Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China
Abstract
1. Introduction
2. Catalogue and Emission Amounts of VOCs in the China Pharmaceutical Industry
2.1. Catalogue of Pharmaceutical VOCs in China
2.2. The Guiding Emission Standards of VOCs in China
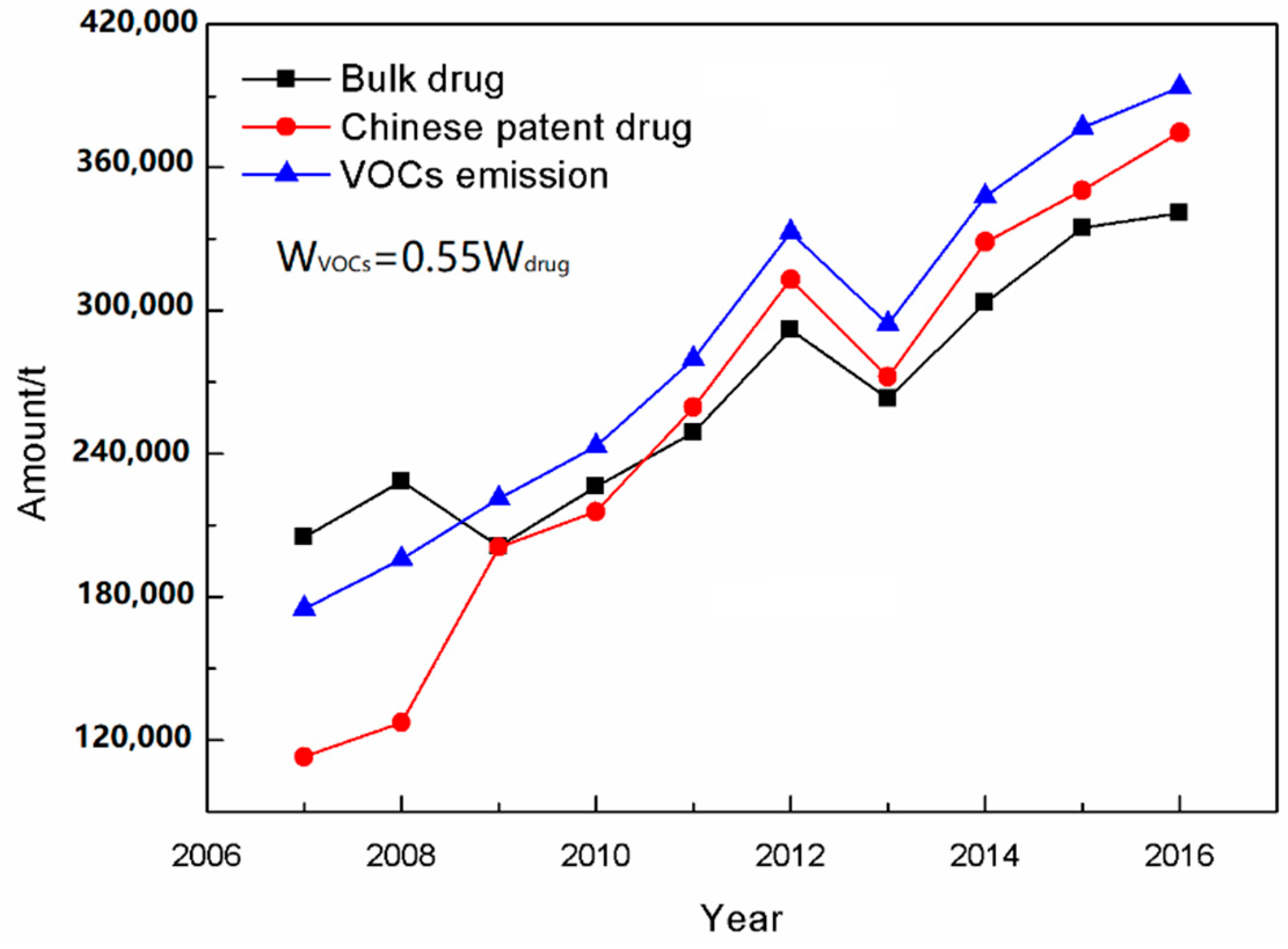
2.3. VOCs Emissions in the Chinese Pharmaceutical Industry
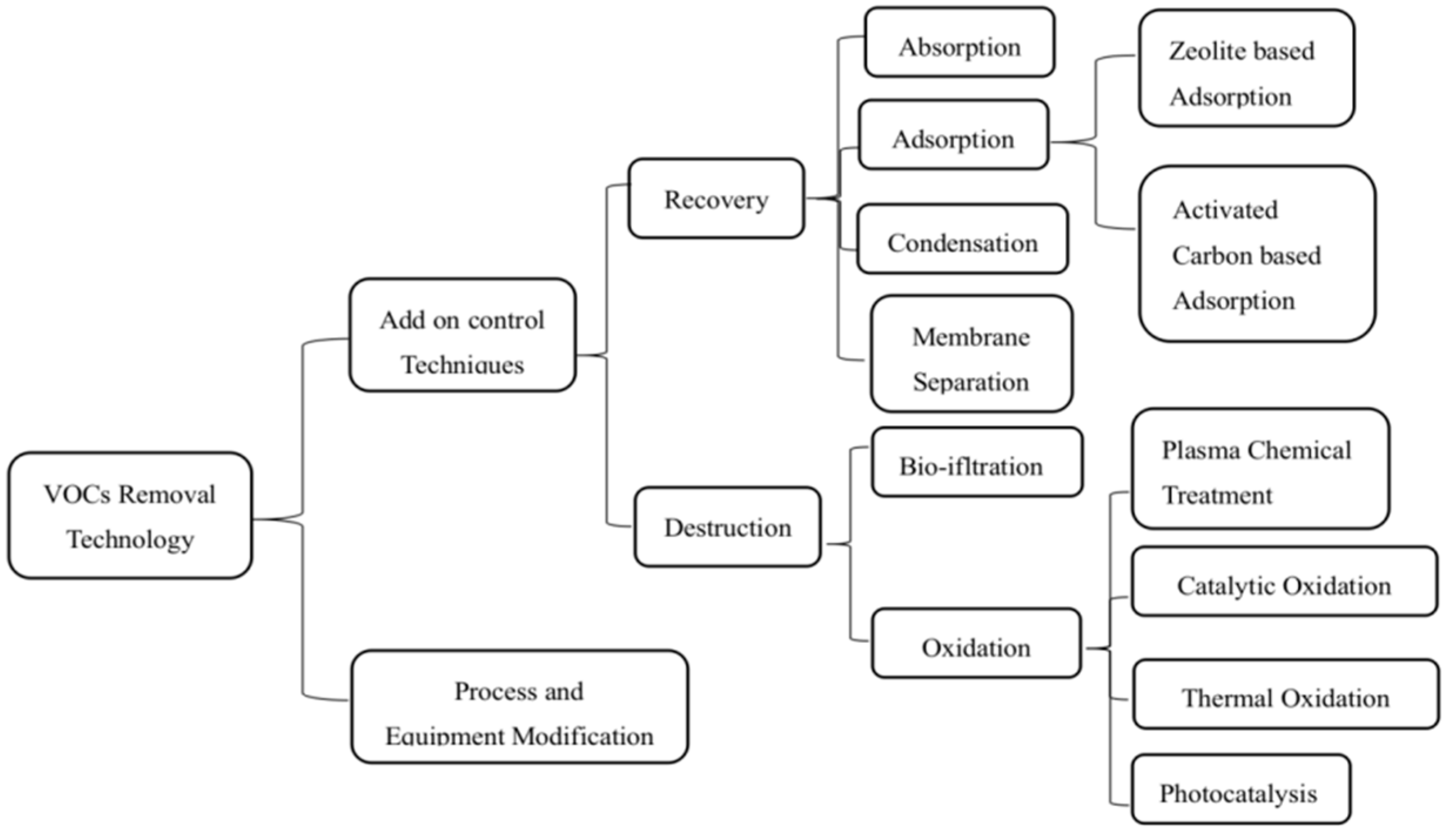
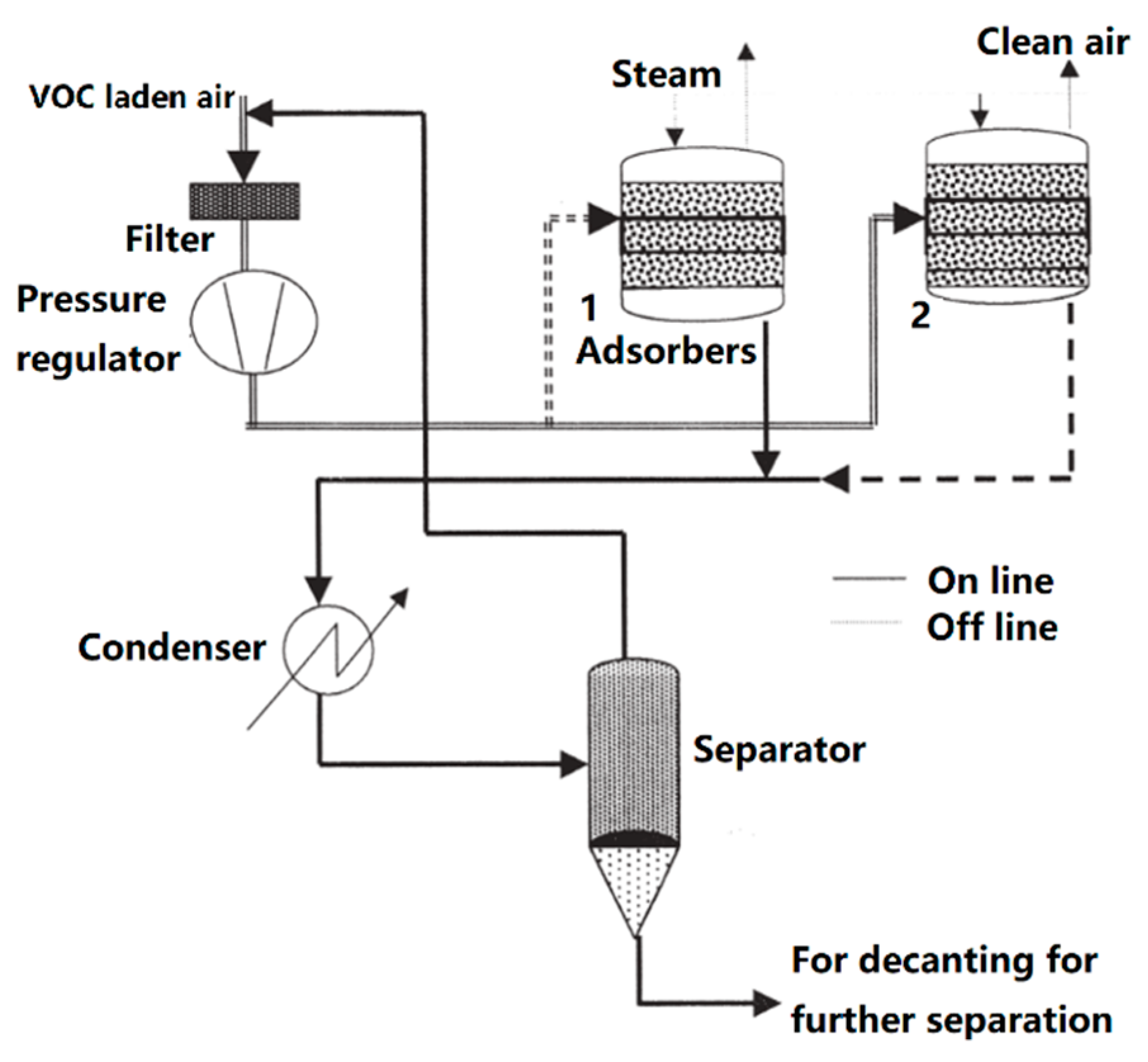
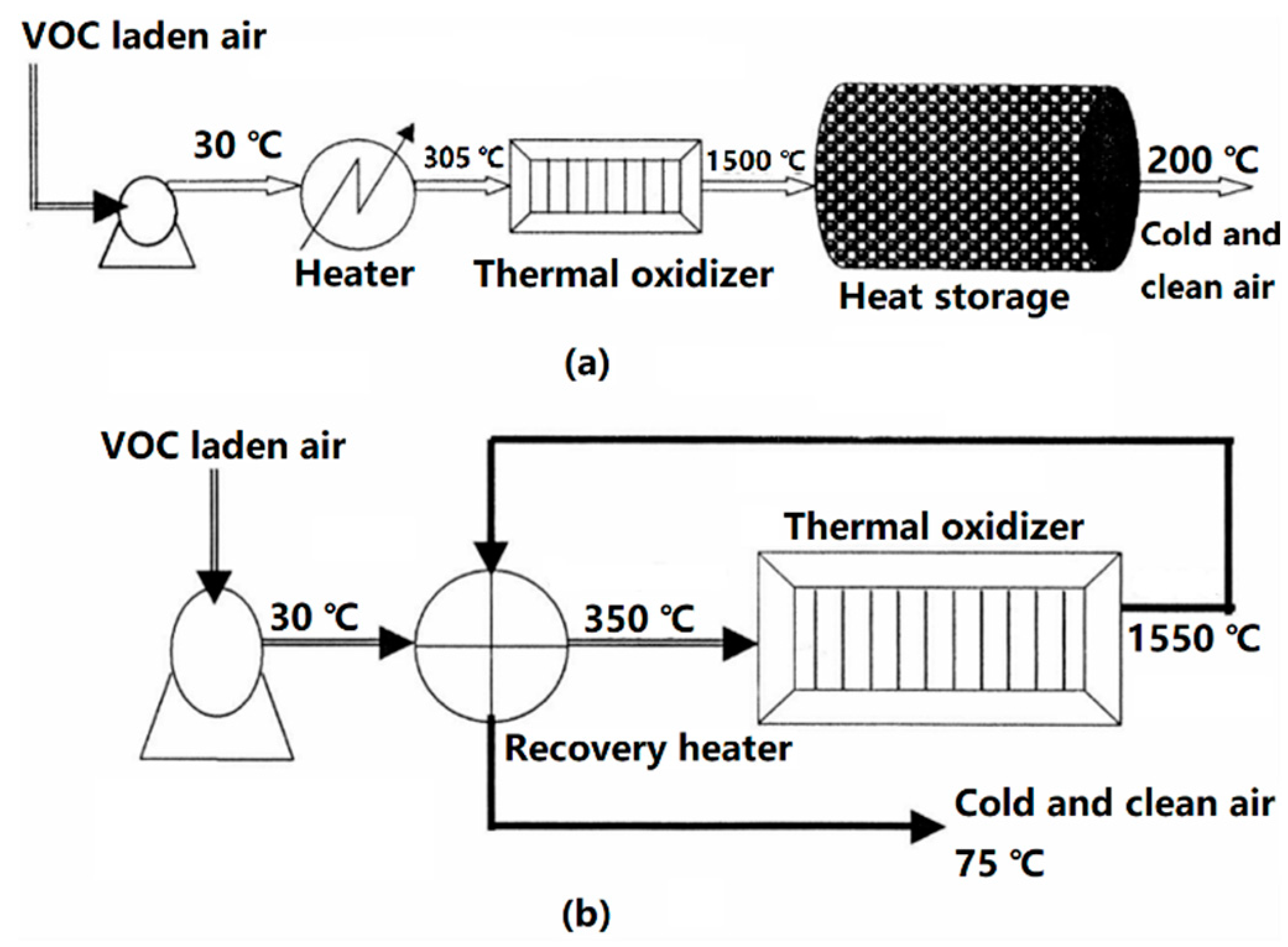
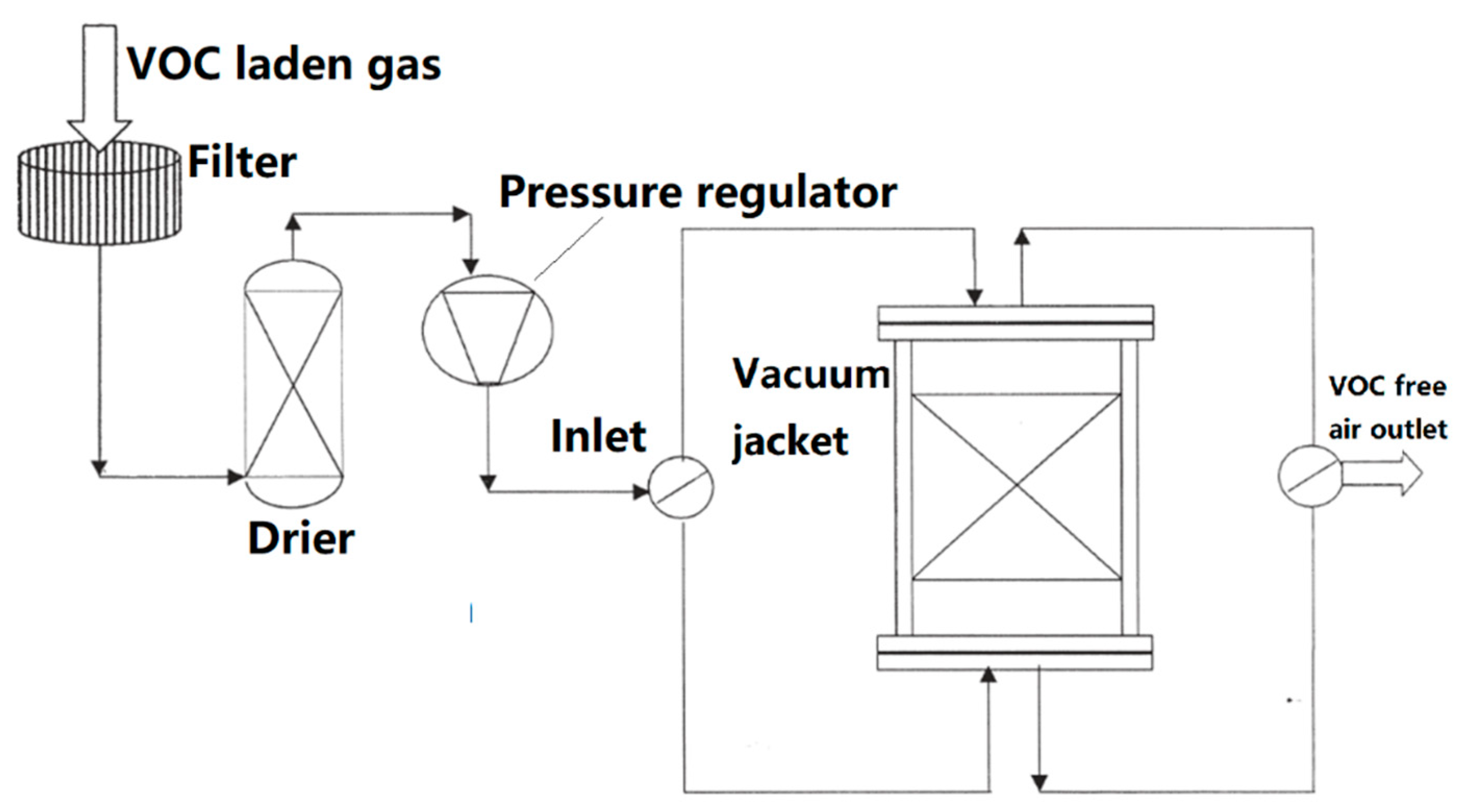
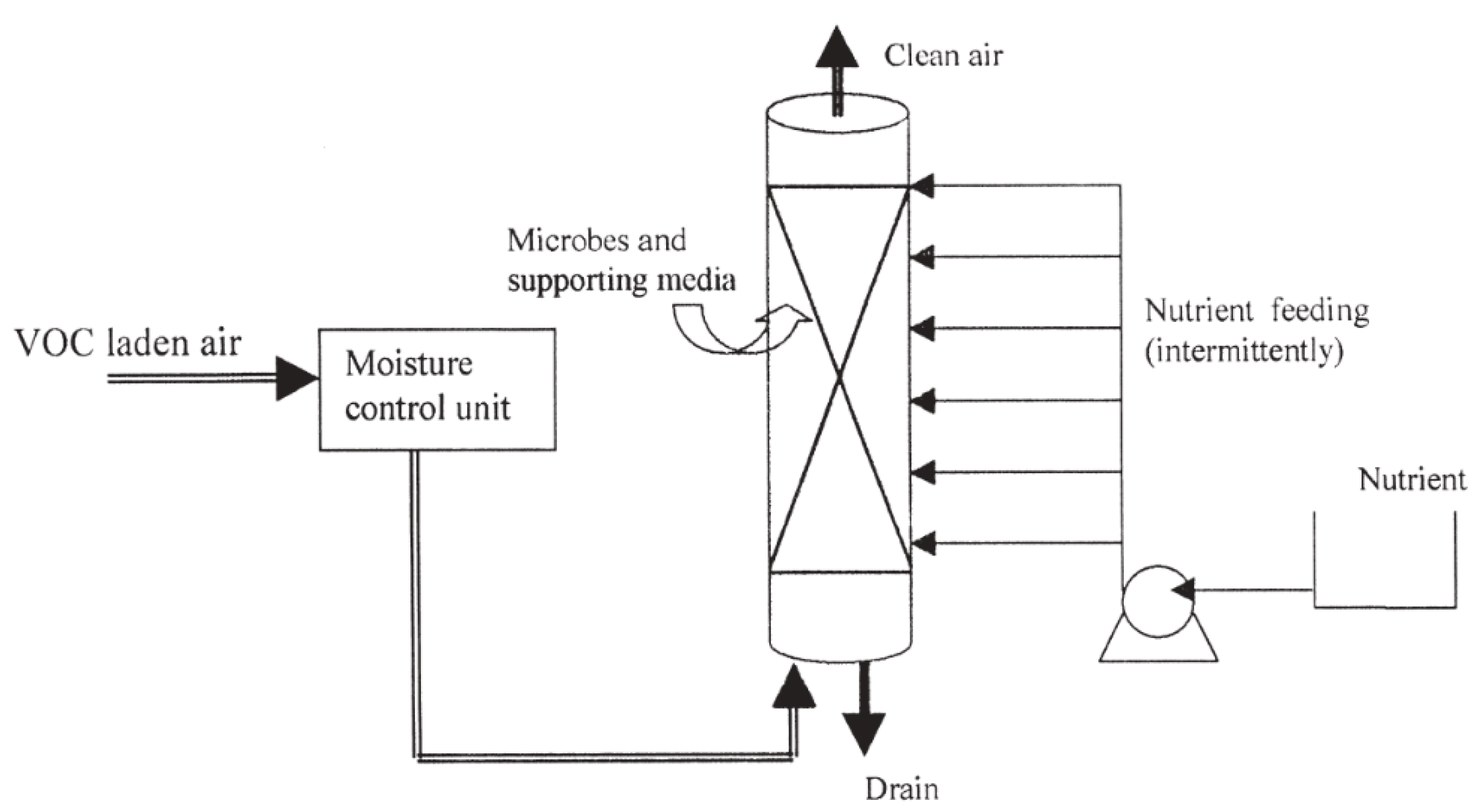
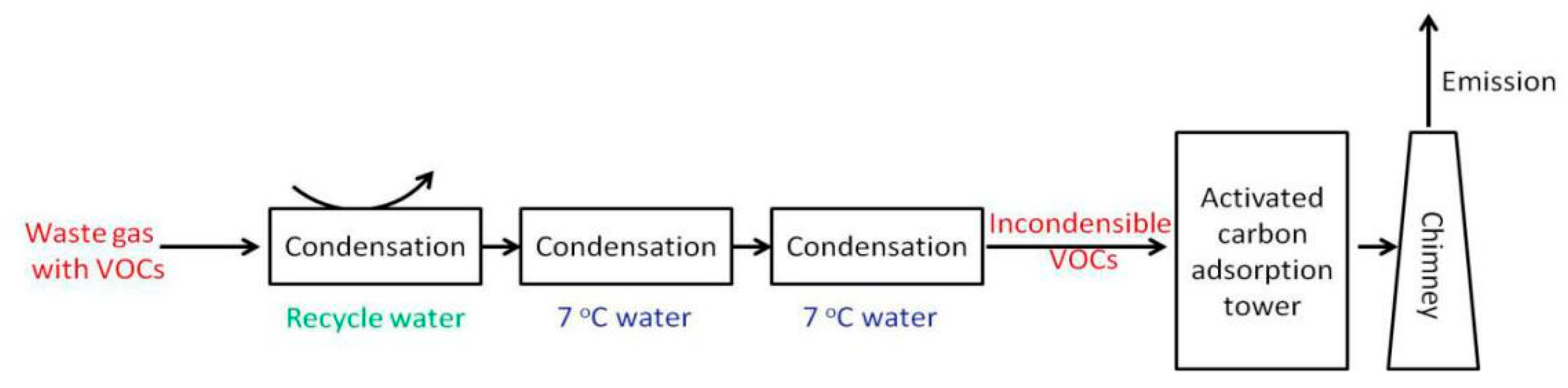
3. The Developing Technologies to Dispel VOCs
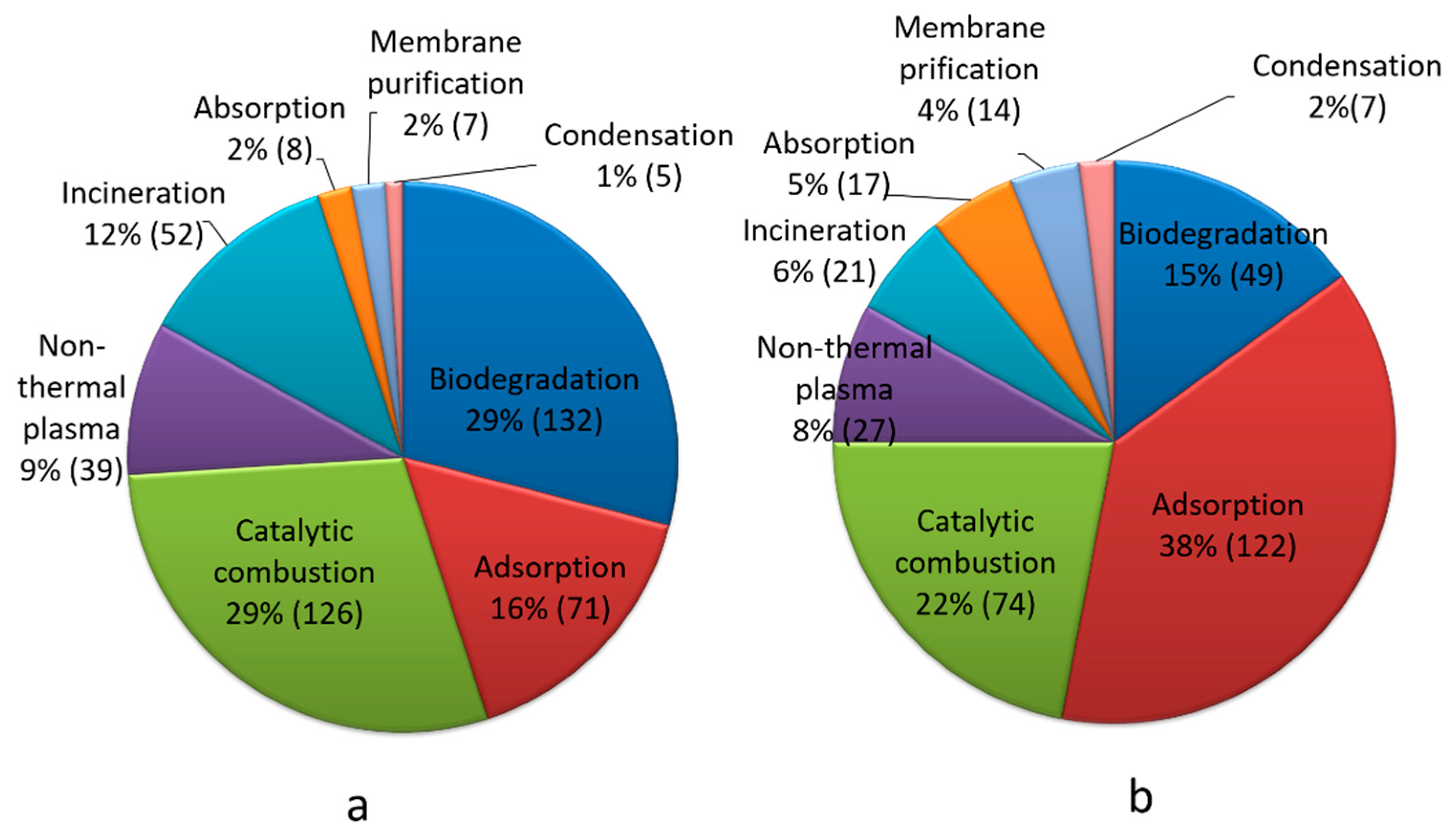
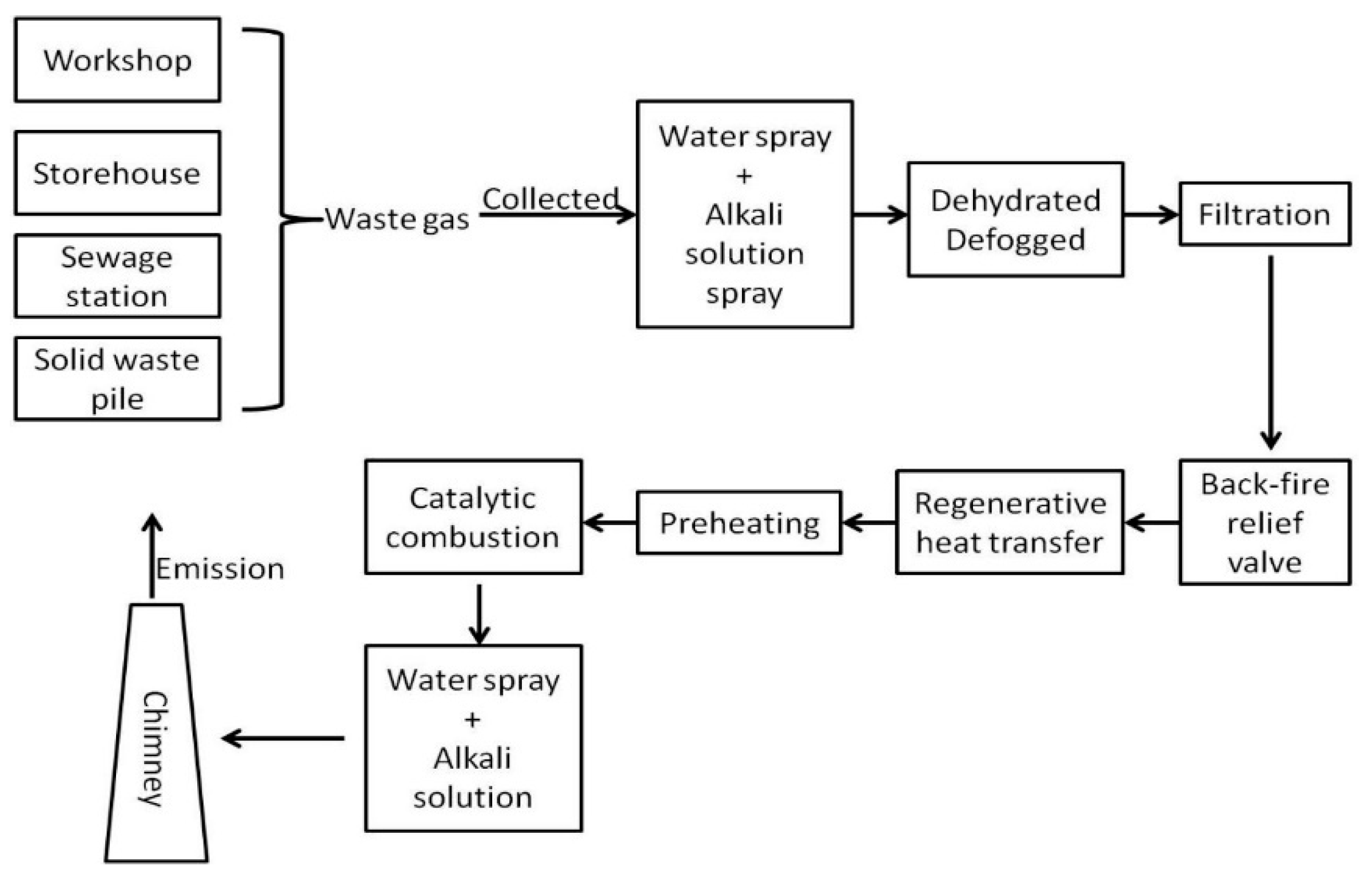
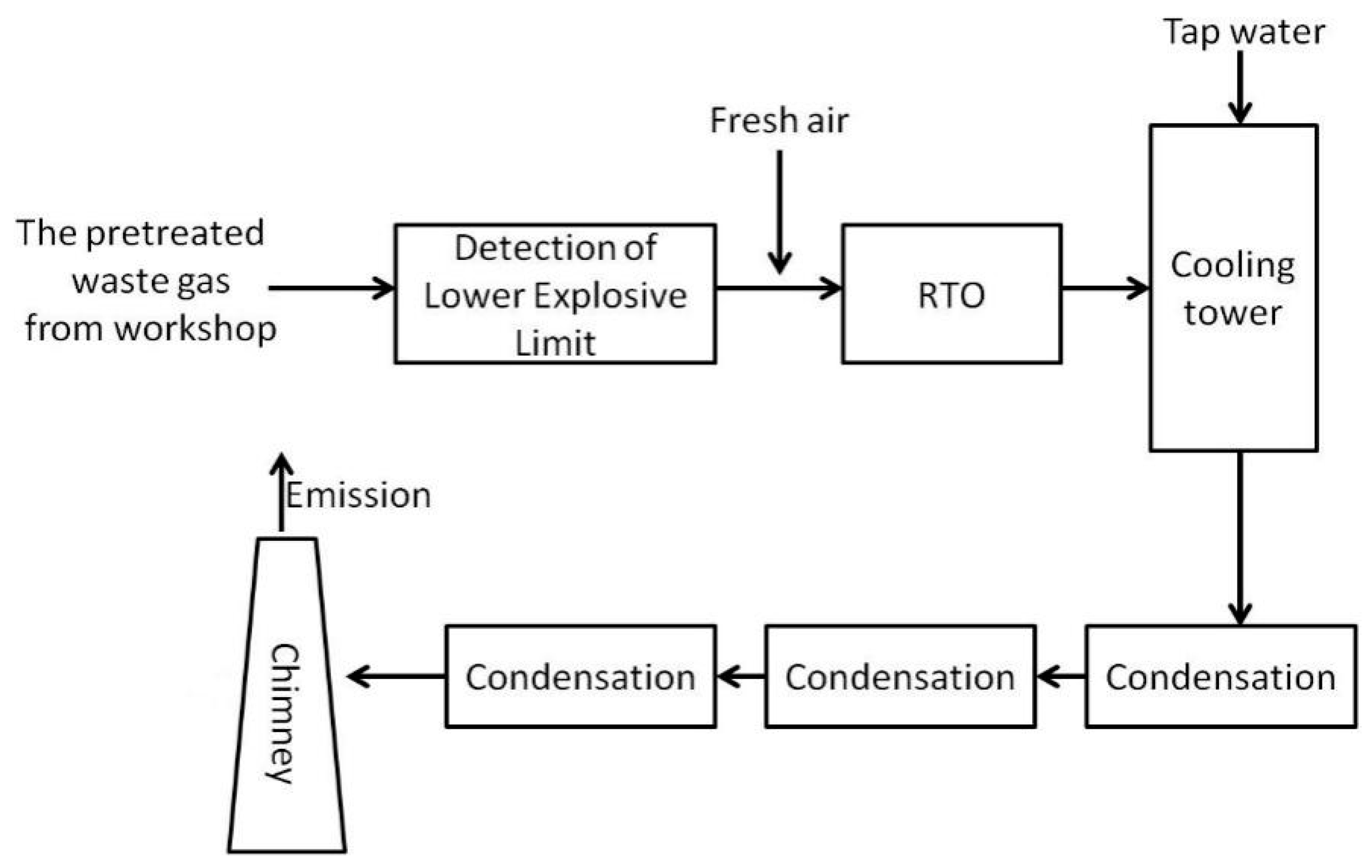
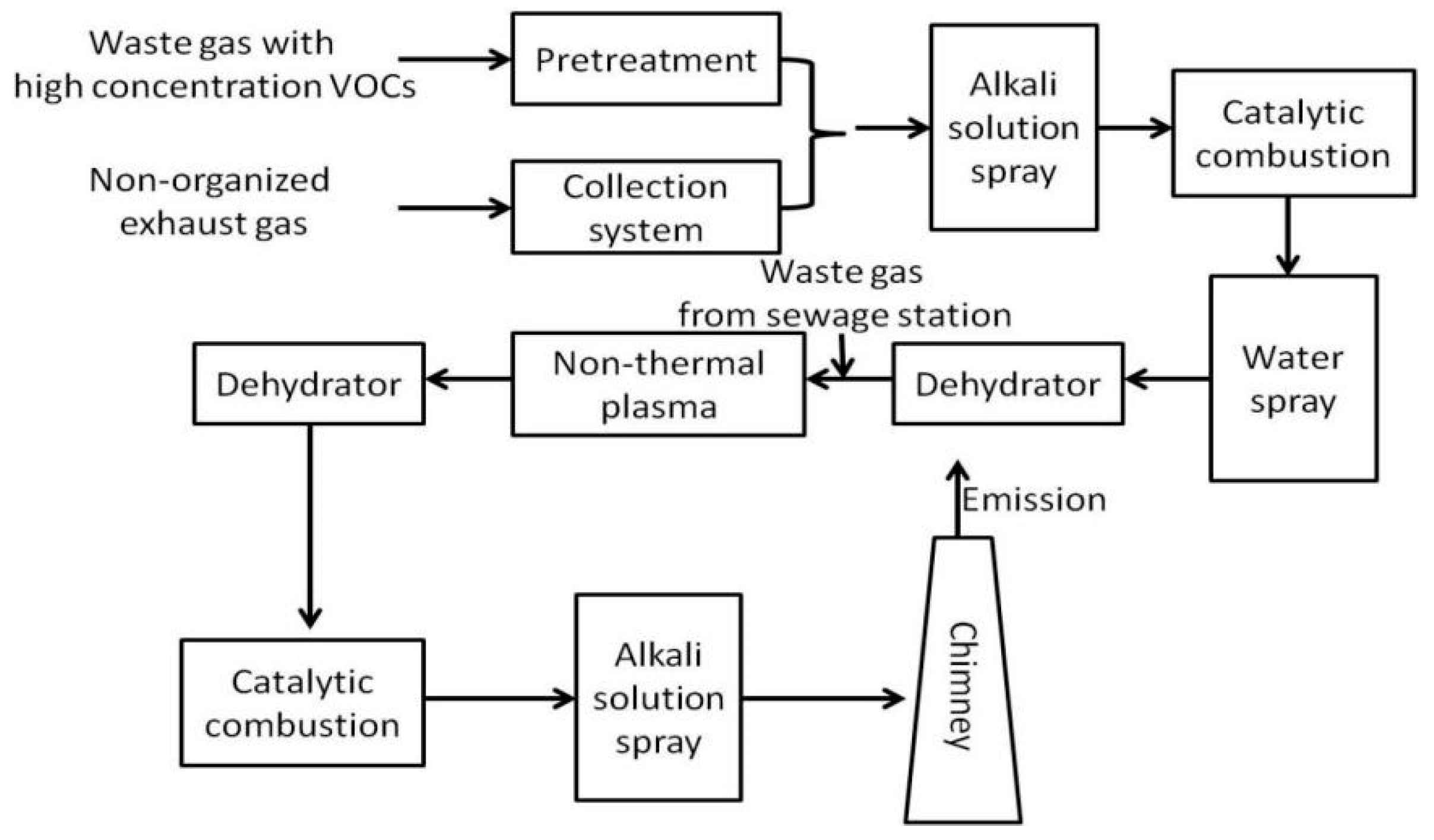
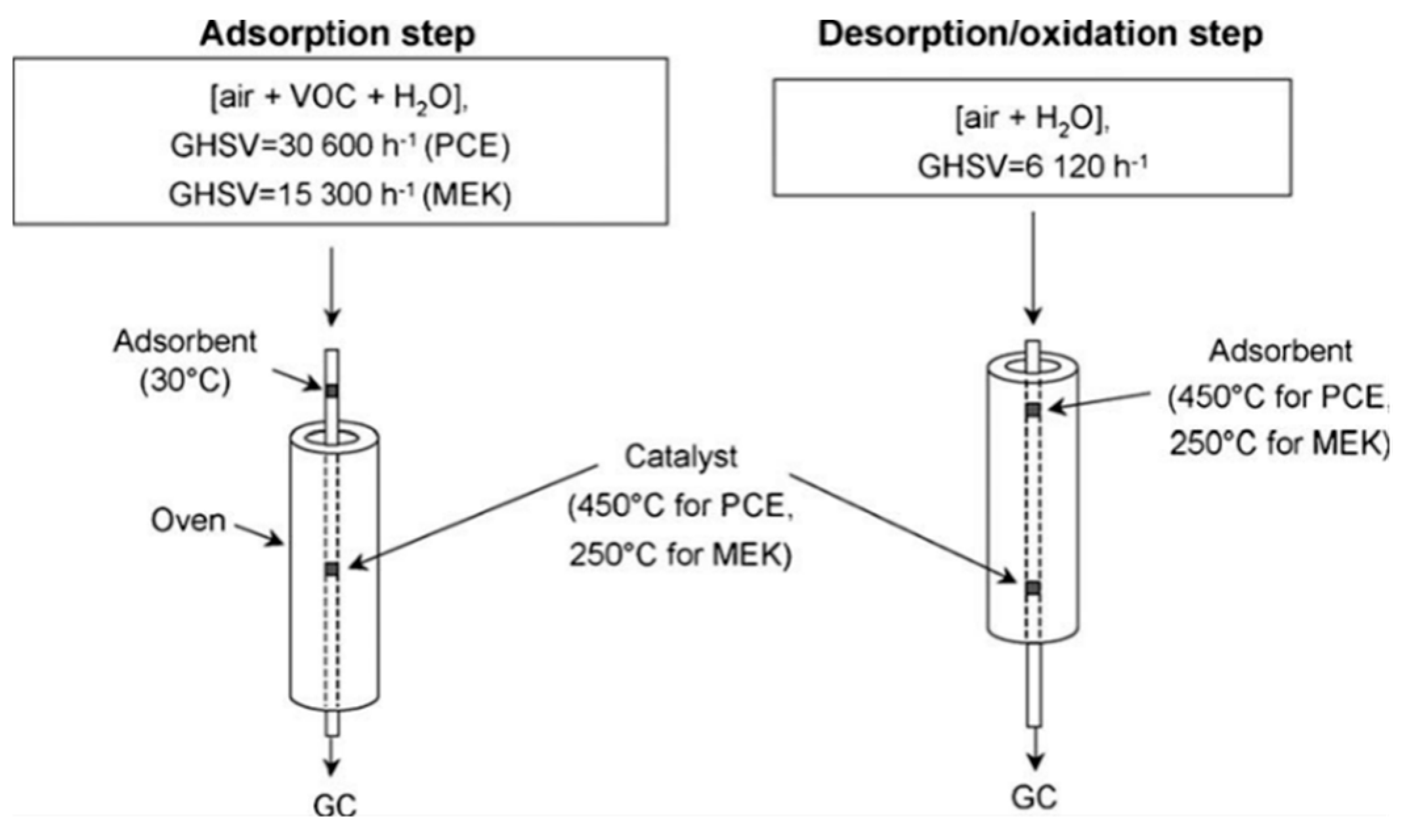
VOCs Elimination Technologies used in China’s Pharmaceutical Industry
4. The Developing Technologies for VOCs Elimination
4.1. Catalytic Combustion
4.1.1. Noble Metal Catalysts
4.1.2. Non-Noble Metal Catalysts
4.1.3. Perovskite Catalysts
4.1.4. Concentrated Oxidation Catalysts
4.1.5. The Influence Factors on Catalytic Performance
4.2. Photocatalytic Oxidation
4.3. Non-Thermal Plasma Process
4.4. Electron Beam Treatment
5. Outlook of the Different Kinds of Technologies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Muhammad, S.K.; Shaikh, A.R.; Mohammad, M.H. Catalytic oxidation of volatile organic compounds (VOCs): A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar]
- Cheng, G. Study on VOCs Emissions Research Present Situation and the Emission Reduction Potential of Key Industries in Hebei Province. Master’s Thesis, Hebei University of Science and Technology, Shijiangzhuang, China, 2016. [Google Scholar]
- Li, Y. Study on Emission Standard of Air Pollutants for Pharmaceutical Industry Chemical Synthesis Products Category. Master’s Thesis, Zhejiang University of Technology, Hangzhou, China, 2016. [Google Scholar]
- Atkinson, R. Gas-phase tropospheric chemistry of volatile organic compounds: 1. Alkanes and alkenes. J. Phys. Chem. Ref. Data 1997, 26, 215–229. [Google Scholar] [CrossRef]
- Fontane, H.; Veillerot, M.; Gallo, J.C.; Guillermo, R. 8th International Symposium on Transport and Air Pollution; Verlag der Technischen Universität Graz: Graz, Austria, 1999. [Google Scholar]
- Rivière, E. CITEPA Report; Fieldwork Inhabitants: Paris, France, 1998. [Google Scholar]
- Duncan, B.N.; Yoshida, Y.; Olson, J.R.; Sillman, S.; Martin, R.V.; Lamsal, L.; Hu, Y.; Pickering, K.E.; Retscher, C.; Allen, D.J.; et al. Application of OMI observations to a space-based indicator of NOx and VOC controls on surface ozone formation. Atmos. Environ. 2010, 44, 2213–2223. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar]
- National Bureau of Statistics of the People’s Republic of China. Available online: http://www.stats.gov.cn/tjsj/ (accessed on 26 May 2020).
- Chen, Y. Study on Current and Future Industrial Emission of Volatile Organic Compounds in China. Master’s Thesis, South China University of Technology, Guangzhou, China, 2011. [Google Scholar]
- Huang, W. Characteristics of Industrial VOCs Emissions and Evaluation of Control Technology in China. Master’s Thesis, Zhejiang University, Hangzhou, China, 2016. [Google Scholar]
- Ministry of Ecological Environment of the People’s Republic of China. Standards for Emissions of Atmospheric Pollutants from Pharmaceutical Industry (Draft for Comments); Ministry of Ecological Environment of the People’s Republic of China: Beijing, China, 2017.
- Lu, Y. Establishment and Application on Assessment System of VOCs’s Control Technology of Pharmaceutical Industry. Master’s Thesis, Hebei University of Science and Technology, Shijiazhuang, China, 2016. [Google Scholar]
- Hu, G. Research of Emission and Control Technology of VOCs and Odorous Gas from Pharmacy Industry in China. Master’s Thesis, Nankai University, Tianjing, China, 2013. [Google Scholar]
- Li, X. The System of Discharge Standards of Pollutants for Pharmaceutical Industry and a case Study. Master’s Thesis, The Chinese Research Academy of Environmental Science, Beijing, China, 2006. [Google Scholar]
- He, J. Fermentology; China Medical Science Press: Beijing, China, 2009. [Google Scholar]
- Wang, Y. Chemical Pharmaceutical Technology; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Zhou, T.; Ma, Q.; Chen, H. Treatment of chemical synthesized pharmaceutical wastewater by hybrid biological reactor. Ind. Water Wastewater 2010, 41, 42–45. [Google Scholar]
- Chen, P. Pharmaceutical Technology; Hubei Science and Technology Press: Wuhan, China, 2008. [Google Scholar]
- Qi, X. Modern Biopharmaceutical Technology; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Cao, G. Pharmaceutical Engineering of Traditional Chinese Medicine; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Wu, C. The thinking and Countermeasures for the Structuring of Learning Seals Team in Chengdu Zhonghui Pharmaceuticals Company. Master’s Thesis, Southwestern University of Finance and Economics, Chengdu, China, 2006. [Google Scholar]
- Chen, Y.; Ye, D.; Liu, X.; Wu, J.; Huang, B.; Zheng, Y. Source tracing and characteristics of industrial VOCs emissions in China. Chin. Environ. Sci. 2012, 32, 48–55. [Google Scholar]
- Song, J. Studies on the Adsorption of VOCs by Activated Carbons and the Structure-Function Relationship. Ph.D. Thesis, Central South University, Changsha, China, 2014. [Google Scholar]
- Khan, F.I.; Ghoshal, A.K. Removal of Volatile Organic Compounds from polluted air. J. Loss Prevent. Proc. Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Parmar, G.R.; Rao, N.N. Emerging Control Technologies for Volatile Organic Compounds. Crit. Rev. Environ. Sci. Technol. 2009, 39, 41–78. [Google Scholar] [CrossRef]
- Shah, R.; Thonon, B.; Benforado, D. Opportunities for heat exchanger applications in environmental systems. Appl. Therm. Eng. 2000, 20, 631–650. [Google Scholar] [CrossRef]
- Dunn, R.F.; El-Halwagi, M.M. Selection of Optimal VOC-condensation Systems. Waste Manag. 1994, 14, 103–113. [Google Scholar] [CrossRef]
- Huang, W.; Shi, L.; Hu, Z.; Zheng, Z. Integrated technology of condensation and adsorption for volatile organic compounds recovery. Chem. Eng. 2012, 6, 13–17. [Google Scholar]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Long, C.; Li, A. Enhanced adsorption and desorption of vocs vapor on novel micro-mesoporous polymeric adsorbents. J. Coll. Interface Sci. 2014, 428, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Serna-Guerrero, R.; Sayari, A. Applications of pore-expanded mesoporoussilica 7, Adsorption of volatile organic compounds. Environ. Sci. Technol. 2007, 41, 4761–4766. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency Clean Air Technology Center. Choosing an adsorption system for VOC: Carbon, zeolite, or polymers? In Proceedings of the SPIE—The International Society for Optical Engineering, Research Triangle Park, NC, USA, 1 May 1999. [Google Scholar]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Removal of hazardous volatile organic compounds from water by vacuum pervaporation with hydrophobic ceramic membranes. J. Membr. Sci. 2015, 474, 11–19. [Google Scholar] [CrossRef]
- Li, Y.X.; Chen, J.Y.; Sun, Y.H. Adsorption of multicomponent volatile organic compounds on semi-coke. Carbon 2008, 46, 858–863. [Google Scholar]
- Komori. Preparation of N-acetylmorpholine. J. Chem. Soc. Jpn. Ind. Chem. 1959, 62, 220–225. [Google Scholar]
- Chang, F.T.; Lin, Y.C.; Bai, H.; Pei, B.S. Adsorption and desorption characteristics of semiconductor volatile organic compounds on the thermal swing honeycomb zeolite concentrator. J. Air Waste Manage. Assoc. 2003, 53, 1384–1390. [Google Scholar] [CrossRef]
- Liu, Y. Study on Ceramic Honeycomb Monolithic Adsorbents for VOCs Adsorption. Master’s Thesis, South China University of Technology, Guangzhou, China, 2015. [Google Scholar]
- Moretti, E.C. Reduce VOC and HAP emissions. Chem. Eng. Prog. 2002, 98, 30–40. [Google Scholar]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, B109, 113–139. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Spivey, J.J. Complete Catalytic Oxidation of Volatile Organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology andapplication: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological methods for odor treatment: A review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manage. 2010, 91, 1039–1054. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; Leys, C.; Geyter N., D.; Morent, R. Abatement of VOCs using packed bed non-thermal plasma reactors: A review. Catalysts 2017, 7, 113. [Google Scholar] [CrossRef]
- Son, Y.S. Decomposition of VOCs and odorous compounds by radiolysis: A critical review. Chem. Eng. J. 2017, 316, 609–622. [Google Scholar] [CrossRef]
- Wang, H. Study on Removal of Miced VOCs in Air by Dielectric Barrier Discharge. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2009. [Google Scholar]
- Hao, J. Control Study of VOCs Regioned Joint Prevention and Control of Atmospheric Pollution. Master’s Thesis, Hebei University of Technology, Shijiazhuang, China, 2012. [Google Scholar]
- Xi, J.; Wu, J.; Hu, H.; Wang, C. Application status of industrial VOCs gas treatment techniques. China Environ. Sci. 2012, 32, 1955–1960. [Google Scholar]
- Hao, Y. Study on The Pharmaceutical and Chemical Industry VOCs and Odor Pollution Characteristics. Master’s Thesis, Hebei University of Science and Technology, Shijiazhung, China, 2014. [Google Scholar]
- Department of Environmental Protection of Zhejiang Province. Standards for Emissions of Atmospheric Pollutants from Pharmaceutical Industry in Zhejiang Province; Department of Environmental Protection of Zhejiang Province: Hangzhou, Chain, 2015–2016.
- Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Quesada-Medina, J. Ionic liquid technology to recover volatile organic compounds (VOCs). J. Hazard. Mater. 2017, 321, 484–499. [Google Scholar] [CrossRef]
- Minella, M.; Baudino, M.; Minero, C. A revised photocatalytic transformation mechanism for chlorinated VOCs: Experimental evidence from C2Cl4 in the gas phase. Catal. Today 2018, 313, 114–121. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. Combustion of non-halogenated volatile organic compounds over group VIII metal catalysts. Appl. Catal. B Environ. 1997, 13, 175–184. [Google Scholar] [CrossRef]
- Tabakova, T.; Ilieva, L.; Petrova, P. Complete benzene oxidation over mono and bimetallic Au–Pd catalysts supported on Fe-modified ceria. Chem. Eng. J. 2015, 260, 133–141. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2018, 357, 258–268. [Google Scholar] [CrossRef]
- Alifanti, M.; Florea, M.; Pârvulescu, V.I. Ceria-based oxides as supports for LaCoO3 perovskite catalysts for total oxidation of VOC. Appl. Catal. B Environ. 2007, 70, 400–405. [Google Scholar] [CrossRef]
- Spinicci, R.; Faticanti, M.; Marini, P.; De Rossi, S.; Porta, P. Catalytic activity of LaMnO3 and LaCoO3 perovskites towards VOCs combustion. J. Mol. Catal. A Chem. 2003, 197, 147–155. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, H.; Li, L.; Zhou, P.; Meng, X.; Guo, M.; Jia, J.; Sun, T. In situ fabrication of highly active γ-MnO2/SmMnO3 catalyst for deep catalytic oxidation of gaseous benzene, ethylbenzene, toluene, and o-xylene. J. Hazard. Mater. 2019, 362, 178–186. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Xu, Y.; Deng, J.; Huang, H.; He, M.; Leung, D.Y.C.; Wu, M.; Liu, S.; Liu, S.; et al. Promotional role of Mn doping on catalytic oxidation of VOCs over mesoporous TiO2 under vacuum ultraviolet (VUV) irradiation. Appl. Catal. B Environ. 2018, 220, 78–87. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Huang, H.; He, M.; Liu, S.; Liu, G.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; et al. Mesoporous TiO2 under VUV irradiation: Enhanced photocatalytic oxidation for VOCs degradation at room temperature. Chem. Eng. J. 2017, 327, 490–499. [Google Scholar] [CrossRef]
- Yadav, H.M.; Jung, S.C.; Kim, J.S. Visible light photocatalyticperformance of in situ synthesized graphite-SiO2–TiO2 composite towards degradation of benzene gas. J. Nanosci. Nanotechnol. 2018, 18, 2032–2036. [Google Scholar] [CrossRef]
- Li, J.; Han, S.; Bai, S.; Han, S.; Song, H.; Pu, Y.; Zhu, X.; Chen, W. Effect of Pt/gamma-Al2O3 catalyst on nonthermal plasma decomposition of benzene and byproducts. Environ. Eng. Sci. 2011, 28, 395–403. [Google Scholar] [CrossRef]
- Pei, W.; Liu, Y.; Deng, J.; Zhang, K.; Hou, Z.; Zhao, X.; Dai, H. Partially embedding Pt nanoparticles in the skeleton of 3DOM Mn2O3: An effective strategy for enhancing catalytic stability in toluene combustion. Appl. Catal. B Environ. 2019, 256, 117814–117824. [Google Scholar] [CrossRef]
- Hosseini, M.; Barakat, T.; Cousin, R. Catalytic performance of core–shell and alloy Pd–Au nanoparticles for total oxidation of VOC: The effect of metal deposition. Appl. Catal. B Environ. 2012, 111, 218–224. [Google Scholar] [CrossRef]
- Lee, D.S.; Chen, Y.W. The mutual promotional effect of Au–Pd/CeO2 bimetallic catalysts on destruction of toluene. J. Taiwan Ins. Chem. Eng. 2013, 44, 40–44. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Lin, M.; Ma, X.; Ge, M. Enhancement effect of acid treatment on Mn2O3 catalyst for toluene oxidation. Catal. Today 2019, 327, 254–261. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Tang, W.; Chen, Y. Catalytic activity of nanometer La1-xSrxCoO3 (x = 0, 0.2) perovskites towards VOCs combustion. Catal. Commun. 2008, 9, 55–59. [Google Scholar] [CrossRef]
- Suárez-Vázquez, S.I.; Gil, S.; García-Vargas, J.M.; Cruz-López, A.; Giroir-Fendler, A. Catalytic oxidation of toluene by SrTi1-XBXO3 (B = Cu and Mn) with dendritic morphology synthesized by one pot hydrothermal route. Appl. Catal. B Environ. 2018, 223, 201–208. [Google Scholar] [CrossRef]
- Guo, M.; Li, K.; Liu, L.; Zhang, H.; Hu, X.; Min, X.; Jia, J.; Sun, T. Resource utilization of spent ternary lithium-ions batteries: Synthesis of highly active manganese-based perovskite catalyst for toluene oxidation. J. Taiwan Inst. Chem. Eng. 2019, 102, 268–275. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Yang, X.; Song, S.; Li, J.; Jing, F.; Chu, W. Enhanced catalytic performances of in situ-assembled LaMnO /δ-MnO2, hetero-structures for toluene combustion. Catal. Today 2019, 327, 19–27. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Pantaleo, G.; Parola, V.L.; Valverde, J.L.; Gil, S.; Liotta, L.F.; Giroir-Fendler, A. The effect of citric acid concentration on the properties of LaMnO3 as a catalyst for hydrocarbon oxidation. Catalysts 2019, 9, 226. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Chen, L.; Yuan, D.; Wang, G.; Meng, X.; Xiao, F.S. Catalytic performance for toluene abatement over Al-rich Beta zeolite supported manganese oxides. Catal. Today 2017, 297, 182–187. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, Z.; Dong, C.; Wang, Y.; Huang, N. Highly catalytic activity of Mn/SBA-15 catalysts for toluene combustion improved by adjusting the morphology of supports. J. Environ. Sci. 2019, 76, 208–216. [Google Scholar] [CrossRef]
- Lee, Y.E.; Chung, W.C.; Chang, M.B. Photocatalytic oxidation of toluene and isopropanol by LaFeO3/black-TiO2. Environ. Sci. Pollut. Res. 2019, 26, 20908–20919. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Semeraro, P.; Ottolini, M.; Salvatore, L.; Marzo, F.; Lovergine, N.; Giancane, G.; Valli, L. SiO2-Coated ZnO Nanoflakes Decorated with Ag Nanoparticles for Photocatalytic Water Oxidation. Chem. Eur. J. 2019, 25, 14123–14132. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, X.; Yi, Z. Photocatalytic oxidation of methane over CuO-decorated ZnO nanocatalysts. J. Mater. Chem. A 2019, 7, 469–475. [Google Scholar] [CrossRef]
- Lu, M.; Huang, R.; Wu, J.; Fu, M.; Chen, L.; Ye, D. On the performance and mechanisms of toluene removal by FeOx/SBA-15-assisted non-thermal plasma at atmospheric pressure and room temperature. Catal. Today 2015, 242, 274–286. [Google Scholar] [CrossRef]
- Nevanperä, T.K.; Ojala, S.; Laitinen, T.; Pitkäaho, S.; Saukko, S.; Keiski, R.L. Catalytic Oxidation of Dimethyl Disulfide over Bimetallic Cu–Au and Pt–Au Catalysts Supported on γ-Al2O3, CeO2, and CeO2–Al2O3. Catalysts 2019, 9, 603. [Google Scholar] [CrossRef]
- Rotter, H.; Landau, M.; Herskowitz, M. Combustion of chlorinated VOC on nanostructured chromia aerogel as catalyst and catalyst support. Environ. Sci. Technol. 2005, 39, 6845–6850. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.; Lu, G. Low-temperature catalytic destruction of chlorinated VOCs over cerium oxide. Catal. Commun. 2007, 8, 1645–1649. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Kasprzyk-Hordern, B. Catalytic ozonation of chlorinated VOCs on ZSM-5 zeolites and alumina: Formation of chlorides. Appl. Catal. B Environ. 2017, 200, 274–282. [Google Scholar] [CrossRef]
- Fei, Z.; Cheng, C.; Chen, H.; Li, L.; Yang, Y.; Liu, Q.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; et al. Construction of uniform nanodots CeO2 stabilized by porous silica matrix for 1,2-dichloroethane catalytic combustion. Chem. Eng. J. 2019, 370, 916–924. [Google Scholar] [CrossRef]
- Stucchi, M.; Galli, F.; Bianchi, C.L.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous photodegradation of VOC mixture by TiO2 powders. Chemosphere 2018, 193, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tu, X.; Mei, D.; Zheng, C.; Zhou, J.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. Investigation of hybrid plasma-catalytic removal of acetone over CuO/-Al2O3 catalysts using response surface method. Chemosphere 2016, 155, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, X.; Zhang, Z. Fabrication of surface hydroxyl modified g-C3N4 with enhanced photocatalytic oxidation activity. Catal. Sci. Technol. 2019, 9, 3979–3993. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, S.; Yuan, S.; Yuan, S.; Bai, T.; Xing, S. Insight into the enhanced activity of Ag/NiOx-MnO2 for catalytic oxidation of o-xylene at low temperatures. Appl. Surf. Sci. 2019, 479, 1262–1269. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, H.; Dong, F.; Zha, F.; Tang, Z. Highly efficient catalytic combustion of o-dichlorobenzene over three-dimensional ordered mesoporous cerium manganese bimetallic oxides: A new concept of chlorine removal mechanism. Mol. Catal. 2019, 463, 119–129. [Google Scholar] [CrossRef]
- He, D.; Zhao, Y.; Yang, S.; Mei, Y.; Yu, J.; Liu, J.; Chen, D.; He, S.; Luo, Y. Enhancement of catalytic performance and resistance to carbonaceous deposit of lanthanum (La) doped HZSM-5 catalysts for decomposition of methyl mercaptan. Chem. Eng. J. 2018, 336, 579–586. [Google Scholar] [CrossRef]
- Liu, N.; Shi, D.; Zhang, R.; Li, Y.; Chen, B. Highly selective catalytic combustion of acrylonitrile towards nitrogen over Cu-modified zeolites. Catal. Today 2018, 332, 201–213. [Google Scholar] [CrossRef]
- Fiorenza, R.; Crisafulli, C.; Condorelli, G.G.; Lupo, F.; Scire, S. Au–Ag/CeO2 and Au–Cu/CeO2 Catalysts for Volatile Organic Compounds Oxidation and CO Preferential Oxidation. Catal. Lett. 2015, 145, 1691–1702. [Google Scholar] [CrossRef]
- Yao, J.; Lu, H.; Xiao, Y.; Hou, B.; Li, D.; Jia, L. Sub-molten salt-acid treatment of LaCoO3 for a highly active catalyst towards propane combustion. Catal. Commun. 2019, 128, 10578–10583. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, X.; Yang, H.; Wang, X.; Zhang, Y.; Zheng, Y.; Xiao, Y.; Jiang, L. Effect of MnO2 morphology on its catalytic performance in lean methane combustion. Mater. Res. Bull. 2018, 111, 338–341. [Google Scholar] [CrossRef]
- Wei, Y.; Ni, L.; Li, M.; Zhao, J. A template-free method for preparation of MnO2 catalysts with high surface areas. Catal. Today 2017, 297, 188–192. [Google Scholar] [CrossRef]
- Sinquin, G.; Petit, C.; Hindermann, J.P.; Kiennemann, A. Study of the formation of LaMO3 (M = Co, Mn) perovskites by propionates precursors: Application to the catalytic destruction of chlorinated VOCs. Catal. Today 2001, 70, 183–196. [Google Scholar] [CrossRef]
- Alberici, R.M.; Jardim, W.F. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl. Catal. B Environ. 1997, 14, 55–68. [Google Scholar] [CrossRef]
- Fujimoto, T.M.; Ponczek, M.; Rochetto, U.L.; Landers, R.; Tomaz, E. Photocatalytic oxidation of selected gas-phase VOCs using UV light, TiO2, and TiO2/Pd. Environ. Sci. Pollut. Res. 2017, 24, 6390–6396. [Google Scholar] [CrossRef]
- Qian, X.; Ren, M.; Yue, D.; Zhu, Y.; Han, Y.; Bian, Z.; Zhao, Y. Mesoporous TiO2 films coated on carbon foam based on waste polyurethane for enhanced photocatalytic oxidation of VOCs. Appl. Catal. B Environ. 2017, 212, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Zhan, Y.; Liu, G.; Wang, X.; Lu, H.; Xiao, L.; Feng, Q.; Leung, D.Y.C. Efficient degradation of gaseous benzene by VUV photolysis combined with ozone-assisted catalytic oxidation: Performance and mechanism. Appl. Catal. B Environ. 2016, 186, 62–68. [Google Scholar] [CrossRef]
- Song, H.; Peng, Y.; Liu, S.; Bai, S.; Hong, X.; Li, J. The Roles of Various Plasma Active Species in Toluene Degradation by Non-thermal Plasma and Plasma Catalysis. Plasma Chem. Plasma Process. 2019, 39, 1469–1482. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Xie, H.; Zhan, W.; Guo, Y. Catalytic combustion of vinyl chloride over Sr doped LaMnO3. Catal. Today 2018, 327, 190–195. [Google Scholar] [CrossRef]
- Kołodziej, A.; Łojewska, J. Optimization of structured catalyst carriers for VOC combustion. Catal. Today 2005, 105, 378–384. [Google Scholar] [CrossRef]
- Joung, H.J.; Kim, J.H.; Oh, J.S.; You, D.W.; Park, H.O.; Jung, K.W. Catalytic oxidation of VOCs over CNT-supported platinum nanoparticles. Appl. Surf. Sci. 2014, 290, 267–273. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 429–436. [Google Scholar] [CrossRef]
- Bedi, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd supported on mesoporous activated carbons with high oxidation resistance as catalysts for toluene oxidation. Appl. Catal. B Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- Usón, L.; Colmenares, M.G.; Hueso, J.L.; Sebastián, V.; Balas, F.; Arruebo, M.; Santamaría, J. VOCs abatement using thick eggshell Pt/SBA-15 pellets with hierarchical porosity. Catal. Today 2014, 227, 179–186. [Google Scholar] [CrossRef]
- Carrillo, A.M.; Carriazo, J.G. Cu and Co oxides supported on halloysite for the total oxidation of toluene. Appl. Catal. B Environ. 2015, 164, 443–452. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Sinev, I.M.; Ojala, S.; Keiski, R.L.; Kustov, M. Adsorptive-catalytic removal of CH3OH, CH3SH, and CH3SSCH3 from air over the bifunctional system noble metals/HZSM-5. Stud. Surf. Sci. Catal. 2007, 170, 1129–1136. [Google Scholar]
- Wang, J. Study on Supported Ruthenium Catalysts for the Catalytic Oxidation of VOCs. Ph.D. Thesis, Institute of Process Engineering, CAS, China, 2016. [Google Scholar]
- Abdelouahab-Reddam, Z.; Mail, R.E.; Coloma, F.; Sepúlveda-Escribano, A. Platinum supported on highly-dispersed ceria on activated carbon for the total oxidation of VOCs. Appl. Catal. A Gen. 2015, 494, 87–94. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Grbic, B.; Radic, N.; Terlecki-Baricevic, A. Kinetics of deep oxidation of n-hexane and toluene over Pt/Al2O3 catalysts: Oxidation of mixture. Appl. Catal. B Environ. 2004, 50, 161–166. [Google Scholar] [CrossRef]
- Patterson, M.J.; Angove, D.E.; Cant, N.W. The effect of carbon monoxide on the oxidation of four C6 to C8 hydrocarbons over platinum, palladium and rhodium. Appl. Catal. B Environ. 2000, 26, 47–57. [Google Scholar] [CrossRef]
- McCabe, R.W.; Mitchell, P.J. Exhaust-catalyst development for methanol-fueled vehicles: 1. A comparative study of methanol oxidation over alumina-supported catalysts containing group 9, 10, and 11 metals. Appl. Catal. 1986, 27, 83–98. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marcì, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4–CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic removal of toluene over Co3O4–CeO2 mixed oxide catalysts: Comparison with Pt/Al2O3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Mitsui, T.; Tsutsui, K.; Matsui, T.; Kikuchi, R.; Eguchi, K. Catalytic abatement of acetaldehyde over oxide-supported precious metal catalysts. Appl. Catal. B Environ. 2008, 78, 158–165. [Google Scholar] [CrossRef]
- Zhang, C.; He, H. A comparative study of TiO2 supported noble metal catalysts for the oxidation of formaldehyde at room temperature. Catal. Today 2007, 126, 345–350. [Google Scholar] [CrossRef]
- Zhang, C.B.; He, H.; Tanaka, K. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Spivey, J.J.; Butt, J.B. Catalyst deactivation during deep oxidation of chlorohydrocarbons. Appl. Catal. A Gen. 1992, 82, 259–275. [Google Scholar] [CrossRef]
- Petrosius, S.C.; Drago, R.S.; Young, V.; Grunewald, G.C. Low-temperature decomposition of some halogenated hydrocarbons using metal oxide/porous carbon catalysts. J. Am. Chem. Soc. 1993, 115, 6131–6137. [Google Scholar] [CrossRef]
- Sedjame, H.J.; Fontaine, C.; Lafaye, G.; Barbier, J.J. On the promoting effect of the addition of ceria to platinum based alumina catalysts for VOCs oxidation. Appl. Catal. B Environ. 2014, 144, 233–242. [Google Scholar] [CrossRef]
- Carabineiro, S.; Chen, X.; Konsolakis, M.; Psarras, A.; Tavares, P.; Orf~ao, J.; Pereira, M.; Figueiredo, J. Catalytic oxidation of toluene on Ce-Co and La-Co mixed oxides synthesized by exotemplating and evaporation methods. Catal. Today 2015, 244, 161–171. [Google Scholar] [CrossRef]
- Carabineiro, S.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.; Orf~ao, J.; Pereira, M.; Figueiredo, J. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Castano, M.H.; Molina, R.; Moreno, S. Catalytic oxidation of VOCs on MnMgAlOx mixed oxides obtained by auto-combustion. J. Mol. Catal. A Chem. 2015, 398, 358–367. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Yan, Y. Fabrication of porous copper/manganese binary oxides modified ZSM-5 membrane catalyst and potential application in the removal of VOCs. Chem. Eng. J. 2014, 254, 133–142. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vázquez, I.; Dejoz, A.; Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts. Appl. Catal. B Environ. 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.C.; Chen, M.; Cao, Y.; He, H.Y.; Fan, K.N. Dry citrate precursor synthesized nanocrystalline cobalt oxide as highly active catalyst for total oxidation of propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Tseng, T.K.; Wang, L.; Ho, C.T.; Chu, H. The destruction of dichloroethane over a g-alumina supported manganese oxide catalyst. J. Hazard. Mater. 2010, 178, 1035–1040. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Rivas, J.A.; Amiridis, M.D. Catalytic oxidation of 1, 2-dichlorobenzene over supported transition metal oxides. J. Catal. 2000, 193, 264–272. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of g-MnO2 as a VOC removal catalyst: Comparison with a noble metal catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Parida, K.; Samal, A. Catalytic combustion of volatile organic compounds on Indian Ocean manganese nodules. Appl. Catal. A Gen. 1999, 182, 249–256. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Huang, A.; Suib, S.L. Total oxidation of volatile organic compounds with hydrophobic cryptomelane-type octahedral molecular sieves. Microporous Mesoporous Mater. 2000, 35–36, 209–217. [Google Scholar] [CrossRef]
- Aguero, F.N.; Scian, A.; Barbero, B.P.; Cadús, L.E. Influence of the support treatment on the behavior of MnOx/Al2O3 catalysts used in VOC combustion. Catal. Lett. 2009, 128, 268–280. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Miranda, B.; Díaz, E.; Ordonez, S.; Vega, A.; Díez, F.V. Oxidation of trichloroethene over metal oxide catalysts: Kinetic studies and correlation with adsorption properties. Chemosphere 2007, 66, 1706–1715. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Chen, S.; Quan, X. The role of lattice oxygen on the activity and selectivity of the OMS-2 catalyst for the total oxidation of toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Cordi, E.M.; O’Neill, P.J.; Falconer, J.L. Transient oxidation of volatile organic compounds on a CuO/Al2O3 catalyst. Appl. Catal. B Environ. 1997, 14, 23–36. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Taylor, S.H. Designing oxidation catalysts. Catal. Today 1999, 49, 105–113. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. The total oxidation of propane over supported Cu and Ce oxides: A comparison of single and binary metal oxides. J. Catal. 2010, 272, 109–120. [Google Scholar] [CrossRef]
- Sinha, A.K.; Suzuki, K. Novel mesoporous chromium oxide for VOCs elimination. Appl. Catal. B Environ. 2007, 70, 417–422. [Google Scholar] [CrossRef]
- Padilla, A.M.; Corella, J.; Toledo, J.M. Total oxidation of some chlorinated hydrocarbons with commercial chromia based catalysts. Appl. Catal. B Environ. 1999, 22, 107–121. [Google Scholar] [CrossRef]
- Yim, S.D.; Chang, K.-H.; Nam, I.S. Deactivation of chromium oxide catalyst for the removal of perchloroethylene (PCE). Stud. Surf. Sci. Catal. 2001, 139, 173–180. [Google Scholar]
- Gorte, R.J. Ceria in catalysis: From automotive applications to the water-gas shift reaction. AlChE J. 2010, 56, 1126–1135. [Google Scholar] [CrossRef]
- Zimmer, P.; Tschope, A.; Birringer, R. Temperature-programmed reaction spectroscopy of ceria-and Cu/ceria-supported oxide catalyst. J. Catal. 2002, 205, 339–345. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Dai, Q.; Wang, Y.; Guo, Y.; Guo, Y. Hierarchical organization and catalytic activity of high-surface-area mesoporous ceria microspheres prepared via hydrothermal routes. ACS Appl. Mater. Interfaces 2010, 2, 838–846. [Google Scholar] [CrossRef]
- Jones, J.; Ross, J.R. The development of supported vanadia catalysts for the combined catalytic removal of the oxides of nitrogen and of chlorinated hydrocarbons from flue gases. Catal. Today 1997, 35, 97–105. [Google Scholar] [CrossRef]
- Delaigle, R.; Debecker, D.P.; Bertinchamps, F.; Gaigneaux, E.M. Revisiting the behaviour of vanadia-based catalysts in the abatement of (chloro)-aromatic pollutants: Towards an integrated understanding. Top. Catal. 2009, 52, 501–516. [Google Scholar] [CrossRef]
- Solsona, B.; Garcia, T.; Aylon, E.; Dejoz, A.M.; Vazquez, I.; Agouram, S.; Davies, T.E.; Taylor, S.H. Promoting the activity and selectivity of high surface area Ni-Ce-O mixed oxides by gold deposition for VOC catalytic combustion. Chem. Eng. J. 2011, 175, 271–278. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over MnOx-CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2008, 84, 303–312. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L.E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Vasile, A.; Bratan, V.; Hornoiu, C.; Caldararu, M.; Ionescu, N.I.; Yuzhakova, T.; Redey, A. Electrical and catalytic properties of cerium etin mixed oxides in CO depollution reaction. Appl. Catal. B Environ. 2013, 140, 25–31. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Li, W.; Chen, Y. Porous Mn-Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Larsson, P.O.; Andersson, A. Complete oxidation of CO, ethanol, and ethyl acetate over copper oxide supported on titania and ceria modified titania. J. Catal. 1998, 179, 72–89. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, Q.; Jiang, Z.; Zhang, Y.; Wang, Y. Preparation and formation mechanism of mesoporous CuOeCeO2 mixed oxides with excellent catalytic performance for removal of VOCs. Microporous Mesoporous Mater. 2008, 113, 427–434. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO-CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Rao, T.; Shen, M.; Jia, L.; Hao, J.; Wang, J. Oxidation of ethanol over Mn-Ce-O and Mn-Ce-Zr-O complex compounds synthesized by solegel method. Catal. Commun. 2007, 8, 1743–1747. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Picasso, G.; Gutierrez, M.; Pina, M.; Herguido, J. Preparation and characterization of Ce-Zr and Ce-Mn based oxides for n-hexane combustion: Application to catalytic membrane reactors. Chem. Eng. J. 2007, 126, 119–130. [Google Scholar] [CrossRef]
- Chen, H.; Sayari, A.; Adnot, A.; Larachi, F. Composition activity effects of Mn-Ce-O composites on phenol catalytic wet oxidation. Appl. Catal. B Environ. 2001, 32, 195–204. [Google Scholar] [CrossRef]
- Yang, P.; Yang, S.; Shi, Z.; Meng, Z.; Zhou, R. Deep oxidation of chlorinated VOCs over CeO2-based transition metal mixed oxide catalysts. Appl. Catal. B Environ. 2015, 162, 227–235. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Liu, S. SnOx-MnOx-TiO2 catalysts with high resistance to chlorine poisoning for low-temperature chlorobenzene oxidation. Appl. Catal. A Gen. 2014, 482, 363–369. [Google Scholar] [CrossRef]
- Rao, G.R.; Sahu, H.R.; Mishra, B.G. Surface and catalytic properties of Cu-Ce-O composite oxides prepared by combustion method. Coll. Surf. A Physicochem. Eng. Asp. 2003, 220, 261–269. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, G.; Zhou, R.; Mao, J.; Chen, Y.; Zheng, X. Studies of pore structure, temperature-programmed reduction performance, and micro-structure of CuO/CeO2 catalysts. Appl. Surf. Sci. 2001, 173, 208–220. [Google Scholar]
- Zheng, X.C.; Wu, S.H.; Wang, S.P.; Wang, S.R.; Zhang, S.M.; Huang, W.P. The preparation and catalytic behavior of copperecerium oxide catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. A Gen. 2005, 283, 217–223. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Zamora, R.; Cadus, L.E.; Fierro, J.L.G. Surface properties and performance for VOCs combustion of LaFe1−yNiyO3 perovskite oxides. J. Solid State Chem. 2008, 181, 905–912. [Google Scholar] [CrossRef]
- Wei, T. Study on Catalytic Combustion of VOCs over Perovskite Catalysts. Master’s Thesis, Zhejiang University of Technology, Zhejiang, China, 2005. [Google Scholar]
- Beauchet, R.; Magnoux, P.; Mijoin, J. Catalytic oxidation of volatile organic compounds (VOCs) mixture (isopropanol/o-xylene) on zeolite catalysts. Catal. Today 2007, 124, 118–123. [Google Scholar] [CrossRef]
- Rachapudi, R.; Chintawar, P.S.; Greene, H.L. Aging and Structure/Activity Characteristics of CR–ZSM-5 Catalysts during Exposure to Chlorinated VOCs. J. Catal. 1999, 185, 58–72. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Iqbal, A.; Rahman, N.R.A. Cu2+ coordinated graphitic carbon nitride (Cu-g-C3N4) nanosheets from melamine for the liquid phase hydroxylation of benzene and VOCs. Appl. Surf. Sci. 2017, 398, 43–55. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Martínez-Triguero, J.; Valencia, S. Cu and Co modified beta zeolite catalysts for the trichloroethylene oxidation. Appl. Catal. B Environ. 2016, 187, 90–97. [Google Scholar] [CrossRef]
- Lopez-Fonseca, R.; Aranzabal, A.; Steltenpohl, P.; Gutierrez-Ortiz, J.I.; Gonzalez Velasco, J.R. Performance of zeolites and product selectivity in the gas-phase oxidation of 1,2-dichloroethane. Catal. Today 2000, 62, 367–377. [Google Scholar] [CrossRef]
- Kullavanijayam, E.; Trimm, D.L.; Cant, N.W. Adsocat: Adsorption/catalytic combustion for VOC and odour control. Stud. Surf. Sci. Catal. 2000, 130, 569–574. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductorelectrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hou, P.; Yang, P.; Cheng, X. BiOBr@SiO2 flower-like nanospheres chemically-bonded on cement-based materials for photocatalysis. Appl. Surf. Sci. 2018, 430, 539–548. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Li, J.; Wang, K.; Zhang, G. Z-scheme g-C3N4@CsxWO3 heterostructure as smart window coating for UV isolating, Vis penetrating, NIR shielding and full spectrum photocatalytic decomposing VOCs. Appl. Catal. B Environ. 2018, 229, 218–226. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of different plasmonic metals on photocatalytic degradation of volatile organic compounds (VOCs) by bentonite/M-TiO2 nanocomposites, under UV/visible light. Appl. Clay Sci. 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Song, S.; Lu, C.; Wu, X.; Jiang, S.; Sun, C.; Le, Z. Strong base g-C3N4 with perfect structure for photocatalytically eliminating formaldehyde under visible-light irradiation. Appl. Catal. B Environ. 2018, 227, 145–152. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2001, 77, 107–116. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Sol–gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Appl. Catal. B Environ. 2006, 63, 60–67. [Google Scholar] [CrossRef]
- Fujishima, A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Minero, C. Surface modified photocatalysts, in environmental photochemistry part III. In The Handbook of Environmental Chemistry; Bahnemann, D.W., Robertson, K.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 35, pp. 23–44. [Google Scholar]
- Tejasvi, R.; Sharma, M.; Upadhyay, K. Passive photo-catalytic destruction of air-borne VOCs in high traffic areas using TiO2-coated flexible PVC sheet. Chem. Eng. J. 2015, 262, 875–881. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Ao, C.H.; Lee, S.C.; Hou, M.F. Enhanced photocatalytic degradation of VOCs using Ln3+–TiO2 catalysts for indoor air purification. Chemosphere 2005, 59, 787–800. [Google Scholar] [CrossRef]
- Héqueta, V.; Raillarda, C.; Debonoa, O.; Thévenet, F.; Locoge, N.; Le Coq, L. Photocatalytic oxidation of VOCs at ppb level using a closed-loop reactor: The mixture effect. Appl. Catal. B Environ. 2018, 226, 473–486. [Google Scholar] [CrossRef]
- Huang, L.; Nakajo, K.; Ozawa, S.; Matsuda, H. Decomposition of dichloromethane in a wire-in-tube pulsed corona reactor. Environ. Sci. Technol. 2001, 35, 1276–1281. [Google Scholar] [CrossRef]
- Norberg, A. Modeling current pulse shape and energy in surface discharges. IEEE Trans. Ind. Appl. 1992, 28, 498–503. [Google Scholar] [CrossRef]
- Rousseau, A.; Dantier, A.; Gatilova, L.; Ionikh, Y.; Röpcke, J.; Tolmachev, Y. On NOx production and volatile organic compound removal in a pulsed microwave discharge in air. Plasma Sour. Sci. Technol. 2005, 14, 70–75. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ramanathan, K.; Lawless, P.A.; Ensor, D.S.; Newsome, J.R.; Plaks, N.; Ramsey, G.H. Control of volatile organic compounds by an ac energized ferroelectric pellet reactor and a pulsed corona reactor. IEEE Trans. Ind. Appl. 1992, 28, 528–534. [Google Scholar] [CrossRef]
- Ding, H.-X.; Zhu, A.-M.; Yang, X.-F.; Li, C.-H.; Xu, Y. Removal of formaldehyde from gas streams via packed-bed dielectric barrier discharge plasmas. J. Phys. D App. Phys. 2005, 38, 4160–4167. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl. Catal. B Environ. 2008, 79, 356–367. [Google Scholar] [CrossRef]
- Futamura, S.; Yamamoto, T.; Lawless, P.A. Towards understanding of VOC decomposition mechanisms using nonthermal plasmas. In Proceedings of the 1995 Thirtieth IAS Annual Meeting IEEE Conference Record of Industry Applications Conference, Orlando, FL, USA, 8–12 October 1995; Volume 2, pp. 1453–1458. [Google Scholar]
- Zheng, C.; Zhu, X.; Gao, X.; Liu, L.; Chang, Q.; Luo, Z.; Cen, K. Experimental study of acetone removal by packed-bed dielectric barrier discharge reactor. J. Ind. Eng. Chem. 2014, 20, 2761–2768. [Google Scholar] [CrossRef]
- Lee, B.Y.; Park, S.H.; Lee, S.C.; Kang, M.; Choung, S.J. Decomposition of benzene by using a discharge plasma-photocatalyst hybrid system. Catal. Today 2004, 93–95, 769–776. [Google Scholar] [CrossRef]
- Gandhi, M.S.; Ananth, A.; Mok, Y.S.; Song, J.I.; Park, K.H. Effect of porosity of α-alumina on non-thermal plasma decomposition of ethylene in a dielectric-packed bed reactor. Res. Chem. Intermed. 2014, 40, 1483–1493. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, N.; Li, J.; Shang, K.; Lu, N.; Wu, Y. Degradation of benzene by bipolar pulsed series surface/packed-bed discharge reactor over MnO2-TiO2/zeolite catalyst. Chem. Eng. J. 2016, 293, 216–224. [Google Scholar] [CrossRef]
- Ogata, A.; Yamanouchi, K.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Oxidation of dilute benzene in an alumina hybrid plasma reactor at atmospheric pressure. Plasma Chem. Plasma Process. 1999, 19, 383–394. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Qin, R.; Zeng, Y.; Qu, R.; Zheng, C.; Tu, X. Plasma-catalytic removal of formaldehyde over Cu–Ce catalysts in a dielectric barrier discharge reactor. Appl. Catal. B Environ. 2015, 170–171, 293–300. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Estimation of CO heats of adsorption on metal surfaces from vibrational spectra. Ind. Eng. Chem. Res. 1996, 35, 3171–3178. [Google Scholar] [CrossRef]
- An, H.T.Q.; Huu, T.P.; Le Van, T.; Cormier, J.M.; Khacef, A. Application of atmospheric non thermal plasma-catalysis hybrid system for air pollution control: Toluene removal. Catal. Today 2011, 176, 474–477. [Google Scholar]
- Futamura, S.; Einaga, H.; Kabashima, H.; Hwan, L.Y. Synergistic effect of silent discharge plasma and catalysts on benzene decomposition. Catal. Today 2004, 89, 89–95. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Xia, Q.; Li, Z. Decomposition of toluene in a plasma catalysis system with NiO, MnO2, CeO2, Fe2O3, and CuO catalysts. Plasma Chem. Plasma Process. 2013, 33, 1073–1082. [Google Scholar] [CrossRef]
- Zhao, D.-Z.; Li, X.-S.; Shi, C.; Fan, H.-Y.; Zhu, A.-M. Low-concentration formaldehyde removal from air using a cycled storage-discharge (CSD) plasma catalytic process. Chem. Eng. Sci. 2011, 66, 3922–3929. [Google Scholar] [CrossRef]
- Ran, L.; Wang, Z.; Wang, X. The effect of Ce on catalytic decomposition of chlorinated methane over RuOx catalysts. Appl. Catal. A gen. 2014, 470, 442–450. [Google Scholar] [CrossRef]
- Asilevi, P.J.; Yi, C.W.; Li, J.; Nawaz, M.I.; Wang, H.J.; Yin, L.; Junli, Z. Decomposition of formaldehyde in strong ionization non-thermal plasma at atmospheric pressure. Int. J. Environ. Sci. Technol. 2020, 17, 765–776. [Google Scholar] [CrossRef]
- Zhu, T.; Li, J.; Jin, Y.; Liang, Y.; Ma, G. Decomposition of benzene by non-thermal plasma processing: Photocatalyst and ozone effect. Int. J. Environ. Sci. Technol. 2008, 5, 375–384. [Google Scholar] [CrossRef]
- Urashima, K.; Kostov, K.G.; Chang, J.S.; Okayasu, Y.; Iwaizumi, T.; Yoshimura, K.; Kato, T. Removal of C2F6 from a semiconductor process flue gas by a ferroelectric packed-bed barrier discharge reactor with an adsorber. IEEE Trans. Ind. Appl. 2001, 37, 1456–1463. [Google Scholar] [CrossRef]
- Ogata, A.; Ito, D.; Mizuno, K.; Kushiyama, S.; Gal, A.; Yamamoto, T. Effect of coexisting components on aromatic decomposition in a packed-bed plasma reactor. Appl. Catal. A Gen. 2002, 236, 9–15. [Google Scholar] [CrossRef]
- Ogata, A.; Shintani, N.; Yamanouchi, K.; Mizuno, K.; Kushiyama, S.; Yamamoto, T. Effect of water vapor on benzene decomposition using a nonthermal-discharge plasma reactor. Plasma Chem. Plasma Process. 2000, 20, 453–467. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Sakai, H.; Washio, M.; Kojima, T. Application of electron beams for the treatment of VOC streams. Ind. Eng. Chem. Res. 2004, 43, 1185–1191. [Google Scholar] [CrossRef]
- Zimek, Z. High power accelerators and processing systems for environmental application. Radiation treatment of gaseous and liquid effluents for contaminant removal. IAEA-TECDOC 2005, 1473, 125–137. [Google Scholar]
- Son, Y.S.; Kim, J.; Kim, J.C. Decomposition of acetaldehyde using an electron beam. Plasma Chem. Plasma Process. 2014, 34, 1233–1245. [Google Scholar] [CrossRef]
- Son, Y.S.; Kim, K.H.; Kim, K.J.; Kim, J.C. Ammonia Decomposition Using Electron Beam. Plasma Chem. Plasma Process. 2013, 33, 617–629. [Google Scholar] [CrossRef]
- Han, D.H.; Stuchinskaya, T.; Won, Y.S.; Park, W.S.; Lim, J.K. Oxidative decomposition of aromatic hydrocarbons by electron beam irradiation. Radiat. Phys. Chem. 2003, 67, 51–60. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hakoda, T.; Hirata, K.; Arai, H. Low energy electron beam treatment of VOCs. Radiat. Phys. Chem. 2000, 57, 485–488. [Google Scholar] [CrossRef]
- Sun, Y.; Chmielewski, A.G.; Licki, J.; Bułka, S.; Zimek, Z. Decomposition of organic compounds in simulated industrial off-gas by using electron beam irradiation. Radiat. Phys. Chem. 2009, 78, 721–723. [Google Scholar] [CrossRef]
- Son, Y.S.; Kim, P.; Park, J.H.; Kim, J.; Kim, J.C. Decomposition of trimethylamine by an electron beam. Plasma Chem. Plasma Process. 2013, 33, 1099–1109. [Google Scholar] [CrossRef]
- Son, Y.S.; Park, J.H.; Kim, P.; Kim, J.C. Oxidation of gaseous styrene by electron beam irradiation. Radiat. Phys. Chem. 2012, 81, 686–692. [Google Scholar] [CrossRef]
- Auslender, V.L.; Ryazantsev, A.A.; Spiridonov, G.A. The use of electron beam for solution of some ecological problems in pulp and paper industry. Radiat. Phys. Chem. 2002, 63, 641–645. [Google Scholar] [CrossRef]
- Son, Y.S.; Kim, J.C. Decomposition of sulfur compounds by radiolysis: I. Influential factors. Chem. Eng. J. 2015, 262, 217–223. [Google Scholar] [CrossRef]
- Son, Y.S.; Jung, I.H.; Lee, S.J.; Kim, J.C. Decomposition of sulfur compounds by a radiolysis: III. A hybrid system and field application. Chem. Eng. J. 2015, 274, 9–16. [Google Scholar] [CrossRef]
- Son, Y.S.; Jung, I.H.; Lee, S.J.; Koutrakis, P.; Kim, J.C. Decomposition of sulfur compounds by radiolysis: II. By-products and mechanisms. Chem. Eng. J. 2015, 269, 27–34. [Google Scholar] [CrossRef]
- Kim, J.C. Factors affecting aromatic VOC removal by electron beam treatment. Radiat. Phys. Chem. 2002, 65, 429–435. [Google Scholar] [CrossRef]

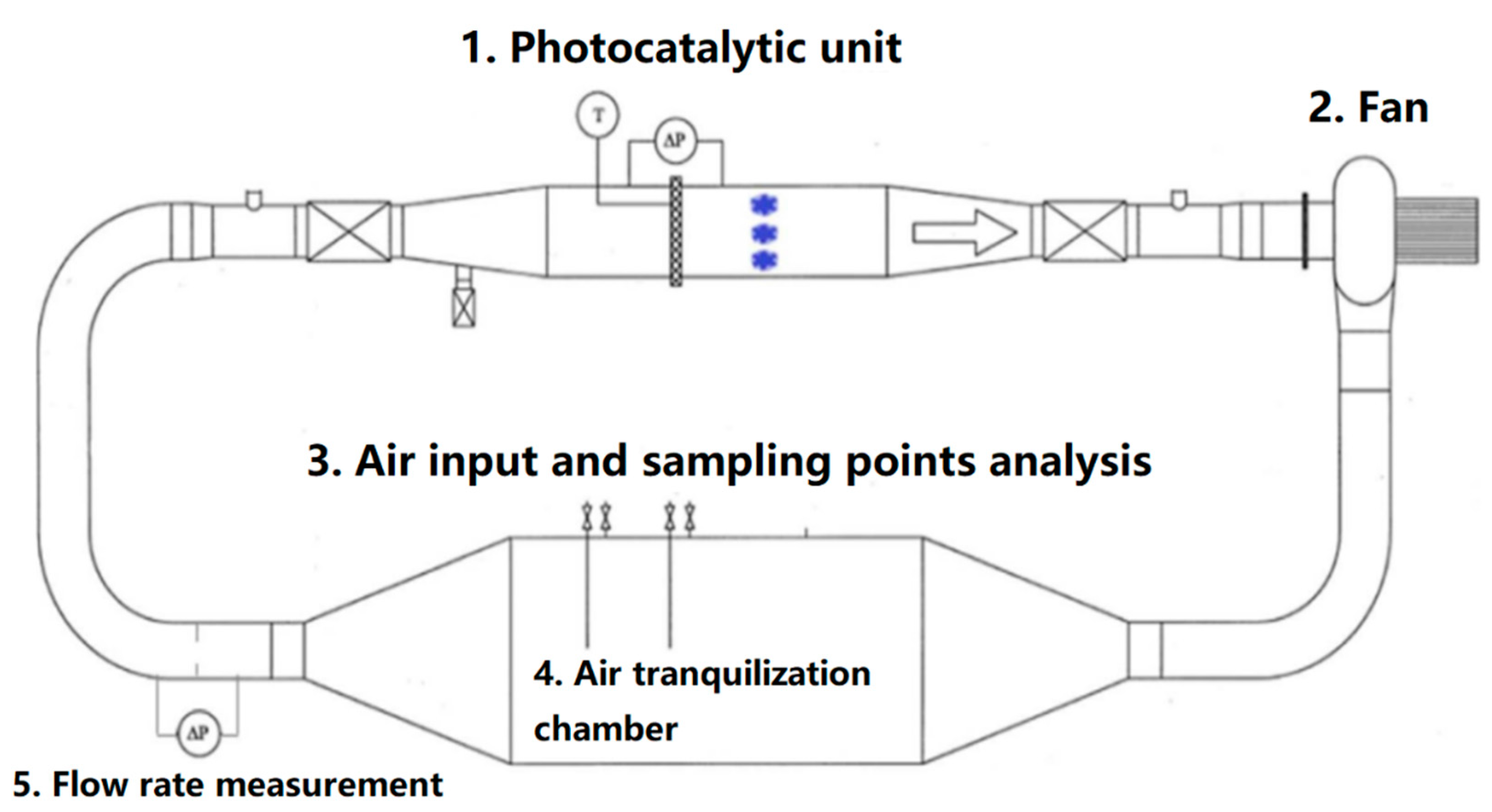
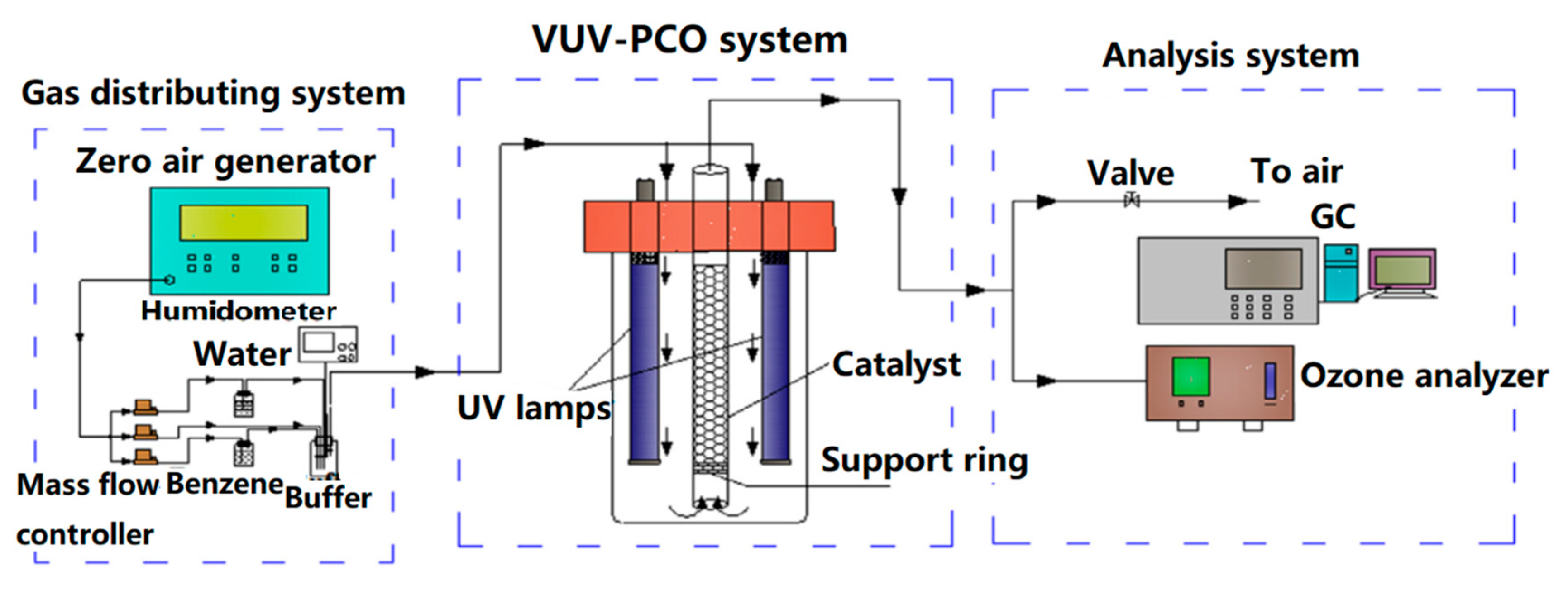
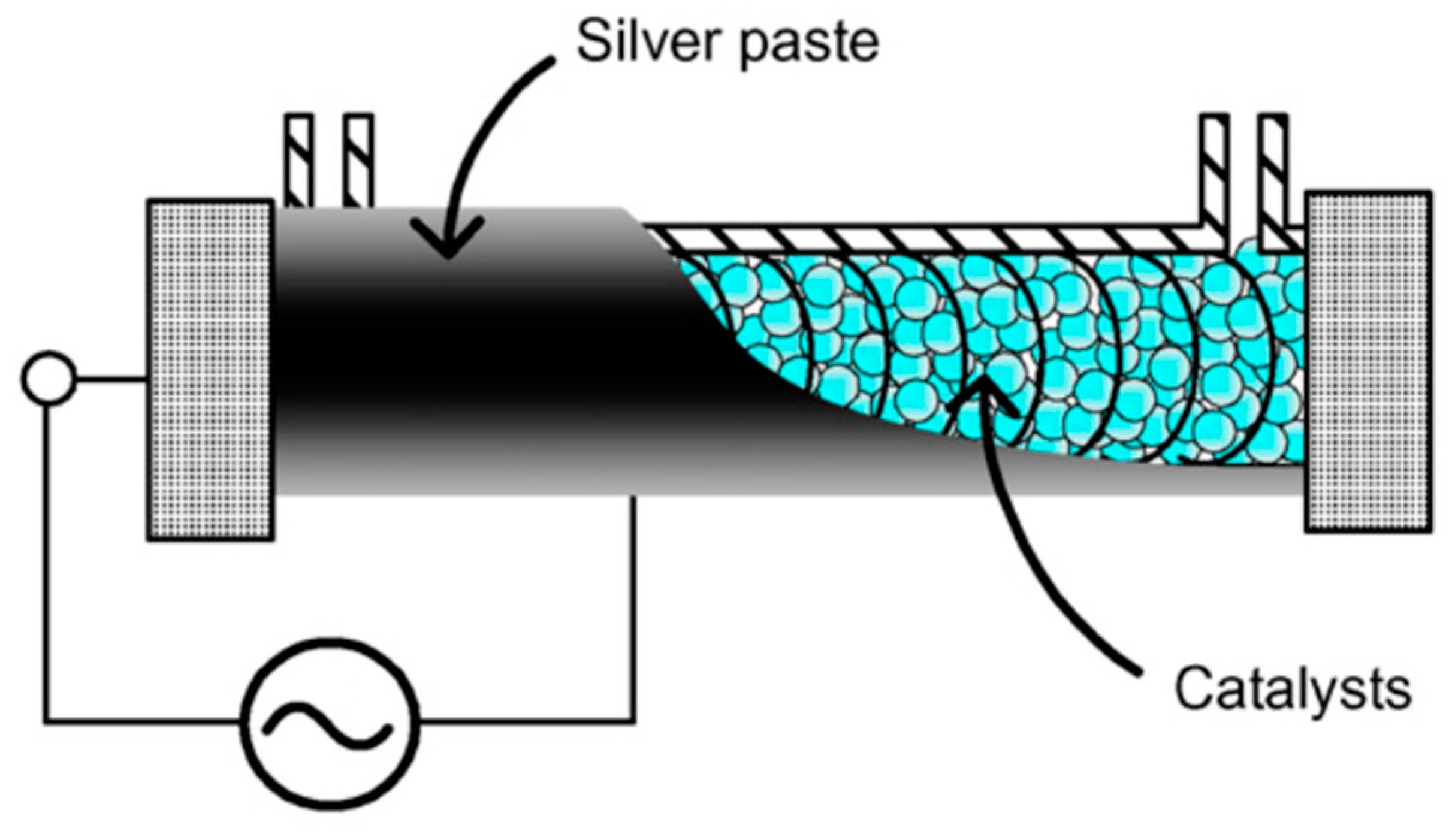
| Pollutants | Limiting Value (mg·m−3) | The Highest Concentration of Selected VOCs Allowed in 1 h for Employees (mg·m−3) |
|---|---|---|
| Benzene | 0.4 | 6 |
| Formaldehyde | 0.2 | 0.1 |
| Trichloroethylene a | 0.1 | 30 |
| Dimethyl sulfate a | 0.5 | 0.5 |
| Dichloromethane a | 4.0 | 0.5 |
| Non-methane organic compounds | 4.0 | - |
| Ozone | 20 | 0.26 |
| Classification | Pollutant | The Limiting Values (mg·m−3) | |
|---|---|---|---|
| General Area | Key Area | ||
| Carcinogens | Trichloroethylene a | 1 | 1 |
| Benzene | 4 | 4 | |
| Formaldehyde | 5 | 5 | |
| Toxic substances | Phosgene | 0.5 | 0.5 |
| HCN | 1.9 | 1.9 | |
| Acrolein | 3 | 3 | |
| Methyl sulfate a | 5 | 5 | |
| Cl2 | 5 | 5 | |
| Photochemically active substances | Toluene | 25 | 15 |
| xylene | 40 | 20 | |
| Dimethyl sulfoxide a | 100 | 50 | |
| Butylene oxide a | 100 | 50 | |
| Other | NH3 | 20 | 10 |
| HCl | 20 | 10 | |
| CH3OH | 50 | 30 | |
| CH2Cl2 a | 75 | 45 |
| Abatement Technologies | VOC Concentration (mg·m−3) | Discharge Rate (m3·h−1) | Temperature (°C) |
|---|---|---|---|
| Adsorption recycling | 100–1.5×104 | <6 × 104 | <45 |
| Preheated catalytic combustion | 3000–1/4 LEL * | <4 × 104 | <500 |
| Thermal storage catalytic combustion | 1000–1/4 LEL | <4 × 104 | <500 |
| Preheated incineration | 3000–1/4 LEL | <4 × 104 | >700 |
| Thermal storage incineration | 1000–1/4 LEL | <4 × 104 | >700 |
| Adsorption concentration | <1500 | 104–1.2 × 105 | <45 |
| Biodegradation | <1000 | <1.2 × 104 | <45 |
| Condensation | 104–105 | <104 | <150 |
| Non-thermal | <500 | <3 × 104 | <80 |
| VOCs | Refer | Catalyst | Catalytic Performance | Remarks | Catalytic Mechanism |
|---|---|---|---|---|---|
| Benzene | [56] | 0.3% Pt/γ-Al2O3 | T95: 220 °C | - | - |
| [57] | Pd-AuFeCeIM | T100: 200 °C | - | - | |
| [58] | Layered copper manganese oxide (LCMO) | T50: 218 °C T90: 240 °C | The introduction of water vapor had no significant effect on the catalyst. The catalyst activity decreased about 30% after SO2 was added. The addition of CO2 has no effect on the catalytic activity of the catalyst. | The larger surface area and the formation of Cu2+-O2−-Mn4+ entities at the interface between CuO and layered MnO2 promoted the CO and VOCs oxidation. | |
| [59] | LaCoO3 | T50: 330 °C | Water vapor has no significant effect on the catalytic performance of catalyst. | Factors improve activity: (i) The larger exposed surface, (ii) the composition of the support provides increased oxygen mobility. | |
| [60] | LaMnO3 LaCoO3 | T50: 301 °C T50: 323 °C | - | - | |
| [61] | γ-MnO2/SmMnO3 | T50: 213 °C T90: 226 °C | - | Toluene-Mn ions+ surface reactive oxygen → benzaldehyde → benzoic acid → chain carboxylic acids → CO2. | |
| [62] | Mn/meso-TiO2 | - | - | Benzene → (splitting of benzene, hydrogenation, H abstraction by OH radicals) six-member ring cyclitols+aldehydes ketones etc. → a class of fulvene (isomerization) →CO2 + H2O. | |
| [63] | Mesoporous TiO2 | - | - | Benzene is mainly degraded by photo-generated electron–hole pairs and hydroxyl radicals. | |
| [64] | Graphite-SiO2-TiO2 | The graphite-SiO2-TiO2 composites exhibited higher photocatalytic activity for degradation of benzene gas under visible light irradiation than that of pure TiO2. | Optimum concentration of graphite facilitates the separation of photogenerated electron–hole pairs for graphite-SiO2-TiO2 composites by visible light. | ||
| [65] | Pt/Al2O3 | - | - | - | |
| Toluene | [66] | 2.3 wt% Pt/3DOM-Mn2O3 | T50: 165 °C T90: 194 °C | - | A first-order reaction mechanism. Toluene → cat. Surface → benzyl alcohol → benzoic acid and benzaldehyde → (rise temperature) maleic anhydride → CO2 and H2O. |
| [67] | Pd(shell)-Au(core)/TiO2 | T50: 220 °C T90: 230 °C | - | Langmuir-Hinshelwood mechanism. | |
| [68] | Au-Pd/CeO2 | T50: 120 °C T90: 150 °C | - | Due to the synergistic effect between Au and Pd nanoclusters, Au-Pd/CeO2 bimetallic catalysts are much better than Au and Pd single metal catalysts. | |
| [69] | Mn2O3 | T50: 231 °C T90: 239 °C | - | Toluene +cat. → benzyl species + active oxygen species → aromatic alkoxide → enzaldehyde → benzoate species+active oxygen specie → maleic anhydrides→CO2. | |
| [58] | Layered copper manganese oxide (LCMO) | T50: 187 °C T90: 207 °C | - | - | |
| [59] | LaCoO3 | T50: 244 °C | - | - | |
| [70] | La0.8Sr0.2CoO3 | T50: < 160 °C T99: 300 °C | - | Partial substitution of strontium (Sr) for lanthanum (La) greatly increased the oxygen vacancies in the surface regions to enhance catalytic activity. | |
| [71] | SrTi1−XCuXO3 SrTi1−XMnXO3 | T50: 343 °C T90: 398 °C T50: 302 °C T90: 335 °C | - | Incorporation of Mn attributed a higher amount of oxygen vacancies in the perovskite surface to promote the toluene conversion. Supra facial mechanism. | |
| [72] | LaMn1−xBxO3 (B = Co, Ni, Cu, Al) | T50: 250 °C 234 °C 226 °C 242 °C T90: none 278 °C 271 °C 318 °C | - | Addition of nickel into LaMnO3 can improve the catalytic oxidation of toluene by the generation of more Mn4+ species and oxygen vacancies and the enhancing reducibility at a low temperature. | |
| [61] | γ-MnO2/SmMnO3 | T50: 187 °C T90: 208 °C | - | - | |
| [73] | LaMnO3/δ-MnO2 | T90: 258 °C | - | - | |
| [74] | LaMnO3 | T50: 258 °C T90: 275 °C | - | - | |
| [75] | MnOx/H-Beta-SDS MnOx/K-Beta-SDS MnOx/Si-Beta | T50: 253, 262, 280 °C T90: 285, 295, 312 °C | - | Mars-Van Krevelen mechanism. Organic molecules + lattice oxygen→oxygen vacancy + CO2 + H2O, oxygen vacancy + O2→lattice oxygen. | |
| [76] | Mn/R-SBA-15 | T98: 240 °C | - | MVK mechanism. Mn2O3-MnO2/R-SBA-15 supply more lattice oxygen species. | |
| [77] | LaFeO3/black-TiO2 | The removal efficiency of black TiO2 and LaFeO3 for toluene was 89% and 98%, respectively, and the removal efficiency for IPA was 90% and 94%, respectively. | - | Cat.+photon (the wavelength shorter than 440 nm)→ electrons + O2 →O2−, O2−+toluene and IPA → CO2 + H2O(g). | |
| [78] | Pd/CeO2/γ-Al2O3 | T98: 205 °C | - | - | |
| [79] | CuO/ZnO Nanocomposite photocatalysts | - | - | Photooxidative activity and stability over ZnO are improved by loading CuO. | |
| [80] | FeOx/SBA-15 | - | Under the condition of 3% Fe loading, the oxidation of toluene is the best one. | (1) Direct removal caused by the collision of electrons or oxidation caused by the gas-phase radicals (O•, OH•, N2•, NO•, NO2•) in gas phase; (2) the reaction between adsorbed toluene or other intermediates and the active species (O•, OH•) on the catalyst surface. O2 might be fixed on the catalyst surface via facile interconversion between Fe2+ and Fe3+ states and then be transported to the toluene or intermediates leading to CO2 formation. | |
| Butanol | [56] | 0.3% Pt/γ-Al2O3 | T95: 200 °C | - | - |
| CH3SSCH3 | [81] | Pt-Au/Ce-Al | T50: 425 °C T90: 480 °C | - | The addition of Au improved the selectivity of ceria-containing catalysts by decreasing the formation of byproducts. This may have had a connection to a lower amount of reactive oxygen after the Au addition. |
| [81] | Cu-Au/Ce-Al | T50: 275 °C T90: 370 °C | -- | - | |
| Chlorobenzene | [82] | Pt/CrOOH | T50: 340 °C T90: 378 °C | - | Platinum accelerates the hydrolysis of Cr-Cl bonds formed at the CrOOH surface and determines the catalyst stability. |
| Dichloroethane | [82] | Pt/CrOOH | T50: 283 °C T90: 317 °C | - | - |
| Dichloromethane | [83] | CeO2 | T90: 260 °C T90: 160 °C | The addition of 3% (v/v) water can obviously inhibit the catalytic decomposition of VOCs on CeO2. | Trichloroethylene + CeO2 →C2HCl→HCl, Cl2, CO2 and trace CO. |
| Dichlorobenzene | [84] | H-ZSM-5 and Na-ZSM-5 | - | - | - |
| Trichloro benzene | [84] | H-ZSM-5 and Na-ZSM-5 | - | - | - |
| DCE | [85] | CeO2@SiO2-400 | T50: 219 °C T90: 275 °C | The conversion of 1 vol% H2O and 3 vol% H2O decreased by 6% and 19%, respectively. | DCE+acid sites→HCl+ VC. (1) VC→reactive carbonium ion→adsorbed alcohol species→acetate species. Or (2) VC→1,1,2-trichloroethane→dichloroethylene formed (subsequent chlorination reactions) →H2O, COx and HCl. |
| Ethylbenzene | [61] | γ-MnO2/SmMnO3 | T50: 201 °C T90: 217 °C | - | - |
| Formaldehyde | [86] | TiO2 | TiO2 degraded almost 100% of formaldehyde or acetaldehyde at a starting concentration of 400–500 ppb with a relative humidity of 40%. | - | The rate-determining step is the adsorption (external diffusion) on the catalysts active sites, thus the higher surface area, the higher the degradation. |
| Acetaldehyde | [86] | TiO2 | - | - | - |
| Acetone | [78] | Pd/CeO2/γ-Al2O3 | T98:220 °C | - | - |
| [87] | CuO/g-Al2O3 | - | 5.0 wt% CuO/g-Al2O3 catalyst has the highest removal rate of acetone, reaching 67.9%. | Both short-lived radicals and acetone/intermediates can be adsorbed on the catalyst surfaces to initiate a series of surface oxidation reactions, forming CO, CO2, H2O, and byproducts. | |
| Ethyl acetate | [78] | Pd/CeO2/γ-Al2O3 | T98:275 °C | - | - |
| Benzoquinone | [88] | g-C3N4 | - | - | OH radicals+phenol→dihydroxycyclohexadienyl radical adducts→phenoxy radicals→H2O(a very slow process), adducts+dissolved O2→dihydroxy intermediates (-HO2) →CO2+H2O. |
| Hydroquinone Catechol | [88] | g-C3N4 | - | - | - |
| O-xylene | [89] | Ag/NiOx-MnO2 | T50: 145 °C T90: 190 °C | The catalytic activity decreased with the addition of water vapor, but recovered after the removal of water vapor. | Mars-Van Krevelen mechanism. (i) Electrophilic Ol +aromatic ring→maleate; (ii) O2+ Cat.→electrophilic oxygen species (O22−, O2−, O−), electrophilic oxygen species+aromatic ring→maleate, carbonate+(O2. O22−, O2−, O− or O2) +Ag→nucleophilic oxygen, nucleophilic oxygen + maleate and carboxylates→CO2 and H2O. |
| [58] | Layered copper manganese oxide (LCMO) | T50: 213 °C T90: 227 °C | - | - | |
| [61] | γ-MnO2/SmMnO3 | T50: 232 °C T90: 250 °C | - | - | |
| O-dichlorobenzene | [90] | CeMn30 | GHSV = 15,000 mL/(g·h) T50: 291 °C T90: 347 °C GHSV= 7500 mL/(g·h) T90: 224 °C GHSV = 30,000 mL/(g·h). T90: 360 °C | The addition of low concentration of water vapor is beneficial to the early reaction, but will weaken the catalytic activity of catalyst. | O-dichlorobenzene molecules+basic oxygen (Mn-O-Ce-Vö, nucleophilic substitution)→HCl-H-O-Ce-Vö+phenyl. Mn-O-Ce weakened HCl-H-O-Ce-Vö and then prevented Deacon reaction and chlorine poisoning. Phenyl+ active lattice oxygen→organic intermediates →CO2+H2O. (Mars-Van Krevelen mechanism). |
| CO | [58] | Layered copper manganese oxide (LCMO) | T50: 62 °C T90: 76 °C | - | - |
| Cyclohexane | [70] | La0.8Sr0.2CoO3 | T50: 180 °C T99: 260 °C | - | - |
| CH3SH | [91] | La(13)/HZSM-5 | T100: 40 °C | - | The La-modified HZSM-5 increased basic sites and displayed better adsorption ability to CH3SH, decreased in strong acid sites and suppressed the formation of coke deposit. |
| C2H3CN | [92] | Cu-ZSM-5(SiO2/Al2O3 = 26) | T90: 325 °C | The catalyst has good resistance to steam poisoning. | Without H2O: C2H3CN-SCC+O2→NCO→oxidation products; with H2O: C2H3CN-SCC+O2 + H2O→NH3→products. |
| Cumene | [84] | H-ZSM-5 and Na-ZSM-5 | - | - | - |
| Isopropanol | [60] | LaMnO3 LaCoO3 | T50: 216 °C 237 °C | - | - |
| [79] | CuO/ZnO Nanocomposite photocatalysts | - | - | Photooxidative activity and stability over ZnO are improved by loading CuO. | |
| [93] | Au-Ag/CeO2 | T50: 105 °C T90: 158 °C | - | MVK redox mechanism. | |
| Propyl alcohol | [60] | LaMnO3 LaCoO3 | T50: 203 °C 222 °C | - | - |
| [70] | La0.8Sr0.2CoO3 | T90: 160 °C | - | - | |
| Propane | [94] | LaCoO3 | T50: 208 °C T90: 238 °C | - | MVK mechanism. |
| Methane | [95] | Nanocubic MnO2 | T50: 293 °C T90: 350 °C | - | CH4+lattice oxygen or surface oxygen vacancies (MnO2-C)→carboxylate species (Langmuir-Hinshelwood route), + active oxygen species→CO2 and H2O. |
| VOCs | [96] | MnO2 | T50: 233 °C T90: 256 °C | -- | Toluene + Mn cations→CO2 and H2O. |
| [97] | LaMnO3 and LaCoO3 | - | - | - | |
| [98] | La3+-TiO2 and Nd3+-TiO2 | 1.2% La3+-TiO2 had the highest photocatalytic activity. | - | - | |
| [99] | TiO2/Pd | The conversion rate of VOCs reached 90% when the residence time was 27 s. | - | - | |
| [100] | MesoTiO2/hydro-CF | - | - | Promotion effects on degradation of gaseous polar acetone come from well crystallized anatase nanocrystals, hydro-CF skeleton for adsorption, and fast mass transportation within the hierarchical frameworks. | |
| [101] | CsX, NaX, and HY | T100: 200 °C | - | Mars-Van Krevelen mechanism, involving several redox steps. | |
| O3 | [102] | Mn/ZSM-5 | O3 can be efficiently decomposed by the Mn/ZSM-5 and used for benzene degradation through the OZCO. | - | Benzene+•OH→phenol→benzoquinone→CO2 + H2O. |
| [103] | CoMnOx/TiO2 | - | When the temperature is 320 °C, the decomposition efficiency of O3 is 98%. | - | |
| Vinyl chloride | [104] | HCl modified La0.5Sr0.5MnO3 | T100: 300 °C | - | Doping and acid treatment obviously promote the active Mn4+ species amount and oxygen activation ability, and affect the chloric by-product distribution. Lower temperature inhibits the Deacon reaction and chlorination. |
| Catalyst | VOCs | Catalytic Performance | Remarks | Catalytic Mechanism |
|---|---|---|---|---|
| 0.3% Pt/γ-Al2O3 | Butanol Benzene | T95: 200 °C 220 °C | - | - |
| Pd-AuFeCeIM | Benzene | T100: 200 °C | - | - |
| Pd(shell)-Au(core)/TiO2 | Toluene | T50: 220 °C T90: 230 °C | - | Langmuir-Hinshelwood mechanism. |
| Au-Pd/CeO2 | Toluene | T50: 120 °C T90: 150 °C | - | Due to the synergistic effect between Au and Pd nanoclusters, Au-Pd/CeO2 bimetallic catalysts are much better than Au and Pd single metal catalysts. |
| Au-Ag/CeO2 | Isopropanol | T50: 105 °C T90: 158 °C | - | MVK redox mechanism. |
| Pt-Au/Ce-Al | CH3SSCH3 | T50: 425 °C T90: 480 °C | - | The addition of Au improved the selectivity of ceria-containing catalysts by decreasing the formation of byproducts due to lower amount of reactive oxygen after the Au addition. |
| Cu-Au/Ce-Al | CH3SSCH3 | T50: 275 °C T90: 370 °C | - | -- |
| Ag/NiOx-MnO2 | O-xylene | T50: 145 °C T90: 190 °C | The catalytic activity decreased with the addition of water vapor, but recovered after the removal of water vapor. | Mars-Van Krevelen mechanism. (i) Electrophilic Ol +aromatic ring→maleate; (ii) O2+ Cat.→electrophilic oxygen species (O22−, O2−, O−), electrophilic oxygen species + aromatic ring→maleate, carbonate+(O2, O22−, O2−, O− or O2) +Ag→nucleophilic oxygen, nucleophilic oxygen + maleate and carboxylates→CO2 and H2O. |
| 2.3 wt% Pt/3DOM-Mn2O3 | Toluene | T50: 165 °C T90: 194 °C | - | A first-order reaction mechanism. Toluene →cat. surface→benzyl alcohol→benzoic acid and benzaldehyde→(rise temperature) maleic anhydride→CO2 and H2O. |
| Refer | Catalyst | VOCs | Catalytic Performance | Remarks |
|---|---|---|---|---|
| [69] | Mn2O3 | Toluene | T50: 231 °C T90: 239 °C | - |
| [82] | Pt/CrOOH | Dichloroethane Chlorobenzene | T50: 283 °C T90: 317 °C T50: 340 °C T90: 378 °C | - |
| [83] | CeO2 | Tetrachloroethylene dichloromethane | T90: 260 °C T90: 160 °C | The addition of 3% (v/v) water can obviously inhibit the catalytic decomposition of VOCs on CeO2. |
| [90] | CeMn30 | O-dichlorobenzene | GHSV = 15,000 mL/(g·h) T50: 291 °C T90: 347 °C GHSV= 7500 mL/(g·h) T90: 224 °C GHSV = 30,000 mL/(g·h) T90: 360 °C | The addition of low concentration of water vapor is beneficial to the early reaction, but will weaken the catalytic activity of the catalyst. |
| [96] | MnO2 | VOCs | T50: 233 °C T90: 256 °C | - |
| [95] | Nanocubic MnO2 | Methane | T50: 293 °C T90: 350 °C | - |
| Refer | Catalyst | VOCs | Catalytic Performance | Remarks | Catalytic Mechanism |
|---|---|---|---|---|---|
| [58] | Layered copper manganese oxide (LCMO) | CO Benzene Toluene O-xylene | T50: 62 °C T90: 76 °C T50: 218 °C T90: 240 °C T50: 187 °C T90: 207 °C T50: 213 °C T90: 227 °C | The introduction of water vapor had no significant effect on the catalyst. The catalyst activity decreased about 30% after SO2 was added. The addition of CO2 has no effect on the catalytic activity of the catalyst. | The larger surface area and the formation of Cu2+-O2−-Mn4+ entities at the interface between CuO and layered MnO2 promoted the CO and VOCs oxidation. |
| [59] | LaCoO3 | Benzene Toluene | T50: 330 °C T50: 244 °C | Water vapor has no significant effect on the catalytic performance of catalyst. | Factors improve activity: (i) The larger exposed surface, (ii) the composition of the support provides increased oxygen mobility. |
| [60] | LaMnO3 LaCoO3 | Isopropanol Benzene Propyl alcohol Isopropanol Benzene Propyl alcohol | T50: 216 °C 301 °C 203 °C T90: 237 °C 323 °C 222 °C | - | - |
| [61] | γ-MnO2/SmMnO3 | Toluene Benzene O-xylene Ethylbenzene | T50: 187 °C T90: 208 °C T50: 213 °C T90: 226 °C T50: 232 °C T90: 250 °C T50: 201 °C T90: 217 °C | - | Toluene-Mn ions+ surface reactive oxygen → benzaldehyde → benzoic acid → chain carboxylic acids → CO2. |
| [70] | La0.8Sr0.2CoO3 | toluene Cyclohexane Propyl alcohol | T50:<160 °C T99: 300 °C T50: 180 °C T99: 260 °C T90: 160 °C | - | Partial substitution of strontium (Sr) for lanthanum (La) greatly increased the oxygen vacancies in the surface regions to enhance the catalytic activity. |
| [71] | SrTi1-XCuXO3 SrTi1-XMnXO3 | Toluene | T50: 343 °C T90: 398 °C T50: 302 °C T90: 335 °C | - | Incorporation of Mn attributed a higher amount of oxygen vacancies in the perovskite surface to promote the toluene conversion. Supra facial mechanism. |
| [72] | LaMn1-xBxO3 (B = Co, Ni, Cu, Al) | Toluene LMLi LMNi LMCo LMAl LMLi LMNi LMCo LMAl | T50: 250 °C 234 °C 226 °C 242 °C T90: none 278 °C 271 °C 318 °C | - | Addition of nickel into LaMnO3 can improve the catalytic oxidation of toluene by generation of more Mn4+ species and oxygen vacancies and the enhancing reducibility at low temperature. |
| [73] | LaMnO3/δ-MnO2 | Toluene | T90: 258 °C | - | - |
| [74] | LaMnO3 | Toluene | T50: 258 °C T90: 275 °C | - | - |
| [85] | CeO2@SiO2-400 | DCE | T50: 219 °C T90: 275 °C | The conversion of 1 vol% H2O and 3 vol% H2O decreased by 6% and 19%, respectively. | DCE+acid sites→HCl+ VC. (1) VC→reactive carbonium ion→adsorbed alcohol species→acetate species. Or (2) VC→1,1,2-trichloroethane→dichloroethylene formed (subsequent chlorination reactions) →H2O, COx and HCl. |
| [94] | LaCoO3 | Propane | T50: 208 °C T90: 238 °C | - | MVK mechanism. |
| [97] | LaMnO3 and LaCoO3 | Chlorinated VOCs | - | - | - |
| [104] | HCl modified La0.5Sr0.5MnO3 | Vinyl chloride | T100: 300 °C | - | Doping and acid treatment obviously promote the active Mn4+ species amount and oxygen activation ability, and affect the chloric byproduct distribution. The lower temperature inhibits the Deacon reaction and chlorination. |
| Refer | Catalyst | VOCs | Catalytic Performance | Remarks | Catalytic Mechanism |
|---|---|---|---|---|---|
| [75] | MnOx/H-Beta-SDS MnOx/K-Beta-SDS MnOx/Si-Beta | Toluene | T50: 253, 262, 280 °C T90: 285, 295, 312 °C | - | Mars-Van Krevelen mechanism. Organic molecules+lattice oxygen→oxygen vacancy+CO2+H2O, oxygen vacancy+O2→lattice oxygen. |
| [84] | H-ZSM-5 and Na-ZSM-5 | Cumene Dichlorobenzene Trichloro benzene | - | - | - |
| [91] | La(13)/HZSM-5 | CH3SH | T100: 40 °C | - | The La-modified HZSM-5 increased basic sites and displayed better adsorption ability to CH3SH, decreased in strong acid sites and suppressed the formation of coke deposit. |
| [92] | Cu-ZSM-5(SiO2/Al2O3 = 26) | C2H3CN | T90: 325 °C | The catalyst has good resistance to steam poisoning. | Without H2O: C2H3CN-SCC+O2→NCO→oxidation products; with H2O: C2H3CN-SCC+O2+ H2O→NH3→products. |
| [101] | CsX, NaX, and HY | VOCs mixture | T100: 200 °C | - | Mars-Van Krevelen mechanism, involving several redox steps. |
| [176] | Mn/R-SBA-15 | Toluene | T98: 240 °C | - | MVK mechanism. Mn2O3-MnO2/R-SBA-15 supply more lattice oxygen species. |
| Refer | Catalyst | VOCs | Catalytic Performance | Remarks | Catalytic Mechanism |
|---|---|---|---|---|---|
| [62] | Mn/meso-TiO2 | Benzene | - | - | Benzene→(splitting of benzene, hydrogenation, H abstraction by OH radicals) six-member ring cyclitols+aldehydes ketones, etc. →a class of fulvene (isomerization)→CO2 +H2O. |
| [63] | Mesoporous TiO2 | Benzene | - | - | Benzene is mainly degraded by photo-generated electron-hole pairs and hydroxyl radicals. |
| [64] | Graphite-SiO2-TiO2 | Benzene | The graphite-SiO2-TiO2 composites exhibited higher photocatalytic activity for degradation of benzene gas under visible light irradiation than that of pure TiO2. | - | Optimum concentration of graphite facilitates the separation of photogenerated electron–hole pairs for graphite-SiO2-TiO2 composites by visible light. |
| [77] | LaFeO3/black-TiO2 | Toluene | The removal efficiency of black-TiO2 and LaFeO3 for toluene was 89% and 98%, respectively, and the removal efficiency for IPA was 90% and 94%, respectively. | - | Cat.+photon (the wavelength shorter than 440 nm)→ electrons +O2→O2−, O2−+toluene and IPA →CO2+H2O(g). |
| [78] | Pd/CeO2/γ-Al2O3 | Toluene Acetone Ethyl acetate | T98: 205 °C 220 °C 275 °C | - | - |
| [79] | CuO/ZnO nanocomposite photocatalysts | Toluene Iopropanol | - | - | Photooxidative activity and stability over ZnO are improved by loading CuO. |
| [86] | TiO2 | Formaldehyde acetaldehyde | TiO2 degraded almost 100% of formaldehyde or acetaldehyde at a starting concentration of 400–500 ppb with a relative humidity of 40%. | - | The rate-determining step is the adsorption (external diffusion) on the catalysts active sites, thus the higher the surface area, the higher the degradation. |
| [88] | g-C3N4 | Benzoquinone Hydroquinone catechol | - | - | OH radicals+phenol→dihydroxycyclohexadienyl radical adducts→phenoxy radicals→H2O (a very slow process), adducts+dissolved O2→dihydroxy intermediates (-HO2) →CO2+H2O. |
| [98] | La3+-TiO2 and Nd3+-TiO2 | VOCs mixture | 1.2% La3+-TiO2 had the highest photocatalytic activity. | - | - |
| [99] | TiO2/Pd | VOCs | The conversion rate of VOCs reached 90% when the residence time was 27 s. | - | - |
| [102] | Mn/ZSM-5 | O3 | O3 can be efficiently decomposed by the Mn/ZSM-5 and used for benzene degradation through the OZCO. | - | Benzene+•OH→phenol→benzoquinone→CO2 +H2O. |
| [100] | Meso-TiO2/hydro-CF | VOCs | - | - | Promotion effects on degradation of gaseous polar acetone come from well crystallized anatase nanocrystals, hydro-CF skeleton for adsorption, and fast mass transportation within the hierarchical frameworks. |
| Refer | Catalyst | VOCs | Catalytic Performance | Remarks | Catalytic Mechanism |
|---|---|---|---|---|---|
| [65] | Pt/Al2O3 | Benzene byproducts | - | - | - |
| [80] | FeOx/SBA-15 | Toluene | - | Under the condition of 3% Fe loading, the oxidation of toluene is the best one. | (1) Direct removal caused by the collision of electrons or oxidation caused by the gas-phase radicals (O•, OH•, N2•, NO•, NO2•) in the gas phase; (2) the reaction between adsorbed toluene or other intermediates and the active species (O•, OH•) on the catalyst surface. O2 might be fixed on the catalyst surface via a facile interconversion between Fe2+ and Fe3+ states and then be transported to the toluene or intermediates leading to CO2 formation. |
| [87] | CuO/g-Al2O3 | Ccetone | - | 5.0 wt% CuO/g-Al2O3 catalyst has the highest removal rate of acetone, reaching 67.9%. | Both short-lived radicals and acetone/intermediates can be adsorbed on the catalyst surfaces to initiate a series of surface oxidation reactions, forming CO, CO2, H2O, and byproducts. |
| [103] | CoMnOx/TiO2 | O3 | - | When the temperature is 320 °C, the decomposition efficiency of O3 is 98%. | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ma, C.; Horlyck, J.; Liu, R.; Yun, J. Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China. Catalysts 2020, 10, 668. https://doi.org/10.3390/catal10060668
Zhou L, Ma C, Horlyck J, Liu R, Yun J. Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China. Catalysts. 2020; 10(6):668. https://doi.org/10.3390/catal10060668
Chicago/Turabian StyleZhou, Lilong, Chen Ma, Jonathan Horlyck, Runjing Liu, and Jimmy Yun. 2020. "Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China" Catalysts 10, no. 6: 668. https://doi.org/10.3390/catal10060668
APA StyleZhou, L., Ma, C., Horlyck, J., Liu, R., & Yun, J. (2020). Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China. Catalysts, 10(6), 668. https://doi.org/10.3390/catal10060668




