Kramers’ Theory and the Dependence of Enzyme Dynamics on Trehalose-Mediated Viscosity
Abstract
1. Trehalose in Biology
2. Trehalose, Water, and Proteins
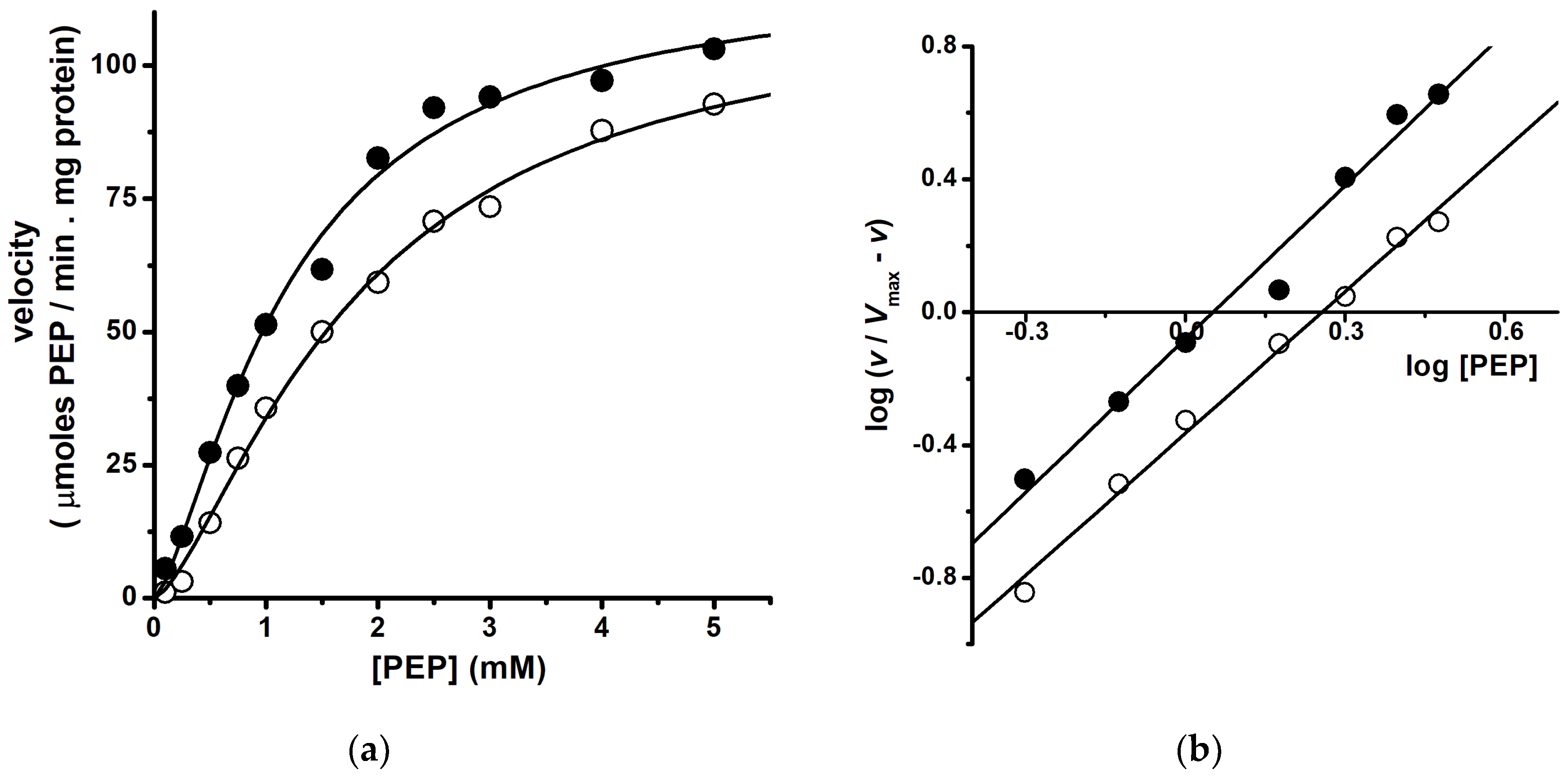
3. Protein Dynamics and Catalysis
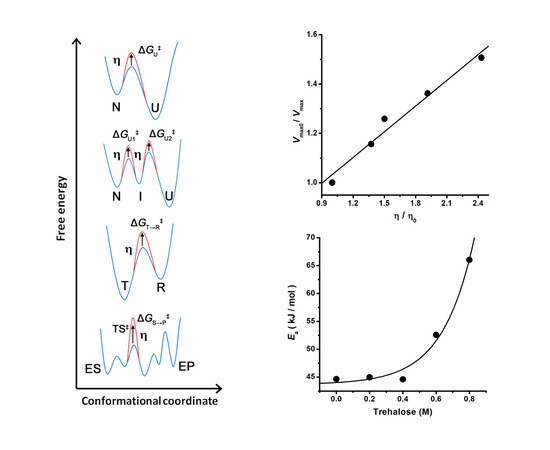
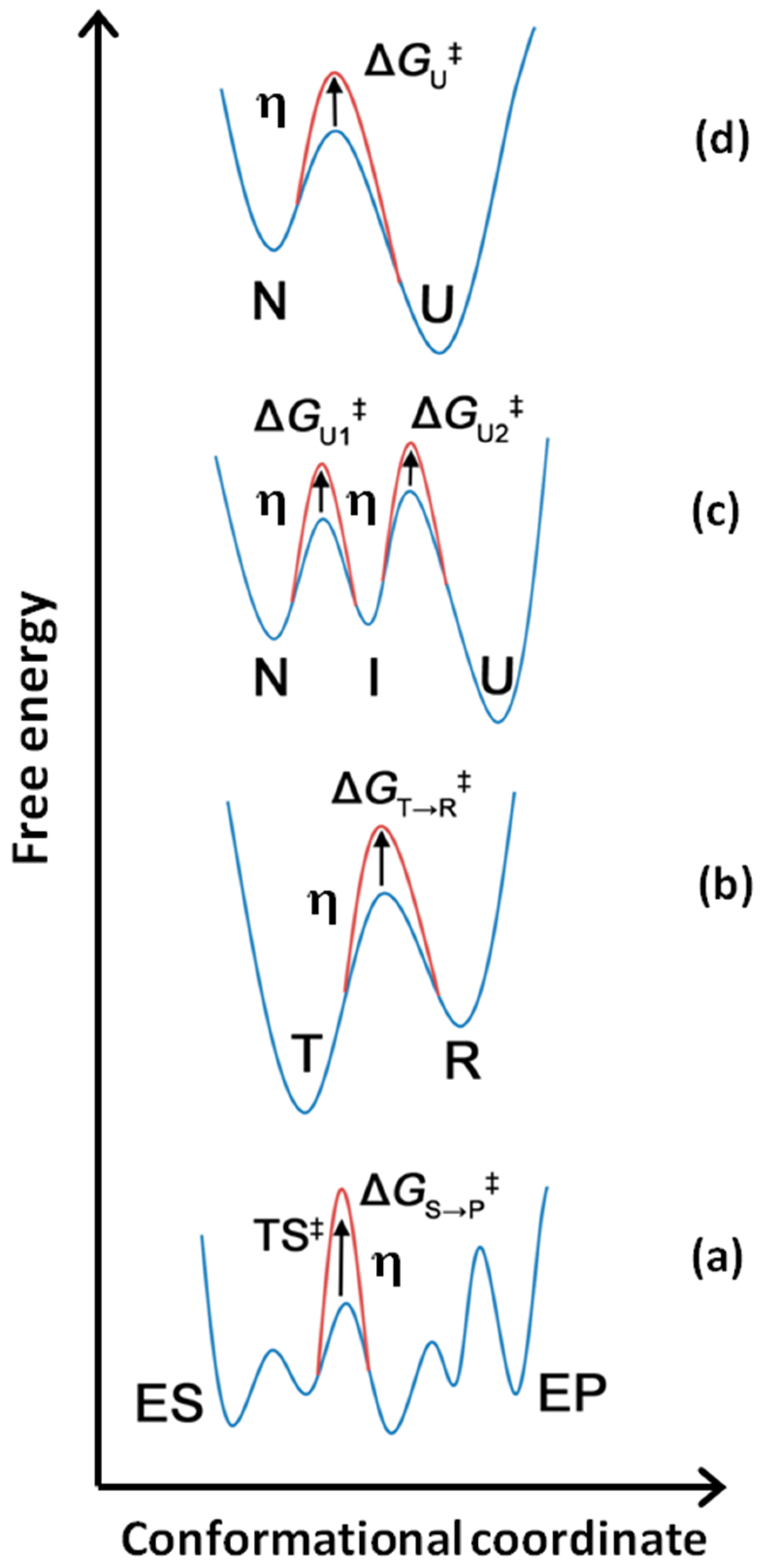
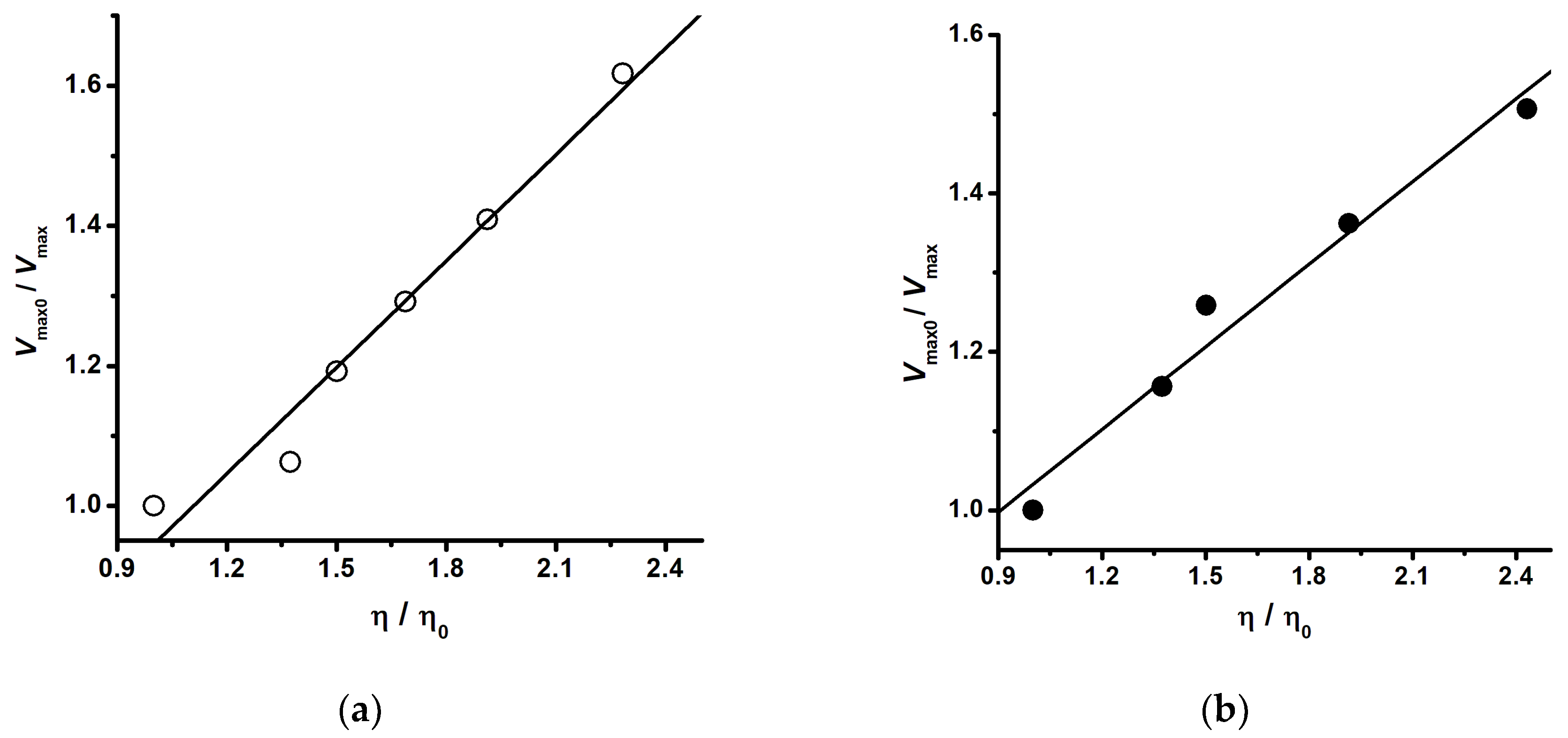
4. Dependence of Enzyme Catalysis on Medium Viscosity (Kramers’ Theory)
5. The Application of Kramers’ Theory on Heat-Mediated Enzyme Inactivation, Protein Folding, and Unfolding
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bodenheimer, F.S. Chapter, V. Asia. In Insects as Human Food; Bodenheimer, F.S., Ed.; Springer: Dordrecht, The Netherlands, 1951; pp. 208–280. ISBN 978-94-017-5767-6. [Google Scholar]
- Leibowitz, J. A new source of trehalose. Nature 1943, 152, 414. [Google Scholar] [CrossRef]
- Gültekin, L.; Shahreyary-Nejad, S. A new trehala-constructing Larinus Dejean (Coleoptera: Curculionidae) from Iran. Zool. Middle East 2015, 61, 246–251. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Thermotolerance in Saccharomyces cerevisiae: The Yin and Yang of trehalose. Trends Biotechnol. 1998, 16, 460–468. [Google Scholar] [CrossRef]
- Richards, A.; Krakowka, S.; Dexter, L.; Schmid, H.; Wolterbeek, A.P.; Waalkens-Berendsen, D.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Elbein, A.D. The metabolism of α,α-trehalose. Adv. Carbohydr. Chem. Biochem. 1974, 30, 227–256. [Google Scholar] [CrossRef]
- Gancedo, C.; Flores, C.L. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004, 4, 351–359. [Google Scholar] [CrossRef]
- Voit, E.O. Biochemical and genomic regulation of the trehalose cycle in yeast: Review of observations and canonical model analysis. J. Theor. Biol. 2003, 223, 55–78. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Thammahong, A.; Puttikamonkul, S.; Perfect, J.R.; Brennan, R.G.; Cramer, R.A. Central role of the trehalose biosynthesis pathway in the pathogenesis of human fungal infections: Opportunities and challenges for therapeutic development. Microbiol. Mol. Biol. Rev. 2017, 81, e00053-16. [Google Scholar] [CrossRef]
- Kalscheuer, R.; Koliwer-Brandl, H. Genetics of mycobacterial trehalose metabolism. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Smallbone, K.; Malys, N.; Messiha, H.L.; Wishart, J.A.; Simeonidis, E. Building a kinetic model of trehalose biosynthesis in Saccharomyces cerevisiae. In Methods in Enzymology; Academic Press Inc.: San Diego, CA, USA, 2011; Volume 500, pp. 355–370. ISBN 978-0-12-385118-5. [Google Scholar]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Di Lernia, I.; De Rosa, M. Trehalose production: Exploiting novel approaches. Trends Biotechnol. 2002, 20, 420–425. [Google Scholar] [CrossRef]
- Crowe, J.H. Anhydrobiosis: An unsolved problem with applications in human welfare. Subcell. Biochem. 2015, 71, 263–280. [Google Scholar] [CrossRef]
- McClements, D.J. Modulation of globular protein functionality by weakly interacting cosolvents. Crit. Rev. Food Sci. Nutr. 2002, 42, 417–471. [Google Scholar] [CrossRef]
- Tunnacliffe, A.; Lapinski, J. Resurrecting Van Leeuwenhoek’s rotifers: A reappraisal of the role of disaccharides in anhydrobiosis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 1755–1771. [Google Scholar] [CrossRef]
- Hengherr, S.; Heyer, A.G.; Köhler, H.R.; Schill, R.O. Trehalose and anhydrobiosis in tardigrades—Evidence for divergence in responses to dehydration. FEBS J. 2008, 275, 281–288. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Guerra, G.; Pardo, J.P.; Uribe, S. Trehalose-mediated protection of the plasma membrane H+-ATPase from Kluyveromyces lactis during freeze-drying and rehydration. Cryobiology 1998, 37, 131–138. [Google Scholar] [CrossRef]
- De Virgilio, C.; Hottiger, T.; Dominguez, J.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 1994, 219, 179–186. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 2017, 65, 975–984.e5. [Google Scholar] [CrossRef]
- Boothby, T.C.; Pielak, G.J. Intrinsically disordered proteins and desiccation tolerance: Elucidating functional and mechanistic underpinnings of anhydrobiosis. BioEssays 2017, 39, 1700119. [Google Scholar] [CrossRef]
- Magalhães, R.S.S.; Popova, B.; Braus, G.H.; Outeiro, T.F.; Eleutherio, E.C.A. The trehalose protective mechanism during thermal stress in Saccharomyces cerevisiae: The roles of Ath1 and Agt1. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Viner, R.I.; Clegg, J.S. Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/α-crystallin protein. Cell Stress Chaperones 2001, 6, 126–135. [Google Scholar] [CrossRef]
- Hu, J.; Jafari, S.; Han, Y.; Grodzinsky, A.J.; Cai, S.; Guo, M. Size- and speed-dependent mechanical behavior in living mammalian cytoplasm. Proc. Natl. Acad. Sci. USA 2017, 114, 9529–9534. [Google Scholar] [CrossRef]
- Luby-Phelps, K. Cytoarchitecture and physical properties of cytoplasm: Volume, viscosity, diffusion, intracellular surface area. In International Review of Cytology; Academic Press Inc.: San Diego, CA, USA, 1999; Volume 192, pp. 189–221. ISBN 978-0-12-364596-8. [Google Scholar]
- Feig, M.; Yu, I.; Wang, P.H.; Nawrocki, G.; Sugita, Y. Crowding in cellular environments at an atomistic level from computer simulations. J. Phys. Chem. B 2017, 121, 8009–8025. [Google Scholar] [CrossRef]
- Welch, G.R.; Somogyi, B.; Matkó, J.; Papp, S. Effect of viscosity on enzyme-ligand dissociation II. Role of the microenvironment. J. Theor. Biol. 1983, 100, 211–238. [Google Scholar] [CrossRef]
- Rampp, M.; Buttersack, C.; Lüdemann, H.-D. c, T-Dependence of the viscosity and the self-diffusion coefficients in some aqueous carbohydrate solutions. Carbohydr. Res. 2000, 328, 561–572. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Muñoz-Clares, R.A.; Uribe, S. Trehalose-mediated inhibition of the plasma membrane H+-ATPase from Kluyveromyces lactis: Dependence on viscosity and temperature. J. Bacteriol. 2002, 184, 4384–4391. [Google Scholar] [CrossRef]
- Uribe, S.; Sampedro, J.G. Measuring solution viscosity and its effect on enzyme activity. Biol. Proced. Online 2003, 5, 108–115. [Google Scholar] [CrossRef]
- Magazù, S.; Maisano, G.; Migliardo, P.; Middendorf, H.D.; Villari, V. Hydration and transport properties of aqueous solutions of α-α-trehalose. J. Chem. Phys. 1998, 109, 1170–1174. [Google Scholar] [CrossRef]
- Wyatt, T.T.; Golovina, E.A.; van Leeuwen, R.; Hallsworth, J.E.; Wösten, H.A.B.; Dijksterhuis, J. A decrease in bulk water and mannitol and accumulation of trehalose and trehalose-based oligosaccharides define a two-stage maturation process towards extreme stress resistance in ascospores of N eosartorya fischeri (A spergillus fischeri). Environ. Microbiol. 2015, 17, 383–394. [Google Scholar] [CrossRef]
- Cicerone, M.T.; Soles, C.L. Fast dynamics and stabilization of proteins: Binary glasses of trehalose and glycerol. Biophys. J. 2004, 86, 3836–3845. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 594, pp. 143–158. ISBN 9780387399744. [Google Scholar]
- Lubchenko, V.; Wolynes, P.G.; Frauenfelder, H. Mosaic energy landscapes of liquids and the control of protein conformational dynamics by glass-forming solvents. J. Phys. Chem. B 2005, 109, 7488–7499. [Google Scholar] [CrossRef]
- Malferrari, M.; Francia, F.; Venturoli, G. Retardation of protein dynamics by trehalose in dehydrated systems of photosynthetic reaction centers. Insights from electron transfer and thermal denaturation kinetics. J. Phys. Chem. B 2015, 119, 13600–13618. [Google Scholar] [CrossRef]
- Malferrari, M.; Savitsky, A.; Lubitz, W.; Möbius, K.; Venturoli, G. Protein immobilization capabilities of sucrose and trehalose glasses: The effect of protein/sugar concentration unraveled by high-field EPR. J. Phys. Chem. Lett. 2016, 7, 4871–4877. [Google Scholar] [CrossRef]
- Zhang, J.; Martinez-Gomez, K.; Heinzle, E.; Wahl, S.A. Metabolic switches from quiescence to growth in synchronized Saccharomyces cerevisiae. Metabolomics 2019, 15, 121. [Google Scholar] [CrossRef]
- Valcourt, J.R.; Lemons, J.M.S.; Haley, E.M.; Kojima, M.; Demuren, O.O.; Coller, H.A. Staying alive. Cell Cycle 2012, 11, 1680–1696. [Google Scholar] [CrossRef]
- Gray, J.V.; Petsko, G.A.; Johnston, G.C.; Ringe, D.; Singer, R.A.; Werner-Washburne, M. “Sleeping Beauty”: Quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004, 68, 187–206. [Google Scholar] [CrossRef]
- Winther, L.R.; Qvist, J.; Halle, B. Hydration and mobility of trehalose in aqueous solution. J. Phys. Chem. B 2012, 116, 9196–9207. [Google Scholar] [CrossRef]
- Shiraga, K.; Adachi, A.; Nakamura, M.; Tajima, T.; Ajito, K.; Ogawa, Y. Characterization of the hydrogen-bond network of water around sucrose and trehalose: Microwave and terahertz spectroscopic study. J. Chem. Phys. 2017, 146, 105102. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Li, W. Protective mechanisms of α,α-trehalose revealed by molecular dynamics simulations. Mol. Simul. 2018, 44, 100–109. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water determines the structure and dynamics of proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- Schirò, G.; Fichou, Y.; Gallat, F.-X.; Wood, K.; Gabel, F.; Moulin, M.; Härtlein, M.; Heyden, M.; Colletier, J.-P.; Orecchini, A.; et al. Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins. Nat. Commun. 2015, 6, 6490. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kataoka, M. Rigidity of protein structure revealed by incoherent neutron scattering. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129536. [Google Scholar] [CrossRef]
- Aggarwal, L.; Biswas, P. Hydration water distribution around intrinsically disordered proteins. J. Phys. Chem. B 2018, 122, 4206–4218. [Google Scholar] [CrossRef]
- Nucci, N.V.; Pometun, M.S.; Wand, A.J. Site-resolved measurement of water-protein interactions by solution NMR. Nat. Struct. Mol. Biol. 2011, 18, 245–250. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Kao, Y.-T.; Qiu, W.; Yang, Y.; Okobiah, O.; Zhong, D. Mapping hydration dynamics around a protein surface. Proc. Natl. Acad. Sci. USA 2007, 104, 18461–18466. [Google Scholar] [CrossRef]
- Houston, P.; Macro, N.; Kang, M.; Chen, L.; Yang, J.; Wang, L.; Wu, Z.; Zhong, D. Ultrafast dynamics of water-protein coupled motions around the surface of eye crystallin. J. Am. Chem. Soc. 2020, 142, 3997–4007. [Google Scholar] [CrossRef]
- Lins, R.D.; Pereira, C.S.; Hünenberger, P.H. Trehalose-protein interaction in aqueous solution. Proteins Struct. Funct. Bioinform. 2004, 55, 177–186. [Google Scholar] [CrossRef]
- Paul, S.S.; Paul, S.S. The influence of trehalose on hydrophobic interactions of small nonpolar solute: A molecular dynamics simulation study. J. Chem. Phys. 2013, 139, 1–9. [Google Scholar] [CrossRef]
- Soper, A.K.; Ricci, M.A.; Bruni, F.; Rhys, N.H.; McLain, S.E. Trehalose in water revisited. J. Phys. Chem. B 2018, 122, 7365–7374. [Google Scholar] [CrossRef]
- Xie, G.; Timasheff, S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997, 64, 25–43. [Google Scholar] [CrossRef]
- Shimizu, S.; Matubayasi, N. Preferential solvation: Dividing surface vs. excess numbers. J. Phys. Chem. B 2014, 118, 3922–3930. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Timasheff, S.N. On the role of surface tension in the stabilization of globular proteins. Protein Sci. 2008, 5, 372–381. [Google Scholar] [CrossRef]
- Timasheff, S.N. Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry 2002, 41, 13473–13482. [Google Scholar] [CrossRef]
- Olsson, C.; Genheden, S.; García Sakai, V.; Swenson, J. Mechanism of trehalose-induced protein stabilization from neutron scattering and modeling. J. Phys. Chem. B 2019, 123, 3679–3687. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Goodman, J.M.; Nerukh, D.; Schumm, S. Self-assembly of trehalose molecules on a lysozyme surface: The broken glass hypothesis. Phys. Chem. Chem. Phys. 2011, 13, 2294–2299. [Google Scholar] [CrossRef]
- Magno, A.; Gallo, P. Understanding the mechanisms of bioprotection: A comparative study of aqueous solutions of trehalose and maltose upon supercooling. J. Phys. Chem. Lett. 2011, 2, 977–982. [Google Scholar] [CrossRef]
- Corradini, D.; Strekalova, E.G.; Eugene Stanley, H.; Gallo, P.; Stanley, H.E.; Gallo, P. Microscopic mechanism of protein cryopreservation in an aqueous solution with trehalose. Sci. Rep. 2013, 3, 1218. [Google Scholar] [CrossRef]
- Giuffrida, S.; Cottone, G.; Bellavia, G.; Cordone, L. Proteins in amorphous saccharide matrices: Structural and dynamical insights on bioprotection. Eur. Phys. J. E 2013, 36, 79. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.C.; Laage, D. Water dynamics in protein hydration shells: The molecular origins of the dynamical perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef] [PubMed]
- GhattyVenkataKrishna, P.K.; Carri, G.A. The effect of complex solvents on the structure and dynamics of protein solutions: The case of lysozyme in trehalose/water mixtures. Eur. Phys. J. E 2013, 36, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabel, F.; Bellissent-Funel, M.C. C-phycocyanin hydration water dynamics in the presence of trehalose: An incoherent elastic neutron scattering study at different energy resolutions. Biophys. J. 2007, 92, 4054–4063. [Google Scholar] [CrossRef]
- Changeux, J.-P. Allostery and the Monod-Wyman-Changeux model after 50 years. Annu. Rev. Biophys. 2012, 41, 103–133. [Google Scholar] [CrossRef]
- Waldauer, S.A.; Stucki-Buchli, B.; Frey, L.; Hamm, P. Effect of viscogens on the kinetic response of a photoperturbed allosteric protein. J. Chem. Phys. 2014, 141, 22D514. [Google Scholar] [CrossRef]
- Eisenmesser, E.Z. Enzyme dynamics during catalysis. Science 2002, 295, 1520–1523. [Google Scholar] [CrossRef]
- Alpert, B.; Rivet, E. Protein Dynamics. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 1–48. ISBN 9780471976707. [Google Scholar]
- Eisenmesser, E.Z.; Millet, O.; Labeikovsky, W.; Korzhnev, D.M.; Wolf-Watz, M.; Bosco, D.A.; Skalicky, J.J.; Kay, L.E.; Kern, D. Intrinsic dynamics of an enzyme underlies catalysis. Nature 2005, 438, 117–121. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Petsko, G.A.; Tsernoglou, D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature 1979, 280, 558–563. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Sligar, S.; Wolynes, P. The energy landscapes and motions of proteins. Science 1991, 254, 1598–1603. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Fenimore, P.W.; Young, R.D. Protein dynamics and function: Insights from the energy landscape and solvent slaving. IUBMB Life 2007, 59, 506–512. [Google Scholar] [CrossRef]
- Henzler-Wildman, K.; Kern, D. Dynamic personalities of proteins. Nature 2007, 450, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Doucet, N.; Chennubhotla, C.; Ramanathan, A.; Narayanan, C. Conformational sub-states and populations in enzyme catalysis. In Methods in Enzymology; Academic Press Inc.: San Diego, CA, USA, 2016; Volume 578, pp. 273–297. ISBN 978-0-12-811107-9. [Google Scholar]
- Callender, R.; Dyer, R.B. The dynamical nature of enzymatic catalysis. Acc. Chem. Res. 2015, 48, 407–413. [Google Scholar] [CrossRef]
- Orellana, L. Large-scale conformational changes and protein function: Breaking the in silico barrier. Front. Mol. Biosci. 2019, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Hammes-Schiffer, S.; Benkovic, S.J. Relating protein motion to catalysis. Annu. Rev. Biochem. 2006, 75, 519–541. [Google Scholar] [CrossRef] [PubMed]
- James, L.C.; Tawfik, D.S. Conformational diversity and protein evolution—A 60-year-old hypothesis revisited. Trends Biochem. Sci. 2003, 28, 361–368. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Geist, A.; Gorin, A. Protein dynamics and enzymatic catalysis: Investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A. Biochemistry 2004, 43, 10605–10618. [Google Scholar] [CrossRef] [PubMed]
- Duff, M.R.; Borreguero, J.M.; Cuneo, M.J.; Ramanathan, A.; He, J.; Kamath, G.; Chennubhotla, S.C.; Meilleur, F.; Howell, E.E.; Herwig, K.W.; et al. Modulating enzyme activity by altering protein dynamics with solvent. Biochemistry 2018, 57, 4263–4275. [Google Scholar] [CrossRef]
- Doshi, U.; McGowan, L.C.; Ladani, S.T.; Hamelberg, D. Resolving the complex role of enzyme conformational dynamics in catalytic function. Proc. Natl. Acad. Sci. USA 2012, 109, 5699–5704. [Google Scholar] [CrossRef]
- Kamerlin, S.C.L.L.; Warshel, A. At the dawn of the 21st century: Is dynamics the missing link for understanding enzyme catalysis. Proteins Struct. Funct. Bioinform. 2010, 78, 1339–1375. [Google Scholar] [CrossRef]
- Kohen, A. Role of dynamics in enzyme catalysis: Substantial versus semantic controversies. Acc. Chem. Res. 2015, 48, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; Kuriyan, J. Molecular dynamics and protein function. Proc. Natl. Acad. Sci. USA 2005, 102, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Rigler, R.; Nilsson, L.; Hahn, U.; Saenger, W. Protein dynamics. A time-resolved fluorescence, energetic and molecular dynamics study of ribonuclease T1. Biophys. Chem. 1987, 26, 247–261. [Google Scholar] [CrossRef]
- Nevin Gerek, Z.; Kumar, S.; Banu Ozkan, S. Structural dynamics flexibility informs function and evolution at a proteome scale. Evol. Appl. 2013, 6, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Tawfik, D.S. Protein dynamism and evolvability. Science 2009, 324, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Meisburger, S.P.; Case, D.A.; Ando, N. Diffuse X-ray scattering from correlated motions in a protein crystal. Nat. Commun. 2020, 11, 1271. [Google Scholar] [CrossRef]
- Grimes, J.M.; Hall, D.R.; Ashton, A.W.; Evans, G.; Owen, R.L.; Wagner, A.; McAuley, K.E.; Von Delft, F.; Orville, A.M.; Sorensen, T.; et al. Where is crystallography going? Acta Crystallogr. Sect. D Struct. Biol. 2018, 74, 152–166. [Google Scholar] [CrossRef]
- Kang, Y.; Gao, X.; Zhou, X.E.; He, Y.; Melcher, K.; Xu, H.E. A structural snapshot of the rhodopsin-arrestin complex. FEBS J. 2016, 283, 816–821. [Google Scholar] [CrossRef][Green Version]
- Shahlaei, M.; Madadkar-Sobhani, A.; Mahnam, K.; Fassihi, A.; Saghaie, L.; Mansourian, M. Homology modeling of human CCR5 and analysis of its binding properties through molecular docking and molecular dynamics simulation. Biochim. Biophys. Acta Biomembr. 2011, 1808, 802–817. [Google Scholar] [CrossRef]
- Schlichting, I.; Berendzen, J.; Chu, K.; Stock, A.M.; Maves, S.A.; Benson, D.E.; Sweet, R.M.; Ringe, D.; Petsko, G.A.; Sligar, S.G. The catalytic pathway of cytochrome P450cam at atomic resolution. Science 2000, 287, 1615–1622. [Google Scholar] [CrossRef]
- Kanai, R.; Ogawa, H.; Vilsen, B.; Cornelius, F.; Toyoshima, C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature 2013, 502, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Inesi, G.; Lewis, D.; Ma, H.; Prasad, A.; Toyoshima, C. Concerted conformational effects of Ca2+ and ATP are required for activation of sequential reactions in the Ca2+ ATPase (SERCA) catalytic cycle. Biochemistry 2006, 45, 13769–13778. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G.; Ikura, M.; Kay, L.E.; Pastor, R.W.; Bax, A. Backbone dynamics of calmodulin studied by nitrogen-15 relaxation using inverse detected two-dimensional NMR spectroscopy: The central helix is flexible. Biochemistry 1992, 31, 5269–5278. [Google Scholar] [CrossRef]
- Diez, M.; Zimmermann, B.; Börsch, M.; König, M.; Schweinberger, E.; Steigmiller, S.; Reuter, R.; Felekyan, S.; Kudryavtsev, V.; Seidel, C.A.M.; et al. Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nat. Struct. Mol. Biol. 2004, 11, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Cui, Y.; Zhang, X.; Liu, X.; Yue, J.; Liu, N.; Jiang, P. Constructing a novel nanodevice powered by δ-free FoF1-ATPase. Biochem. Biophys. Res. Commun. 2006, 350, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; McCammon, J.A. Molecular dynamics simulations of biomolecules. Nat. Struct. Biol. 2002, 9, 646–652. [Google Scholar] [CrossRef]
- Lindahl, E.R. Molecular dynamics simulations. In Molecular Modeling of Proteins (Methods in Molecular Biology); Kukol, A., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 443, pp. 3–23. ISBN 978-1-58829-864-5. [Google Scholar]
- Náray-Szabó, G. Protein Modelling; Náray-Szabó, G., Ed.; Springer: Heidelberg, Germany, 2014; ISBN 978-3-319-09975-0. [Google Scholar]
- Schlee, S.; Klein, T.; Schumacher, M.; Nazet, J.; Merkl, R.; Steinhoff, H.-J.; Sterner, R. Relationship of catalysis and active site loop dynamics in the (βα) 8 -barrel enzyme indole-3-glycerol phosphate synthase. Biochemistry 2018, 57, 3265–3277. [Google Scholar] [CrossRef]
- Beece, D.; Eisenstein, L.; Frauenfelder, H.; Good, D.; Marden, M.C.; Reinisch, L.; Reynolds, A.H.; Sorensen, L.B.; Yue, K.T.; Marden, M.C.; et al. Solvent viscosity and protein dynamics. Biochemistry 1980, 19, 5147–5157. [Google Scholar] [CrossRef]
- Caliskan, G.; Mechtani, D.; Roh, J.H.; Kisliuk, A.; Sokolov, A.P.; Azzam, S.; Cicerone, M.T.; Lin-Gibson, S.; Peral, I. Protein and solvent dynamics: How strongly are they coupled? J. Chem. Phys. 2004, 121, 1978–1983. [Google Scholar] [CrossRef]
- Caliskan, G.; Kisliuk, A.; Tsai, A.M.; Soles, C.L.; Sokolov, A.P. Protein dynamics in viscous solvents. J. Chem. Phys. 2003, 118, 4230–4236. [Google Scholar] [CrossRef]
- Pan, X.; Schwartz, S.D. Conformational heterogeneity in the michaelis complex of lactate dehydrogenase: An analysis of vibrational spectroscopy using Markov and hidden Markov models. J. Phys. Chem. B 2016, 120, 6612–6620. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Xie, X.S.; Bagchi, B. Two-dimensional reaction free energy surfaces of catalytic reaction: Effects of protein conformational dynamics on enzyme catalysis. J. Phys. Chem. B 2008, 112, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Świderek, K.; Tuñón, I.; Martí, S.; Moliner, V. Protein conformational landscapes and catalysis. Influence of active site conformations in the reaction catalyzed by L-lactate dehydrogenase. ACS Catal. 2015, 5, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Nussinov, R. Enzyme dynamics point to stepwise conformational selection in catalysis. Curr. Opin. Chem. Biol. 2010, 14, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, D.; Hyeon, C.; Zhuravlev, P.I.; Lorimer, G.H. Symmetry, rigidity, and allosteric signaling: From monomeric proteins to molecular machines. Chem. Rev. 2019, 119, 6788–6821. [Google Scholar] [CrossRef]
- Campitelli, P.; Modi, T.; Kumar, S.; Ozkan, S.B. The role of conformational dynamics and allostery in modulating protein evolution. Annu. Rev. Biophys. 2020, 49, 267–288. [Google Scholar] [CrossRef]
- Puchkov, E.O. Intracellular viscosity: Methods of measurement and role in metabolism. Biochem. Suppl. Ser. A Membr. Cell Biol. 2013, 7, 270–279. [Google Scholar] [CrossRef]
- Luby-Phelps, K. The physical chemistry of cytoplasm and its influence on cell function: An update. Mol. Biol. Cell 2013, 24, 2593–2596. [Google Scholar] [CrossRef]
- Chung, S.Y.; Lerner, E.; Jin, Y.; Kim, S.; Alhadid, Y.; Grimaud, L.W.; Zhang, I.X.; Knobler, C.M.; Gelbart, W.M.; Weiss, S. The effect of macromolecular crowding on single-round transcription by Escherichia coli RNA polymerase. Nucleic Acids Res. 2019, 47, 1440–1450. [Google Scholar] [CrossRef]
- Rickey Welch, G.; Somogyi, B.; Damjanovich, S. The role of protein fluctuations in enzyme action: A review. Prog. Biophys. Mol. Biol. 1982, 39, 109–146. [Google Scholar] [CrossRef]
- Pal, N.; Wu, M.; Lu, H.P. Probing conformational dynamics of an enzymatic active site by an in situ single fluorogenic probe under piconewton force manipulation. Proc. Natl. Acad. Sci. USA 2016, 113, 15006–15011. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, H.; Chen, G.; Berendzen, J.; Fenimore, P.W.; Jansson, H.; McMahon, B.H.; Stroe, I.R.; Swenson, J.; Young, R.D. A unified model of protein dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Schlitter, J. Viscosity dependence of intramolecular activated processes. Chem. Phys. 1988, 120, 187–197. [Google Scholar] [CrossRef]
- Doster, W. Viscosity scaling and protein dynamics. Biophys. Chem. 1983, 17, 97–103. [Google Scholar] [CrossRef]
- Gavish, B.; Werber, M.M.; Gavish, B. Viscosity-dependent structural fluctuations in enzyme catalysis. Biochemistry 1979, 18, 1269–1275. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Bokhari, S.A.; Afzal, A.J.; Singh, S. A novel thermodynamic relationship based on Kramers theory for studying enzyme kinetics under high viscosity. IUBMB Life 2004, 56, 403–407. [Google Scholar] [CrossRef]
- Ajito, S.; Hirai, M.; Iwase, H.; Shimizu, N.; Igarashi, N.; Ohta, N. Protective action of trehalose and glucose on protein hydration shell clarified by using X-ray and neutron scattering. Phys. B Condens. Matter 2018, 551, 249–255. [Google Scholar] [CrossRef]
- Kramers, H.A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 1940, 7, 284–304. [Google Scholar] [CrossRef]
- Gavish, B. Position-dependent viscosity effects on rate coefficients. Phys. Rev. Lett. 1980, 44, 1160–1163. [Google Scholar] [CrossRef]
- Fanghänel, J. Enzymatic catalysis of the peptidyl bond rotation: Are transition state formation and enzyme dynamics directly linked? Angew. Chem. Int. Ed. 2003, 42, 490–492. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell. Biochem. 2004, 256, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Meza, J.M.; Sampedro, J.G. Trehalose mediated inhibition of lactate dehydrogenase from rabbit muscle. The application of Kramers’ theory in enzyme catalysis. J. Phys. Chem. B 2018, 122, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Hilge, M.; Siegal, G.; Vuister, G.W.; Güntert, P.; Gloor, S.M.; Abrahams, J.P. ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase. Nat. Struct. Biol. 2003, 10, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Berczi, A.; Moller, I.M. Control of the activity of plant plasma membrane MgATPase by the viscosity of the aqueous phase. Physiol. Plant. 1993, 89, 409–415. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Ruskyn, O.I.; Saburova, E.A. Kinetics of the lactate dehydrogenase reaction in high-viscosity media. Biochim. Biophys. Acta 1989, 998, 196–203. [Google Scholar] [CrossRef]
- Pocker, Y.; Janjic, N. Origin of viscosity effects in carbonic anhydrase catalysis. Kinetic studies with bulky buffers at limiting concentrations. Biochemistry 1988, 27, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Asai, H. The effects of solvent viscosity on the kinetic parameters of myosin and heavy meromyosin ATPase. J. Bioenerg. Biomembr. 1977, 9, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Malyan, A.N. The effect of medium viscosity on kinetics of ATP hydrolysis by the chloroplast coupling factor CF1. Photosynth. Res. 2016, 128, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Rosenberg, A. The coupling of catalytically relevant conformational fluctuations in subtilisin BPN′ to solution viscosity revealed by hydrogen isotope exchange and inhibitor binding. Biophys. Chem. 1991, 41, 289–299. [Google Scholar] [CrossRef]
- Kyushiki, H.; Ikai, A. The effect of solvent viscosity on the rate-determining step of fatty acid synthetase. Proteins Struct. Funct. Genet. 1990, 8, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Goguadze, N.G.; Hammerstad-Pedersen, J.M.; Khoshtariya, D.E.; Ulstrup, J. Conformational dynamics and solvent viscosity effects in carboxypeptidase-A-catalyzed benzoylglycylphenyllactate hydrolysis. Eur. J. Biochem. 1991, 200, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sashi, P.; Bhuyan, A.K. Viscosity dependence of some protein and enzyme reaction rates: Seventy-five years after Kramers. Biochemistry 2015, 54, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Bakhtina, M.; Lee, S.; Wang, Y.; Dunlap, C.; Lamarche, B.; Tsai, M.D. Use of viscogens, dNTPαS, and rhodium(III) as probes in stopped-flow experiments to obtain new evidence for the mechanism of catalysis by DNA polymerase β. Biochemistry 2005, 44, 5177–5187. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.B.L.; Wells, S.A.; Prentice, E.J.; Kwok, A.; Liang, L.L.; Arcus, V.L.; Pudney, C.R. A complete thermodynamic analysis of enzyme turnover links the free energy landscape to enzyme catalysis. FEBS J. 2017, 284, 2829–2842. [Google Scholar] [CrossRef]
- Okada, A. Fractional power dependence of mean lifetime of electron transfer reaction on viscosity of solvent. J. Chem. Phys. 1999, 111, 2665–2677. [Google Scholar] [CrossRef]
- Ivković-Jensen, M.M.; Kostić, N.M. Effects of viscosity and temperature on the kinetics of the electron- transfer reaction between the triplet state of zinc cytochrome c and cupriplastocyanin. Biochemistry 1997, 36, 8135–8144. [Google Scholar] [CrossRef]
- Feng, C.; Kedia, R.V.; Hazzard, J.T.; Hurley, J.K.; Tollin, G.; Enemark, J.H. Effect of solution viscosity on intramolecular electron transfer in sulfite oxidase. Biochemistry 2002, 41, 5816–5821. [Google Scholar] [CrossRef]
- Curtis, J.E.; Dirama, T.E.; Carri, G.A.; Tobias, D.J. Inertial suppression of protein dynamics in a binary glycerol-trehalose glass. J. Phys. Chem. B 2006, 110, 22953–22956. [Google Scholar] [CrossRef]
- Walser, R.; van Gunsteren, W.F. Viscosity dependence of protein dynamics. Proteins Struct. Funct. Genet. 2001, 42, 414–421. [Google Scholar] [CrossRef]
- Perkins, J.; Edwards, E.; Kleiv, R.; Weinberg, N. Molecular dynamics study of reaction kinetics in viscous media. Mol. Phys. 2011, 109, 1901–1909. [Google Scholar] [CrossRef]
- Damjanovich, S.; Bot, J.; Somogyi, B.; Sümegi, J. Effect of glycerol on some kinetic parameters of phosphorylase b. BBA Enzymol. 1972, 284, 345–348. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Z.; Shi, R.; Zhan, L.; Hu, W.; Xiang, H.; Xie, Q. Effects of temperature and additives on the thermal stability of glucoamylase from Aspergillus niger. J. Microbiol. Biotechnol. 2015, 25, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Paz-Alfaro, K.J.; Ruiz-Granados, Y.G.; Uribe-Carvajal, S.; Sampedro, J.G. Trehalose-mediated thermal stabilization of glucose oxidase from Aspergillus niger. J. Biotechnol. 2009, 141, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Satyanarayana, T. Purification and kinetics of a raw starch-hydrolyzing, thermostable, and neutral glucoamylase of the thermophilic mold Thermomucor indicae-seudaticae. Biotechnol. Prog. 2003, 19, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Faber-Barata, J.; Sola-Penna, M. Opposing effects of two osmolytes—trehalose and glycerol—on thermal inactivation of rabbit muscle 6-phosphofructo-1-kinase. Mol. Cell. Biochem. 2005, 269, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Lerbret, A.; Affouard, F.; Hédoux, A.; Krenzlin, S.; Siepmann, J.; Bellissent-Funel, M.-C.C.; Descamps, M. How strongly does trehalose interact with lysozyme in the solid state? Insights from molecular dynamics simulation and inelastic neutron scattering. J. Phys. Chem. B 2012, 116, 11103–11116. [Google Scholar] [CrossRef] [PubMed]
- Zelent, B.; Bialas, C.; Gryczynski, I.; Chen, P.; Chib, R.; Lewerissa, K.; Corradini, M.G.; Ludescher, R.D.; Vanderkooi, J.M.; Matschinsky, F.M. Tryptophan Fluorescence Yields and Lifetimes as a Probe of Conformational Changes in Human Glucokinase. J. Fluoresc. 2017, 27, 1621–1631. [Google Scholar] [CrossRef]
- Priev, A.; Almagor, A.; Yedgar, S.; Gavish, B. Glycerol decreases the volume and compressibility of protein interior. Biochemistry 1996, 35, 2061–2066. [Google Scholar] [CrossRef]
- Almagor, A.; Priev, A.; Barshtein, G.; Gavish, B.; Yedgar, S. Reduction of protein volume and compressibility by macromolecular cosolvents: Dependence on the cosolvent molecular weight. Biochim. Biophys. Acta—Protein Struct Mol. Enzymol. 1998, 1382, 151–156. [Google Scholar] [CrossRef]
- Somogyi, B.; Karasz, F.E.; Trón, L.; Couchma, P.R. The effect of viscosity on the apparent decomposition rate on enzyme-ligand complexes. J. Theor. Biol. 1978, 74, 209–216. [Google Scholar] [CrossRef]
- Yedgar, S.; Tetreau, C.; Gavish, B.; Lavalette, D. Viscosity dependence of O2 escape from respiratory proteins as a function of cosolvent molecular weight. Biophys. J. 1995, 68, 665–670. [Google Scholar] [CrossRef]
- Lavalette, D.; Tetreau, C. Viscosity-dependent energy barriers and equilibrium conformational fluctuations in oxygen recombination with hemerythrin. Eur. J. Biochem. 1988, 177, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Findsen, E.W.; Friedman, J.M.; Ondrias, M.R. Effect of solvent viscosity on the heme-pocket dynamics of photolyzed (carbonmonoxy) hemoglobin. Biochemistry 1988, 27, 8719–8724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jain, R.; Kumar, R. Viscosity-dependent structural fluctuation of the M80-containing Ω-loop of horse ferrocytochrome c. Chem. Phys. 2013, 418, 57–64. [Google Scholar] [CrossRef]
- Kleinert, T.; Doster, W.; Leyser, H.; Petry, W.; Schwarz, V.; Settles, M. Solvent composition and viscosity effects on the kinetics of CO binding to horse myoglobin. Biochemistry 1998, 37, 717–733. [Google Scholar] [CrossRef]
- Rivera-Moran, M.A. The Viscosity of Trehalose Solutions and Its Effect on Enzyme Kinetics of Pyruvate Kinase from Geobacillus Stearothermophilus. Master’s Thesis, Licenciatura en Biofísica, Instituto de Física, Universidad Autónoma de San Luis Potosí, San Luis Potosí, México, 2014. [Google Scholar]
- Hottiger, T.; De Virgilio, C.; Hall, M.N.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur. J. Biochem. 1994, 219, 187–193. [Google Scholar] [CrossRef]
- Uversky, V.N.; Dunker, A.K. Intrinsically Disordered Protein Analysis; Methods in Molecular Biology; Uversky, V.N., Dunker, A.K., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 895, ISBN 978-1-61779-926-6. [Google Scholar]
- Takagi, A.; Kamijo, M.; Ikeda, S. Darier disease. J. Dermatol. 2016, 43, 275–279. [Google Scholar] [CrossRef]
- Pace, C.N.; Hebert, E.J.; Shaw, K.L.; Schell, D.; Both, V.; Krajcikova, D.; Sevcik, J.; Wilson, K.S.; Dauter, Z.; Hartley, R.W.; et al. Conformational stability and thermodynamics of folding of ribonucleases Sa, Sa2 and Sa3. J. Mol. Biol. 1998, 279, 271–286. [Google Scholar] [CrossRef]
- Pradeep, L.; Udgaonkar, J.B. Diffusional barrier in the unfolding of a small protein. J. Mol. Biol. 2007, 366, 1016–1028. [Google Scholar] [CrossRef]
- Santoro, M.M.; Bolen, D.W. Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl alpha-chymotrypsin using different denaturants. Biochemistry 1988, 27, 8063–8068. [Google Scholar] [CrossRef]
- Ghosh, K.; De Graff, A.M.R.; Sawle, L.; Dill, K.A. Role of proteome physical chemistry in cell behavior. J. Phys. Chem. B 2016, 120, 9549–9563. [Google Scholar] [CrossRef] [PubMed]
- Ajito, S.; Iwase, H.; Takata, S.; Hirai, M. Sugar-mediated stabilization of protein against chemical or thermal denaturation. J. Phys. Chem. B 2018, 122, 8685–8697. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, J.G.; Cortés, P.; Muñoz-Clares, R.A.; Fernández, A.; Uribe, S. Thermal inactivation of the plasma membrane H+-ATPase from Kluyveromyces lactis. Protection by trehalose. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1544, 64–73. [Google Scholar] [CrossRef]
- Sekiguchi, S.; Hashida, Y.; Yasukawa, K.; Inouyey, K. Stabilization of bovine intestine alkaline phosphatase by sugars. Biosci. Biotechnol. Biochem. 2012, 76, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Mu, H.; Lü, Z.R.; Yin, S.J.; Si, Y.X.; Zhou, S.M.; Zhang, F.; Hu, W.J.; Meng, F.G.; Zhou, H.M.; et al. Trehalose has a protective effect on human brain-type creatine kinase during thermal denaturation. Appl. Biochem. Biotechnol. 2011, 165, 476–484. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Galli, G.; Laganà, G.; Leuzzi, U.; Magazù, S.; Migliardo, F.; Galtieri, A.; Telling, M.T.F. Stabilization effects of kosmotrope systems on ornithine carbamoyltransferase. Int. J. Biol. Macromol. 2009, 45, 120–128. [Google Scholar] [CrossRef]
- Neves, V.A.; Da Silva, M.A. Polyphenol oxidase from yacon roots (Smallanthus sonchifolius). J. Agric. Food Chem. 2007, 55, 2424–2430. [Google Scholar] [CrossRef]
- Baptista, R.P.; Cabral, J.M.S.; Melo, E.P. Trehalose delays the reversible but not the irreversible thermal denaturation of cutinase. Biotechnol. Bioeng. 2000, 70, 699–703. [Google Scholar] [CrossRef]
- Meyer-Fernandes, J.R.; Arrese, E.L.; Wells, M.A. Allosteric effectors and trehalose protect larval Manduca sexta fat body glycogen phosphorylase B against thermal denaturation. Insect Biochem. Mol. Biol. 2000, 30, 473–478. [Google Scholar] [CrossRef]
- Liyaghatdar, Z.; Emamzadeh, R.; Rasa, S.M.M.; Nazari, M. Trehalose radial networks protect Renilla luciferase helical layers against thermal inactivation. Int. J. Biol. Macromol. 2017, 105, 66–73. [Google Scholar] [CrossRef]
- Sola-Penna, M.; Meyer-Fernandes, J.R. Protective role of trehalose in thermal denaturation of yeast pyrophosphatase. Zeitschrift für Naturforsch. Sect. C J. Biosci. 1994, 49, 327–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barreca, D.; Laganà, G.; Magazù, S.; Migliardo, F.; Gattuso, G.; Bellocco, E. FTIR, ESI-MS, VT-NMR and SANS study of trehalose thermal stabilization of lysozyme. Int. J. Biol. Macromol. 2014, 63, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.K.; Prakash, V. Thermal stability of α-amylase in aqueous cosolvent systems. J. Biosci. 2009, 34, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xin, Y.; Yang, H.; Zhang, L.; Xia, X.; Tong, Y.; Chen, Y.; Wang, W. Thermal inactivation of xanthine oxidase from Arthrobacter M3: Mechanism and the corresponding thermostabilization strategy. Bioprocess Biosyst. Eng. 2014, 37, 719–725. [Google Scholar] [CrossRef]
- Gheibi, N.; Saboury, A.A.; Haghbeen, K.; Moosavi-Movahedi, A.A. The effect of some osmolytes on the activity and stability of mushroom tyrosinase. J. Biosci. 2006, 31, 355–362. [Google Scholar] [CrossRef]
- Guerrero-Mendiola, C.; Oria-Hernández, J.; Ramírez-Silva, L. Kinetics of the thermal inactivation and aggregate formation of rabbit muscle pyruvate kinase in the presence of trehalose. Arch. Biochem. Biophys. 2009, 490, 129–136. [Google Scholar] [CrossRef]
- Felix, C.F.; Moreira, C.C.; Oliveira, M.S.; Sola-Penna, M.; Meyer-Fernandes, J.R.; Scofano, H.M.; Ferreira-Pereira, A. Protection against thermal denaturation by trehalose on the plasma membrane H+-ATPase from yeast. Synergetic effect between trehalose and phospholipid environment. Eur. J. Biochem. 1999, 266, 660–664. [Google Scholar] [CrossRef]
- Ruiz-Granados, Y.; De La Cruz-Torres, V.; Sampedro, J. The oligomeric state of the plasma membrane H+-ATPase from Kluyveromyces lactis. Molecules 2019, 24, 958. [Google Scholar] [CrossRef]
- Zoldák, G.; Zubrik, A.; Musatov, A.; Stupák, M.; Sedlák, E. Irreversible thermal denaturation of glucose oxidase from Aspergillus niger is the transition to the denatured state with residual structure. J. Biol. Chem. 2004, 279, 47601–47609. [Google Scholar] [CrossRef]
- Tang, X.; Pikal, M.J. Measurement of the kinetics of protein unfolding in viscous systems and implications for protein stability in freeze-drying. Pharm. Res. 2005, 22, 1176–1185. [Google Scholar] [CrossRef]
- Baptista, R.P.; Pedersen, S.; Cabrita, G.J.M.; Otzen, D.E.; Cabral, J.M.S.; Melo, E.P. Thermodynamics and mechanism of cutinase stabilization by trehalose. Biopolymers 2008, 89, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.M.; Pande, V.S. Solvent viscosity dependence of the protein folding dynamics. J. Phys. Chem. B 2008, 112, 6221–6227. [Google Scholar] [CrossRef]
- Klimov, D.K.; Thirumalai, D. Viscosity dependence of the folding rates of proteins. Phys. Rev. Lett. 1997, 79, 317–320. [Google Scholar] [CrossRef]
- Qiu, L.; Hagen, S.J. A limiting speed for protein folding at low solvent viscosity. J. Am. Chem. Soc. 2004, 126, 3398–3399. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.J. Solvent viscosity and friction in protein folding dynamics. Curr. Protein Pept. Sci. 2010, 11, 385–395. [Google Scholar] [CrossRef]
- de Sancho, D.; Sirur, A.; Best, R.B. Molecular origins of internal friction effects on protein-folding rates. Nat. Commun. 2014, 5, 4307. [Google Scholar] [CrossRef]
- Sekhar, A.; Vallurupalli, P.; Kay, L.E. Folding of the four-helix bundle FF domain from a compact on-pathway intermediate state is governed predominantly by water motion. Proc. Natl. Acad. Sci. USA 2012, 109, 19268–19273. [Google Scholar] [CrossRef]
- Paz-Alfaro, K.J. Thermal Inactivation of Glucose Oxidase from Aspergillus niger. Stabilization by Trehalose. Master’s Thesis, Licenciatura en Nutrición, Universidad Autónoma del Estado de Hidalgo, Pachuca, Hidalgo, México, 2009. [Google Scholar]
- Pace, C.N. Measuring and increasing protein stability. Trends Biotechnol. 1990, 8, 93–98. [Google Scholar] [CrossRef]
- Niranjani, G.; Murugan, R. Theory on the mechanism of DNA renaturation: Stochastic nucleation and zipping. PLoS ONE 2016, 11, e0153172. [Google Scholar] [CrossRef]
- Sikorav, J.L.; Orland, H.; Braslau, A. Mechanism of thermal renaturation and hybridization of nucleic acids: Kramers’ process and universality in watson-crick base pairing. J. Phys. Chem. B 2009, 113, 3715–3725. [Google Scholar] [CrossRef][Green Version]
- Lannan, F.M.; Mamajanov, I.; Hud, N.V. Human telomere sequence DNA in water-free and high-viscosity solvents: G-quadruplex folding governed by Kramers rate theory. J. Am. Chem. Soc. 2012, 134, 15324–15330. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, N.F.; Holmstrom, E.D.; Nesbitt, D.J. Tests of Kramers’ theory at the single-molecule level: Evidence for folding of an isolated RNA tertiary interaction at the viscous speed limit. J. Phys. Chem. B 2018, 122, 8796–8804. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Denesyuk, N.A.; Thirumalai, D. Frictional effects on RNA folding: Speed limit and Kramers turnover. J. Phys. Chem. B 2018, 122, 11279–11288. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampedro, J.G.; Rivera-Moran, M.A.; Uribe-Carvajal, S. Kramers’ Theory and the Dependence of Enzyme Dynamics on Trehalose-Mediated Viscosity. Catalysts 2020, 10, 659. https://doi.org/10.3390/catal10060659
Sampedro JG, Rivera-Moran MA, Uribe-Carvajal S. Kramers’ Theory and the Dependence of Enzyme Dynamics on Trehalose-Mediated Viscosity. Catalysts. 2020; 10(6):659. https://doi.org/10.3390/catal10060659
Chicago/Turabian StyleSampedro, José G., Miguel A. Rivera-Moran, and Salvador Uribe-Carvajal. 2020. "Kramers’ Theory and the Dependence of Enzyme Dynamics on Trehalose-Mediated Viscosity" Catalysts 10, no. 6: 659. https://doi.org/10.3390/catal10060659
APA StyleSampedro, J. G., Rivera-Moran, M. A., & Uribe-Carvajal, S. (2020). Kramers’ Theory and the Dependence of Enzyme Dynamics on Trehalose-Mediated Viscosity. Catalysts, 10(6), 659. https://doi.org/10.3390/catal10060659