@β-Glc_Doxi). Regardless of the impact that the size of the MNPs could have, the results correlate with those previously demonstrated by Correa et al. [
13] where the MNPs were found to have a profound effect on the silica matrix formation irrespective of the size of the aminated mediator of the Si synthesis. The values for polydispersity index of all the preparations vary around 0.3, indicating a homogeneous population of particles with a low probability of agglomeration.
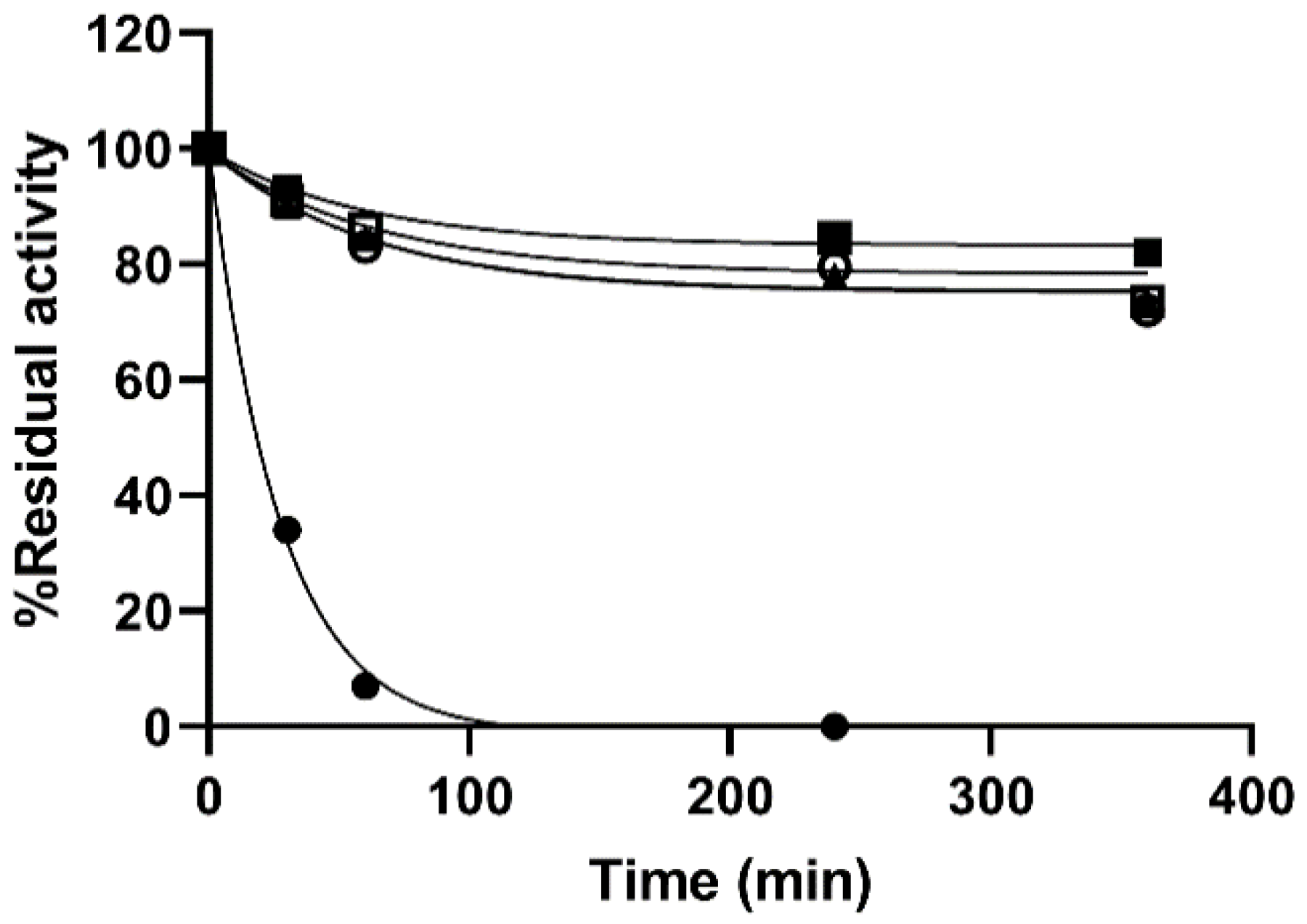
The thermal stability at 75 °C of the hybrid biocatalysts was then tested (
Figure 3). Compared to the soluble enzyme, the immobilized preparations were dramatically more thermostable as the soluble enzyme lost 50% of its activity in the first 20 min of the experiment while all immobilized biocatalysts retained more than 90% of its initial activity after 350 min under the same conditions.
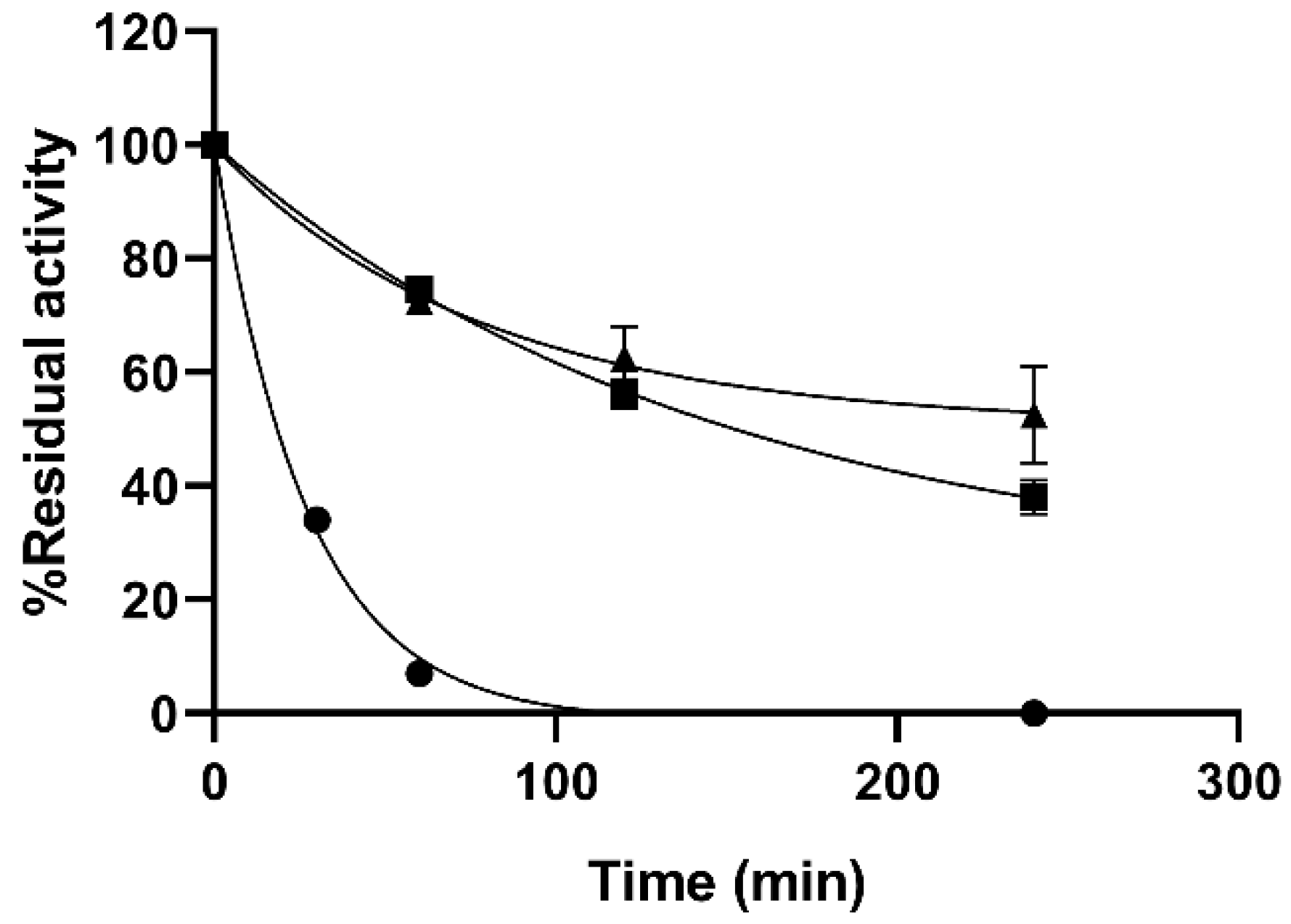
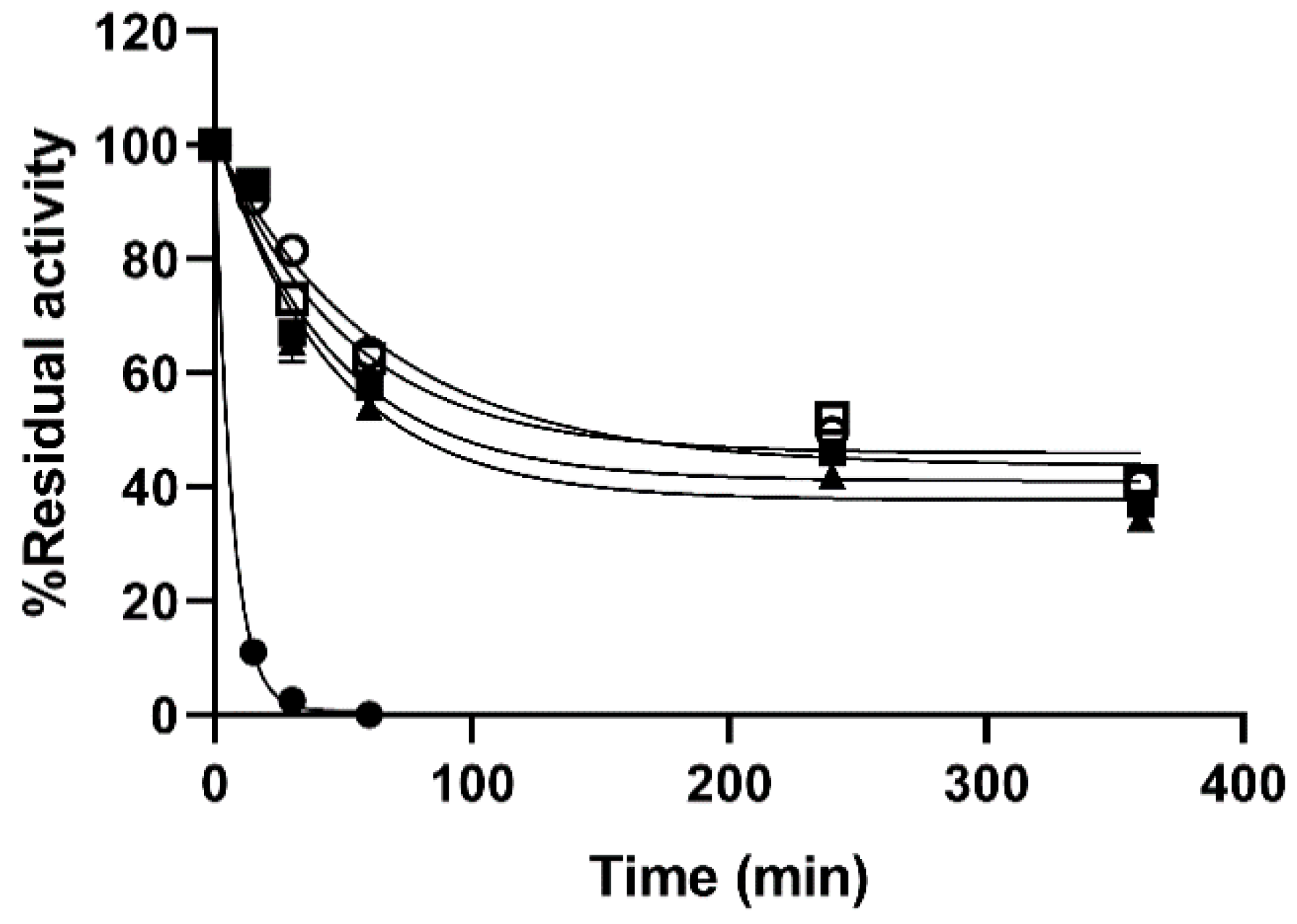
Even increasing the T to 80 °C the immobilized preparations preserved 40% of their initial activity after 6 h whereas the soluble enzyme lost 90% of its initial activity within 11 min of experiment (
Figure 4). Further increasing of the T of the thermal stability experiments was not possible due loss of colloidal stability of the particles. Under the conditions tested the CP of the immobilized preparations were 10 times superior to those of the soluble enzyme (98.23 µmoles of hydrolyzed 4-MUG/g catalyst for the Si@β-GlcDOxi and 10.68 µmoles of hydrolyzed 4-MUG/g catalyst for β-Glc in 350 min).
The enhanced stability of β-GIc particles provides a greater versatility for their use in a wide range of applications. The improved stability of the immobilized enzyme could be a consequence of the constraints imposed by both the rigidity of the covalent attachment to the MNPs and the multiple interactions with the Si matrix that has proven in many instances to have a protective effect on the stability of enzymes [
9,
32]. Moreover, given the multimeric structure of the enzyme, it is expected that entrapment or crosslinking of its subunits significantly improve its stability as subunit dissociation is often the first step in multimeric enzyme denaturation [
33]. Moreover, Spr has been shown to induce positive changes in the secondary and tertiary structures of enzymes that lead to a superior stability [
34]. It was not possible to establish a different stabilization factor for the Si alone and the combined Si-MNPs materials as both preparations showed outstanding stability in the conditions tested compared to the soluble enzyme. However, MNPs provides the obvious advantage of ease of separation after desired applications and could also serve as a mean to increase the enzymatic activity via magnetic hyperthermia [
13].
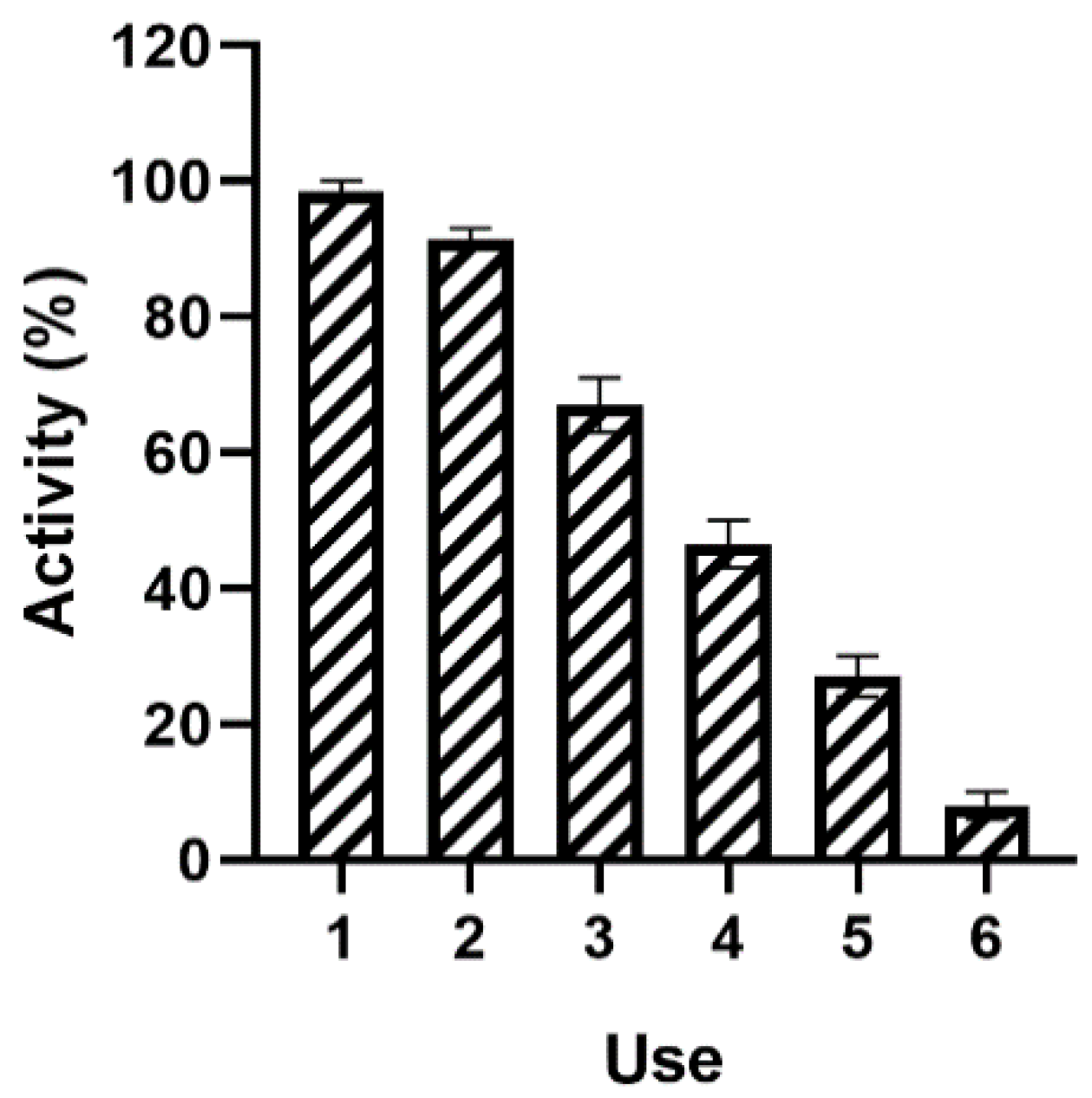
Finally, the operational stability of the BioSi@β-Glc_DOxi was assessed after several enzymatic cycles. In all the studied cycles, the immobilized enzyme was magnetically separated and assessed for its remnant catalytic activity. The enzyme preserved activity up to six reuses (
Figure 5). However, a significant loss in activity was observed considering that the experiment was carried out at 37 °C for 10 min for each cycle. Although it was proven that the preparation was thermostable, the pH of the experiment may have affected the performance of the enzyme. In fact, an analysis of the pH stability of both the β-Glc and BioSi@β-Glc_DOxi demonstrated a low pH stability at acidic pHs (
Figure S1 (Supplementary Materials)). For pH 3.0, pH 4.0, and pH 5.5 no activity was recovered after 1 h of incubation for the soluble or immobilized enzyme. Even at pH 7, only 17.7 ± 0.1% of the initial activity was recovered for BioSi@β-Glc_DOxi and 11.4 ± 6.3 % of the β-Glc was active. For higher pHs, the immobilized preparation showed a superior stability. Albeit the good activity that this enzyme has at acidic pH we have proven its poor stability under these conditions. Further experiments at shorter time periods would have to be conducted to assess whether the immobilization has provided stabilization at acidic pHs as was the case for basic pHs. Moreover, an effect of the salts used during the experiments cannot be ruled out. In spite of the activity loss over the cycles, the possibility of reusing the biocatalyst using magnetic separation reinforces the idea of a combined material with added advantages of each of its components. Moreover, our results demonstrated that the hybrid nanocomposites prepared herein, surpass a previous report in thermo stabilization of the enzyme via immobilization [
35]. In this work, a PEI-glutaraldehyde derivatized nylon net was used to attach the enzyme and results showed that the preparation suffered a drop in initial activity of more than 90% at 75 °C in the first 20 min of experiment.
3. Materials and Methods
3.1. Materials
Patella vulgata limpets β-glucuronidase enzyme (EC 3.2.1.31), 4-Nitrophenyl β-ᴅ-glucuronide (pNPG), 4-methyl-umbelliferyl-β-d-glucuronide (4-MUG) (MDL MFCD09039280), polyethyleneimine (PEI) MW 1300, and poly-lysine MW. 70,000–150,000, N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and N-Hydroxysuccinimide 98% (NHS) were from Sigma Aldrich (Missouri, EEUU). Bicinconinic acid (BCA), column PD-10 (Sephadex G 25) were from GE Healthcare’s Life Sciences (Illinois, EEUU), tetramethyl orthosilicate (TMOS) and dibasic sodium phosphate, 2-ethanesulfonic acid (MES) were from Merck (New Jersey, EEUU), 200 nm fluidMAG-PAA (MNP PAA), 100 nm fluidMAG-Amine (MNP Amine), and fluidMAG-D (MNP D) were from Chemicell (Berlin, Germany). All other chemicals used were analytical grade reagents.
3.2. Enzymatic Assay
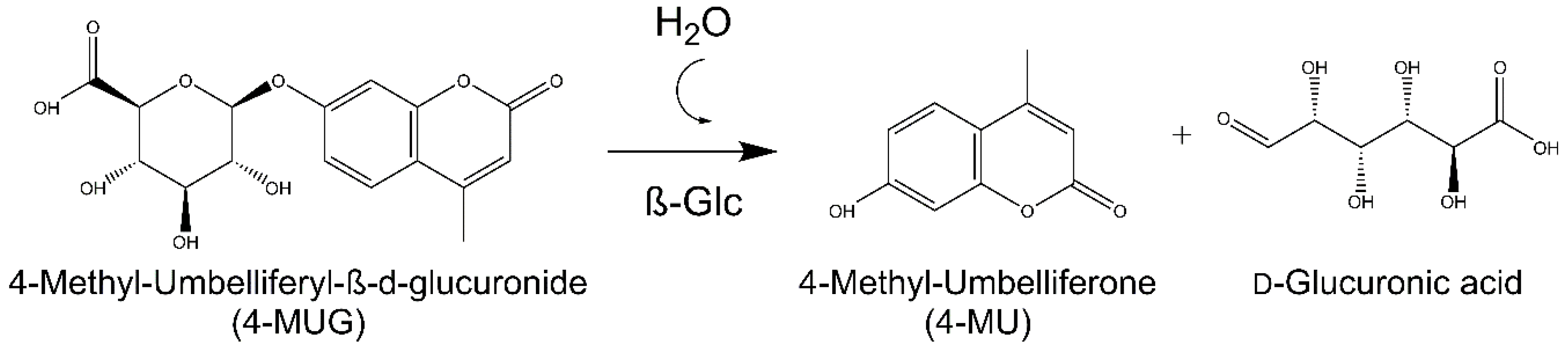
Enzymatic activity was evaluated with a fluorogenic substrate, 4-methyl-umbelliferyl-β-d-glucuronide (4-MUG) (MW 352.29 g/mol). The substrate when cleaved by β-Glc generates the fluorophore 4-methylumbelliferone (4-MU) (
Scheme 4). The excitation and emission wavelengths used to perform the activity measurements were 321 and 447, respectively.
For the enzymatic assay with the fluorescent substrate, 225 µL of 10 mM MES buffer pH 5.5, 20 µL of enzymatic sample and 5 µL of a 0.01 mM 4-MUG solution were mixed. It was incubated for 10 min at 37 °C in agitation and centrifuged at 13,500 rpm for 5 min. The supernatant was then plated in a 96-well fluorescence plate in a Tecan (Männedorf, Switzerland) plate reader at 37 °C. Using the Magellan 7.2 data analysis software, the relative units of fluorescence per minute (RFU/min) were obtained and used to calculate the units of enzymatic activity. First, a calibration curve of the 4-MU fluorophore, relative fluorescence units (RFU) as a function of concentration (mM) was performed. The slope obtained in the calibration curve is used to calculate the reaction rate. The reaction rate is obtained in mM/min; therefore, the corresponding calculations are made to obtain the units of enzyme activity per milliliter (IU/mL).
The enzyme unit is defined as the amount of enzyme that catalyzes the conversion of 1 µmol of substrate per minute per milliliter of enzyme under the defined test conditions.
3.3. Immobilization in Biomimetic Silica
3.3.1. Physical Entrapment in Biomimetic Si-Polyethyleneimine (PEI)
400 µL of sodium phosphate buffer 0.1 M, pH 7.0, 50 µL of enzyme (0.57 mg), 100 µL of 10% polyethyleneimine solution (PEI) and 100 µL of a hydrolyzed TMOS solution prepared by diluting TMOS in hydrochloric acid (1 mM) to a final concentration of 1 M were added to a one-pot synthesis. The mixture was kept standing at 25 °C for 5 min. It was then centrifuged at 13,500 rpm for 5 min and the supernatant was collected. To remove ionically absorbed proteins on the surface of the nanoparticles in the silica matrix, the suspension was washed with 10 mM sodium phosphate buffer containing 150 mM NaCl pH 7.2. It was then centrifuged at 13,500 rpm for 5 min. The supernatant was discarded and resuspended in 600 µL of 0.1 M sodium phosphate buffer pH 8.0. This preparation was stored at 4 °C. The enzymatic suspension was measured using the aforementioned enzymatic assay. The immobilization yield and expressed activity was calculated.
Immobilization percentage was defined as:
Expressed activity was defined as:
3.3.2. Physical Entrapment in Biomimetic Si-Poly-Lysine (PL)
For the final synthesis, we used 100 µL of sodium phosphate buffer 0.1 M, pH 7.0, 50 µL of enzyme (0.57 mg), 100 µL of poly-lysine (3 mg/mL) and 100 µL of hydrolyzed TMOS prepared as in a. The mixture was kept standing at 25 °C for 5 min. It was then centrifuged at 13,500 rpm for 5 min and the supernatant was collected. To remove ionically absorbed proteins on the surface of the nanoparticles in the silica matrix, the suspension was washed with 10 mM sodium phosphate buffer containing 150 mM NaCl pH 7.2. It was then centrifuged at 13,500 rpm for 5 min. The supernatant was discarded and resuspended in 600 µL of 0.1 M sodium phosphate buffer pH 8.0. This preparation was stored at 4 °C. The enzymatic suspension was measured using the aforementioned enzymatic assay.
3.3.3. Physical Entrapment in Biomimetic Si-Spermidine (Spr)
Similarly, 400 µL of sodium phosphate buffer 0.1 M, pH 7.0, 50 µL of enzyme (0.57 mg), 100 µL of Spermidine and 100 µL of hydrolyzed TMOS prepared as in a. were added to a one-pot synthesis. The mixture was kept standing at 25 °C for 5 min. It was then centrifuged at 13,500 rpm for 5 min and the supernatant was collected and to the suspension 10 mM sodium phosphate buffer containing 150 mM NaCl pH 7.2 wash was performed to remove ionically absorbed proteins on the surface of the nanoparticles in the silica matrix. It was then centrifuged at 13,500 rpm for 5 min. The supernatant was discarded and resuspended in 600 µL of 0.1 M sodium phosphate buffer pH 8.0. This preparation was stored at 4 °C. The enzymatic suspension was measured using the aforementioned enzymatic assay.
3.3.4. Co-Entrapment of MNPs in Si
A first washing step of the nanoparticles was carried out to remove detergent residues that come in the commercial solution: 100 µL of a 25 mg/mL fluidMAG-PAA carboxylated nanoparticles solution were washed thrice, with distilled water. A one-pot synthesis was carried out where 400 µL of 0.1 M sodium phosphate pH 8.0, 100 µL of a 12 mM spermidine solution (Spr) pH 8.0, 50 µL of β-Glc (0.57 mg), 20 µL of MNPs_PAA and 100 µL of hydrolyzed TMOS prepared as in a. were mixed and incubated at 25 °C for 5 min. The resulting preparation was glued to the magnet of the magnetic rack, the supernatant was collected and then the suspension was resuspended in 10 mM sodium phosphate buffer containing 150 mM NaCl pH 7.2 to remove ionically absorbed proteins on the matrix surface of Si. Finally, it was resuspended in 300 µL of 0.1 M sodium phosphate buffer pH 8.0 and stored at 4 °C during the experiment.
3.4. Immobilization of β-Glc on MNPs and Co-Entrapment in Si
3.4.1. On MNPs PAA
To carry out the immobilization of β-Glc in the commercial magnetite nanoparticles from Chemicell (Berlin, Germany), a first washing step of the nanoparticles was carried out to remove detergent residues that come in the commercial solution. First, 100 µL of a 25 mg/mL fluidMAG-PAA carboxylated nanoparticles solution were washed thrice, with distilled water.
For the covalent immobilization on the MNPs an activation was performed. The nanoparticles were resuspended in 125 µL of EDC solution (5 mg) and NHS (7.5 mg) in 10 mM MES pH 5.5 for 30 min at 37 °C with stirring. (EDC (MW = 171.91 g/mol) and NHS (MW = 115.09 g/mol)). Then, it was washed twice with 10 mM MES buffer pH 5.5 for 5 min. Subsequently, the enzyme binding stage was performed by incubating the MNPs with 250 µL of the enzyme (0.49 mg/mL) in 10 mM MES pH 5.5 for 60 min at 37 °C with constant agitation. Then, a wash with 10 mM MES pH 5.5 was performed for 5 min.
In the case of covalent bonding, an additional step was required to remove the enzyme that had not been covalently bound, but was ionically absorbed, the nanoparticle suspension was incubated with enzyme in 250 µL of 10 mM MES pH 5.5 with 300 mM NaCl for 15 min at 37 °C with stirring. Finally, a wash with the aforementioned buffer was performed, and the supernatant and suspension were stored at 4 °C.
The co-entrapment was carried out as mentioned above but instead of the soluble β-Glc and MNPs added separately, 50 µL of the conjugate fluidMAG-Amine-GA-β-Glc separated using the magnetic rack and resuspended in 50 µL of 0.1 M sodium phosphate buffer pH 7.0 was added to the synthesis.
3.4.2. On MNPs Amine
FluidMag-Amine MNPs were functionalized with glutaraldehyde. 100 µL of a 25 mg/mL of fluidMAG-Amine MNPs were incubated with 1% GA for 1 h at 25 °C. The MNPs were then separated using a magnetic rack and the supernatant was discarded. To this suspension of MNPS Amine activated with glutaraldehyde, 50 µL of β-Glc (0.57 mg) was added and incubated for 30 min at 25 °C.
The MNP Amineβ-Glc conjugate was separated using the magnetic rack and the solution was resuspended in 50 µL of 0.1 M sodium phosphate buffer pH 7.0. This mixture was then entrapped in Si using the aforementioned protocol.
3.4.3. On MNPs D
A mild oxidation of the 100 µL of fluidMAG-D MNPs, dextran functionalized MNPs, was carried out in the presence of 1 mg/mL of sodium periodate for 1 h in the dark. 20 µL of enzyme (0.57 mg) was then added and incubated at 25 °C for 1 h. The suspension was separated using a magnetic rack and the activity was measured. The co-entrapment was carried out as mentioned above. Post entrapment, the suspension was resuspended in 0.1 M sodium bicarbonate buffer pH 10 containing sodium borohydride 0.1 mg/mL and was kept in constant agitation for 30 min at 25 °C. Following which it was centrifuged at 13,500 rpm for 5 min and washed to remove excess sodium borohydride from the suspension. Finally, the mixture was resuspended in 0.1 M sodium phosphate buffer pH 7.0.
A control was carried out wherein the _MNP DOxi β-Glc conjugate once oxidation and immobilization onto the MNP was achieved, the reduction procedure was carried out as mentioned above to the MNPs.
3.5. Dynamic Light Scattering (DLS) and Z-Potential Measurements
The measurements were performed on a Zetasizer NanoPlus instrument at 25 °C. Each sample was prepared by diluting the sample (1:100,000) with milliQ water of which 1 mL was added to a cuvette. The measurement was repeated 50 times, with a combination of 2 runs per measurement.
3.6. Thermal Stability Experiments
The thermal stability activity measurements were carried out at different incubation times to measure the stability of the immobilized enzyme against the soluble enzyme. Both samples were subjected to 70, 75, or 80 °C in a thermoblock. Aliquots were analyzed at different intervals and the residual activity was calculated as follows:
where
are the IU at a time point and
is the initial activity in IU. Biocatalysts inactivation was modelled using the software GraphPad Prism 8.4.0 (San Diego, CA, USA).
The stability factor (SF) was the parameter used for a quantitative comparison of the stability of the biocatalysts. The coefficient of determination, R2, was determined in each case. In order to consider both the activity of the immobilized biocatalysts and their stability, the lumped parameter catalytic potential (CP) was defined:
CP was assessed evaluating the thermal stability of the immobilized biocatalysts under non-reactive conditions at 70, 75, or 80 °C in 25 mM phosphate buffer pH 7.0. aexp represents the expressed specific activity in the immobilized catalysts and tf the time for catalyst replacement. In this case, tf is 350 min.
3.7. pH Stability Experiments
pH stability experiments were carried out incubating 20 µL (24 mg) of enzymatic sample (soluble or immobilized suspension) for 1 h at 25 °C in 1 mL of the following buffers: 25 mM sodium acetate pH 3.0, pH 4.0, and pH 5.5, 25 mM sodium phosphate buffer pH 7.0 and pH 8.0, 25 mM sodium bicarbonate pH 9.0 and pH 10.0. Residual activity was calculated as in Equation (3) after measuring the enzymatic activity as previously described.
3.8. Reuse of Nanohybrid by Magnetic Separation
The reusability of the immobilized enzyme nanohybrids was studied by repeated usage for 6 enzymatic cycles. Enzymatic reactions using 225 µL of 10 mM MES buffer pH 5.5, 20 µL of enzymatic sample and 5 µL of a 0.01 mM 4-MUG were incubated for 10 min at 37 °C in agitation and centrifuged at 13,500 rpm for 5 min. The supernatant was then plated in a 96-well fluorescence plate in a Tecan (Männedorf, Switzerland) plate reader at 37 °C. Using the Magellan 7.2 data analysis software, the relative units of fluorescence per minute (RFU/min) were obtained and used to calculate the units of enzymatic activity.
Between each cycle, the nanohybrids were carefully separated using a magnetic separator (Chemicell, MagnetoPURE BIG SIZE) and then resuspended in the reaction mixture. The reactions were measured as previously described. The activity determined during the first cycle was considered 100% for the calculation of remaining percentage activity after each use.