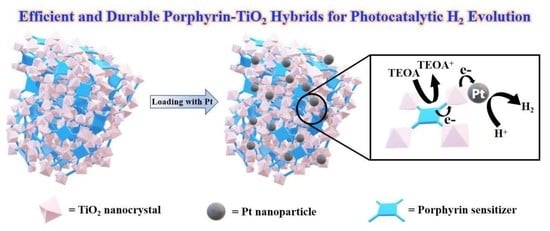
Porous Hybrid Materials Based on Mesotetrakis(Hydroxyphenyl) Porphyrins and TiO2 for Efficient Visible-Light-Driven Hydrogen Production
Abstract
1. Introduction
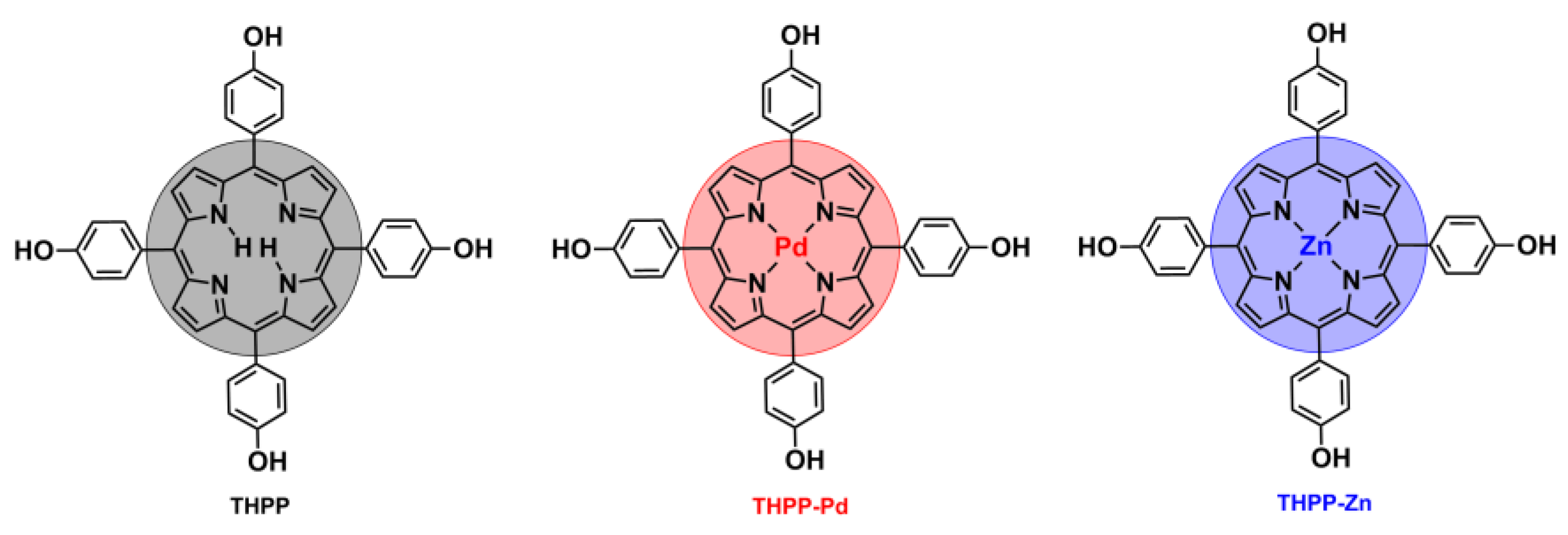
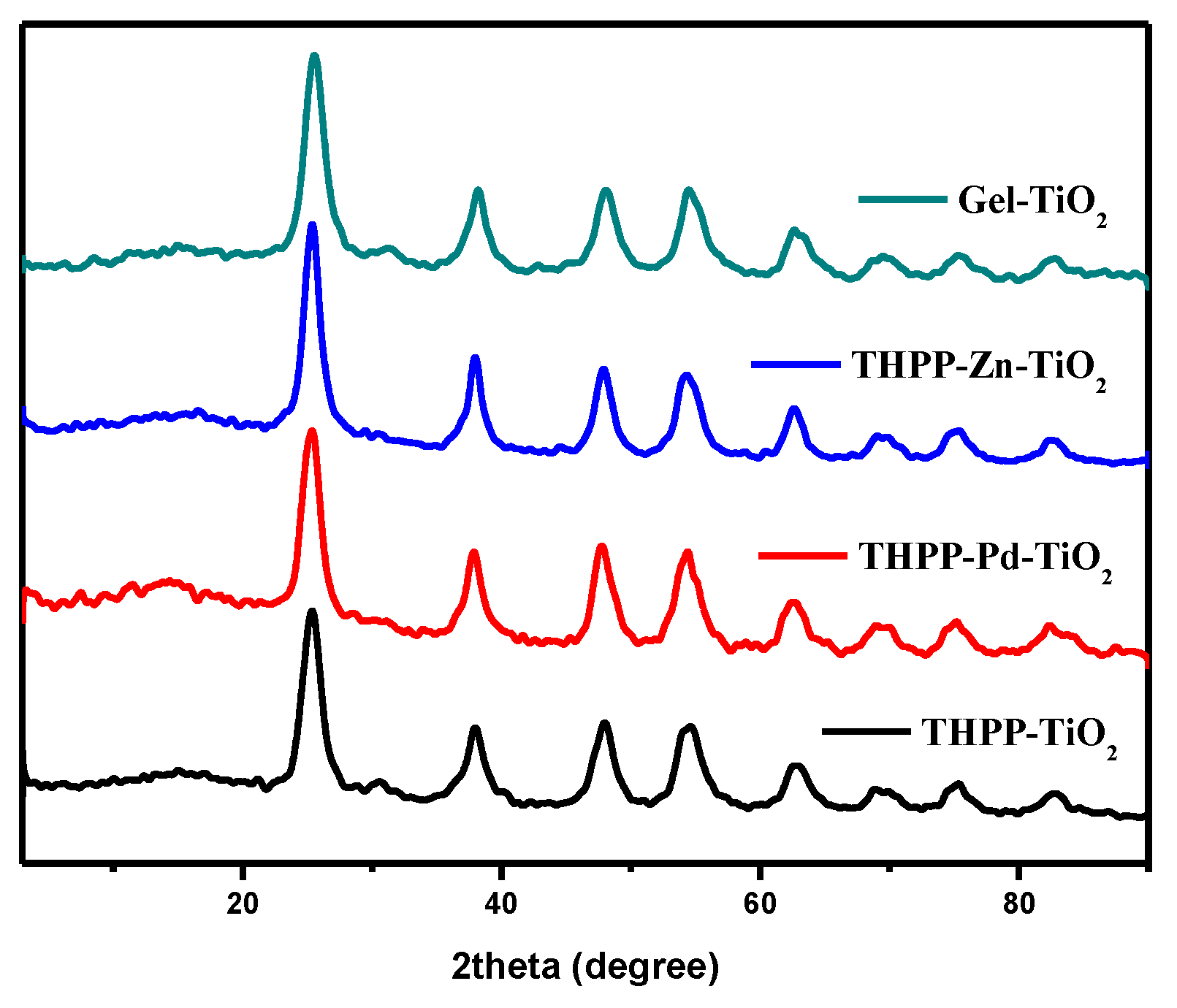
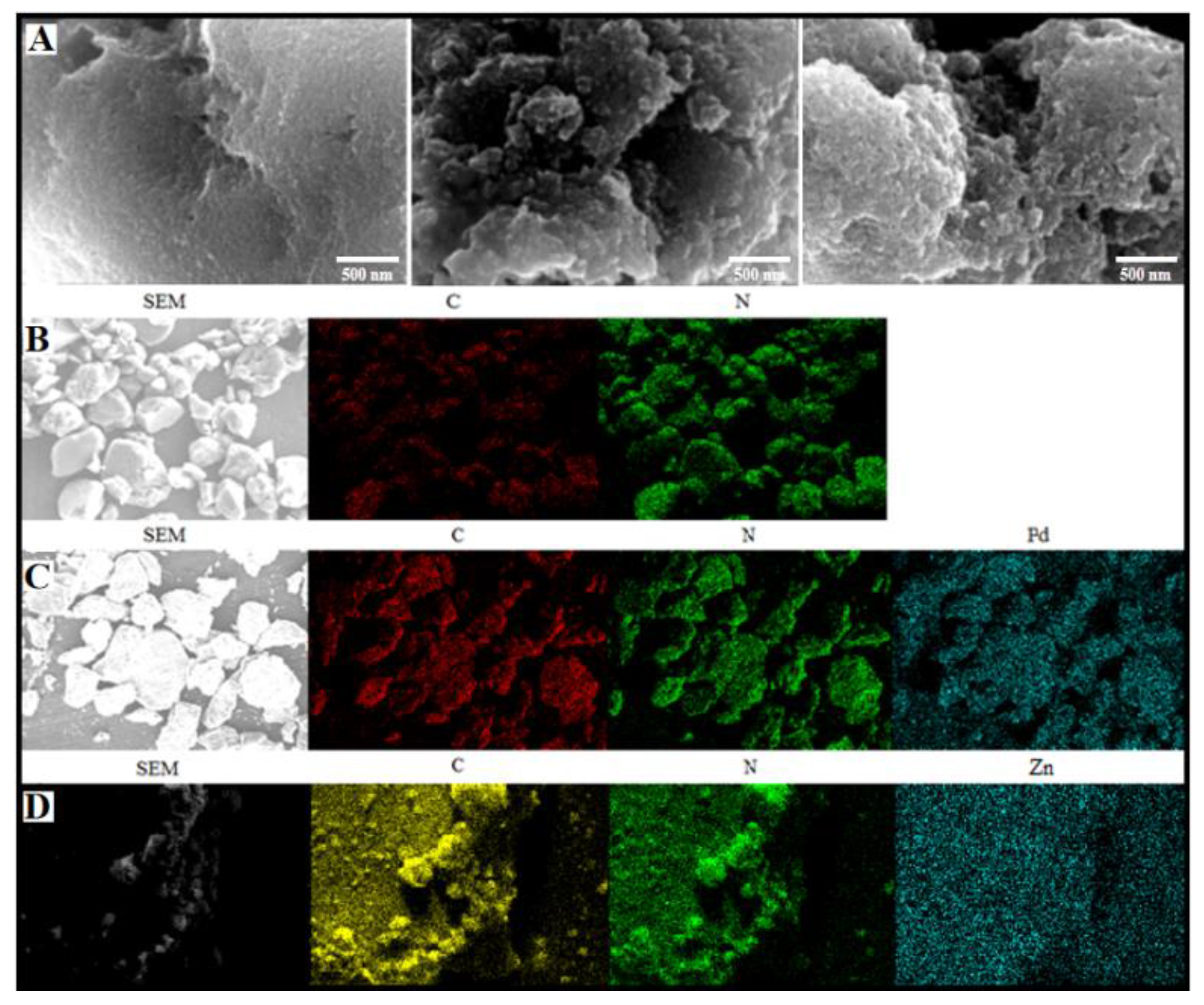
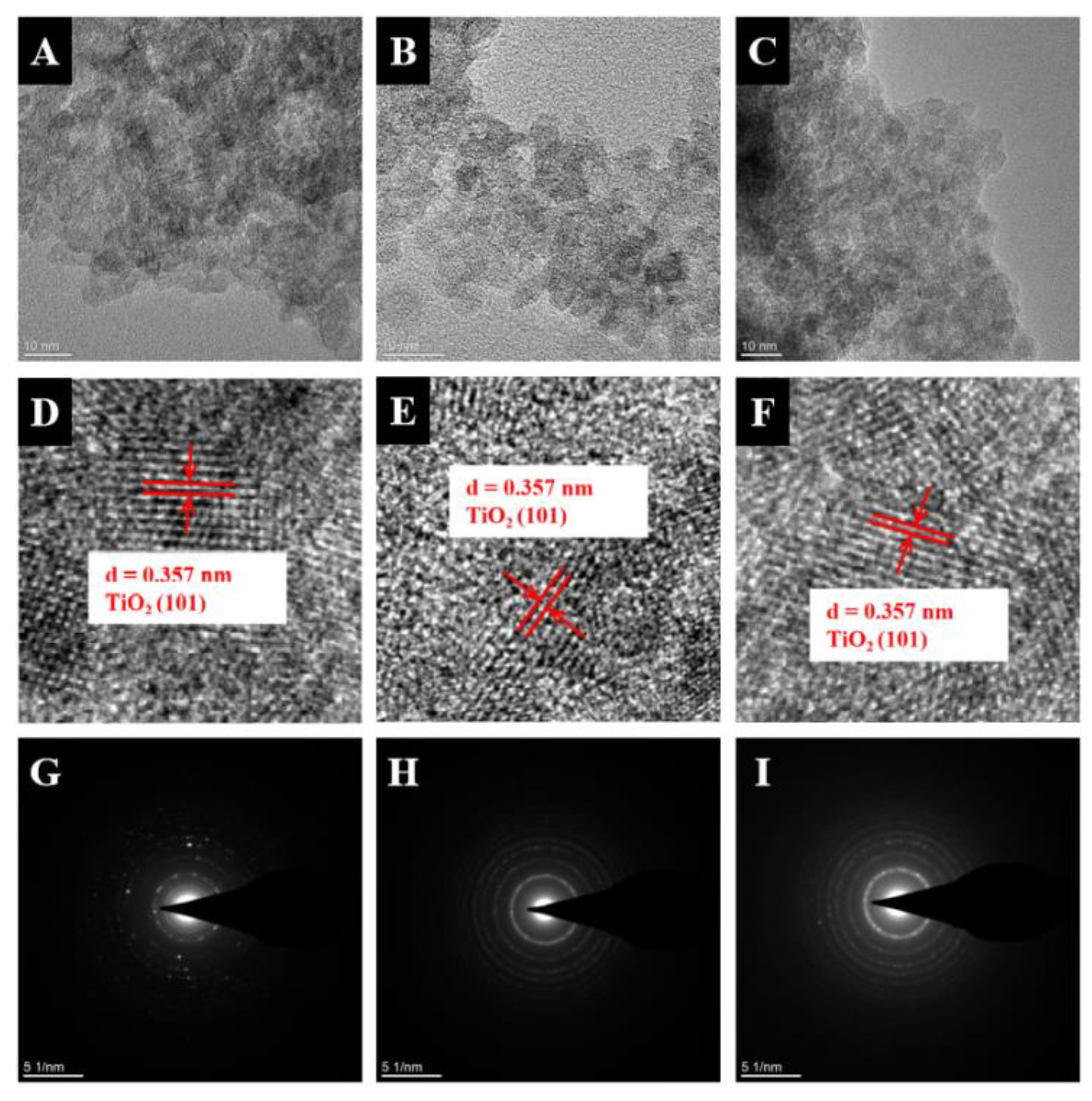
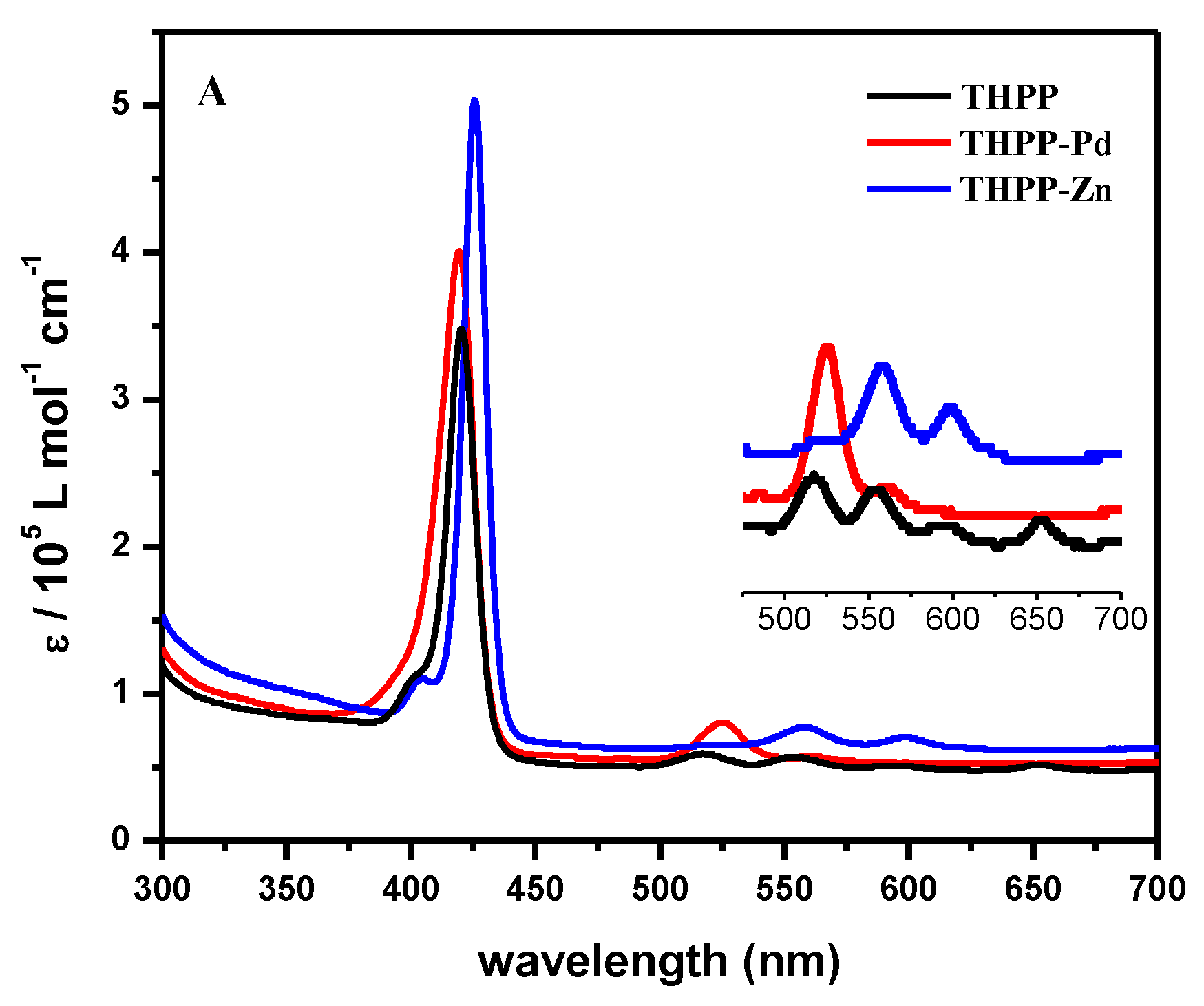
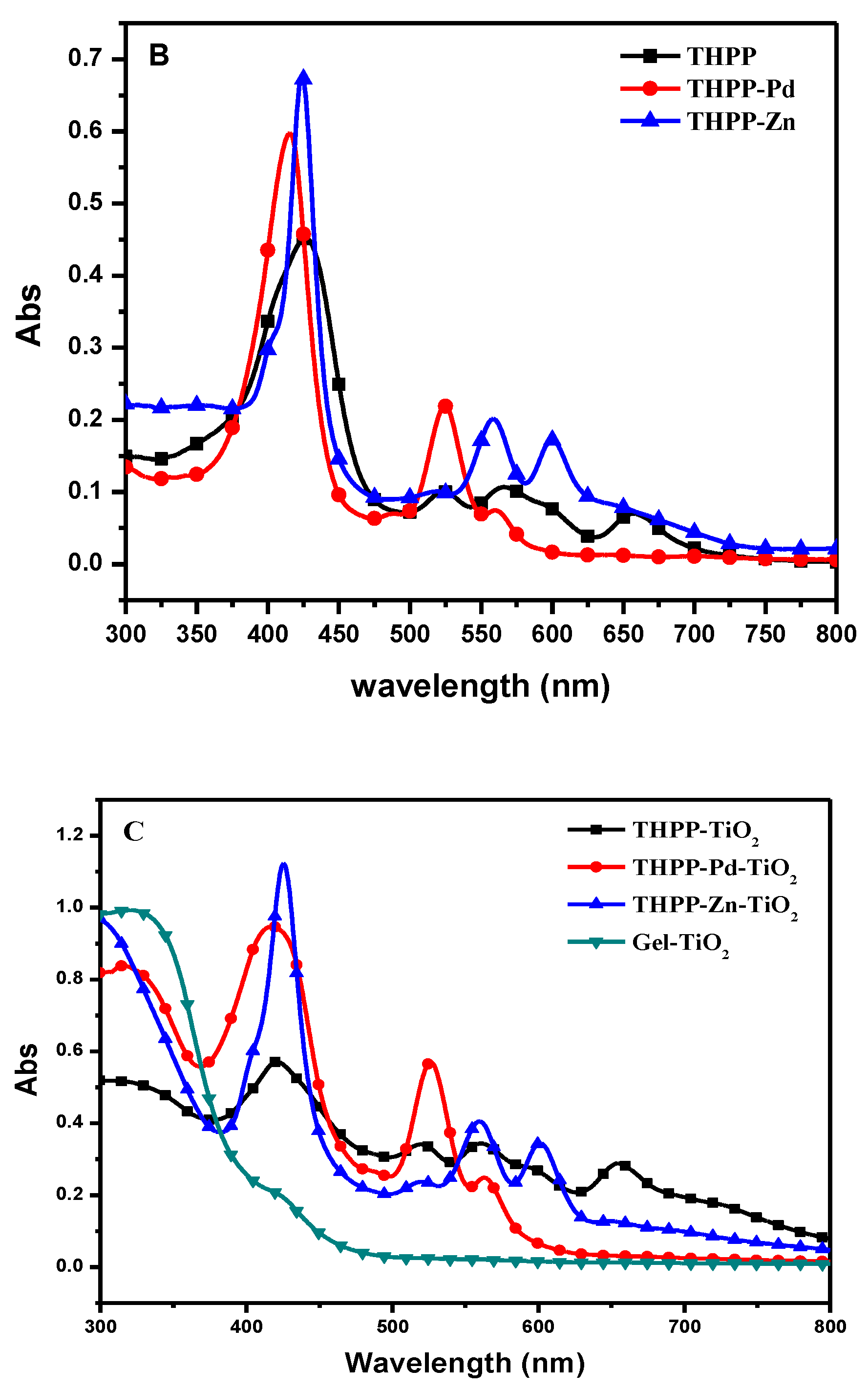
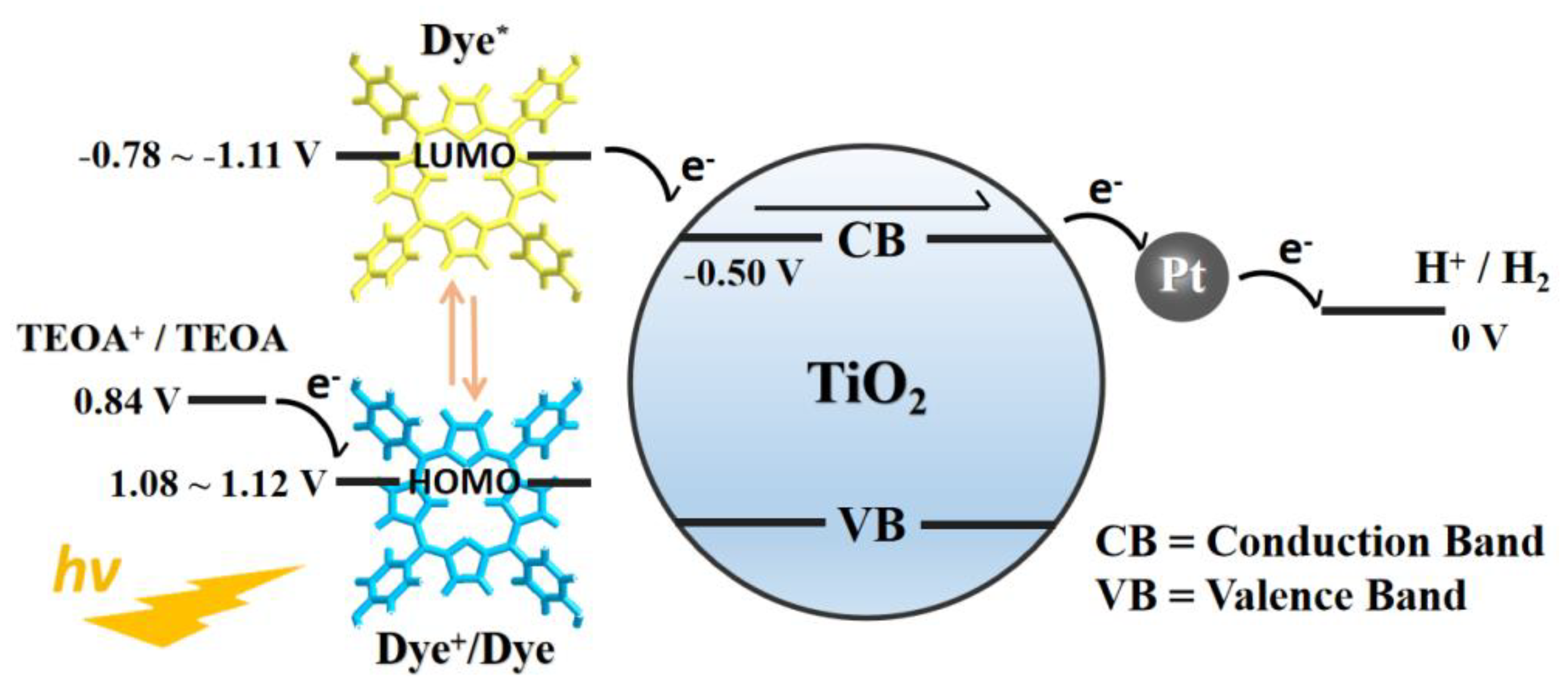
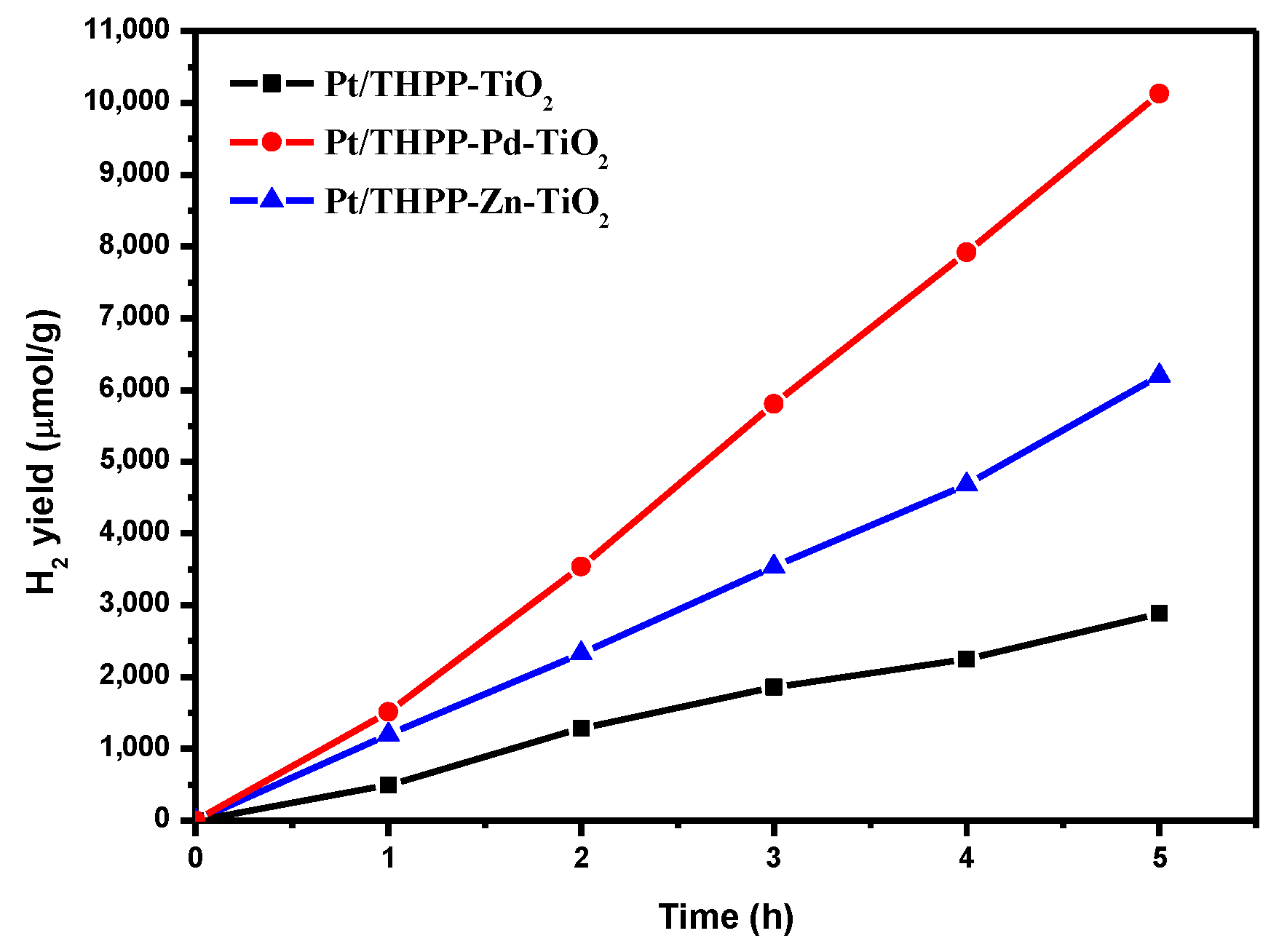
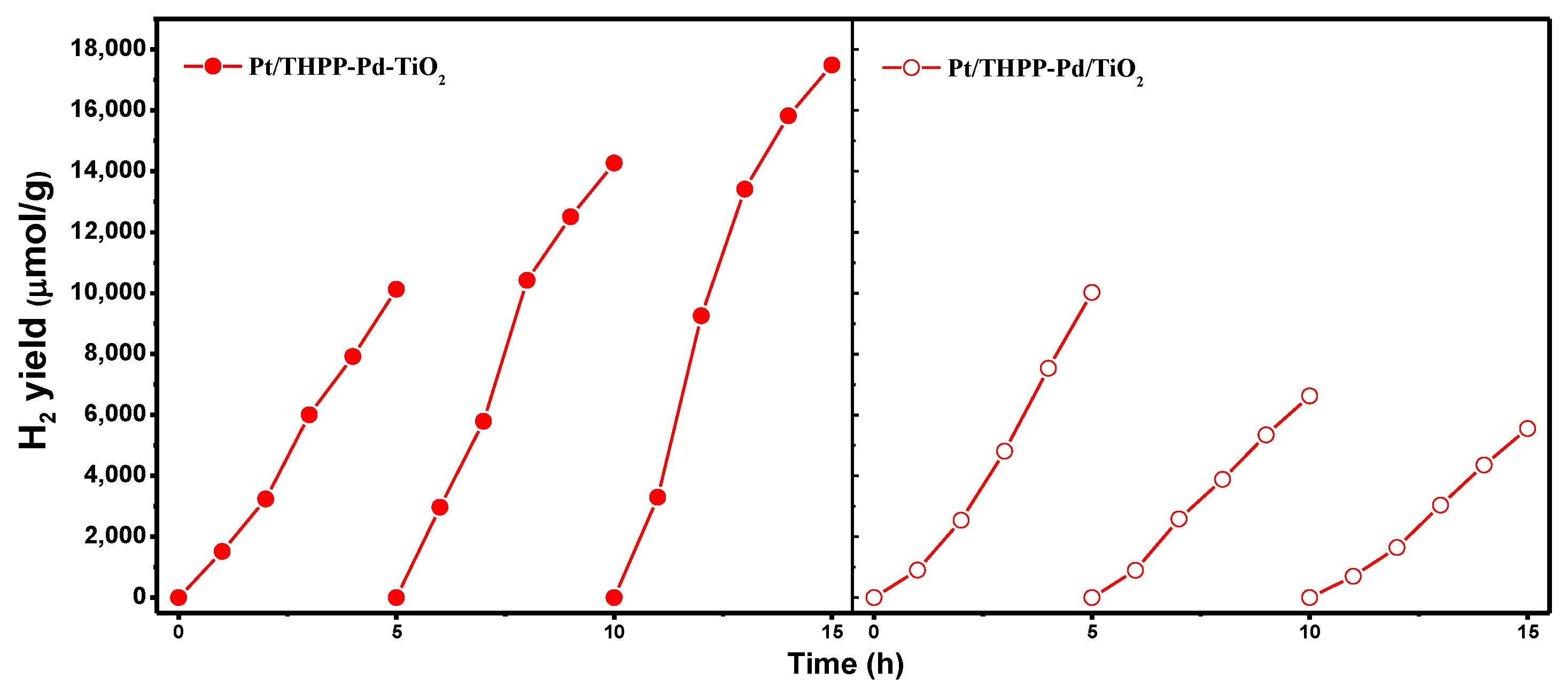
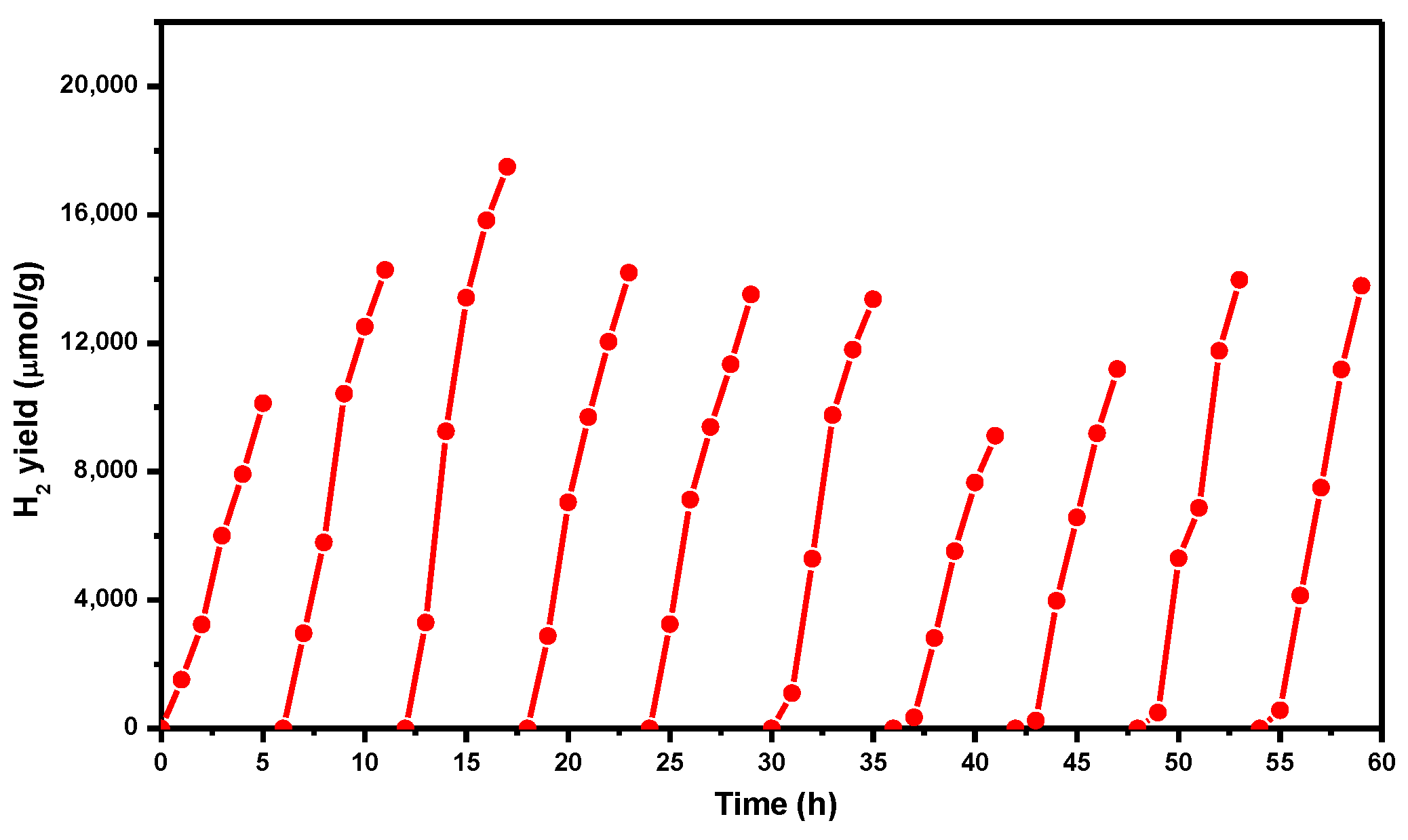
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.D. Recent advances and emerging trends in photo-electrochemical solar energy conversion. Adv. Energy Mater. 2019, 9, 1802877. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferna’ndez-Garcı’a, M.; Colo’n, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Miyoshi, A.; Nishioka, S.; Maeda, K. Water splitting on rutile TiO2-based photocatalysts. Chem. Eur. J. 2018, 24, 18204–18219. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Zou, J.; Bullock, J.; Wallace, G.G. Emerging approach in semiconductor photocatalysis: Towards 3D architectures for efficient solar fuels generation in semi-artificial photosynthetic systems. J. Photochem. Photobiol. C 2019, 39, 142–160. [Google Scholar] [CrossRef]
- Cecconi, B.; Manfredi, N.; Montini, T.; Fornasiero, P.; Abbotto, A. Dye-sensitized solar hydrogen production: The emerging role of metal-free organic sensitizers. Eur. J. Org. Chem. 2016, 31, 5194–5215. [Google Scholar] [CrossRef]
- Ho, P.-Y.; Wang, Y.; Yiu, S.-C.; Yu, W.-H.; Ho, C.-L.; Huang, S. Starburst triarylamine donor-based metal-free photosensitizers for photocatalytic hydrogen production from water. Org. Lett. 2017, 5, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Duvva, N.; Rao, V.N.; Venkatakrishnan, S.M.; Giribabu, L.; Pal, U. Tetrathiafulvalene scaffold-based sensitizer on hierarchical porous TiO2: Efficient light-harvesting material for hydrogen production. J. Phys. Chem. C 2019, 123, 70–81. [Google Scholar] [CrossRef]
- Su, R.; Tiruvalam, R.; Logsdail, A.J.; He, Q.; Downing, C.A.; Jensen, M.T.; Dimitratos, N.; Kesavan, L.; Wells, P.P.; Bechstein, R.; et al. Designer titania-supported Au-Pd nanoparticles for efficient photocatalytic hydrogen production. ACS Nano 2014, 8, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Navarro, R.M.; Alvarez-Galvan, M.C.; Villoria de la Mano, J.A.; Al-Zahrani, S.M.; Fierro, J.L.G. A framework for visible-light water splitting. Energy Environ. Sci. 2010, 3, 1865–1882. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-sensitized solar cells: Systematic molecular optimization, coadsorption and cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef]
- Mukherjee, G.; Thote, J.; Aiyappa, H.B.; Kandambeth, S.; Banerjee, S.; Vanka, K.; Banerjee, R. A porous porphyrin organic polymer (PPOP) for visible light triggered hydrogen production. Chem. Commun. 2017, 53, 4461–4464. [Google Scholar] [CrossRef] [PubMed]
- Koposova, E.; Liu, X.; Pendin, A.A.; Thiele, B.; Shumilova, G.; Ermolenko, Y.; Offenhäusser, A.; Mourzina, Y. Influence of Meso-substitution of the Porphyrin ring on enhanced hydrogen evolution in a photochemical system. J. Phys. Chem. C 2016, 120, 13873–13890. [Google Scholar] [CrossRef]
- Huang, J.-F.; Lei, Y.; Xiao, L.-M.; Chen, X.-L.; Zhong, Y.-H.; Qin, S.; Liu, J.-M. Photocatalysts for H2 generation from starburst triphenylamine/carbazole donor-based metal-free dyes and porous anatase TiO2 cube. ChemSusChem 2020, 13, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, J.; Zhang, W.; Xu, R. Carbon nitride nanosheets for photocatalytic hydrogen evolution: Remarkably enhanced activity by dye sensitization. Catal. Sci. Technol. 2013, 3, 1703–1711. [Google Scholar] [CrossRef]
- Min, S.X.; Lu, G.X. Dye-sensitized reduced graphene oxide photocatalysts for highly efficient visible-light-driven water reduction. J. Phys. Chem. C 2011, 115, 13938–13945. [Google Scholar] [CrossRef]
- Chen, Q.; Marco, N.D.; Yang, Y.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the spotlight: The organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Pan, Z.; Yao, L.; Zhai, J.; Yao, X.; Chen, H. Interfacial coupling effect in organic/inorganic nanocomposites with high energy density. Adv. Mater. 2018, 30, 1705662. [Google Scholar]
- Chen, Y.; Shi, J. Chemistry of mesoporous organosilica in nanotechnology: Molecularly organic-inorganic hybridization into frameworks. Adv. Mater. 2016, 28, 3235–3272. [Google Scholar] [CrossRef]
- Gobbi, M.; Orgiu, E.; Samorì, P. When 2D materials meet molecules: Opportunities and challenges of hybrid organic/inorganic van der waals heterostructures. Adv. Mater. 2018, 30, 1706103. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K.; Mishima, K. Preparation of poly(methyl methacrylate)-TiO2 nanoparticle composites by pseudo-dispersion polymerization of methyl methacrylate in supercritical CO2. J. Supercrit. Fluids 2009, 49, 256–264. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Bhat, T.S.; Mali, S.S.; Korade, S.D.; Shaikh, J.S.; Karanjkar, M.M.; Hong, C.K.; Kim, J.H.; Patil, P.S. Mesoporous architecture of TiO2 microspheres via controlled template assisted route and their photoelectrochemical properties. J. Mater. Sci. Mater. El. 2017, 28, 304–316. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The advancements in sol-gel method of doped-TiO2 photocatalysts. Appl. Catal. A 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Guo, X.; Dai, J.; Xiang, J. Fast sol-gel method to prepare mesoporous TiO2 with high photocatalytic activity. Nano 2015, 10, 1550001. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Liu, Q.; Peng, T.; Li, R.; Zhou, S. Effects of the central metal ions on the photosensitization of metalloporphyrins over carbon nitride for visible-light-responsive H2 production. Appl. Surf. Sci. 2019, 464, 255–261. [Google Scholar] [CrossRef]
- Liao, M.-S.; Scheiner, S. Electronic structure and bonding in metal porphyrins, metal = Fe, Co, Ni, Cu, Zn. J. Chem. Phys. 2002, 117, 205–219. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Huang, J.-F.; Shen, M.-H.; Liu, J.-M.; Huang, L.-B.; Zhong, Y.-H.; Qin, S.; Guo, J.; Su, C.-Y. A porous hybrid material based on calixarene dye and TiO2 demonstrating high and stable photocatalytic performance. J. Mater. Chem. A 2019, 7, 19852–19861. [Google Scholar] [CrossRef]
- Zhong, Y.-H.; Lei, Y.; Huang, J.-F.; Xiao, L.-M.; Chen, X.-L.; Luo, T.; Qin, S.; Guo, J.; Liu, J.-M. Design of an alkalinous pyridyl acceptor-based calix[4]arene dye and synthesis of stable calixarene-TiO2 porous hybrid materials for efficient photocatalysis. J. Mater. Chem. A 2020, 8, 8883–8891. [Google Scholar] [CrossRef]
- Sun, H.; Hoffman, M.Z. Reductive quenching of the excited states of ruthenium(II) complexes containing 2,2’-bipyridine, 2,2’-bipyrazine and 2,2’-bipyrimidine ligands. J. Phys. Chem. A 1994, 98, 11719–11726. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Schäfer, B.; Smolentsev, G.; Uhlig, J.; Nazarenko, E.; Guthmuller, J.; Kuhnt, C.; Wächtler, M.; Dietzek, B.; Sundström, V.; et al. Palladium versus platinum: The metal in the catalytic center of a molecular photocatalyst determines the mechanism of the hydrogen production with visible light. Angew. Chem. Int. Ed. 2015, 54, 5044–5048. [Google Scholar] [CrossRef]
- Tschierlei, S.; Presselt, M.; Kuhnt, C.; Yartsev, A.; Pascher, T.; Sundström, V.; Karnahl, M.; Schwalbe, M.; Schäfer, B.; Rau, S.; et al. Photophysics of an intramolecular hydrogen-evolving Ru-Pd photocatalyst. Chem. Eur. J. 2009, 15, 7678–7688. [Google Scholar] [CrossRef]
- Dempsey, J.L.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 2009, 42, 1995–2004. [Google Scholar] [CrossRef]
- Manbeck, G.F.; Brewer, K.J. Photoinitiated electron collection in polyazine chromophores coupled to water reduction catalysts for solar H2 production. Coord. Chem. Rev. 2013, 257, 1660–1675. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-Y.; Huang, J.-F.; Lei, Y.; Qin, S.; Liu, J.-M. Porous Hybrid Materials Based on Mesotetrakis(Hydroxyphenyl) Porphyrins and TiO2 for Efficient Visible-Light-Driven Hydrogen Production. Catalysts 2020, 10, 656. https://doi.org/10.3390/catal10060656
Huang L-Y, Huang J-F, Lei Y, Qin S, Liu J-M. Porous Hybrid Materials Based on Mesotetrakis(Hydroxyphenyl) Porphyrins and TiO2 for Efficient Visible-Light-Driven Hydrogen Production. Catalysts. 2020; 10(6):656. https://doi.org/10.3390/catal10060656
Chicago/Turabian StyleHuang, Li-Yuan, Jian-Feng Huang, Yang Lei, Su Qin, and Jun-Min Liu. 2020. "Porous Hybrid Materials Based on Mesotetrakis(Hydroxyphenyl) Porphyrins and TiO2 for Efficient Visible-Light-Driven Hydrogen Production" Catalysts 10, no. 6: 656. https://doi.org/10.3390/catal10060656
APA StyleHuang, L.-Y., Huang, J.-F., Lei, Y., Qin, S., & Liu, J.-M. (2020). Porous Hybrid Materials Based on Mesotetrakis(Hydroxyphenyl) Porphyrins and TiO2 for Efficient Visible-Light-Driven Hydrogen Production. Catalysts, 10(6), 656. https://doi.org/10.3390/catal10060656