Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization
Abstract
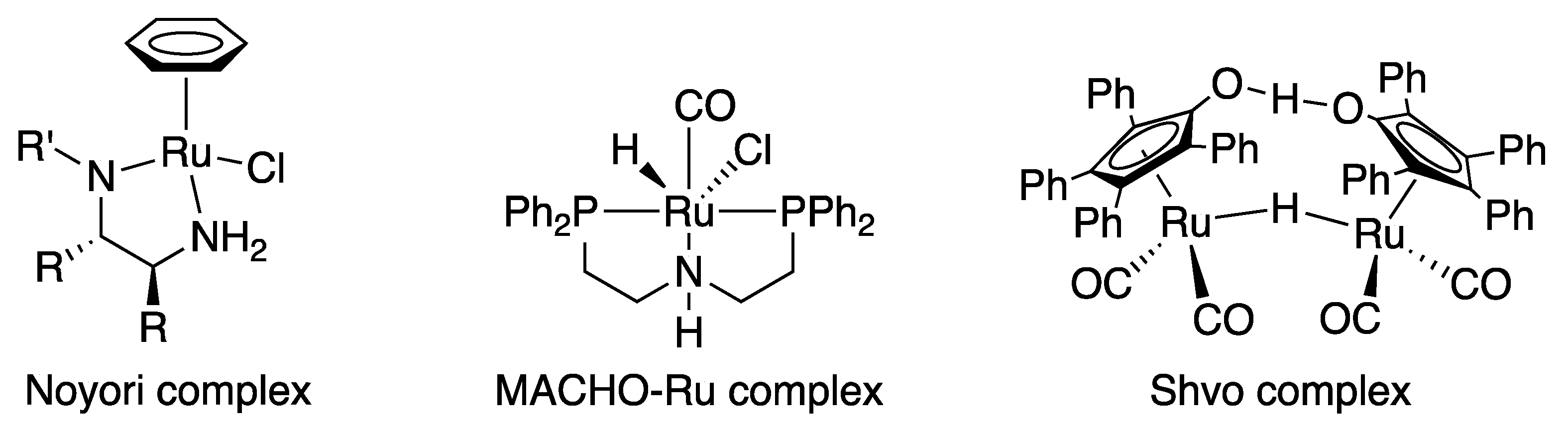
1. Introduction
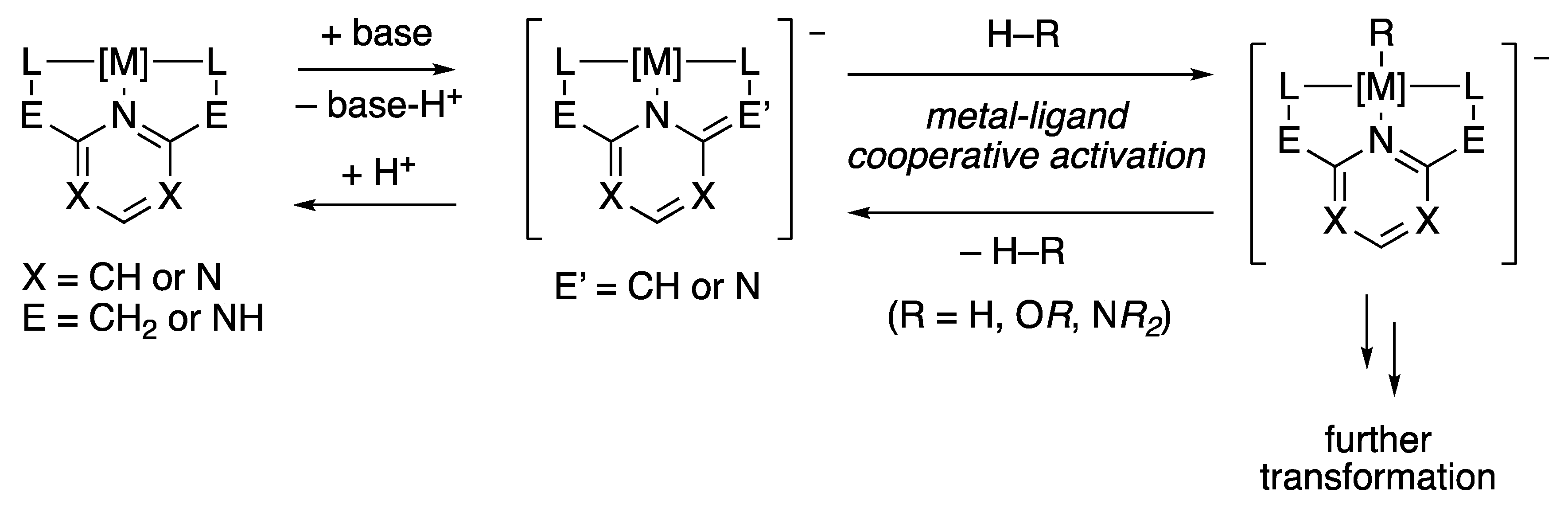
2. Pincer-Type Ligand Enabling Proton Uptake and Release on Side Arm
2.1. Hydrogenation Reactions
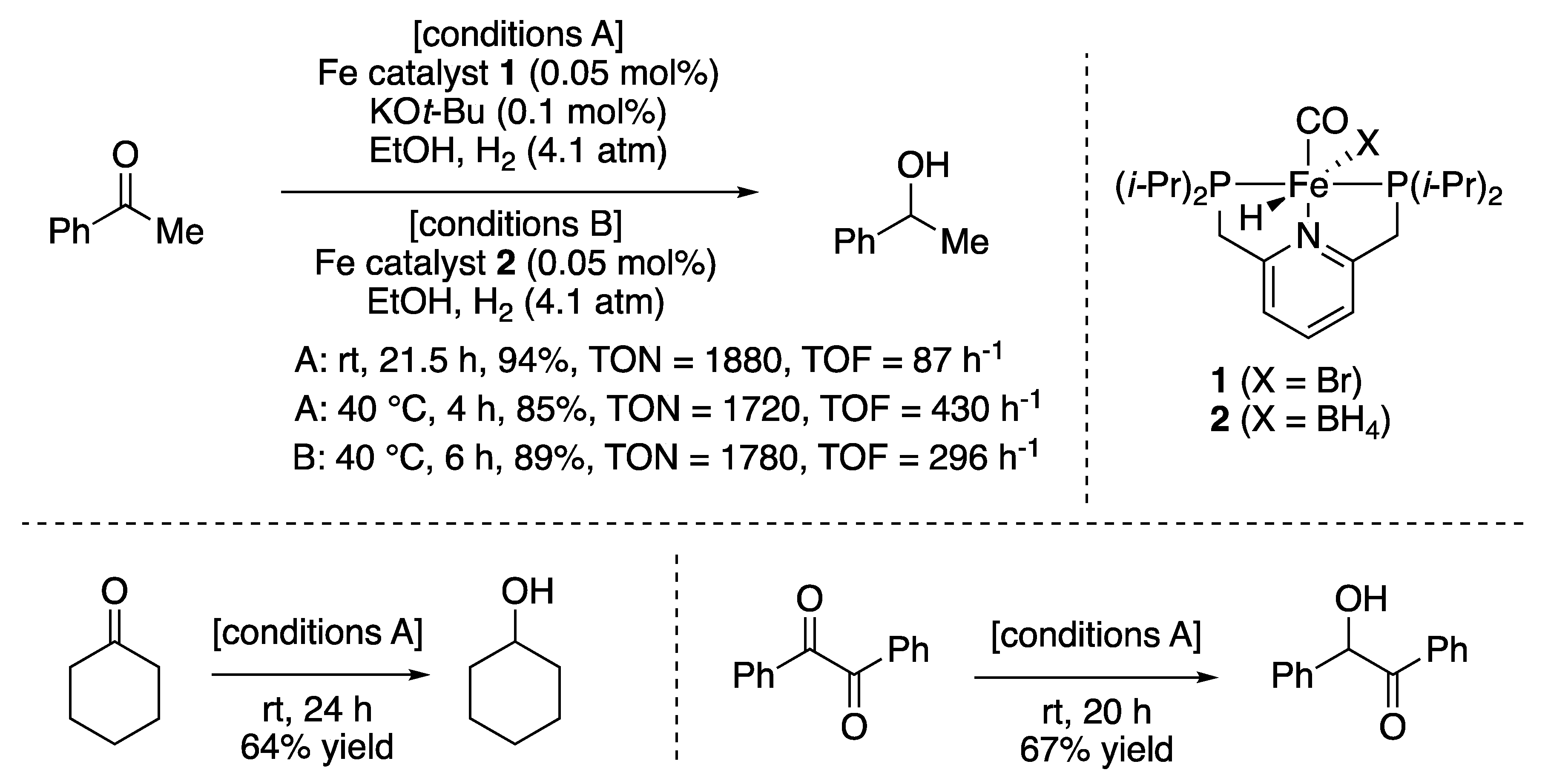
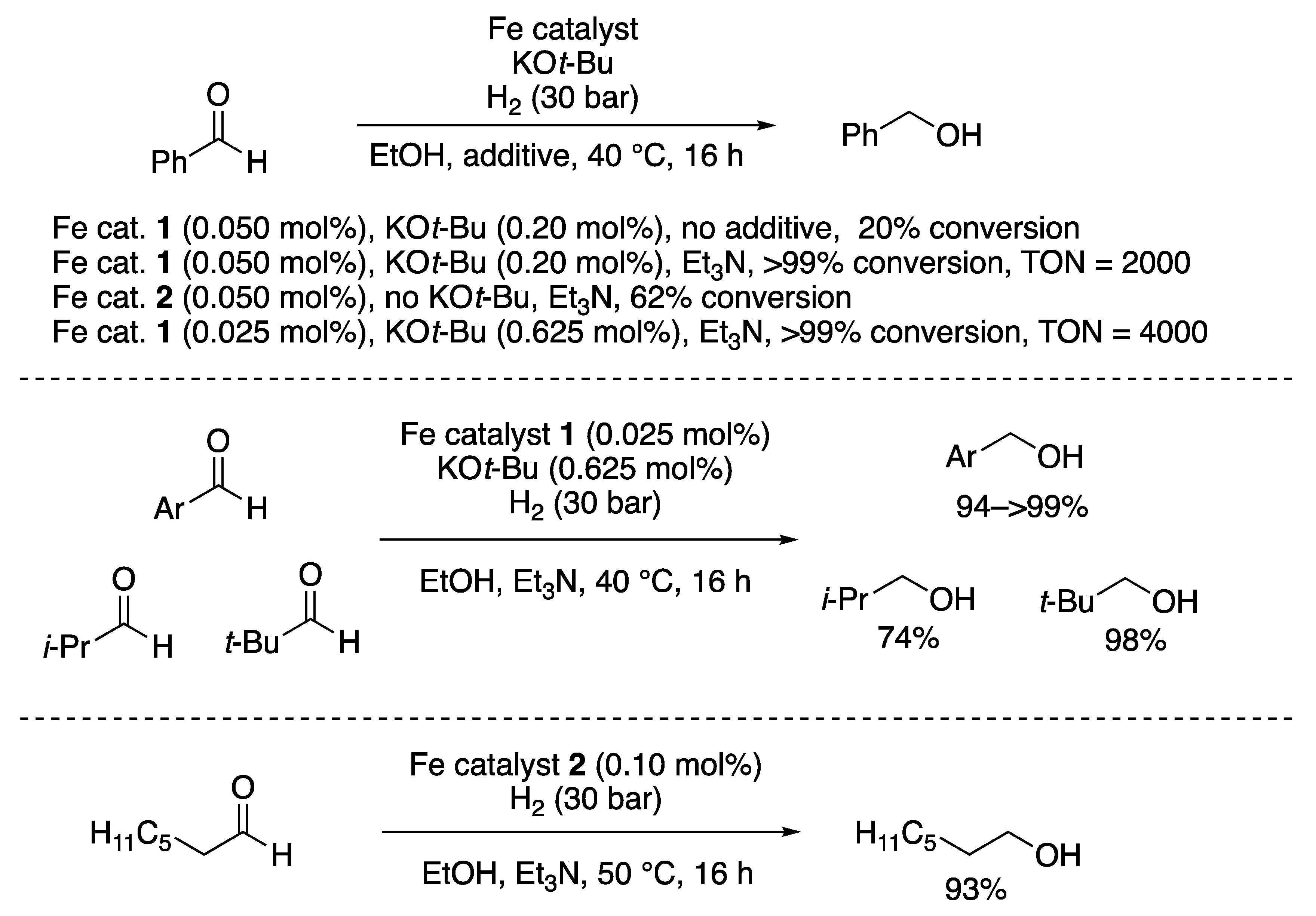
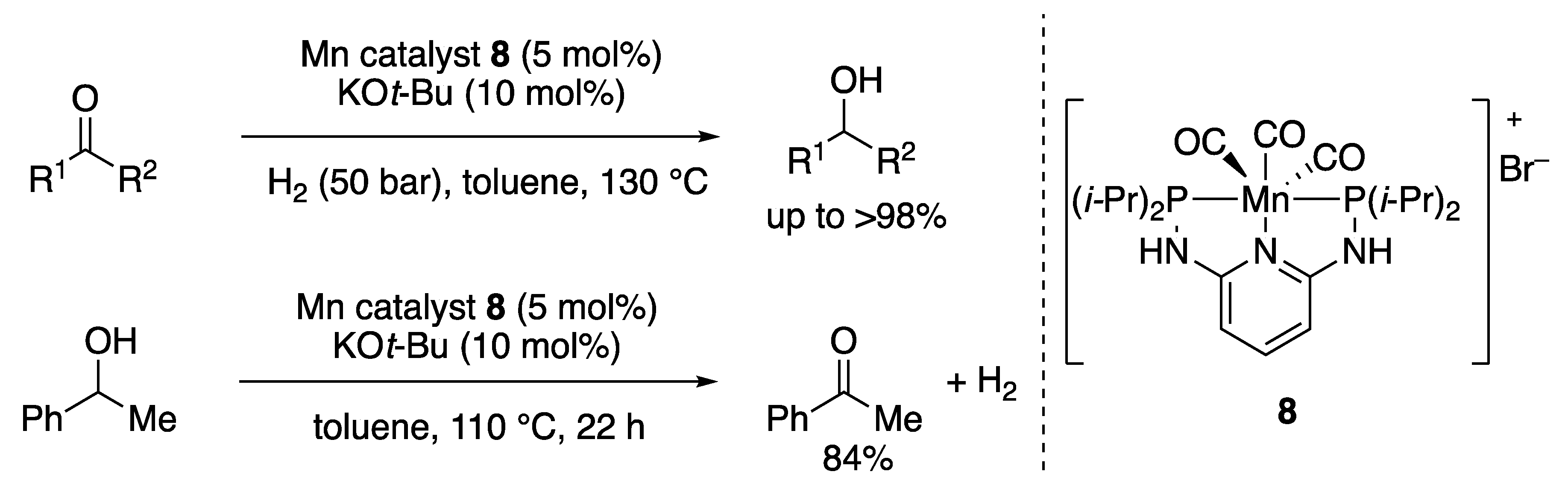
2.1.1. Hydrogenations of Ketone and Aldehydes
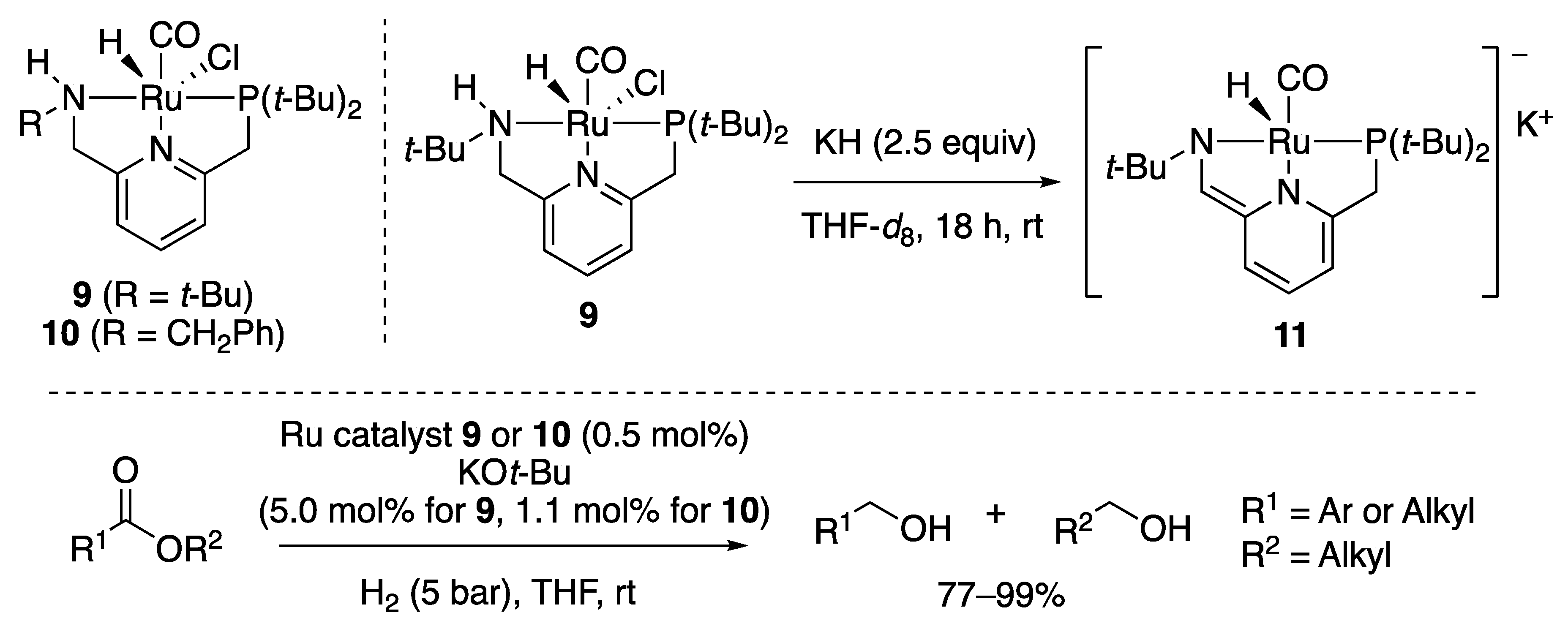
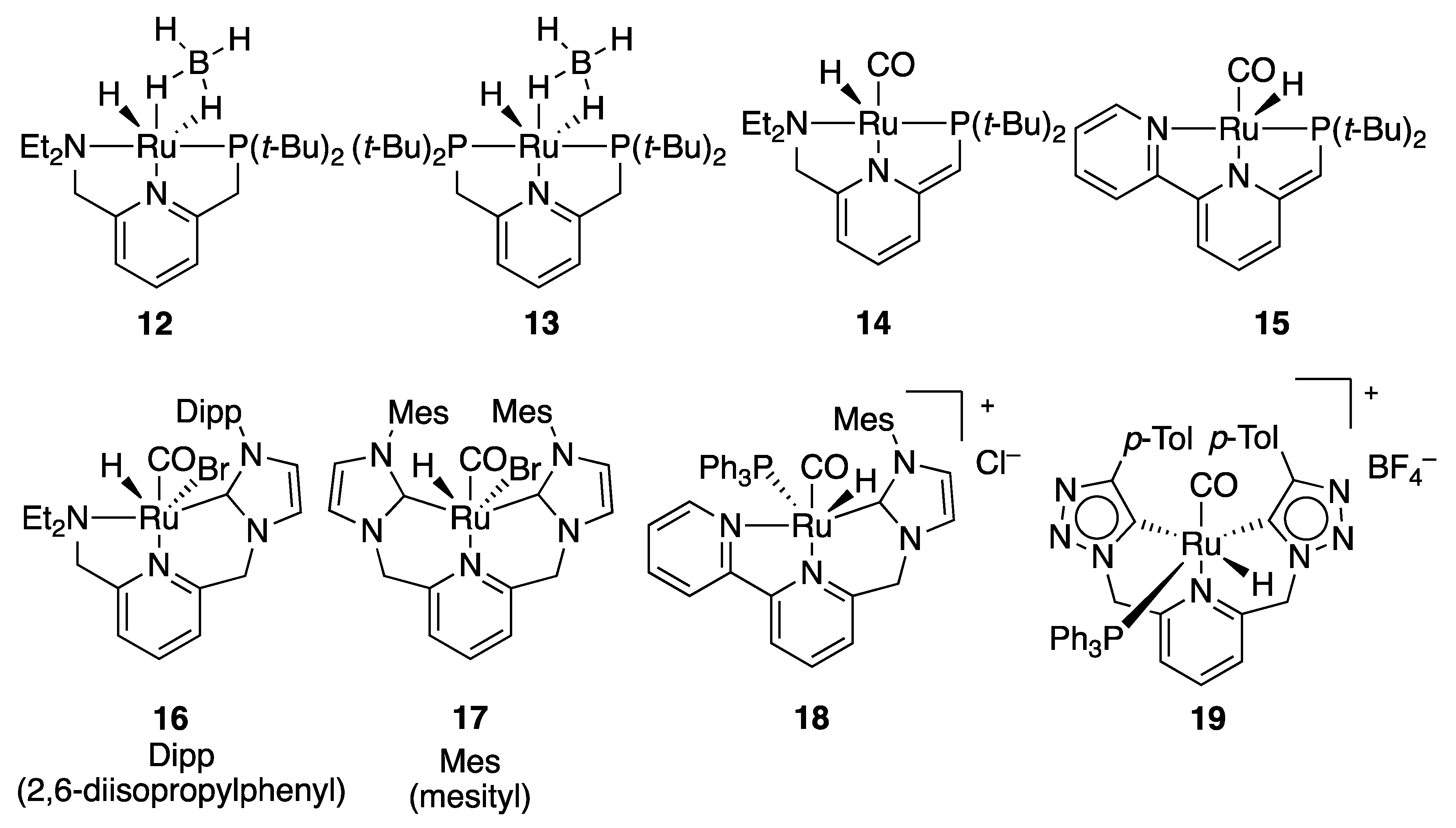
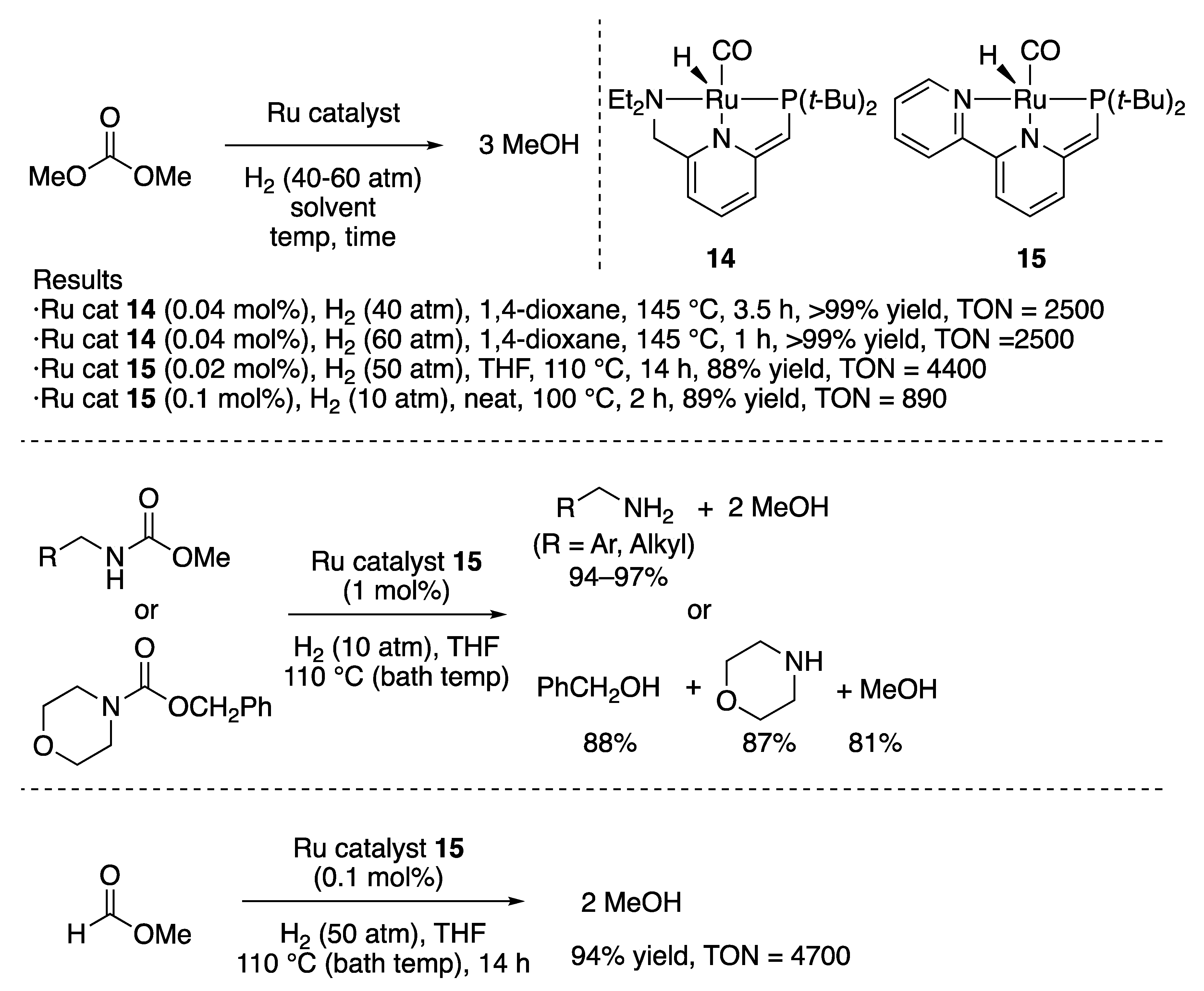
2.1.2. Ester Hydrogenation
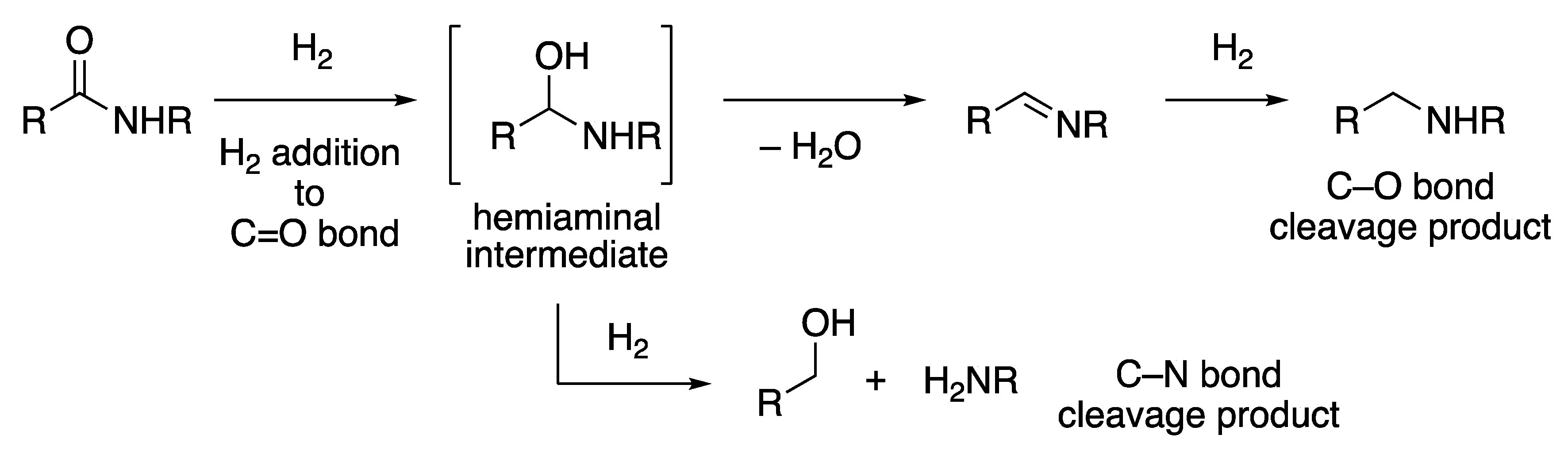
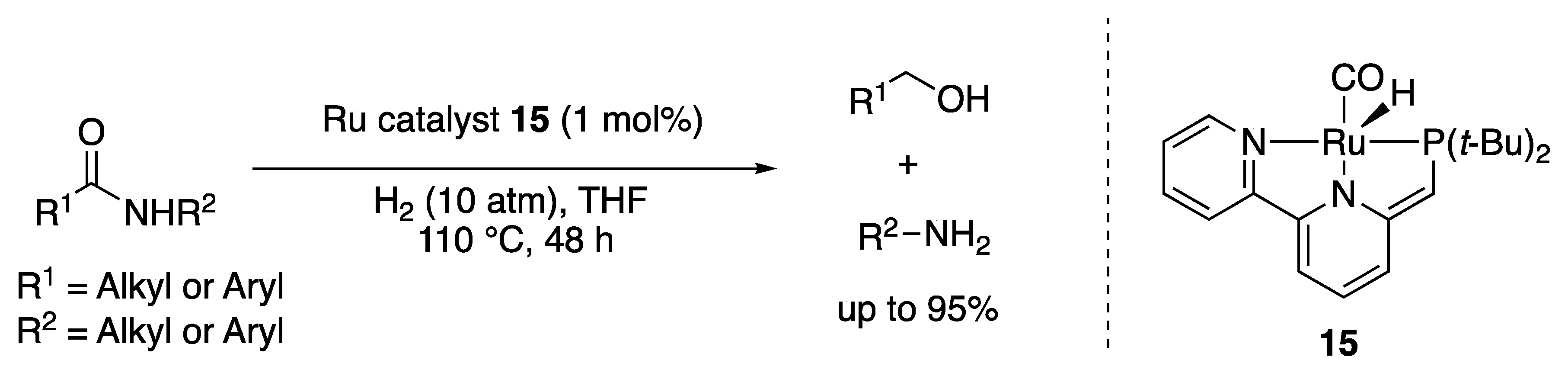
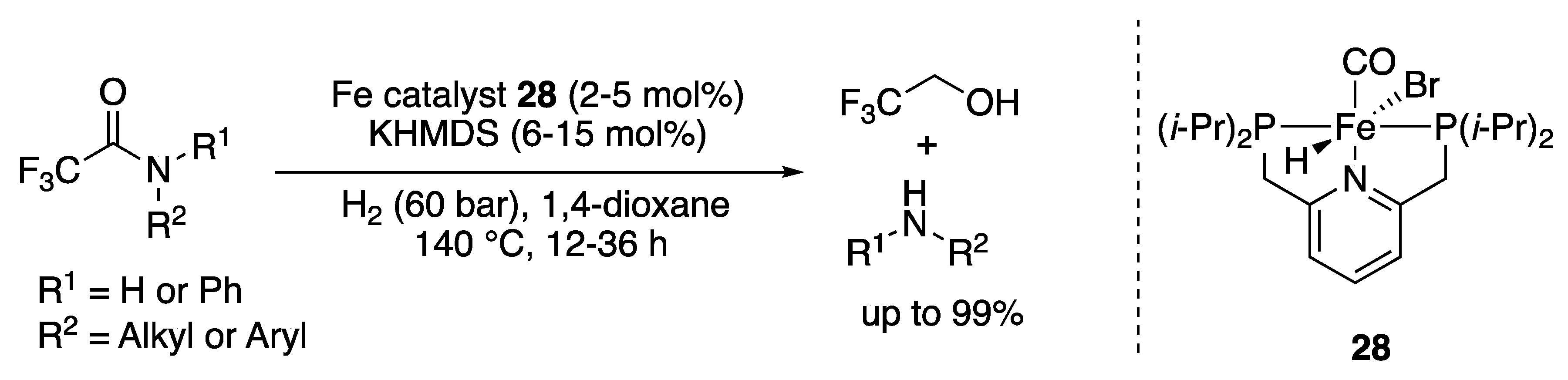
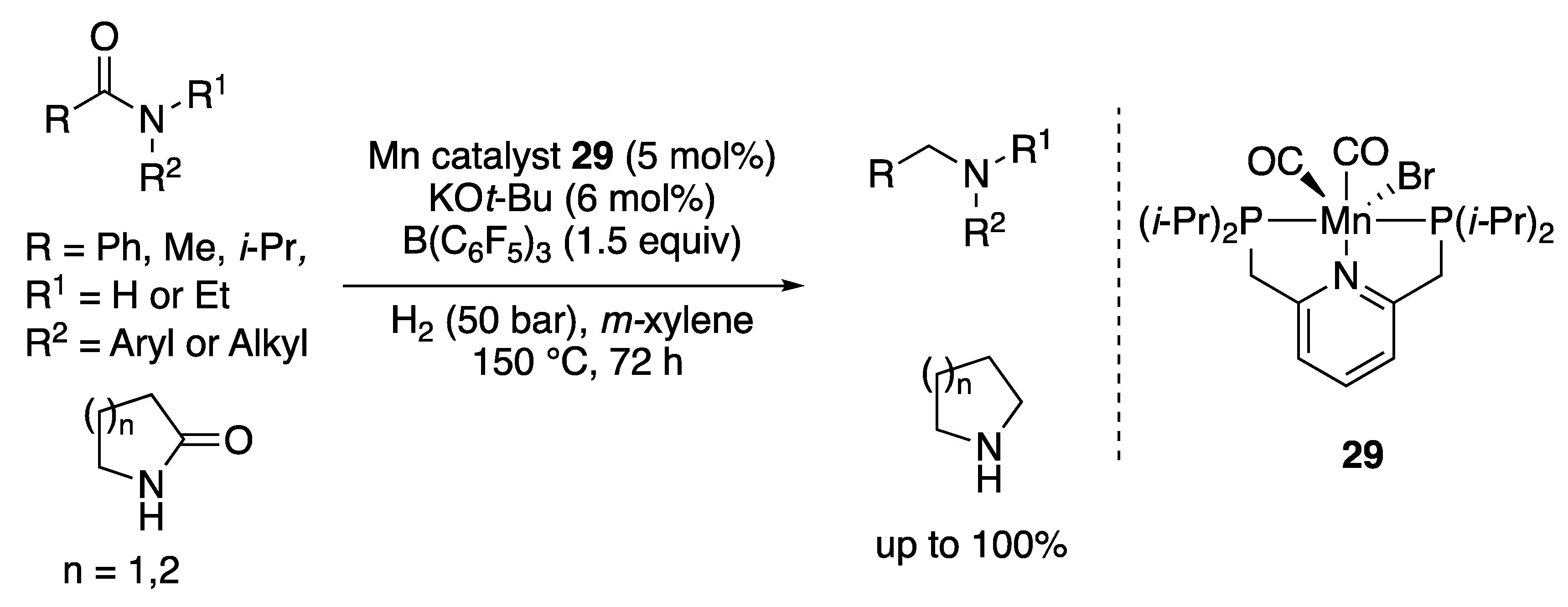
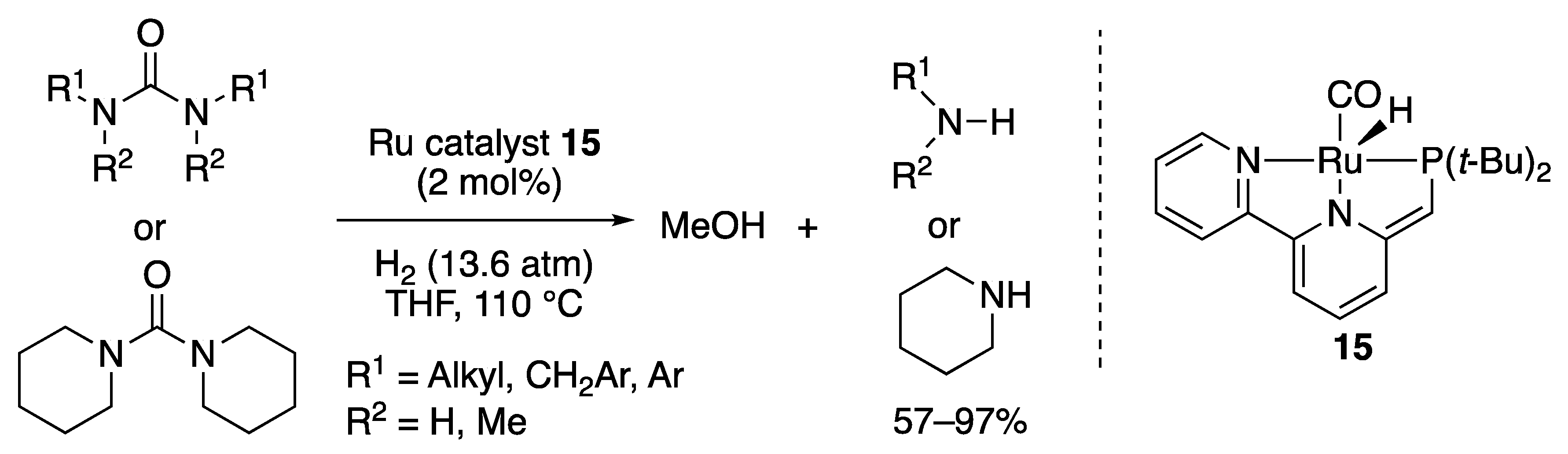
2.1.3. Amide Hydrogenation
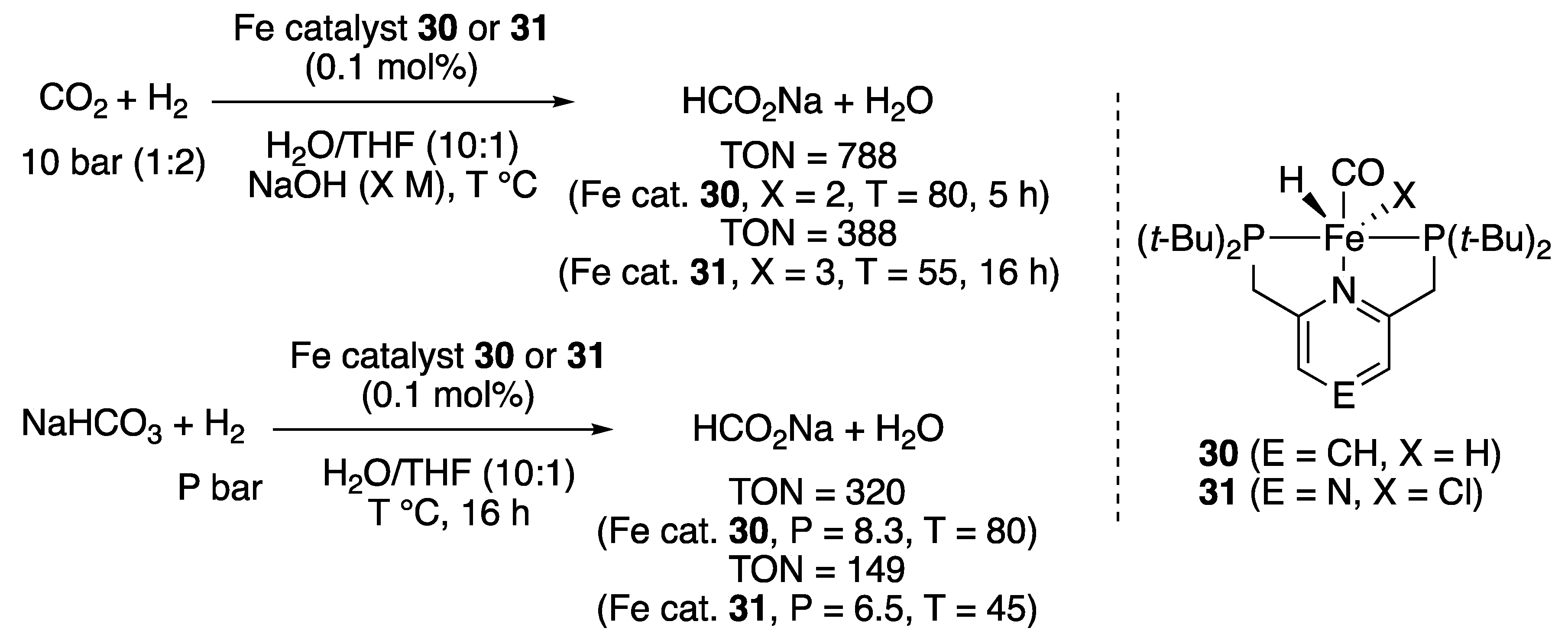
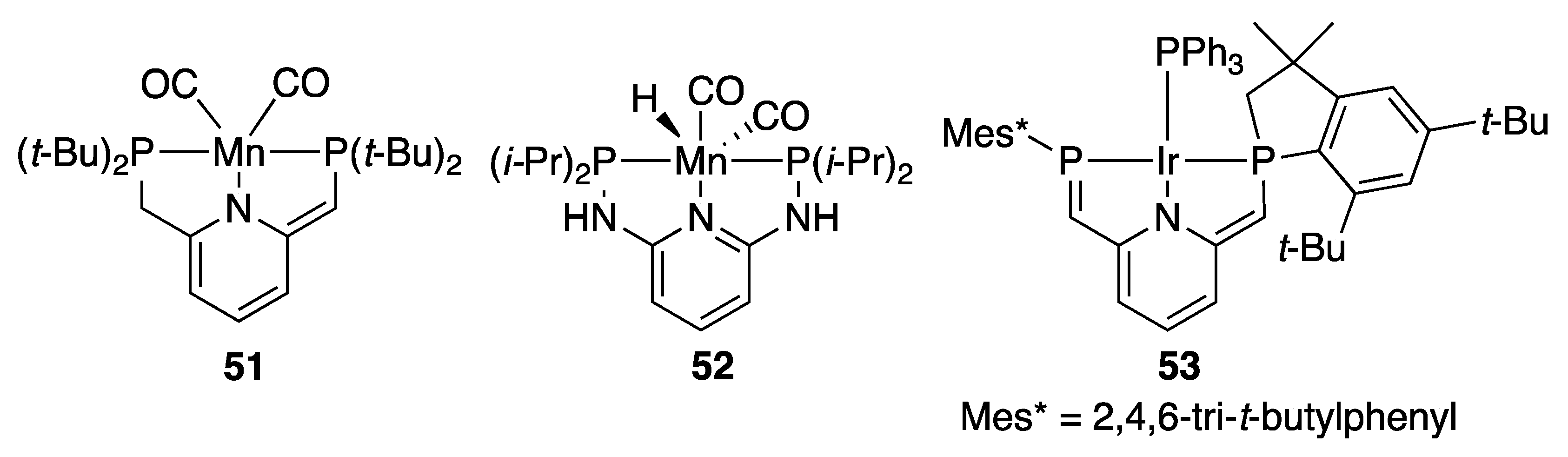
2.1.4. Carbonate, Carbamate, Urea, and Formate Hydrogenation
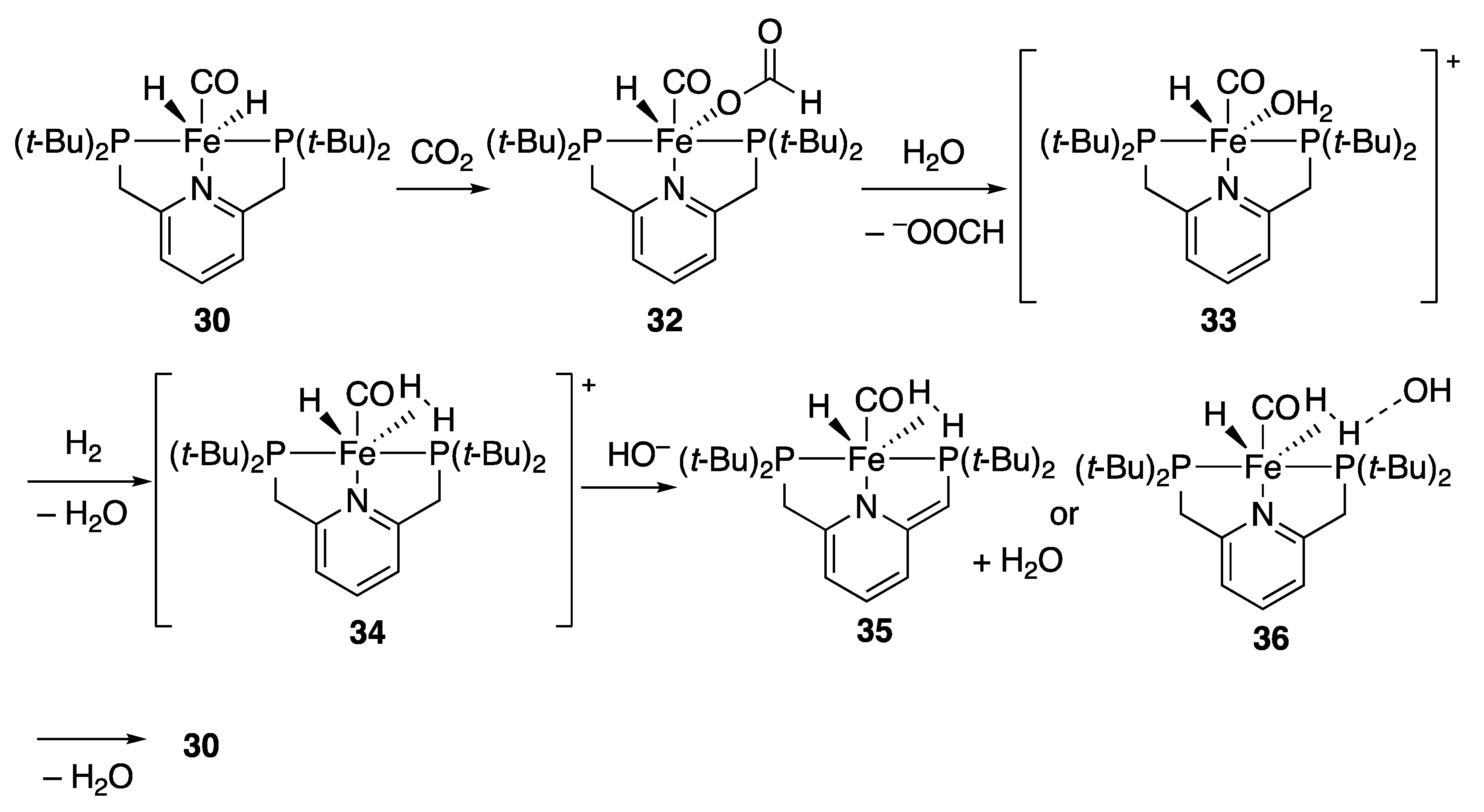
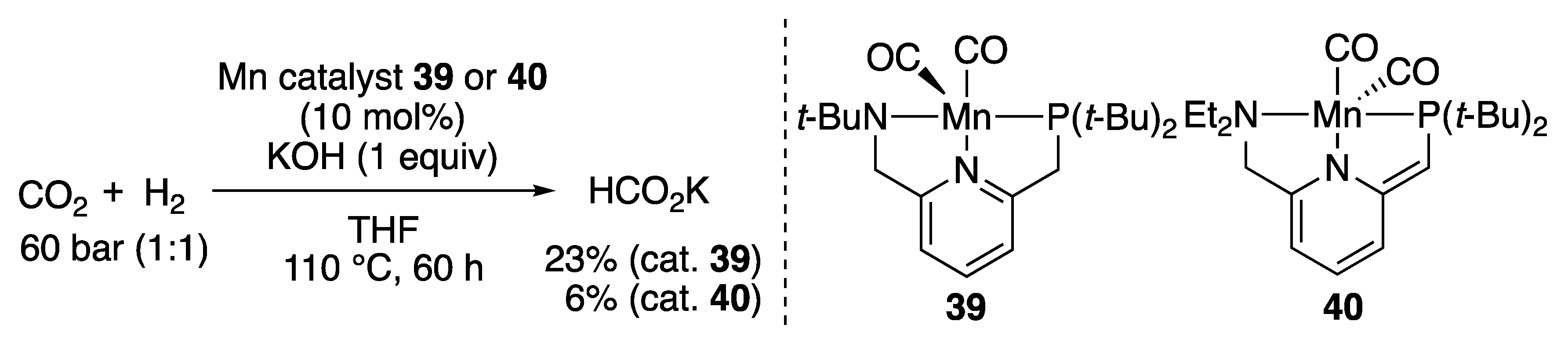
2.1.5. CO2 Hydrogenation
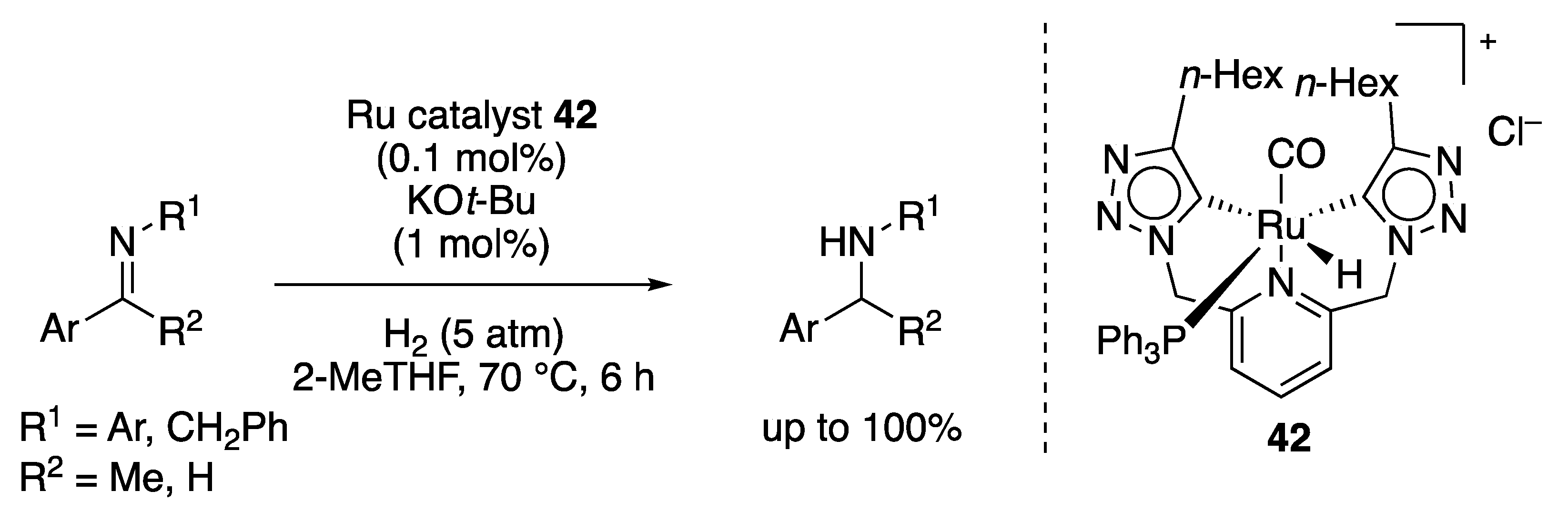
2.1.6. Other Hydrogenation Reactions
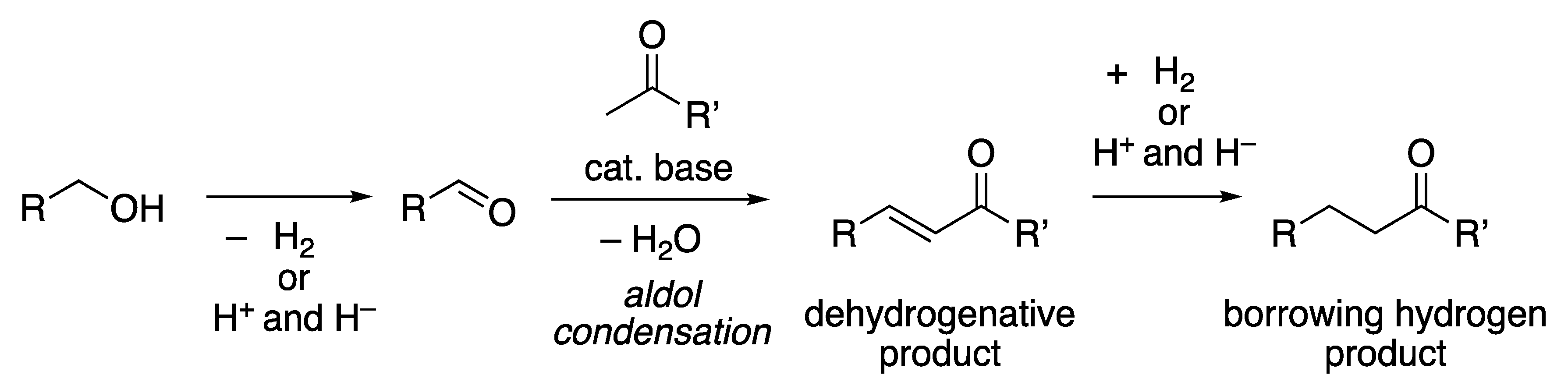
2.2. Dehydrogenation
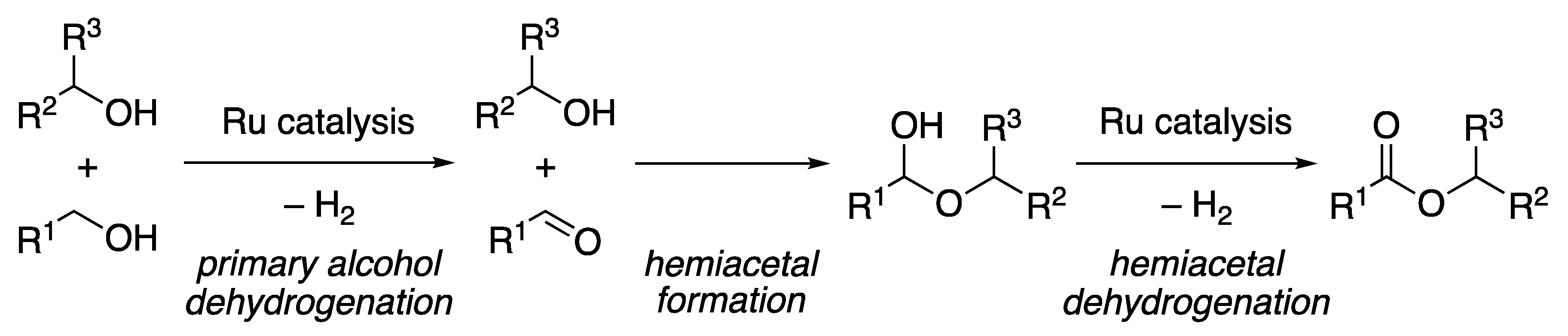
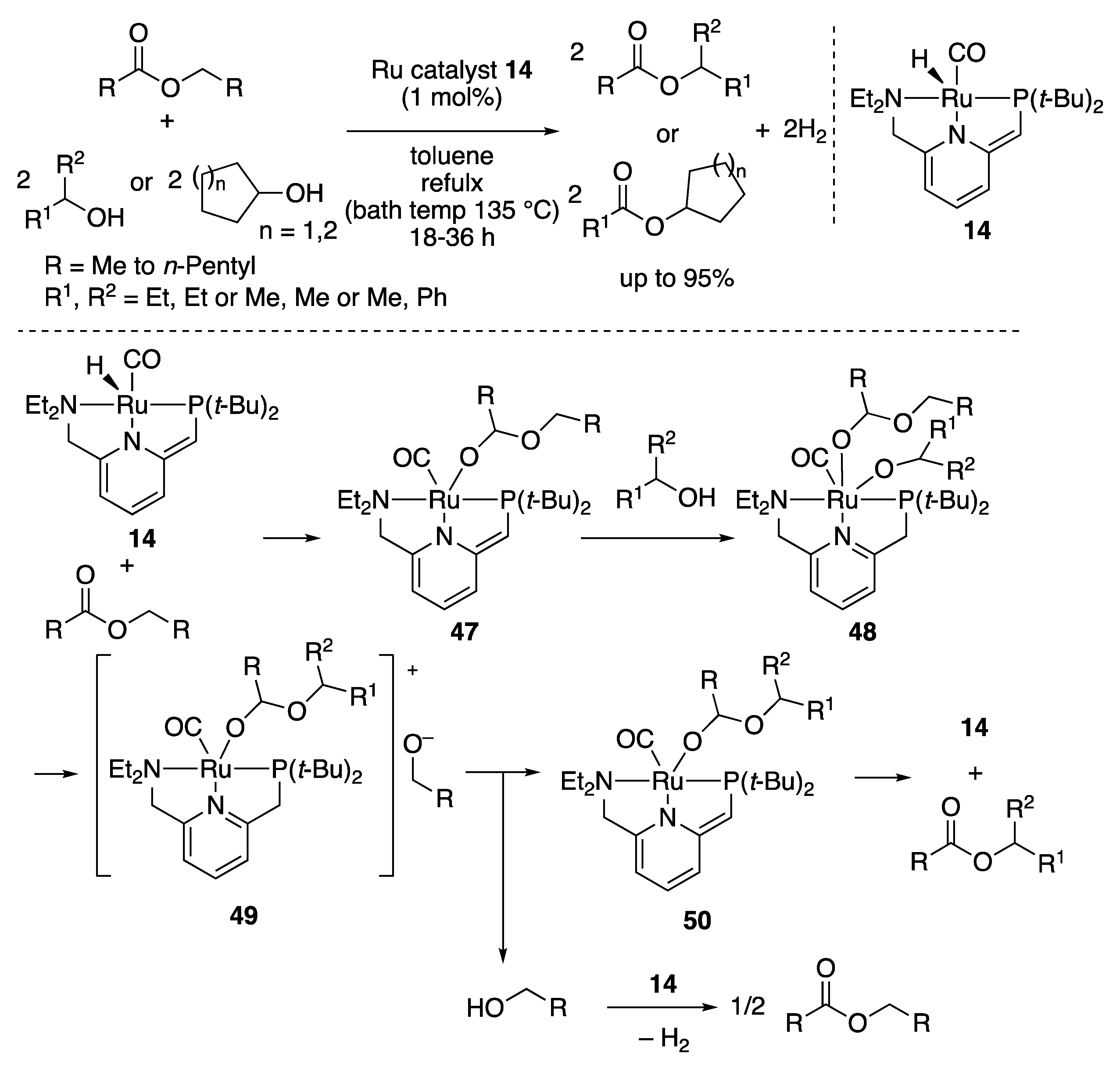
2.2.1. Ester Formation
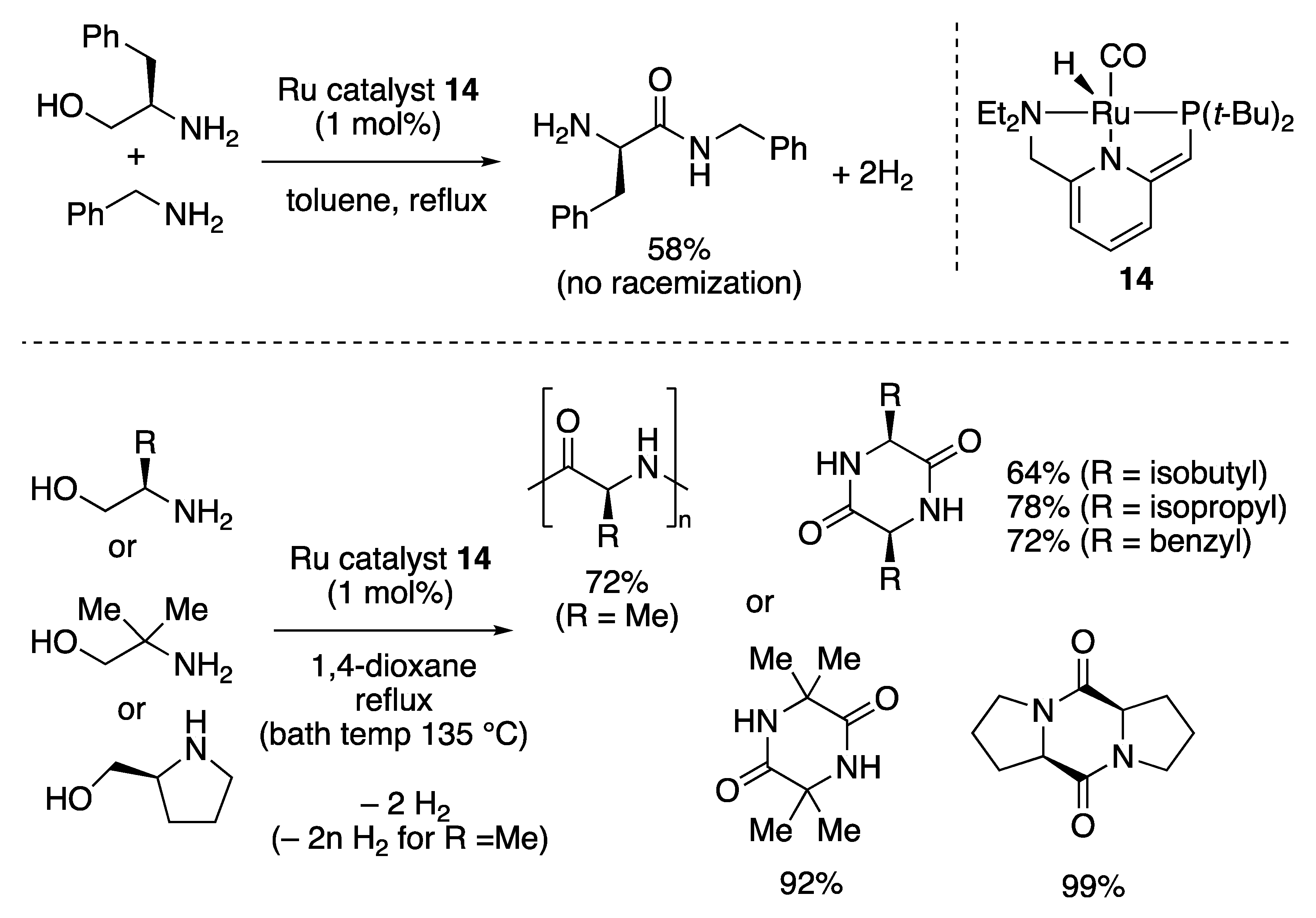
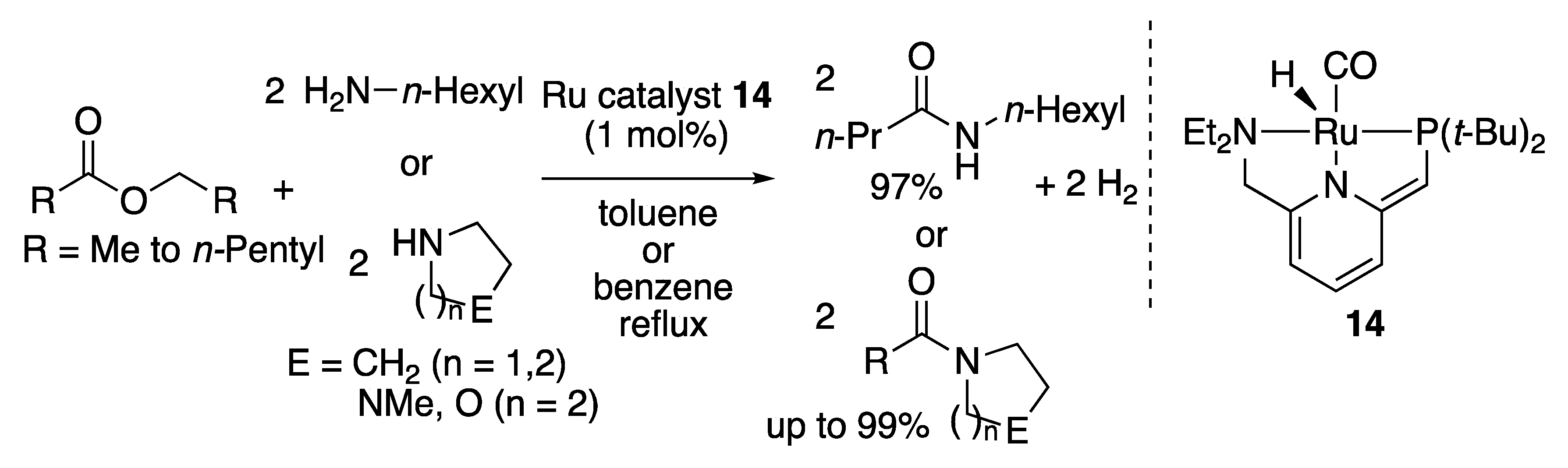
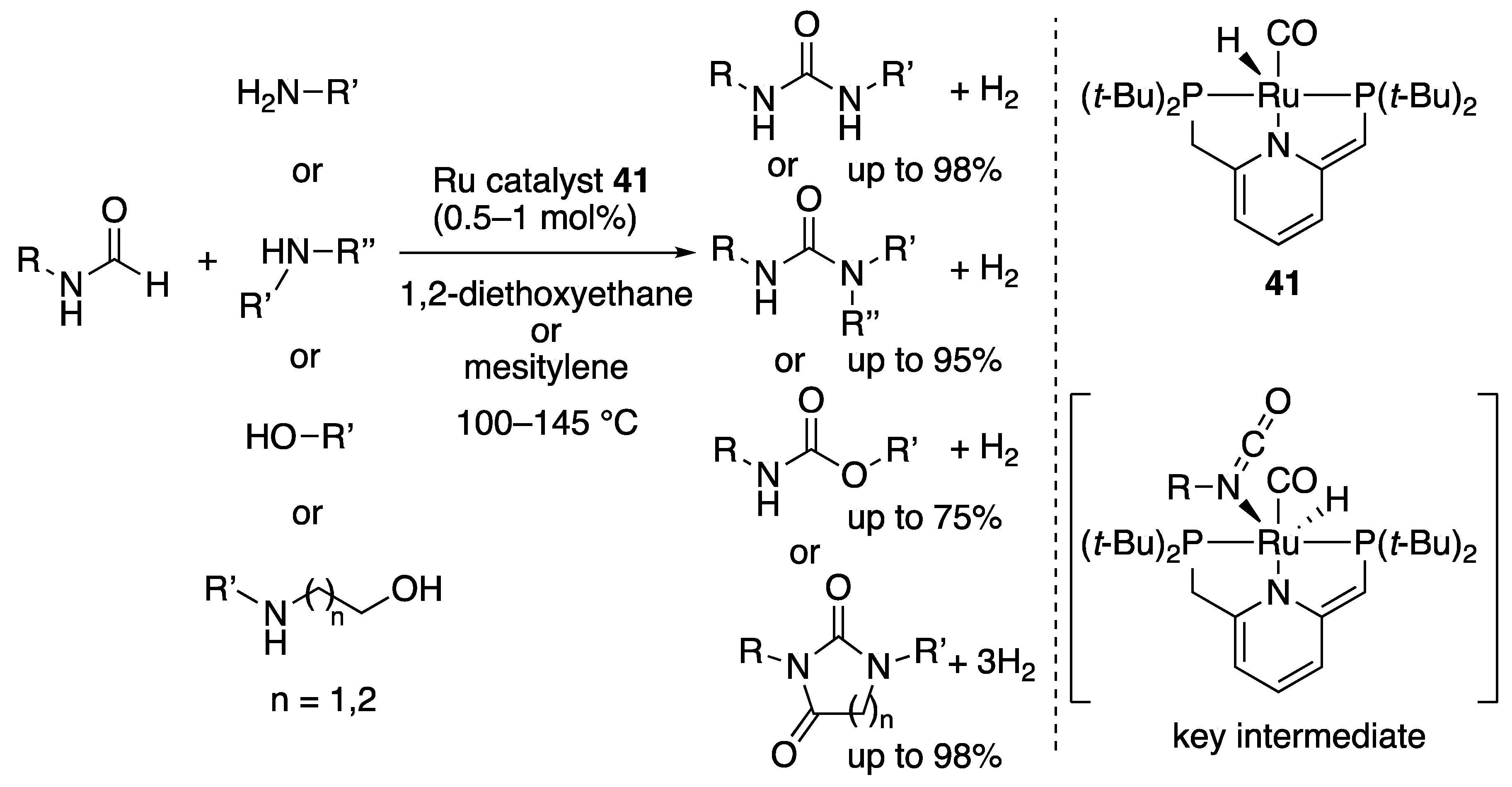
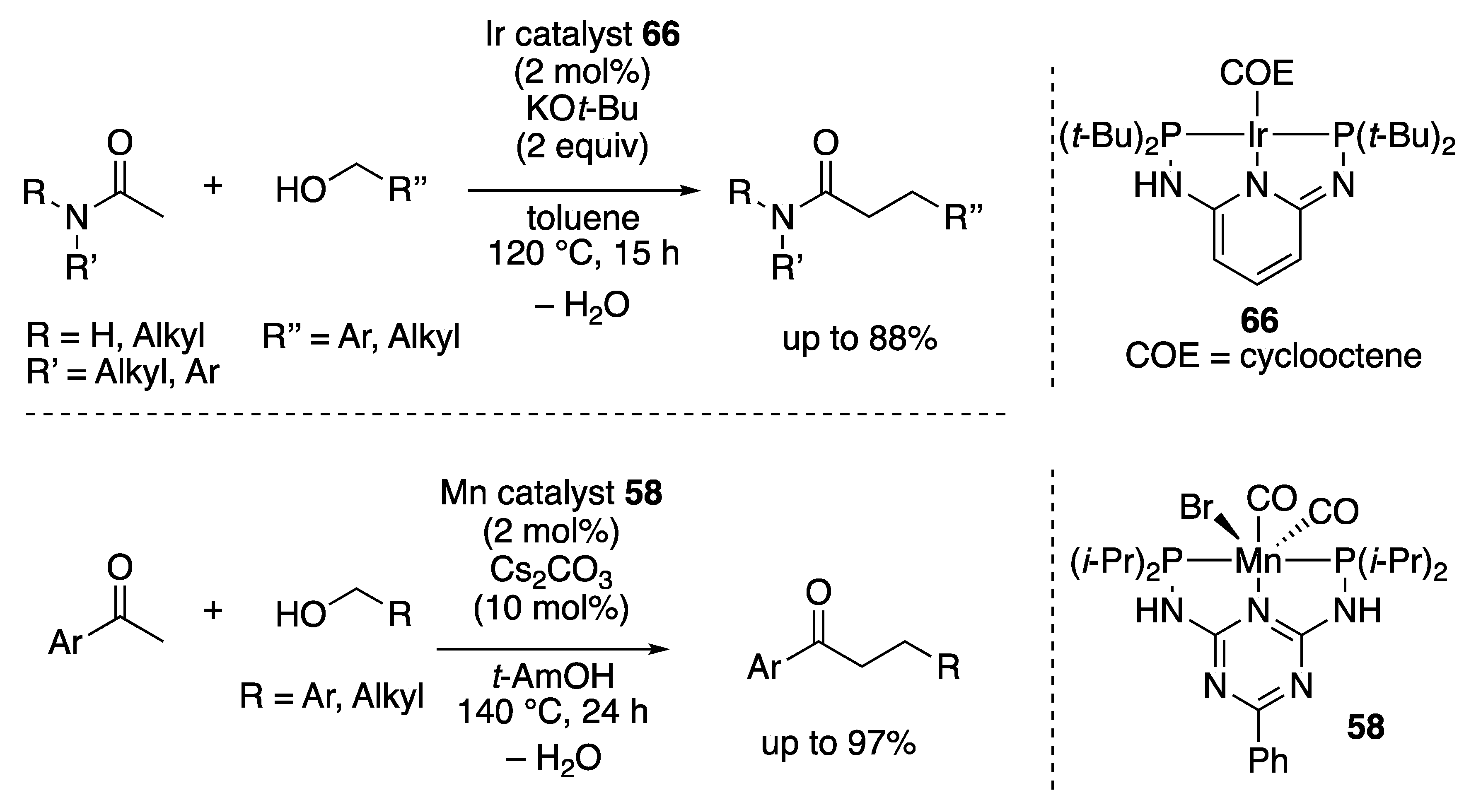
2.2.2. Amide Formation
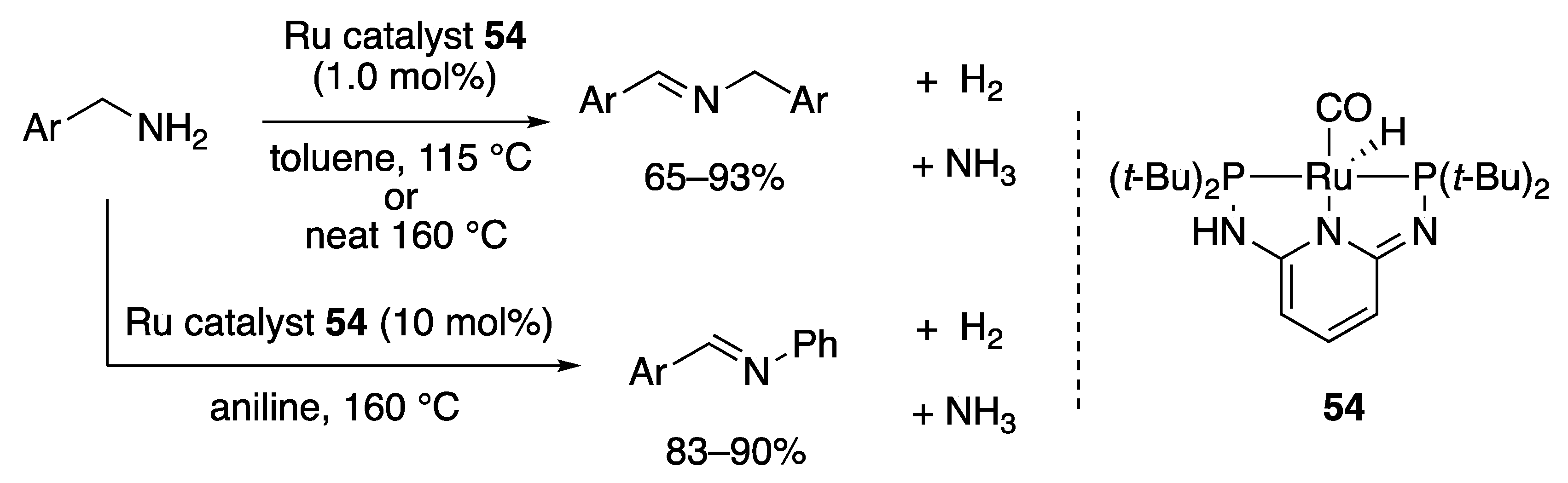
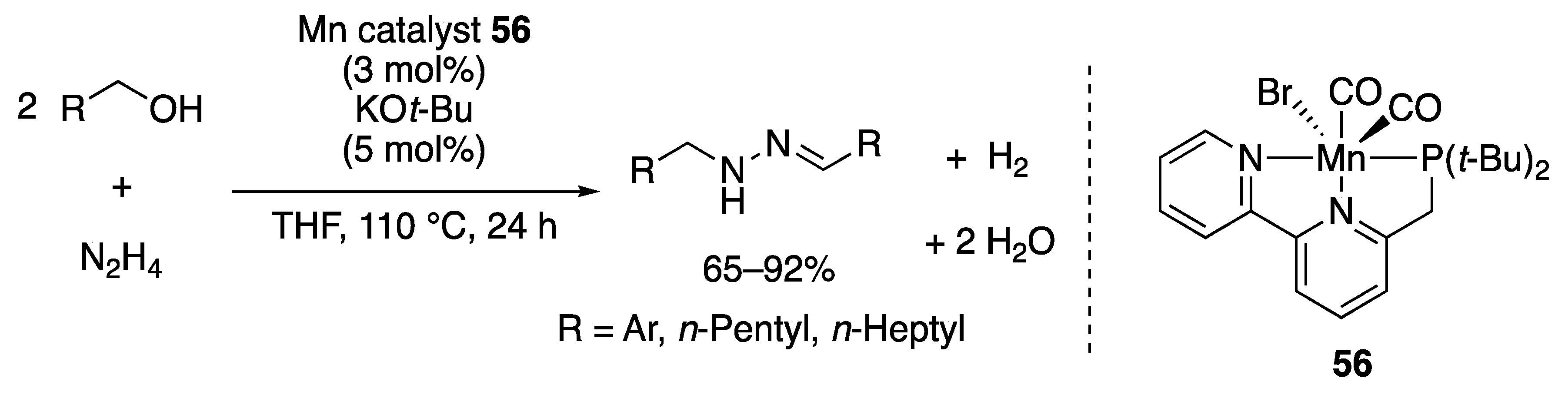
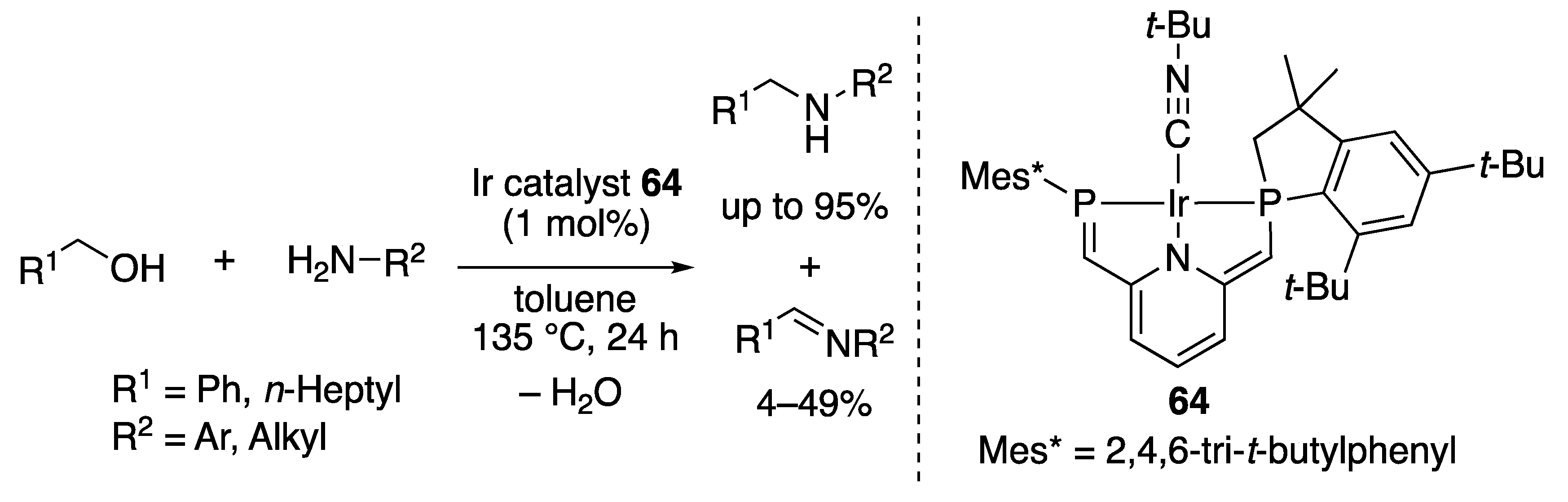
2.2.3. Imine Formation
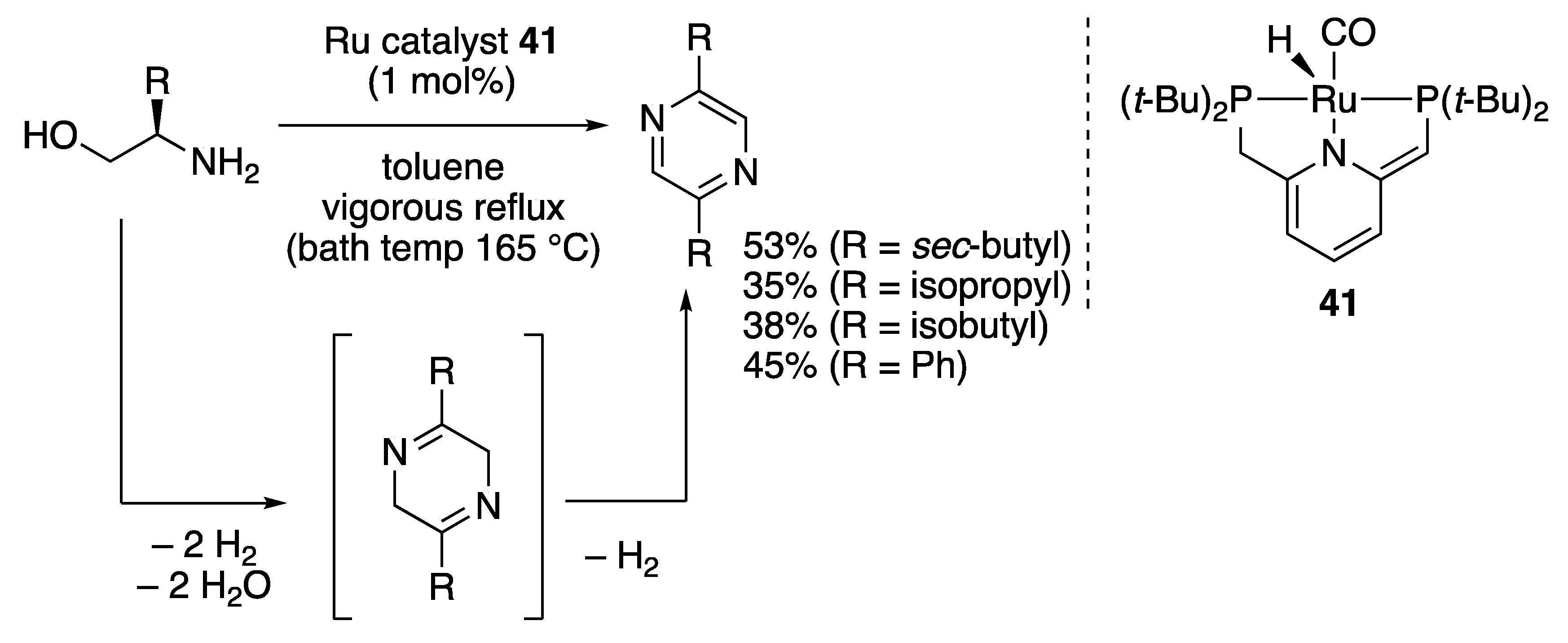
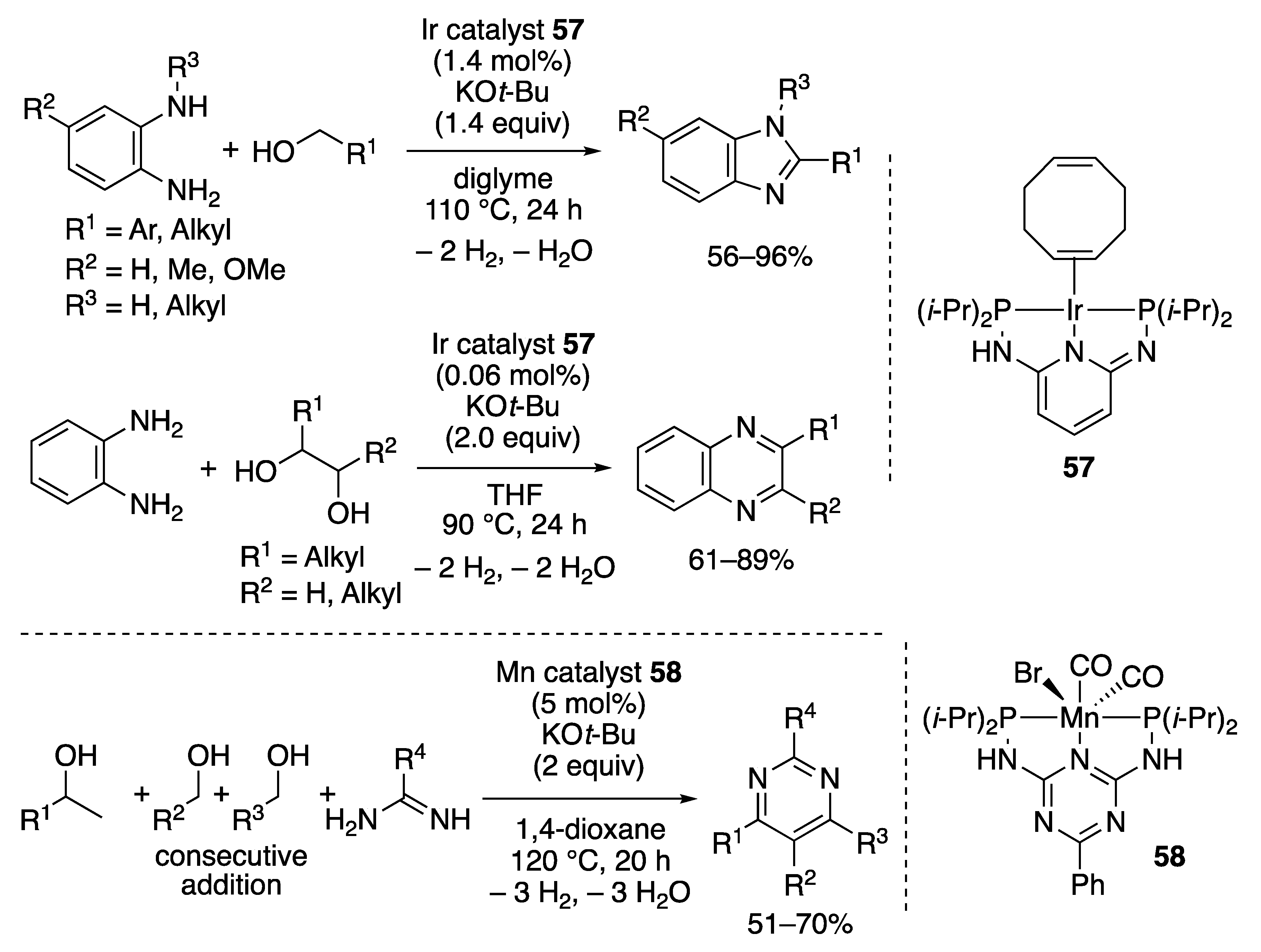
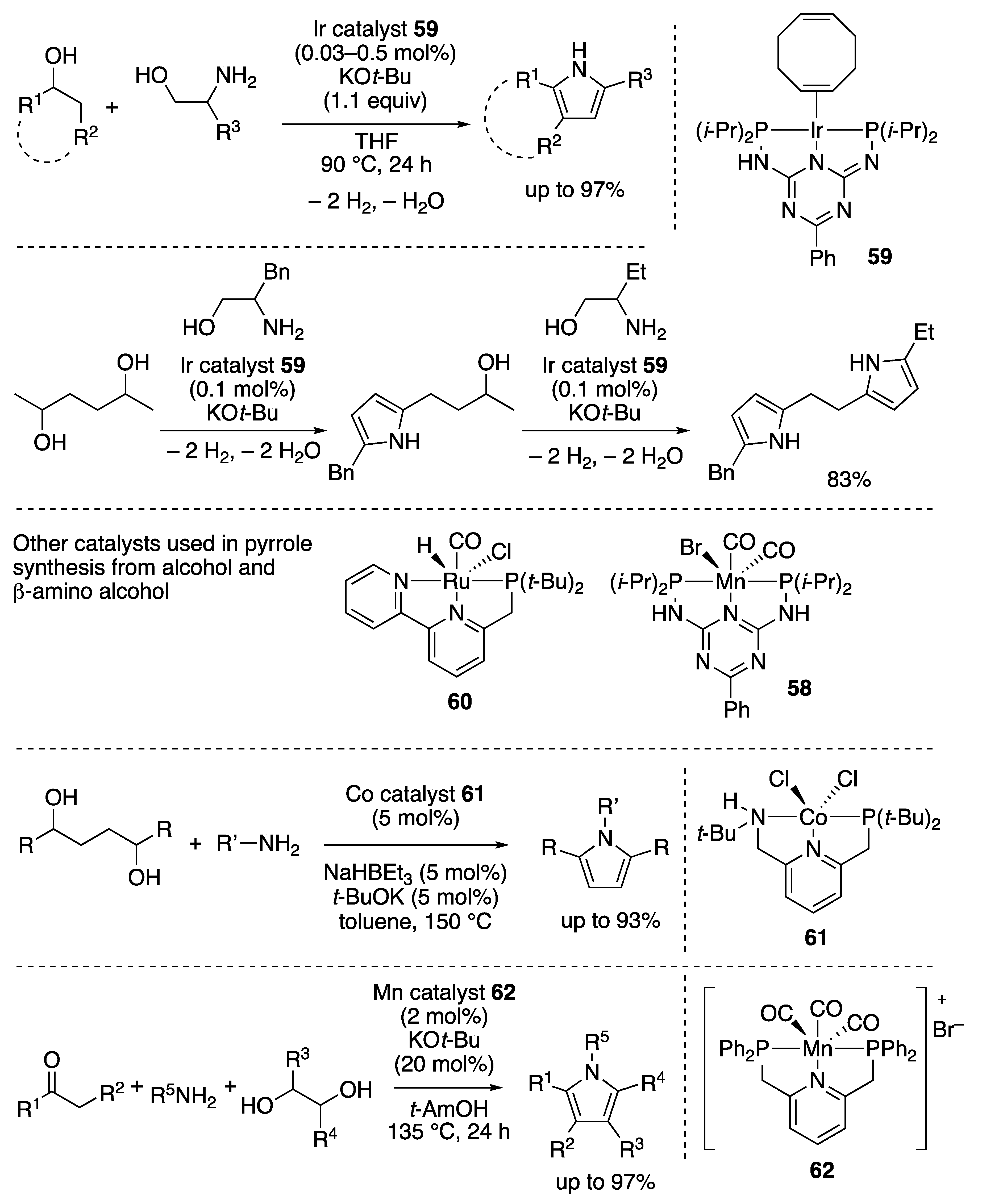
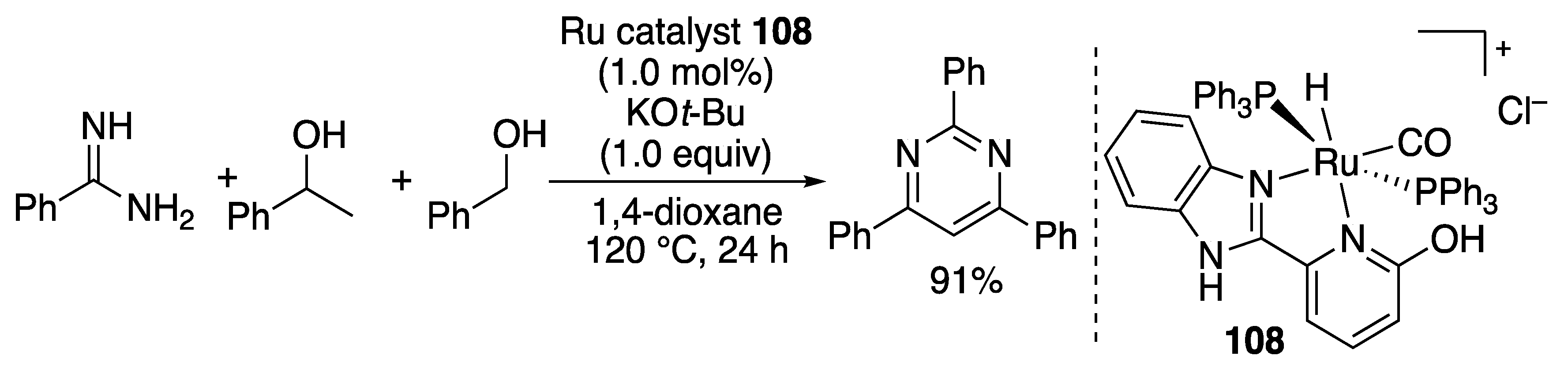
2.2.4. N-Heterocycle Formation
2.2.5. C–C Bond Formation Involving Alcohol Dehydrogenation
2.2.6. Other Dehydrogenative Transformations
2.3. Transfer Hydrogenation and Borrowing Hydrogen Reactions
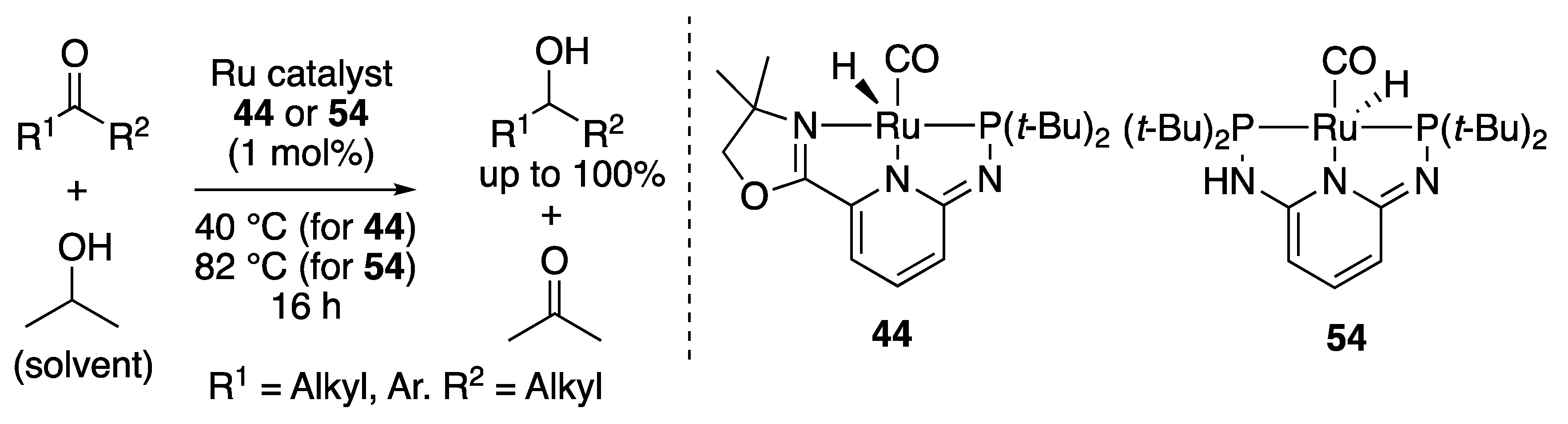
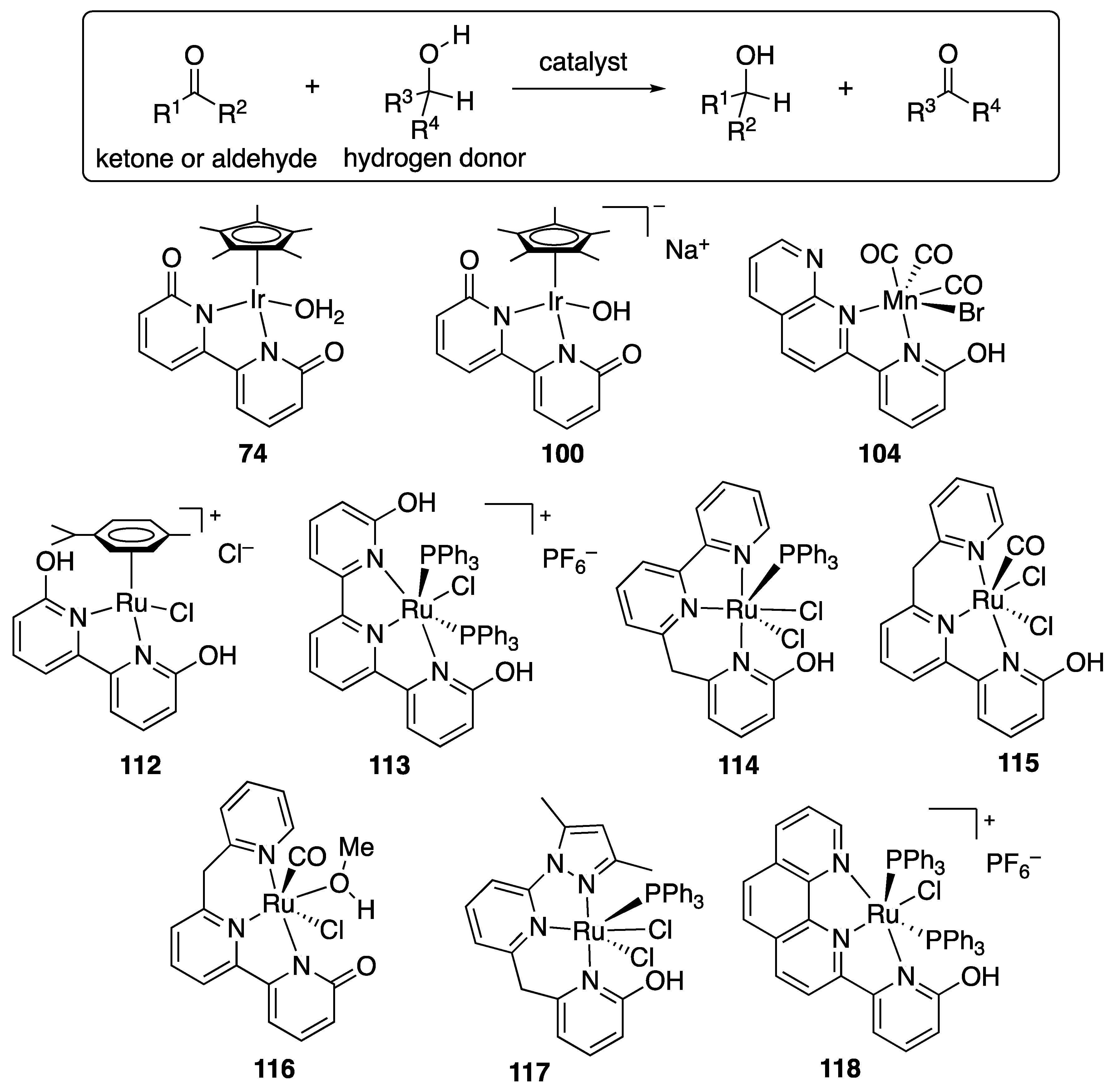
2.3.1. Transfer Hydrogenation of Ketones
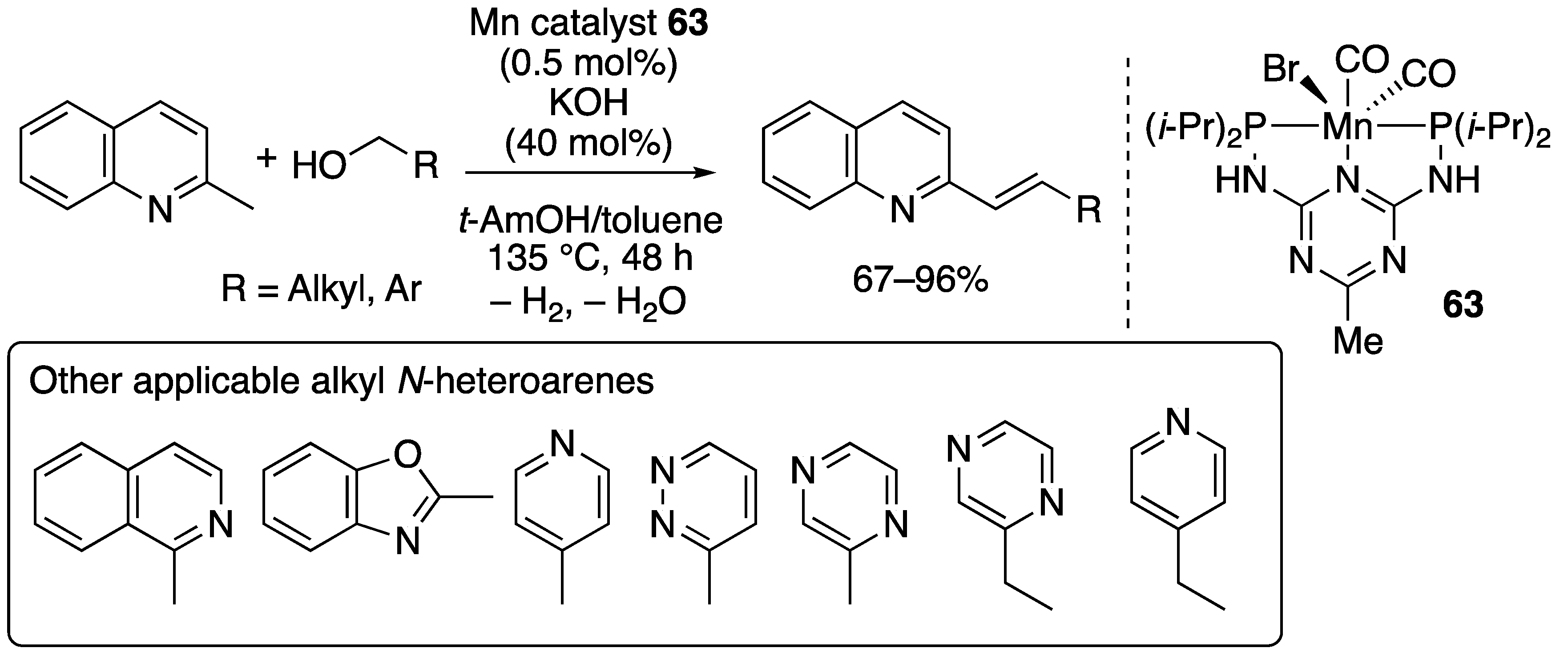
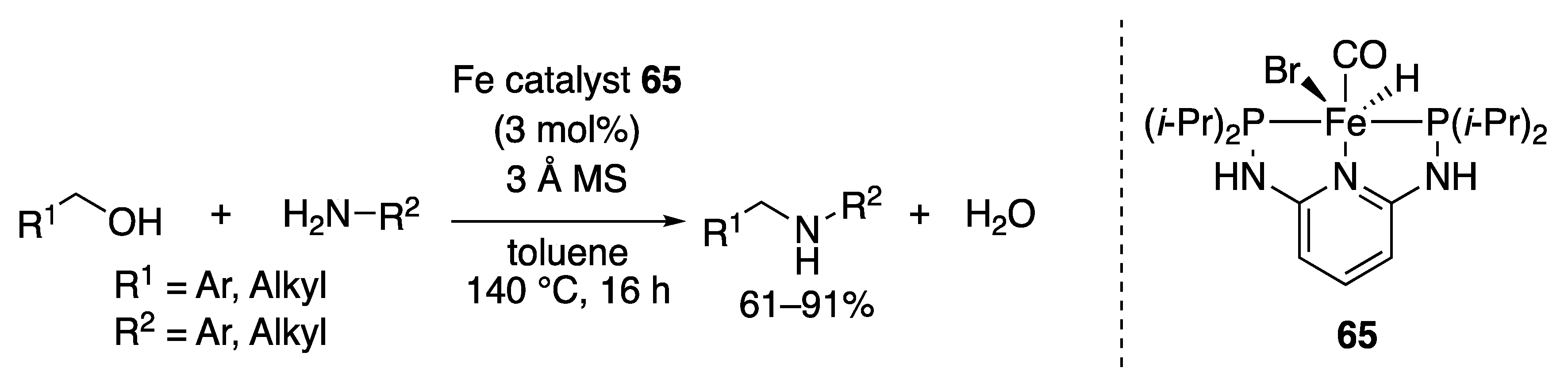
2.3.2. N-Alkylation of Amines by the Borrowing Hydrogen Mechanism
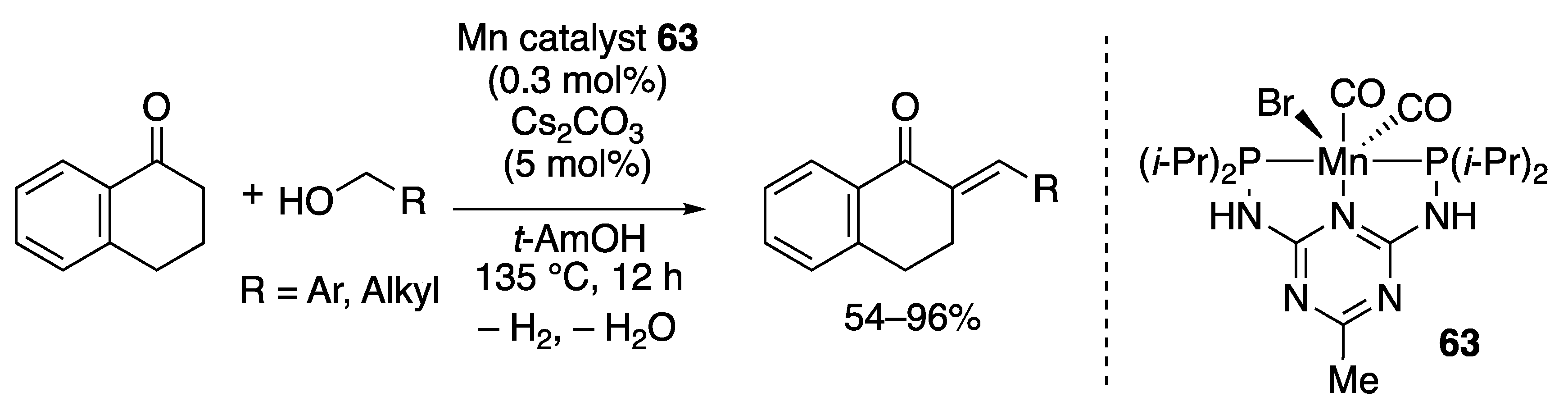
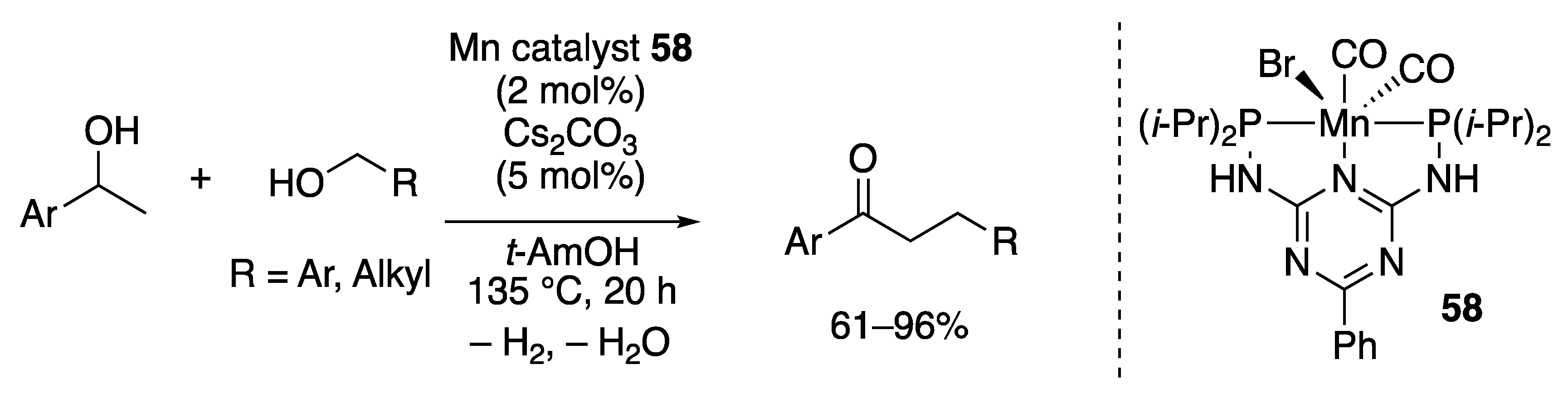
2.3.3. C–C Bond-forming Alkylation by the Borrowing Hydrogen Mechanism
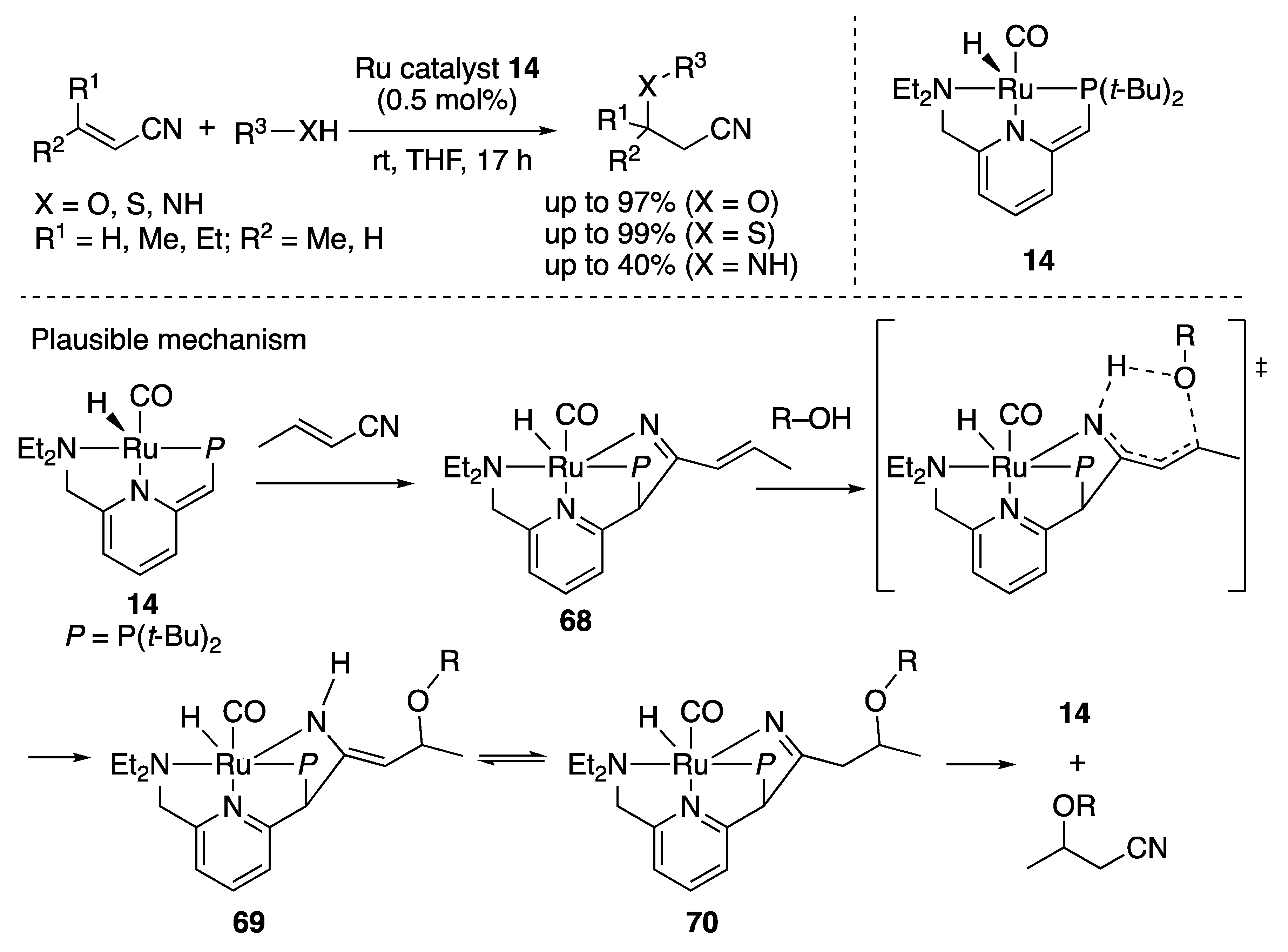
2.4. Nitrile Activation on the Ligand Skeleton
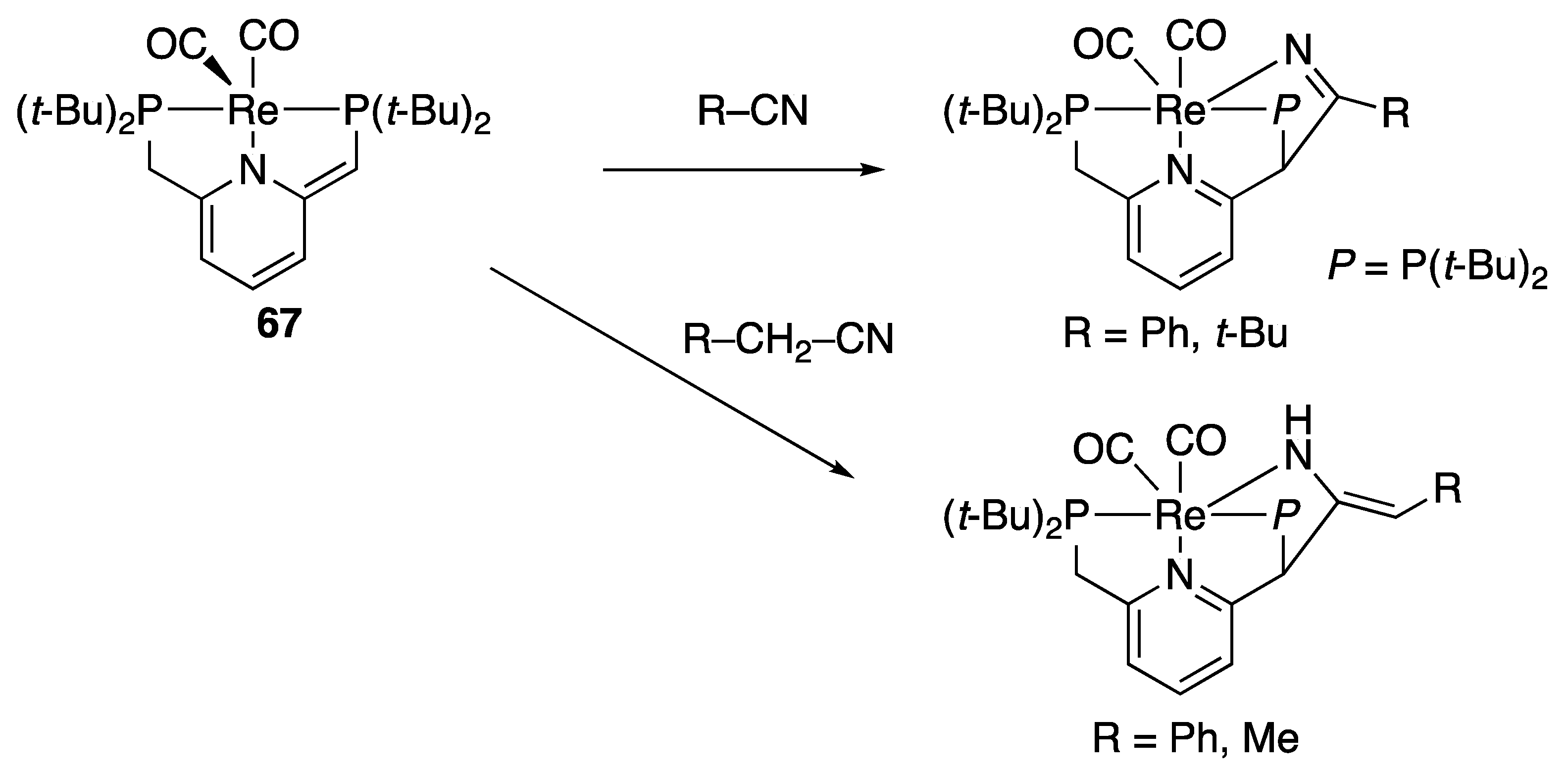
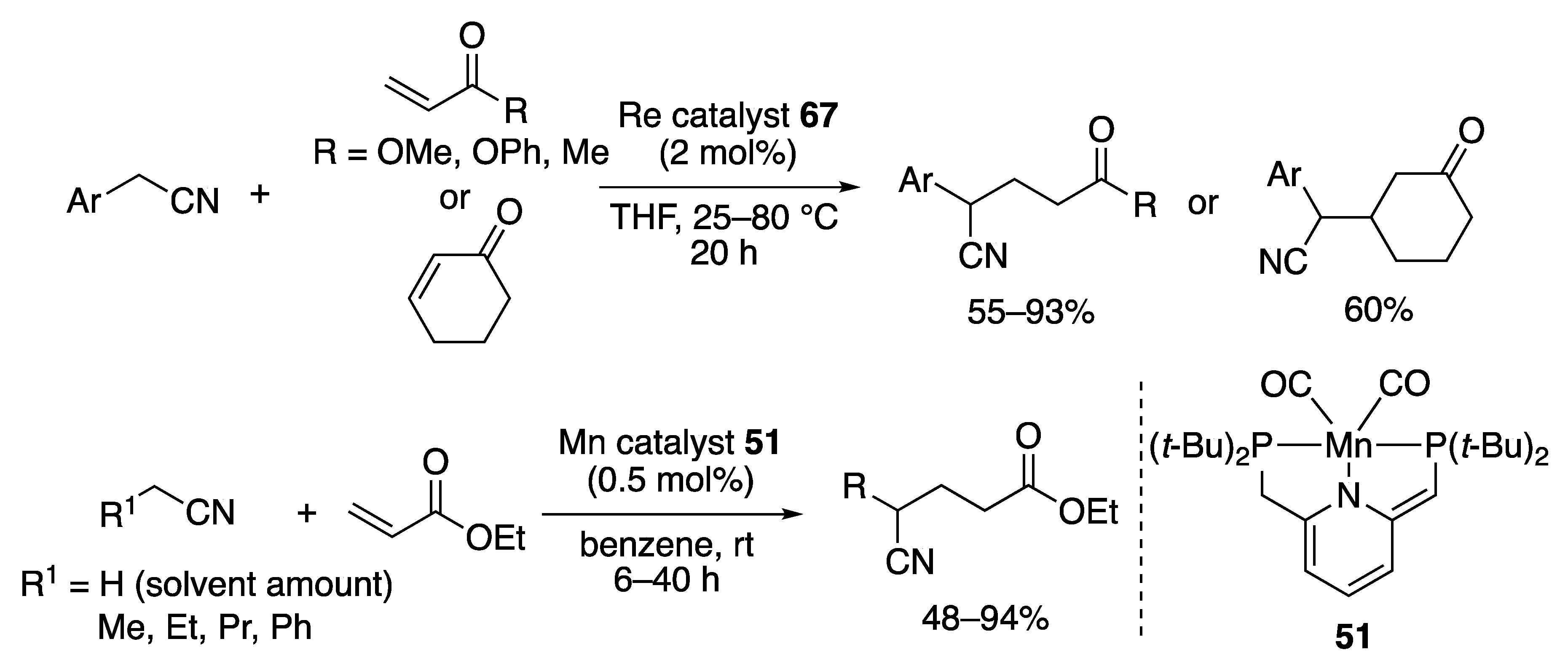
2.4.1. Michael Additions of Aliphatic Nitriles
2.4.2. Michael Addition to α,β-Unsaturated Nitriles
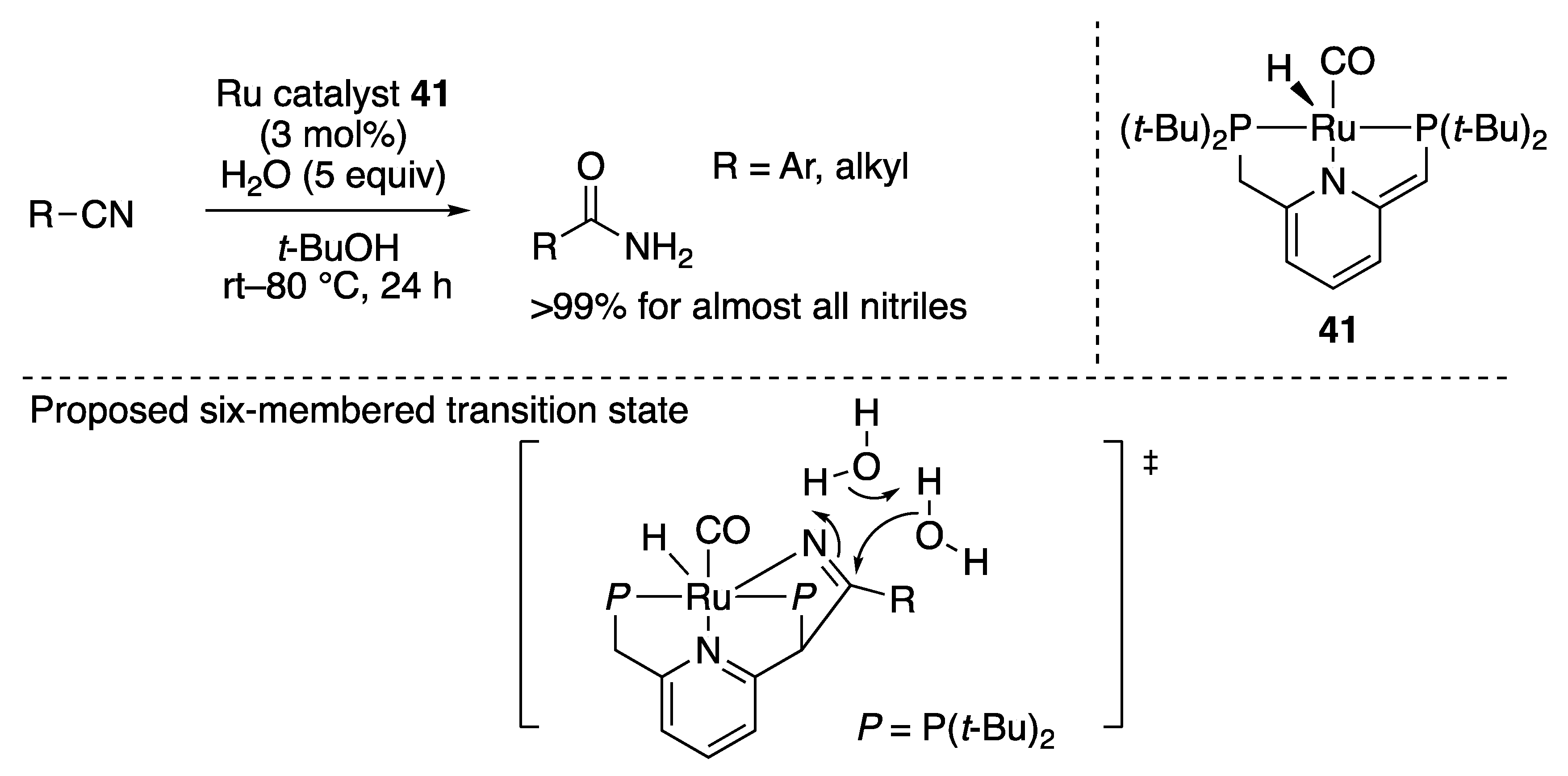
2.4.3. Nitrile Hydration
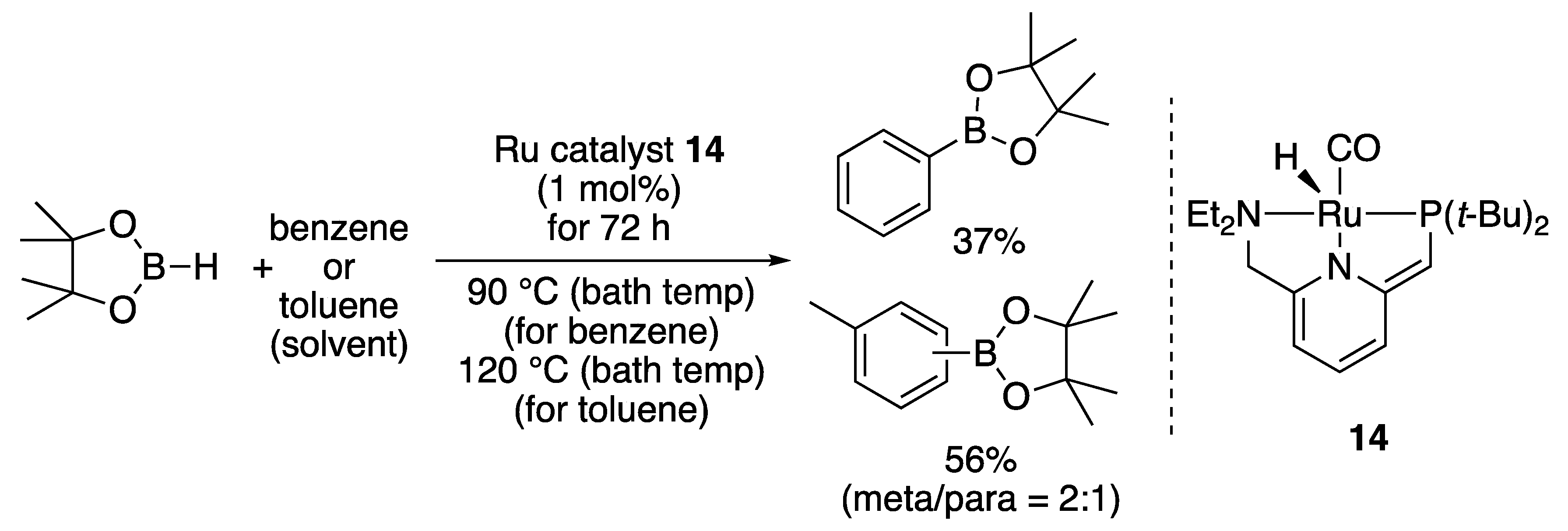
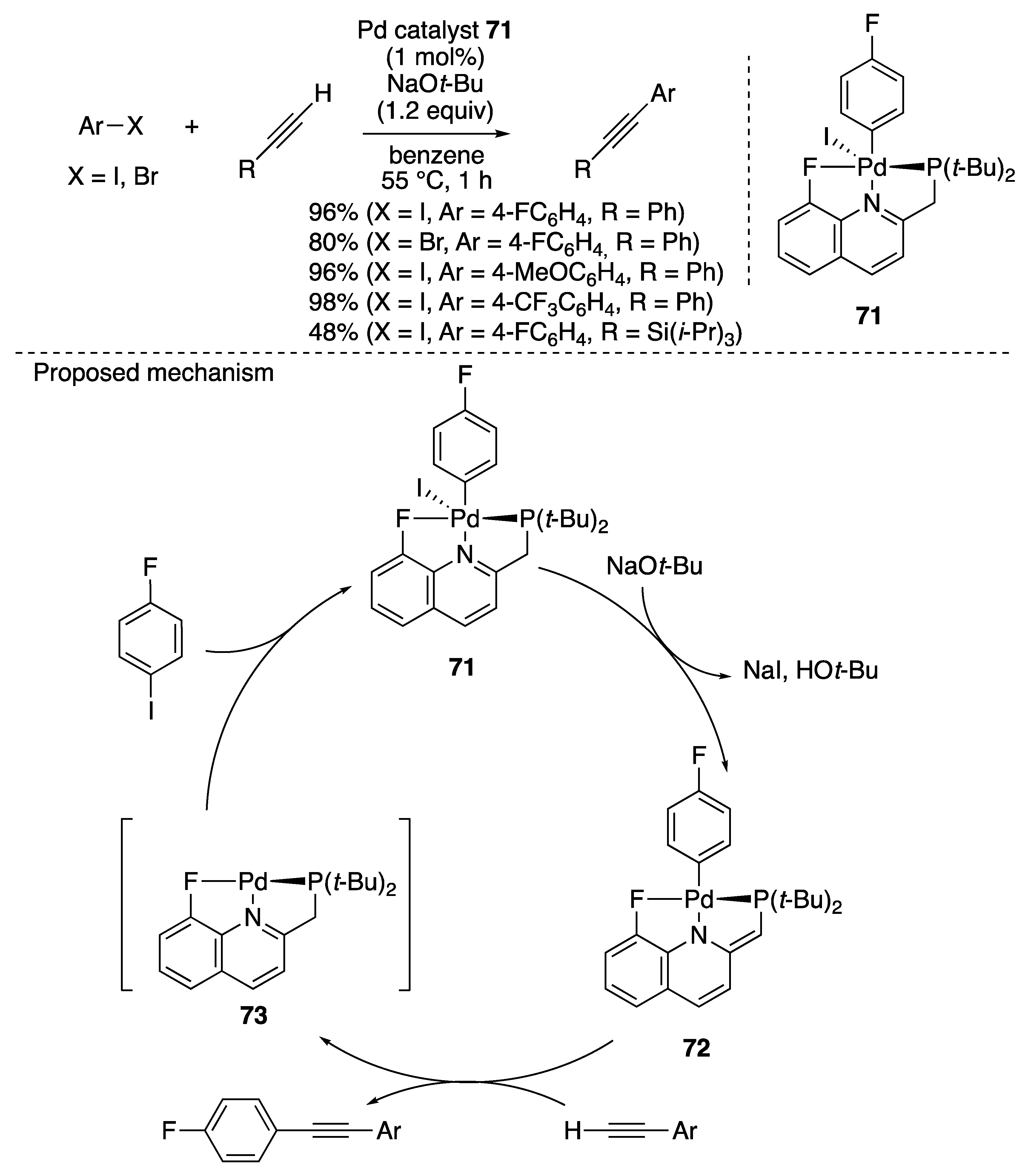
2.5. Miscellaneous
3. Hydroxypyridine-Based Ligands
3.1. Hydrogenation
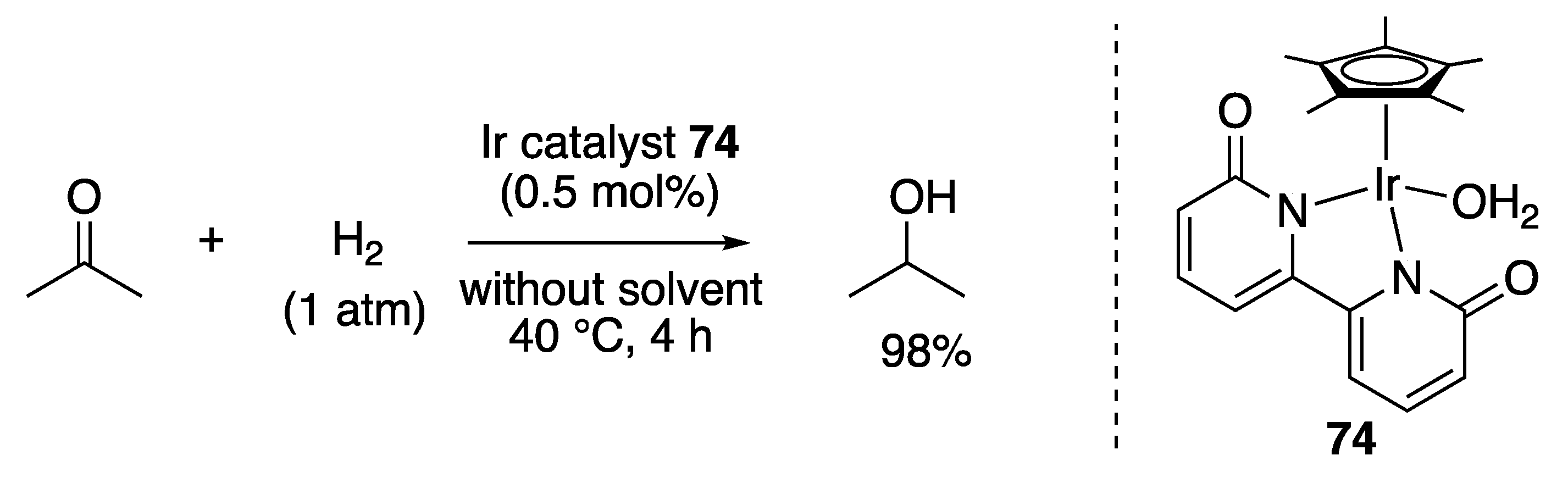
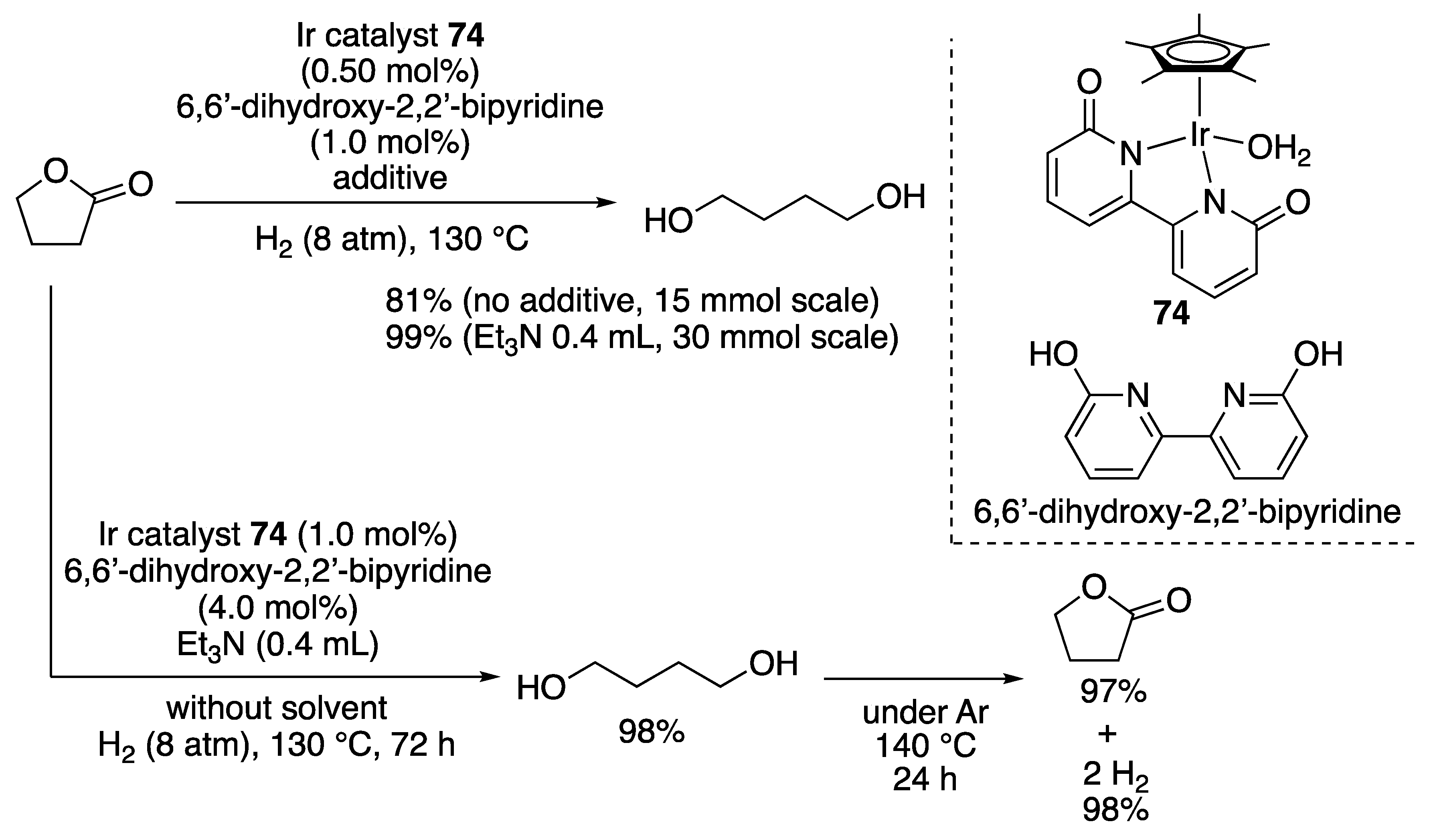
3.1.1. Ketone and Ester Hydrogenation
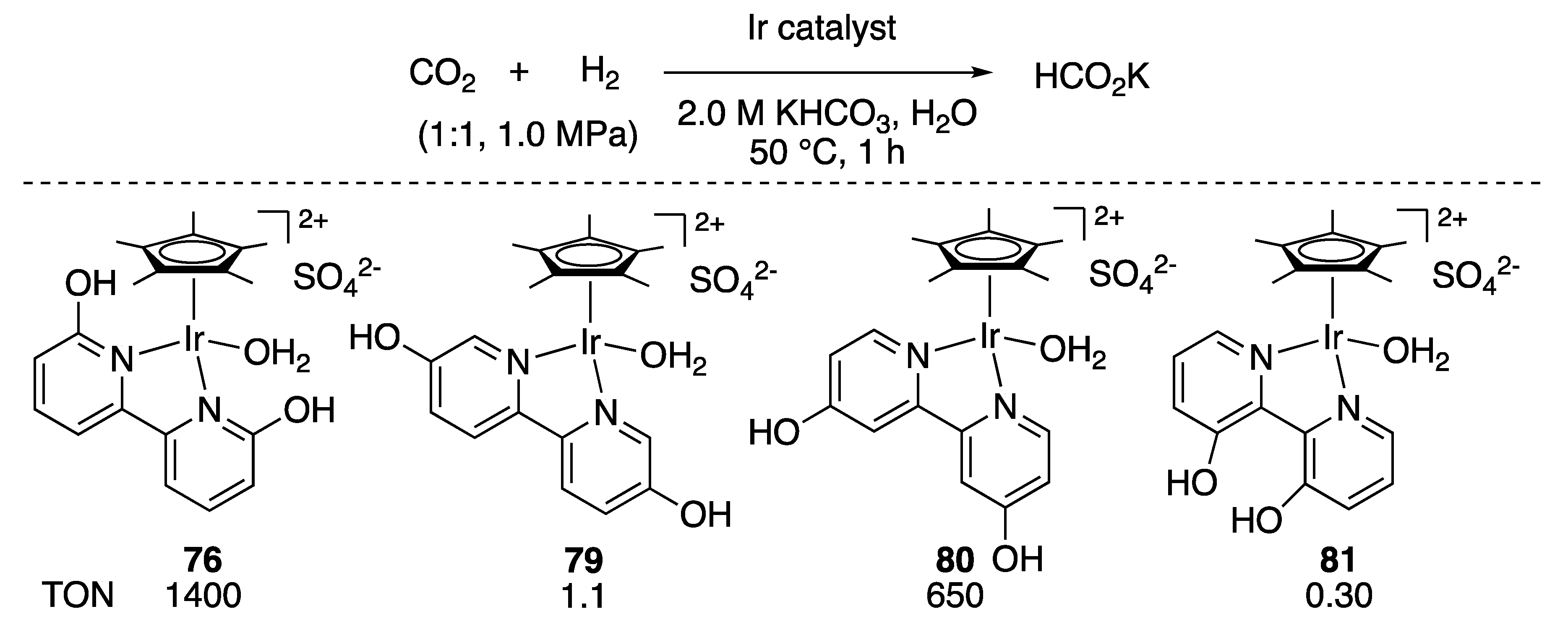
3.1.2. CO2 Hydrogenation
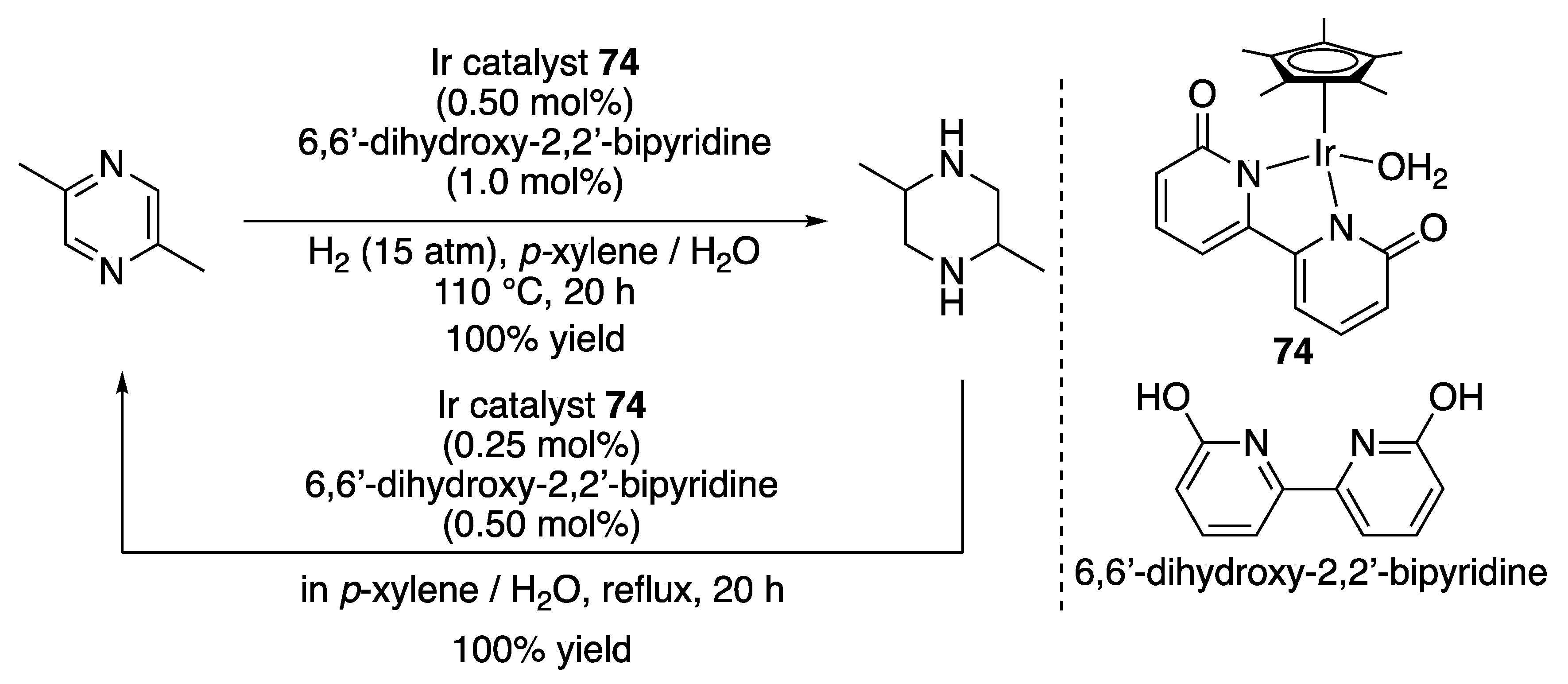
3.1.3. N-Heterocycle Hydrogenation
3.2. Dehydrogenation
3.2.1. Alcohol Dehydrogenative Oxidation
Ketone and Aldehyde Formation
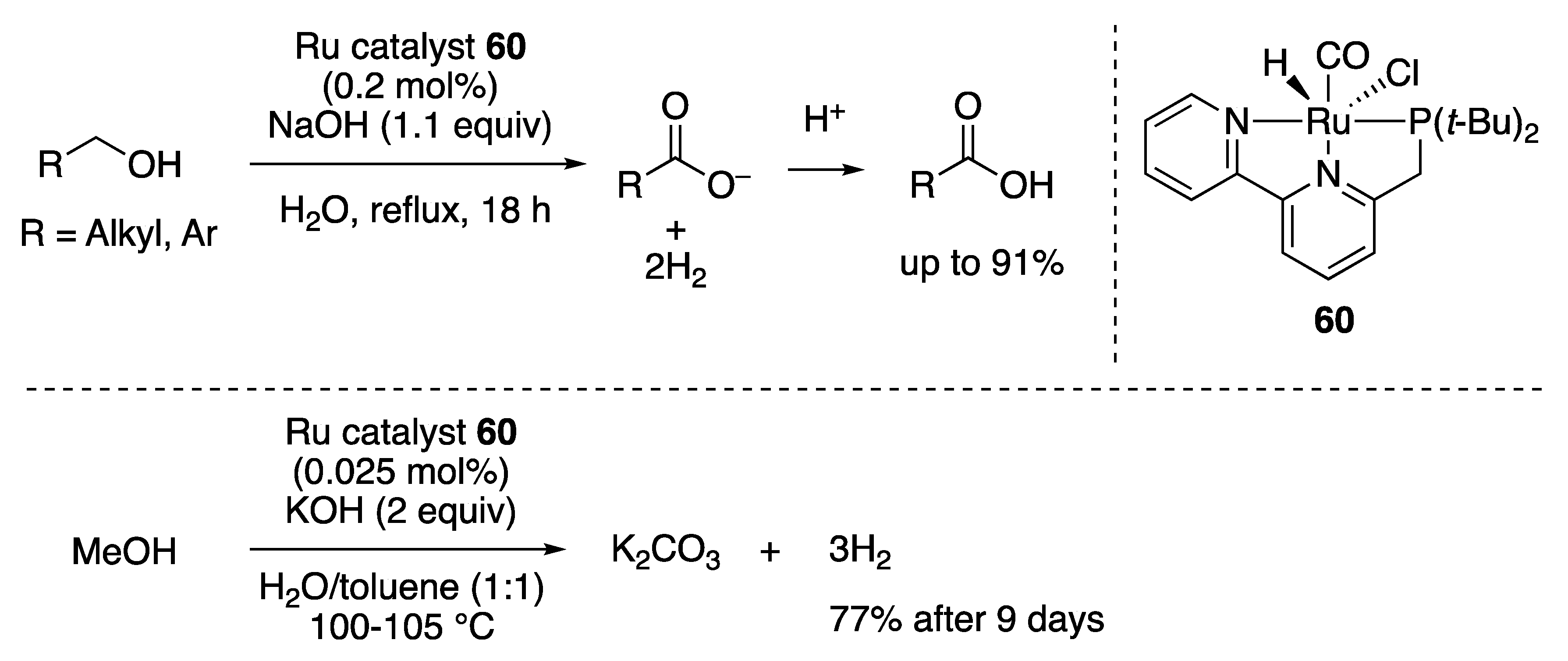
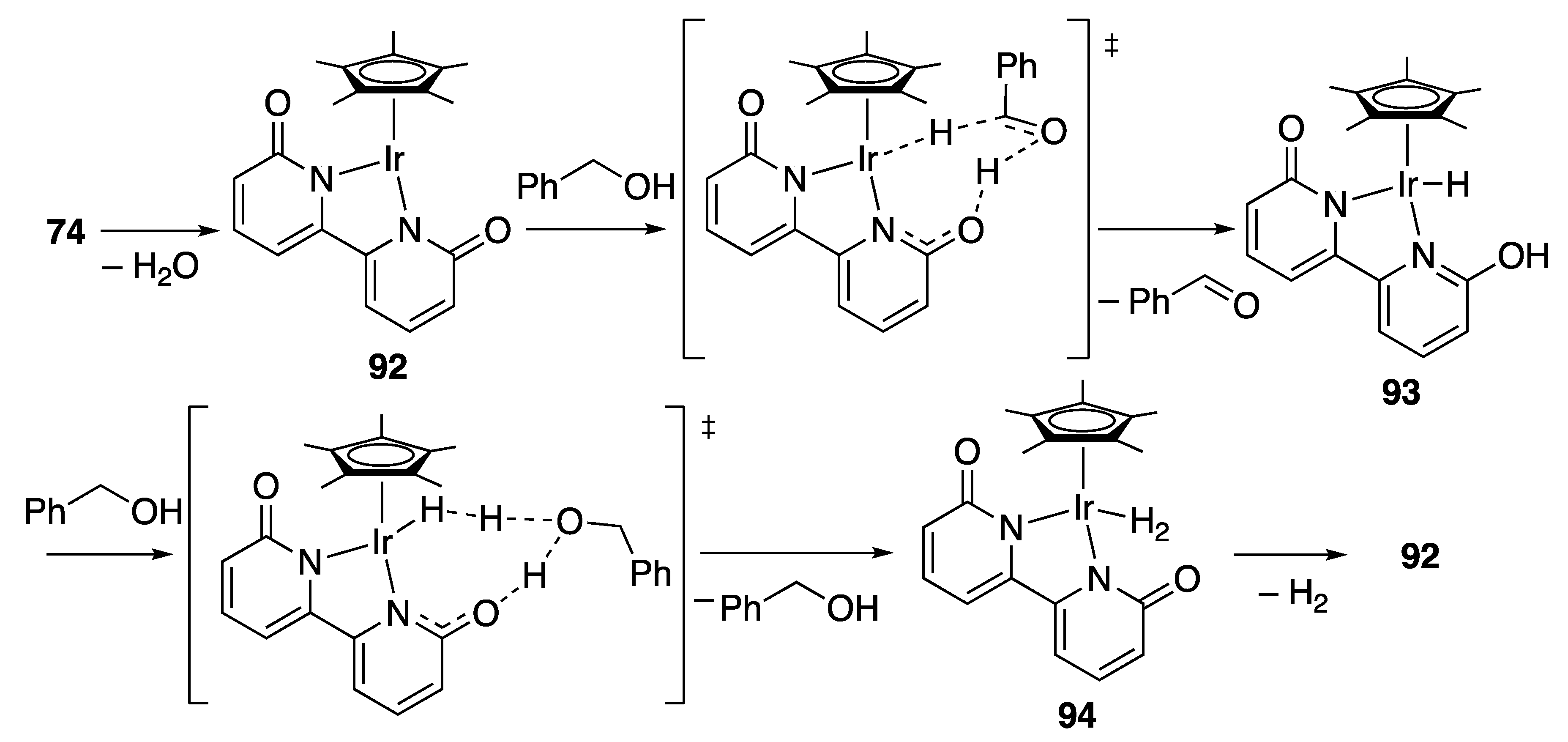
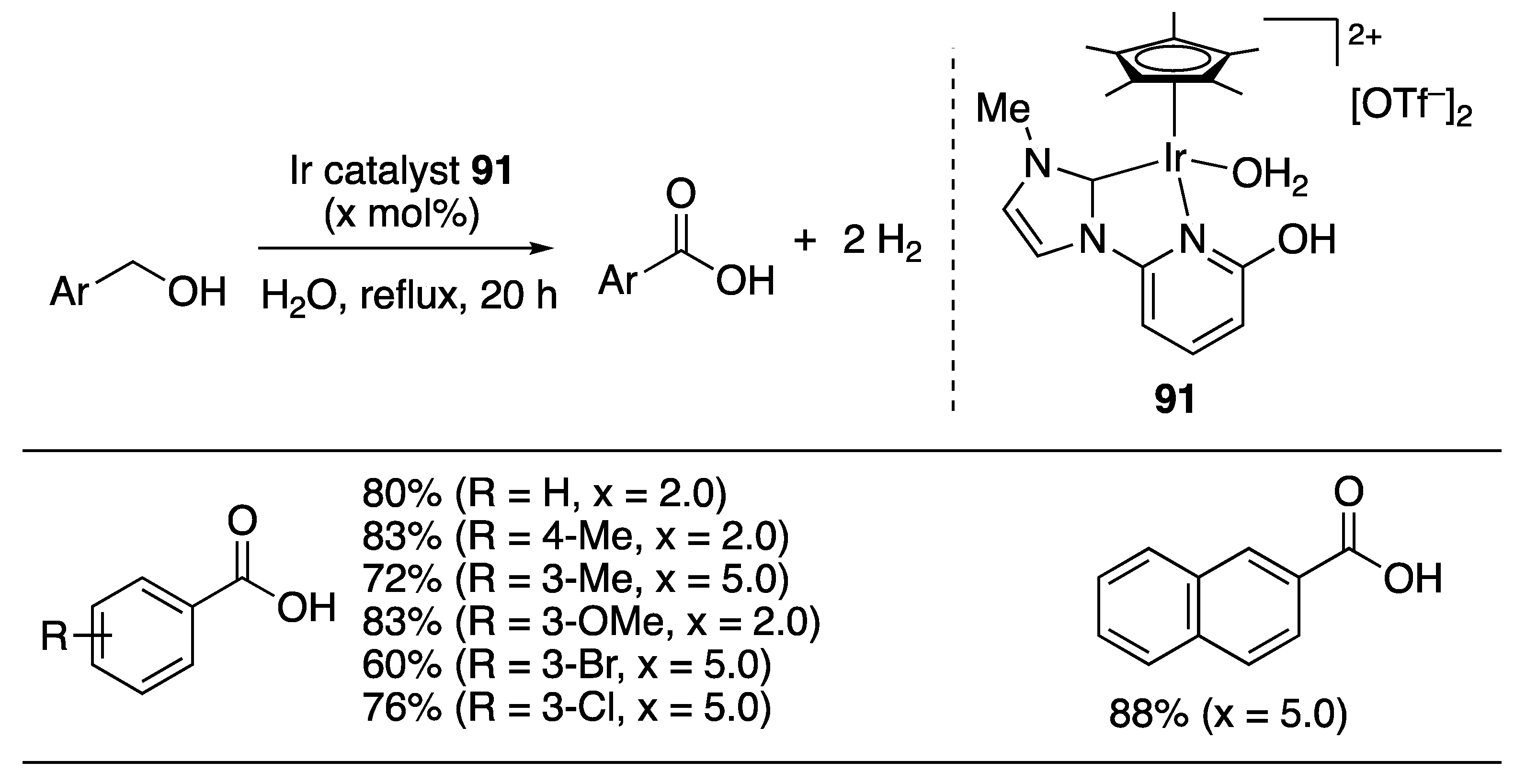
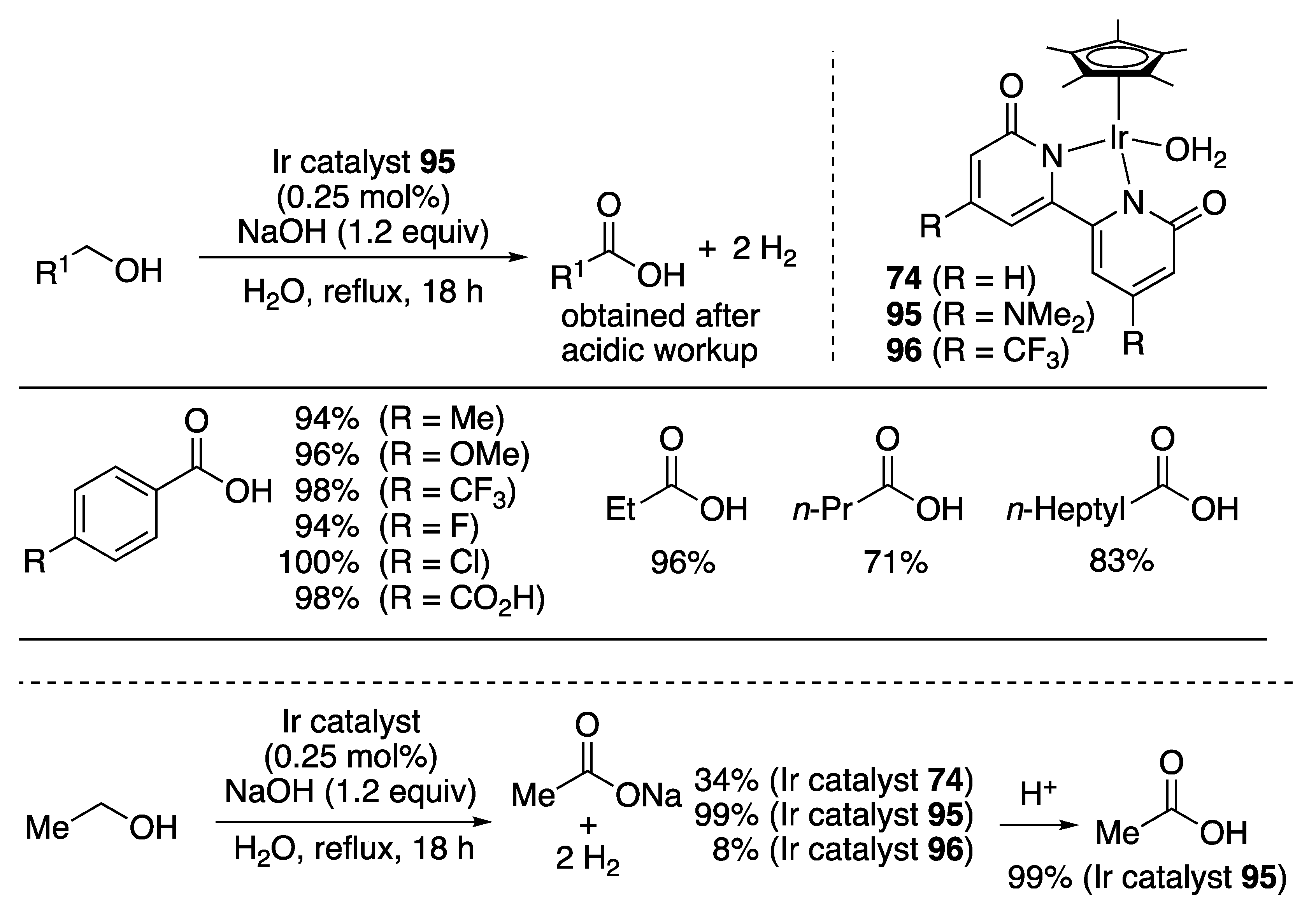
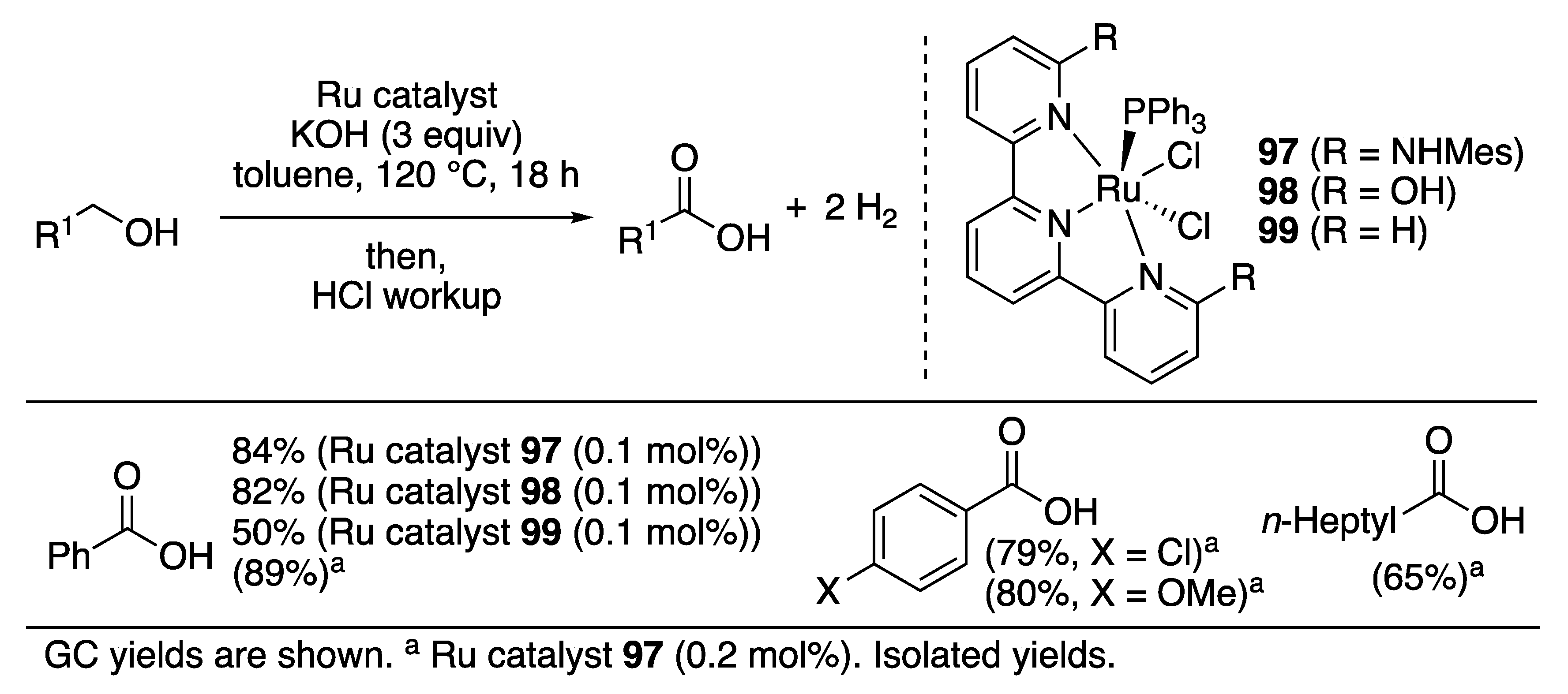
Carboxylic Acid Formation
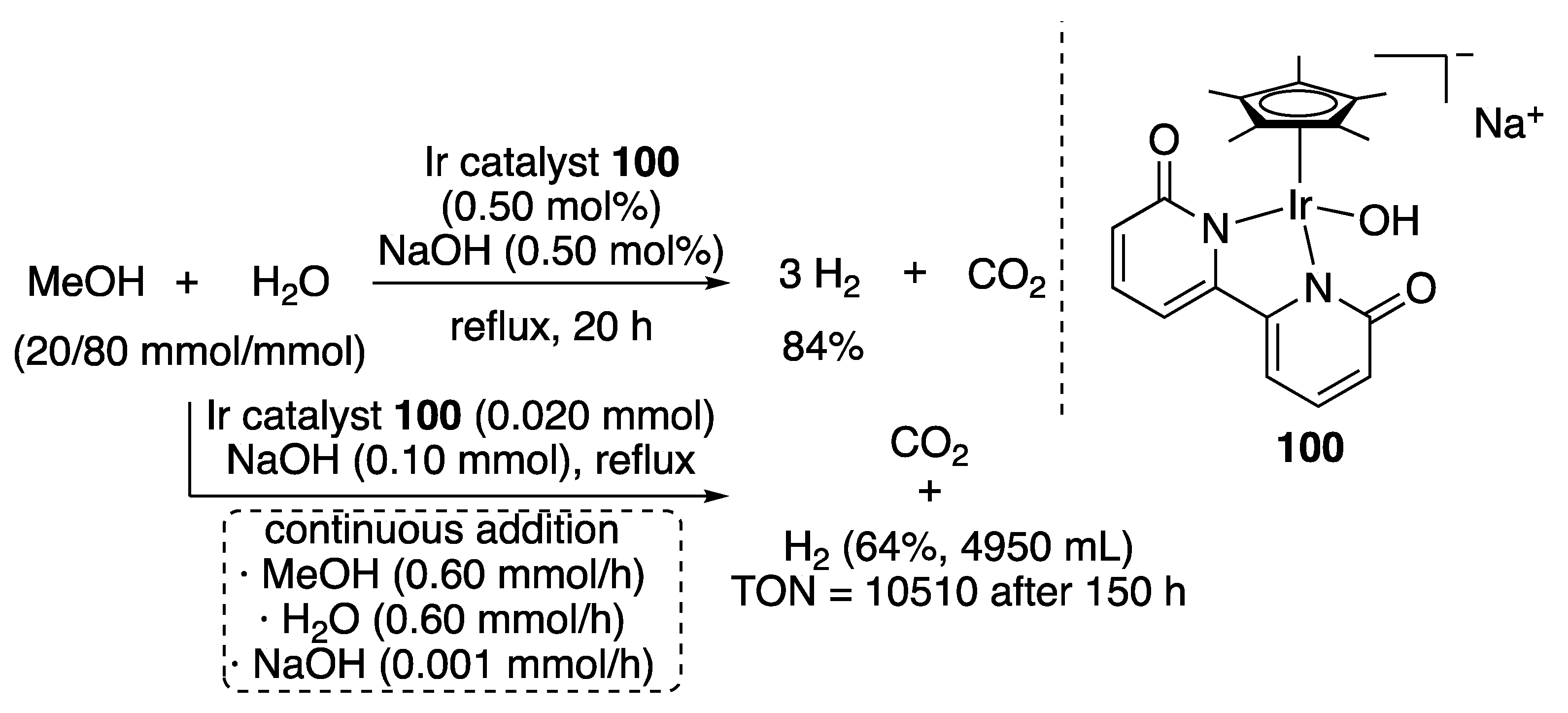
CO2 Formation from Aqueous Methanol
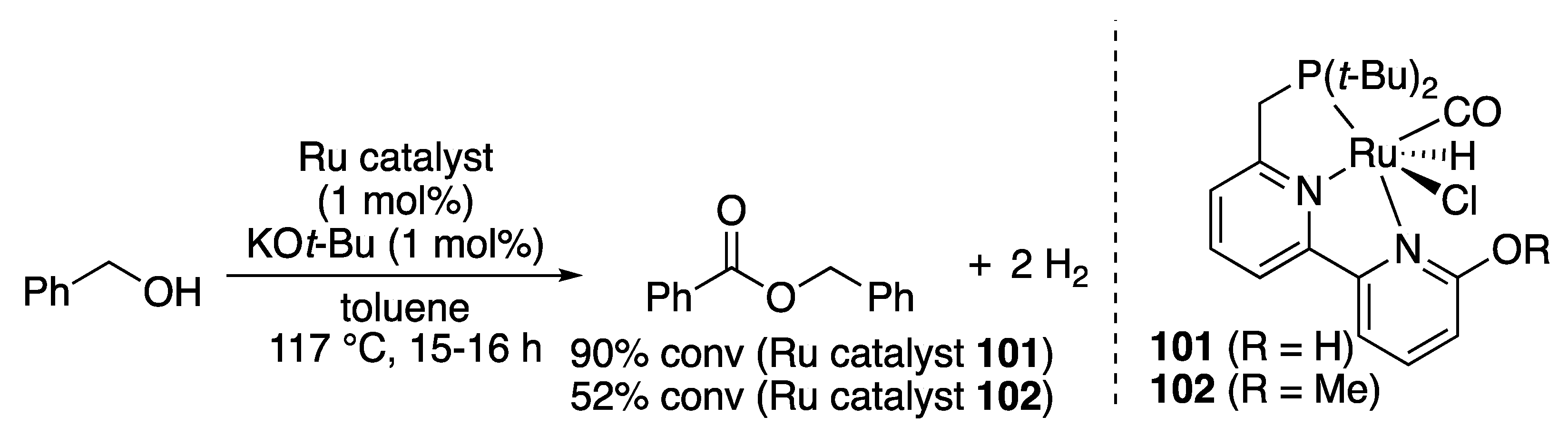
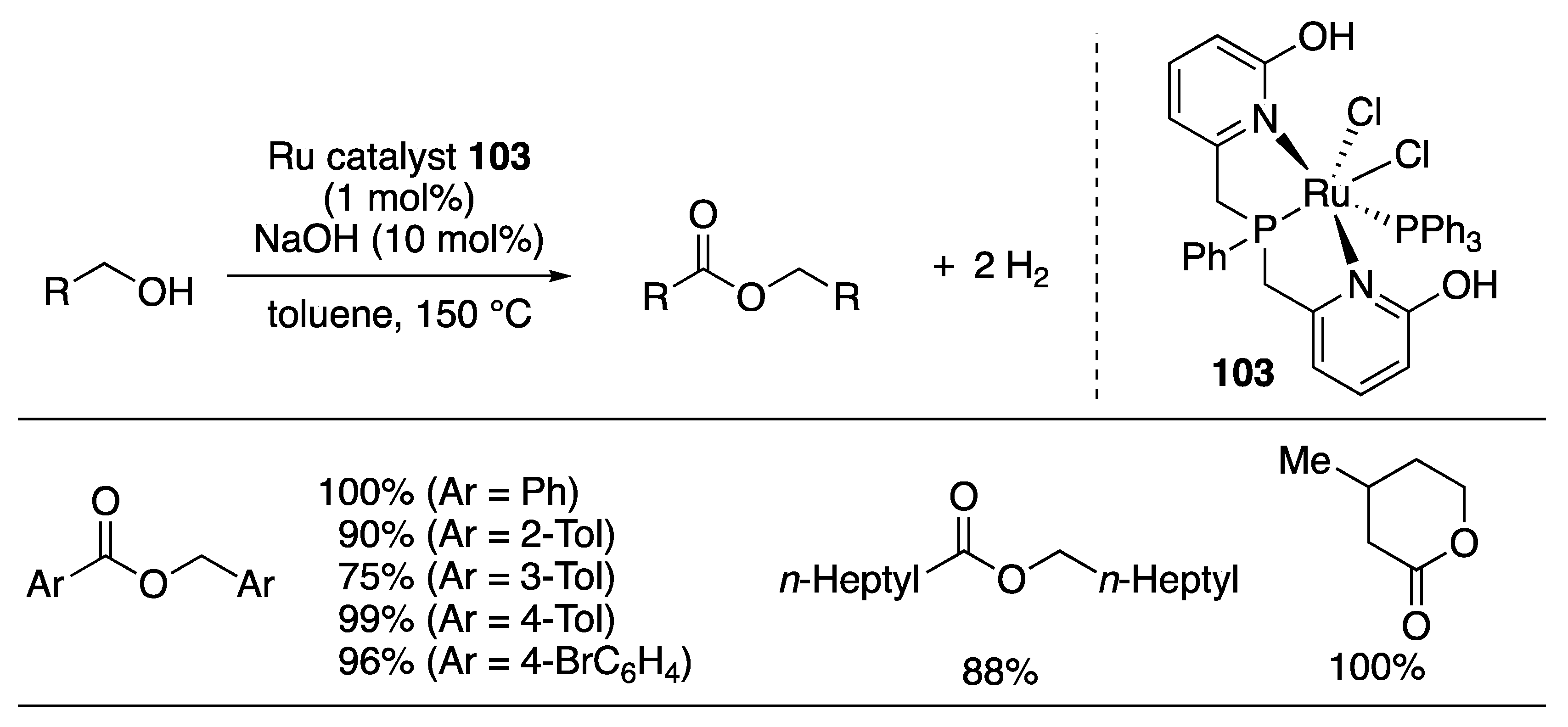
Ester Formation
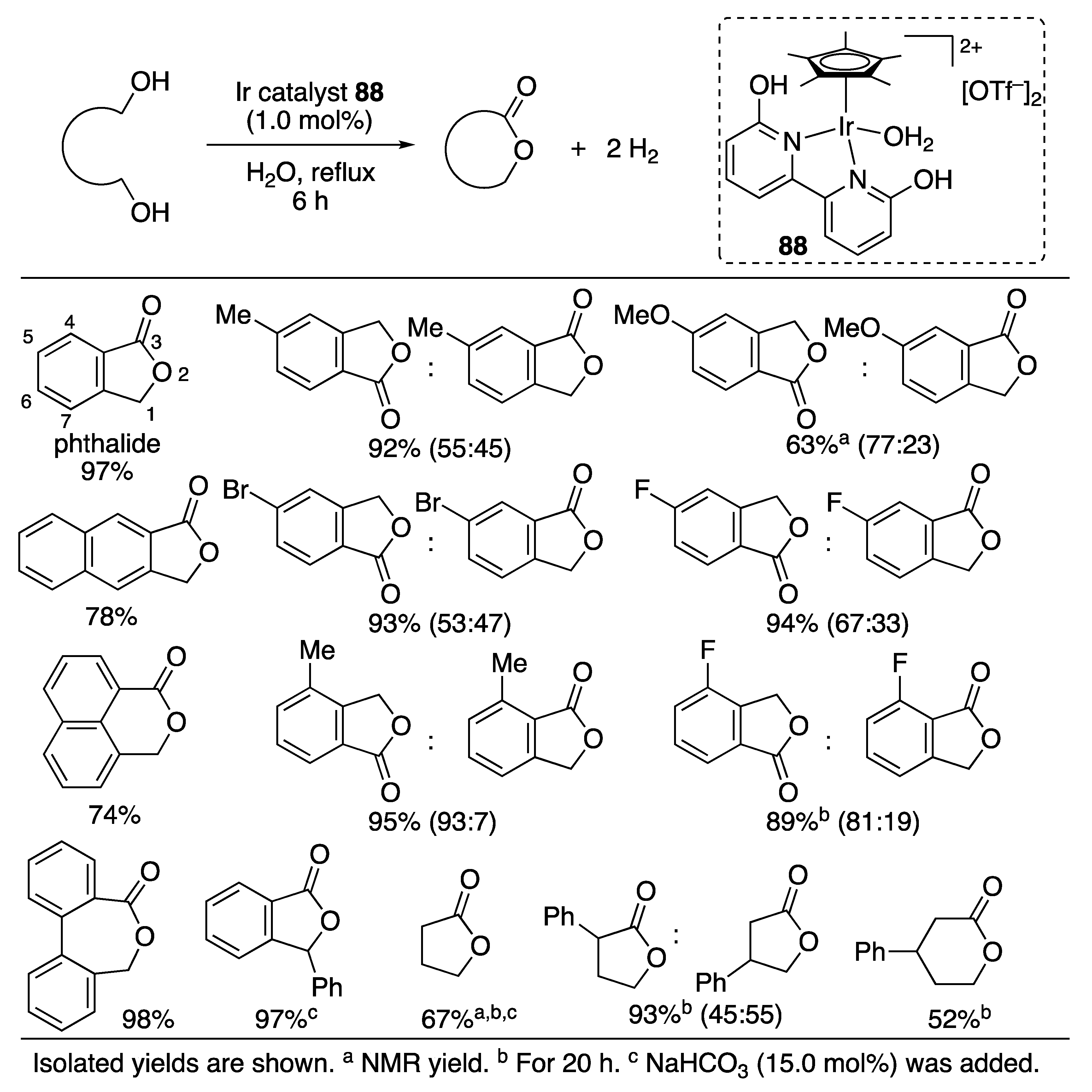
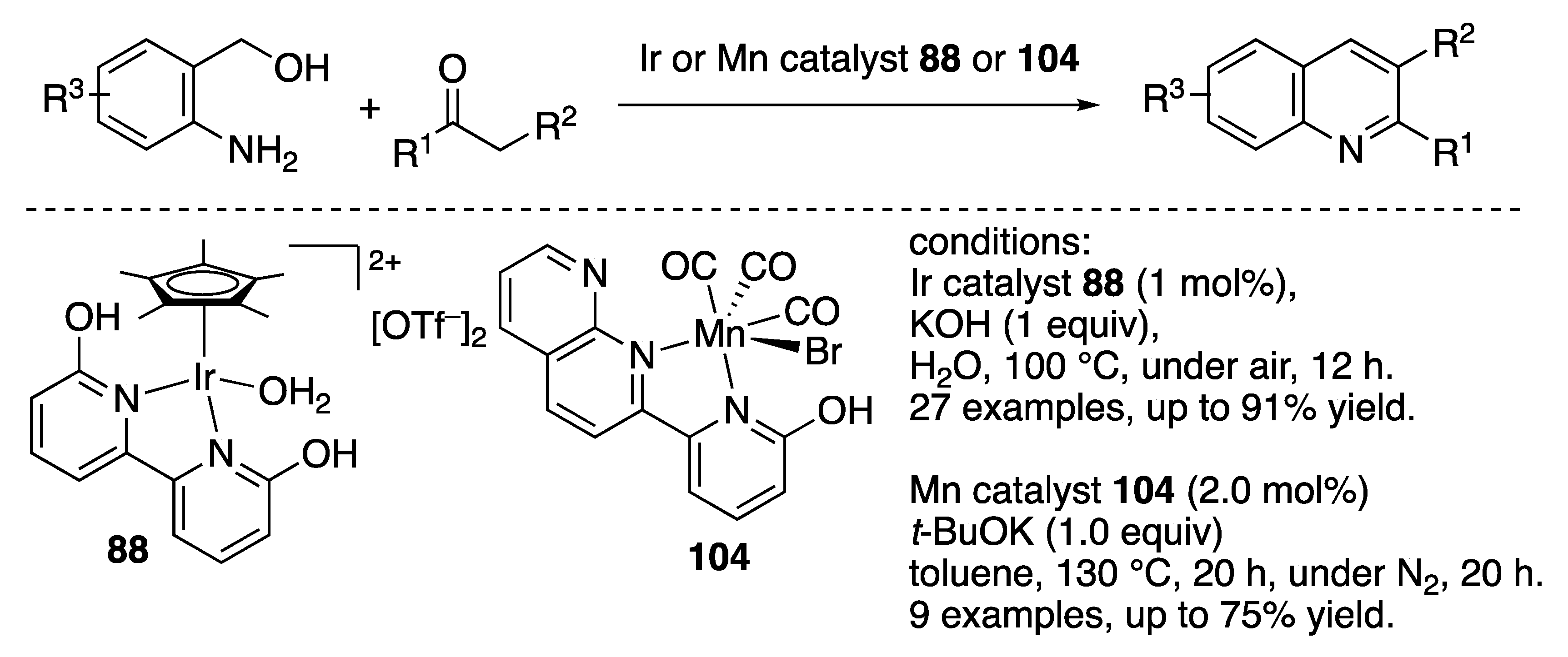
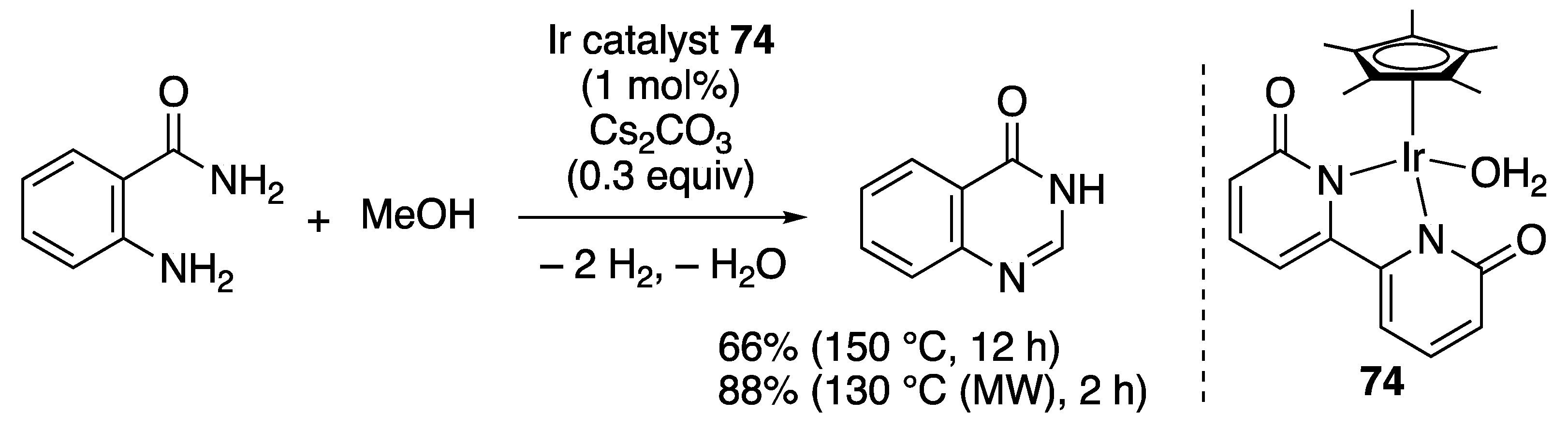
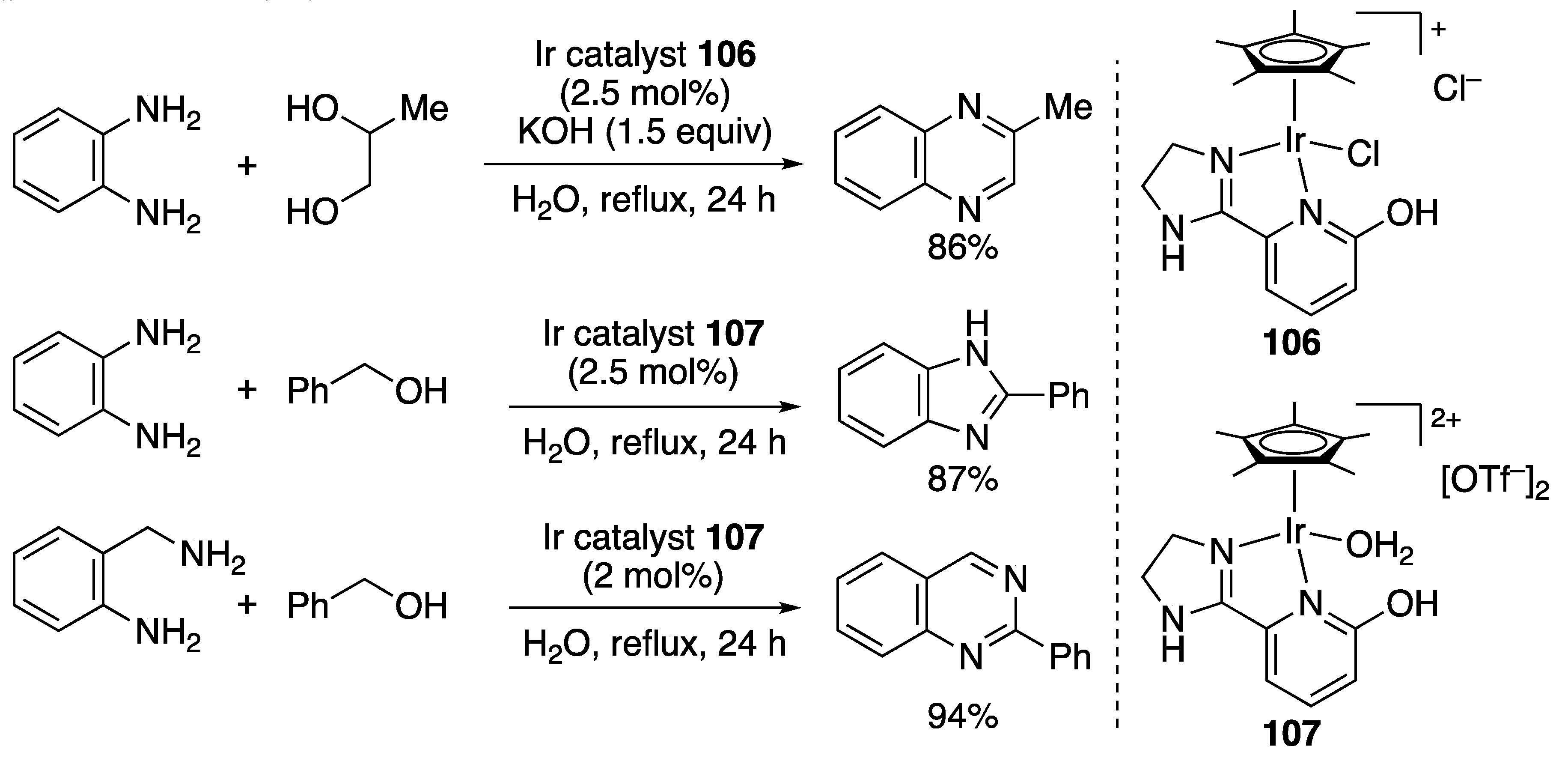
N-Heterocycle Formation
3.2.2. Formic Acid Dehydrogenation
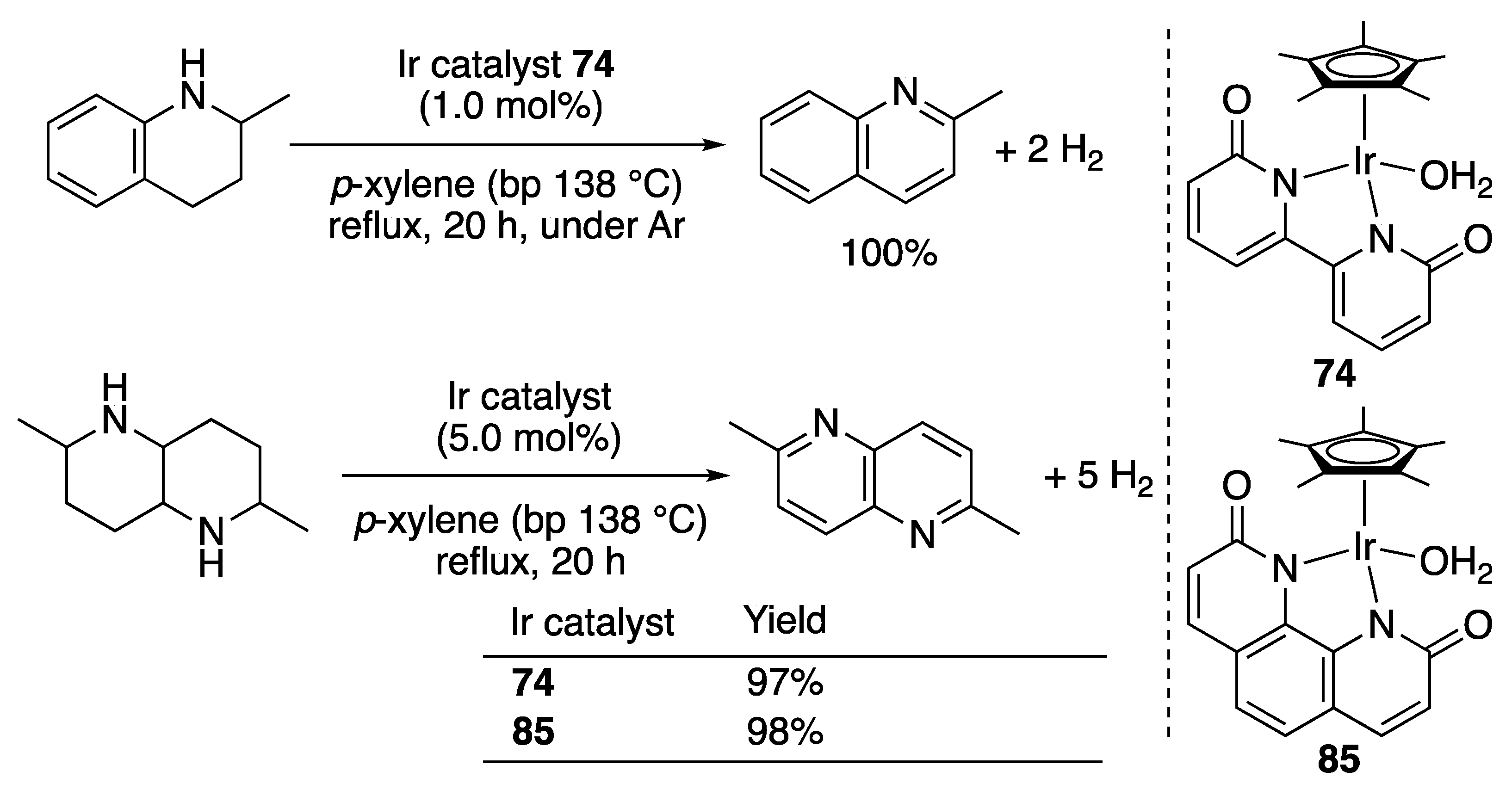
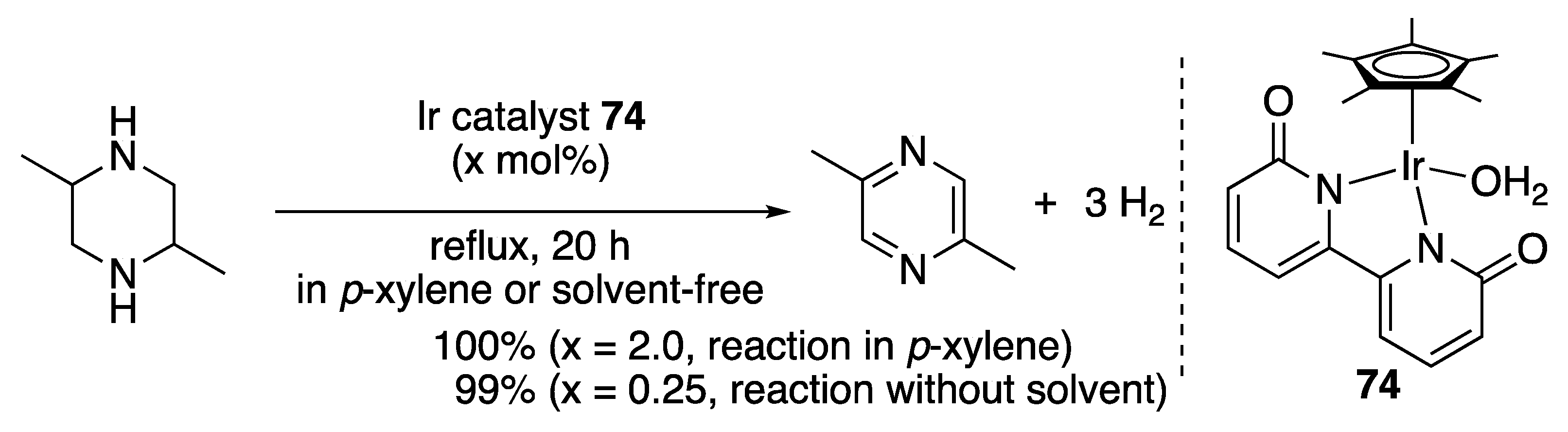
3.2.3. N-Heterocycle Dehydrogenation
3.3. Transfer Hydrogenation and Borrowing Hydrogen Reactions
3.3.1. Transfer Hydrogenation of Ketones and Aldehydes
3.3.2. Transfer Dehydrogenation of Alcohols
3.3.3. C–C Bond-Forming Alkylation Based on Borrowing Hydrogen Methodology
3.4. Miscellaneous
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsuji, J. Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis; John Wiley & Sons: New York, NY, USA, 2000; ISBN 978-0471634980. [Google Scholar]
- Hegedus, L.S. Transition Metals in the Synthesis of Complex Organic Molecules; University Science Books: Mill Valley, CA, USA, 1994; ISBN 978-0935702286. [Google Scholar]
- Trincado, M.; Grützmacher, H. Cooperating Ligands, in Catalysis, in Cooperative Catalysis: Designing Efficient Catalysts for Synthesis; Peters, R., Ed.; Chapter 3; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-33689-0. [Google Scholar]
- Grützmacher, H. Cooperating Ligands in Catalysis. Angew. Chem. Int. Ed. 2008, 47, 1814–1818. [Google Scholar] [CrossRef]
- Haack, K.-J.; Hashiguchi, S.; Fujii, A.; Ikariya, T.; Noyori, R. The Catalyst Precursor, Catalyst, and Intermediate in the RuII-Promoted Asymmetric Hydrogen Transfer between Alcohols and Ketones. Angew. Chem. Int. Ed. 1997, 36, 285–288. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Fujii, A.; Takehara, J.; Ikariya, T.; Noyori, R. Asymmetric Transfer Hydrogenation of Aromatic Ketones Catalyzed by Chiral Ruthenium(II) Complexes. J. Am. Chem. Soc. 1995, 117, 7562–7563. [Google Scholar] [CrossRef]
- Noyori, R.; Hashiguchi, S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Accounts Chem. Res. 1997, 30, 97–102. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Fujita, K. Ligand Platforms in Homogeneous Catalytic Reactions with Metals: Practice and Applications for Green Organic Transformations; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1118203514. [Google Scholar]
- Blum, Y.; Shvo, Y. Catalytically Reactive Ruthenium Intermediates in the Homogeneous Oxidation of Alcohols to Esters. Isr. J. Chem. 1984, 24, 144–148. [Google Scholar] [CrossRef]
- Blum, Y.; Czarkie, D.; Rahamim, Y.; Shvo, Y. (Cyclopentadienone)ruthenium Carbonyl Complexes – A New Class of Homogeneous Hydrogenation Catalysts. Organometallics 1985, 8, 1459–1461. [Google Scholar] [CrossRef]
- Blum, Y.; Shvo, Y.; Chodosh, D.F. Structure of (η4-Ph4C4CO)(CO)3Ru – A Catalyst Precursor in H-Transfer and Dehydrogenation Reactions of Alcohols. Inorg. Chim. Acta 1985, 97, L25–L26. [Google Scholar] [CrossRef]
- Shvo, Y.; Czarkie, D.; Rahamim, Y.; Chodosh, D.F. A New Group of Ruthenium Complexes: Structure and Catalysis. J. Am. Chem. Soc. 1986, 108, 7400–7402. [Google Scholar] [CrossRef]
- Conley, B.L.; Pennington-Boggio, M.K.; Boz, E.; Williams, T.J. Discovery, Applications, and Catalytic Mechanisms of Shvo’s Catalyst. Chem. Rev. 2010, 110, 2294–2312. [Google Scholar] [CrossRef]
- Karvembu, R.; Prabhakaran, R.; Natarajan, K. Shvo’s Diruthenium Complex: A Robust Catalyst. Coord. Chem. Rev. 2005, 249, 911–918. [Google Scholar] [CrossRef]
- Morales-Morales, D. (Ed.) Pincer Compounds: Chemistry and Applications; Elsevier: Amsterdam, The Netherland, 2018; ISBN 978-0-128-12932-6. [Google Scholar]
- van Koten, G.; Gossage, R.A. (Eds.) The Privileged Pincer-Metal Platform: Coordination Chemistry & Applications; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-22927-0. [Google Scholar]
- van Koten, G.; Milstein, D. (Eds.) Organometallic Pincer Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-31081-2. [Google Scholar]
- Lawrence, M.A.; Green, K.-A.; Nelson, P.N.; Lorraine, S.C. Review: Pincer ligands—Tunable, versatile and applicable. Polyhedron 2018, 143, 11–27. [Google Scholar] [CrossRef]
- Szabó, K.J.; Wendt, O.F. (Eds.) Pincer and Pincer-Type Complexes: Applications in Organic Synthesis and Catalysis; Wiley-VCH: Weinheim, Germany, 2014; ISBN 978-3-527-68130-3. [Google Scholar]
- Valdés, H.; García-Eleno, M.A.; Canseco-Gonzalez, D.; Morales-Morales, D. Recent Advances in Catalysis with Transition-Metal Pincer Compounds. ChemCatChem 2018, 10, 3136–3172. [Google Scholar] [CrossRef]
- Singleton, J.T. The Uses of Pincer Complexes in Organic Synthesis. Tetrahedron 2003, 59, 1837–1857. [Google Scholar] [CrossRef]
- Albrecht, M.; Lindner, M.M. Cleavage of Unreactive Bonds with Pincer Metal Complexes. Dalton Trans. 2011, 40, 8733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hall, M.B. Computational Mechanistic Studies on Reactions of Transition Metal Complexes with Noninnocent Pincer Ligands: Aromatization–Dearomatization or Not. ACS Catal. 2015, 5, 1895–1913. [Google Scholar] [CrossRef]
- Milstein, D. Metal–Ligand Cooperation by Aromatization–Dearomatization as a Tool in Single Bond Activation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140189. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Bond Activation by Metal-Ligand Cooperation: Design of “Green” Catalytic Reactions Based on Aromatization-Dearomatization of Pincer Complexes. In Organometallics in Process Chemistry; Springer Science and Business Media LLC: Berlin, Germany, 2011; Volume 37, pp. 55–84. ISBN 978-3-642-20731-0. [Google Scholar]
- Higashi, T.; Kusumoto, S.; Nozaki, K. Cleavage of Si–H, B–H, and C–H Bonds by Metal–Ligand Cooperation. Chem. Rev. 2019, 119, 10393–10402. [Google Scholar] [CrossRef]
- Khusnutdinova, J.R.; Milstein, D. Metal-Ligand Cooperation. Angew. Chem. Int. Ed. 2015, 54, 12236–12273. [Google Scholar] [CrossRef]
- Li, H.; Zheng, B.; Huang, K.-W. A New Class of PN3-Pincer Ligands for Metal–Ligand Cooperative Catalysis. Coord. Chem. Rev. 2015, 293, 116–138. [Google Scholar] [CrossRef]
- Li, H.; Gonçalves, T.P.; Lupp, D.; Huang, K.-W. PN3(P)-Pincer Complexes: Cooperative Catalysis and Beyond. ACS Catal. 2019, 9, 1619–1629. [Google Scholar] [CrossRef]
- Clarke, M.L.; Roff, G.J. Homogeneous Hydrogenation of Aldehydes, Ketones, Imines and Carboxylic Acid Derivatives: Chemoselectivity and Catalytic Activity. In The Handbook of Homogeneous Hydrogenation; Wiley: Hoboken, NJ, USA, 2008; Volume 1, pp. 413–454. ISBN 978-3-527-61938-2. [Google Scholar]
- Wu, X.; Xiao, J. Reduction of C=O to CHOH by Metal-Catalyzed Hydrogenation and Transfer Hydrogenation. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherland, 2014; Volume 8, pp. 198–273. ISBN 978-0-080-97743-0. [Google Scholar]
- Blaser, H.-U.; Spindler, F. Reduction of C=N to CHNH by Metal-Catalyzed Hydrogenation and Transfer Hydrogenation. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherland, 2014; Volume 8, pp. 274–299. ISBN 978-0-080-97743-0. [Google Scholar]
- Milstein, D. Discovery of Environmentally Benign Catalytic Reactions of Alcohols Catalyzed by Pyridine-Based Pincer Ru Complexes, Based on Metal–Ligand Cooperation. Top. Catal. 2010, 53, 915–923. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Metal–Ligand Cooperation by Aromatization–Dearomatization: A New Paradigm in Bond Activation and “Green” Catalysis. Acc. Chem. Res. 2011, 44, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; MacArthur, A.H.R.; Brookhart, M.; Goldman, A. Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes. Chem. Rev. 2011, 111, 1761–1779. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, E.; Milstein, D. Hydrogenation of Polar Bonds Catalyzed by Ruthenium-Pincer Complexes. In Ruthenium in Catalysis; Dixneuf, P.H., Bruneau, C., Eds.; Springer: Berlin, Germany, 2014; pp. 19–43. ISBN 978-3-319-08482-4. [Google Scholar]
- Younus, H.A.; Su, W.; Ahmad, N.; Chen, S.; Verpoort, F. Ruthenium Pincer Complexes: Synthesis and Catalytic Applications. Adv. Synth. Catal. 2015, 357, 283–330. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Bond Activation and Catalysis by Ruthenium Pincer Complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Guan, H. Synthesis and Catalytic Applications of Iron Pincer Complexes. Comments Inorg. Chem. 2011, 32, 88–112. [Google Scholar] [CrossRef]
- Zell, T.; Milstein, D. Hydrogenation and Dehydrogenation Iron Pincer Catalysts Capable of Metal–Ligand Cooperation by Aromatization/Dearomatization. Acc. Chem. Res. 2015, 48, 1979–1994. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharya, P.; Dai, H.; Guan, H. Nickel and Iron Pincer Complexes as Catalysts for the Reduction of Carbonyl Compounds. Acc. Chem. Res. 2015, 48, 1995–2003. [Google Scholar] [CrossRef]
- Garbe, M.; Junge, K.; Beller, M. Homogeneous Catalysis by Manganese-Based Pincer Complexes. Eur. J. Org. Chem. 2017, 2017, 4344–4362. [Google Scholar] [CrossRef]
- Mukherjee, A.; Milstein, D. Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes. ACS Catal. 2018, 8, 11435–11469. [Google Scholar] [CrossRef]
- Li, W.; Xie, J.; Lin, H.; Zhou, Q.-L. Highly Efficient Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone Catalyzed by Iridium Pincer Complexes. Green Chem. 2012, 14, 2388–2390. [Google Scholar] [CrossRef]
- Sánchez, P.; Hernández-Juarez, M.; Álvarez, E.; Paneque, M.; Rendón, N.; Suárez, A. Synthesis, Structure and Reactivity of Pd and Ir Complexes Based on New Lutidine-Derived NHC/Phosphine Mixed Pincer Ligands. Dalton Trans. 2016, 45, 16997–17009. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Leitus, G.; Ben-David, Y.; Milstein, D. Efficient Hydrogenation of Ketones Catalyzed by an Iron Pincer Complex. Angew. Chem. Int. Ed. 2011, 50, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Iron, M.A.; Konstantinovski, L.; Diskin-Posner, Y.; Leitus, G.; Ben-David, Y.; Milstein, D. Iron Borohydride Pincer Complexes for the Efficient Hydrogenation of Ketones under Mild, Base-Free Conditions: Synthesis and Mechanistic Insight. Chem. Eur. J. 2012, 18, 7196–7209. [Google Scholar] [CrossRef] [PubMed]
- Butschke, B.; Feller, M.; Diskin-Posner, Y.; Milstein, D. Ketone Hydrogenation Catalyzed by a New Iron(ii)–PNN Complex. Catal. Sci. Technol. 2016, 6, 4428–4437. [Google Scholar] [CrossRef]
- Zell, T.; Ben-David, Y.; Milstein, D. Highly Efficient, General Hydrogenation of Aldehydes Catalyzed by PNP Iron Pincer Complexes. Catal. Sci. Technol. 2015, 5, 822–826. [Google Scholar] [CrossRef]
- Bruneau-Voisine, A.; Wang, D.; Roisnel, T.; Darcel, C.; Sortais, J.-B. Hydrogenation of Ketones with a Manganese PN3P Pincer Pre-Catalyst. Catal. Commun. 2017, 92, 1–4. [Google Scholar] [CrossRef]
- Grey, R.A.; Pez, G.P.; Wallo, A. Anionic Metal Hydride Catalysts. 2. Application to the Hydrogenation of Ketones, Aldehydes, Carboxylic Acid Esters, and Nitriles. J. Am. Chem. Soc. 1981, 103, 7536–7542. [Google Scholar] [CrossRef]
- Teunissen, H.T. Homogeneous Ruthenium Catalyzed Hydrogenation of Esters to Alcohols‡. Chem. Commun. 1998, 1367–1368. [Google Scholar] [CrossRef]
- Nomura, K.; Ogura, H.; Imanishi, Y. Ruthenium Catalyzed Hydrogenation of Methyl Phenylacetate under Low Hydrogen Pressure. J. Mol. Catal. A Chem. 2002, 178, 105–114. [Google Scholar] [CrossRef]
- Zhang, J.; Leitus, G.; Ben-David, Y.; Milstein, D. Efficient Homogeneous Catalytic Hydrogenation of Esters to Alcohols. Angew. Chem. Int. Ed. 2006, 45, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Fogler, E.; Garg, J.A.; Hu, P.; Leitus, G.; Shimon, L.J.W.; Milstein, D. System with Potential Dual Modes of Metal-Ligand Cooperation: Highly Catalytically Active Pyridine-Based PNNH-Ru Pincer Complexes. Chem. Eur. J. 2014, 20, 15727–15731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Balaraman, E.; Leitus, G.; Milstein, D. Electron-Rich PNP- and PNN-Type Ruthenium(II) Hydrido Borohydride Pincer Complexes. Synthesis, Structure, and Catalytic Dehydrogenation of Alcohols and Hydrogenation of Esters. Organometallics 2011, 30, 5716–5724. [Google Scholar] [CrossRef]
- Krall, E.M.; Klein, T.W.; Andersen, R.J.; Nett, A.J.; Glasgow, R.W.; Reader, D.S.; Dauphinais, B.C.; Mc Ilrath, S.P.; Fischer, A.A.; Carney, M.J.; et al. Controlled Hydrogenative Depolymerization of Polyesters and Polycarbonates Catalyzed by Ruthenium(II) PNN Pincer Complexes. Chem. Commun. 2014, 50, 4884–4887. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Koehler, C.; Tan, R.; Annibale, V.T.; Song, D. Ester Hydrogenation Catalyzed by Ru-CNN Pincer Complexes. Chem. Commun. 2011, 47, 8349–8351. [Google Scholar] [CrossRef]
- Filonenko, G.; Cosimi, E.; Lefort, L.; Conley, M.P.; Copéret, C.; Lutz, M.; Hensen, E.J.; Pidko, E. Lutidine-Derived Ru-CNC Hydrogenation Pincer Catalysts with Versatile Coordination Properties. ACS Catal. 2014, 4, 2667–2671. [Google Scholar] [CrossRef]
- Fogler, E.; Balaraman, E.; Ben-David, Y.; Leitus, G.; Shimon, L.J.W.; Milstein, D. New CNN-Type Ruthenium Pincer NHC Complexes. Mild, Efficient Catalytic Hydrogenation of Esters. Organometallics 2011, 30, 3826–3833. [Google Scholar] [CrossRef]
- Sluijter, S.N.; Korstanje, T.J.; van der Vlugt, J.I.; Elsevier, C.J. Mechanistic Insights into Catalytic Carboxylic Esters Hydrogenation with Cooperative Ru(II)-bis{1,2,3-triazolylidene}pyridine Pincer Complexes. J. Organomet. Chem. 2017, 845, 30–37. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Qu, S.; Zheng, B.; He, L.; Lai, Z.; Wang, Z.-X.; Huang, K.-W. Hydrogenation of Esters Catalyzed by Ruthenium PN3-Pincer Complexes Containing an Aminophosphine Arm. Organometallics 2014, 33, 4152–4155. [Google Scholar] [CrossRef]
- Zell, T.; Ben-David, Y.; Milstein, D. Unprecedented Iron-Catalyzed Ester Hydrogenation. Mild, Selective, and Efficient Hydrogenation of Trifluoroacetic Esters to Alcohols Catalyzed by an Iron Pincer Complex. Angew. Chem. Int. Ed. 2014, 53, 4685–4689. [Google Scholar] [CrossRef]
- Hirosawa, C.; Wakasa, N.; Fuchikami, T. Hydrogenation of Amides by the Use of Bimetallic Catalysts Consisting of Group 8 to 10, and Group 6 or 7 Metals. Tetrahedron Lett. 1996, 37, 6749–6752. [Google Scholar] [CrossRef]
- Núñez Magro, A.A.; Eastham, G.R.; Cole-Hamilton, D.J. The Synthesis of Amines by the Homogeneous Hydrogenation of Secondary and Primary Amides. Chem. Commun. 2007, 3154–3156. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G.; Papworth, A.J.; Philipps, C.; Smith, A.M.; Whyman, R. Selective Hydrogenation of Amides using Ruthenium/Molybdenum Catalysts. Adv. Synth. Catal. 2010, 352, 869–883. [Google Scholar] [CrossRef]
- Beamson, G.; Papworth, A.J.; Philipps, C.; Smith, A.M.; Whyman, R. Selective Hydrogenation of Amides Using Rh/Mo Catalysts. J. Catal. 2010, 269, 93–102. [Google Scholar] [CrossRef]
- Ito, M.; Ootsuka, T.; Watari, R.; Shiibashi, A.; Himizu, A.; Ikariya, T. Catalytic Hydrogenation of Carboxamides and Esters by Well-Defined Cp*Ru Complexes Bearing a Protic Amine Ligand. J. Am. Chem. Soc. 2011, 133, 4240–4242. [Google Scholar] [CrossRef]
- John, J.M.; Bergens, S.H. A Highly Active Catalyst for the Hydrogenation of Amides to Alcohols and Amines. Angew. Chem. Int. Ed. 2011, 50, 10377–10380. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Held, I.E.; Oishi, S.; Naruto, M.; Saito, S. Catalytic Hydrogenation of Unactivated Amides Enabled by Hydrogenation of Catalyst Precursor. Tetrahedron Lett. 2013, 54, 2674–2678. [Google Scholar] [CrossRef]
- Kita, Y.; Higuchi, T.; Mashima, K. Hydrogenation of Amides Catalyzed by a Combined Catalytic System of a Ru Complex with a Zinc Salt. Chem. Commun. 2014, 50, 11211–11213. [Google Scholar] [CrossRef] [PubMed]
- John, J.M.; Loorthuraja, R.; Antoniuk, E.; Bergens, S.H. Catalytic Hydrogenation of Functionalized Amides under Basic and Neutral Conditions. Catal. Sci. Technol. 2015, 5, 1181–1186. [Google Scholar] [CrossRef]
- Shi, L.; Tan, X.; Long, J.; Xiong, X.; Yang, S.; Xue, P.; Lv, H.; Zhang, X. Direct Catalytic Hydrogenation of Simple Amides: A Highly Efficient Approach from Amides to Amines and Alcohols. Chem. Eur. J. 2016, 23, 546–548. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Alberico, E.; Drexler, H.-J.; Baumann, W.; Junge, K.; Junge, H.; Beller, M. Efficient Base-Free Hydrogenation of Amides to Alcohols and Amines Catalyzed by Well-Defined Pincer Imidazolyl–Ruthenium Complexes. ACS Catal. 2015, 6, 47–54. [Google Scholar] [CrossRef]
- Rezayee, N.M.; Samblanet, D.C.; Sanford, M.S. Iron-Catalyzed Hydrogenation of Amides to Alcohols and Amines. ACS Catal. 2016, 6, 6377–6383. [Google Scholar] [CrossRef]
- Schneck, F.; Assmann, M.; Balmer, M.; Harms, K.; Langer, R. Selective Hydrogenation of Amides to Amines and Alcohols Catalyzed by Improved Iron Pincer Complexes. Organometallics 2016, 35, 1931–1943. [Google Scholar] [CrossRef]
- Papa, V.; Cabrero-Antonino, J.R.; Alberico, E.; Spanneberg, A.; Junge, K.; Junge, H.; Beller, M. Efficient and Selective Hydrogenation of Amides to Alcohols and Amines Using a Well-Defined Manganese–PNN Pincer Complex. Chem. Sci. 2017, 8, 3576–3585. [Google Scholar] [CrossRef]
- Balaraman, E.; Gnanaprakasam, B.; Shimon, L.J.W.; Milstein, D.; Boopathy, G. Direct Hydrogenation of Amides to Alcohols and Amines under Mild Conditions. J. Am. Chem. Soc. 2010, 132, 16756–16758. [Google Scholar] [CrossRef]
- Barrios-Francisco, R.; Balaraman, E.; Diskin-Posner, Y.; Leitus, G.; Shimon, L.J.W.; Milstein, D. PNN Ruthenium Pincer Complexes Based on Phosphinated 2,2′-Dipyridinemethane and 2,2′-Oxobispyridine. Metal–Ligand Cooperation in Cyclometalation and Catalysis. Organometallics 2013, 32, 2973–2982. [Google Scholar] [CrossRef]
- Hu, P.; Fogler, E.; Diskin-Posner, Y.; Iron, M.A.; Milstein, D. A Novel Liquid Organic Hydrogen Carrier System Based on Catalytic Peptide Formation and Hydrogenation. Nat. Commun. 2015, 6, 6859. [Google Scholar] [CrossRef]
- Hu, P.; Ben-David, Y.; Milstein, D. Rechargeable Hydrogen Storage System Based on the Dehydrogenative Coupling of Ethylenediamine with Ethanol. Angew. Chem. Int. Ed. 2015, 55, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Janes, T.; Espinosa-Jalapa, N.Á.; Milstein, D. Selective Hydrogenation of Cyclic Imides to Diols and Amines and Its Application in the Development of a Liquid Organic Hydrogen Carrier. J. Am. Chem. Soc. 2018, 140, 7453–7457. [Google Scholar] [CrossRef] [PubMed]
- Garg, J.A.; Chakraborty, S.; Ben-David, Y.; Milstein, D. Unprecedented Iron-Catalyzed Selective Hydrogenation of Activated Amides to Amines and Alcohols. Chem. Commun. 2016, 52, 5285–5288. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-Q.; Chakraborty, S.; Nerush, A.; Oren, D.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Highly Selective, Efficient Deoxygenative Hydrogenation of Amides Catalyzed by a Manganese Pincer Complex via Metal–Ligand Cooperation. ACS Catal. 2018, 8, 8014–8019. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, D.; Rivetti, F.; Romano, U. Oxidative Carbonylation of Methanol to Dimethyl Carbonate (DMC): A New Catalytic System. J. Organomet. Chem. 1995, 488, C15–C19. [Google Scholar] [CrossRef]
- Delledonne, D.; Rivetti, F.; Romano, U. Developments in the Production and Application of Dimethylcarbonate. Appl. Catal. A Gen. 2001, 221, 241–251. [Google Scholar] [CrossRef]
- Sakakura, T.; Kohno, K. The Synthesis of Organic Carbonates from Carbon Dioxide. Chem. Commun. 2009, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.; Chol, J.C.; Sakakura, T. Halogen-Free Process for the Conversion of Carbon Dioxide to Urethanes by Homogeneous Catalysis. Chem. Commun. 2001, 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.; Choi, J.-C.; Sakakura, T. Nickel-Catalyzed Dehydrative Transformation of CO2 to Urethanes. Green Chem. 2004, 6, 524. [Google Scholar] [CrossRef]
- Du, X.-L.; Jiang, Z.; Su, D.S.; Wang, J.-Q. Research Progress on the Indirect Hydrogenation of Carbon Dioxide to Methanol. ChemSusChem 2016, 9, 315. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Balaraman, E.; Gunanathan, C.; Zhang, J.; Shimon, L.J.W.; Milstein, D. Efficient Hydrogenation of Organic Carbonates, Carbamates and Formates Indicates Alternative Routes to Methanol Based on CO2 and CO. Nat. Chem. 2011, 3, 609–614. [Google Scholar] [CrossRef]
- Huff, C.A.; Sanford, M.S. Cascade Catalysis for the Homogeneous Hydrogenation of CO2 to Methanol. J. Am. Chem. Soc. 2011, 133, 18122–18125. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, J.R.; Garg, J.A.; Milstein, D. Combining Low-Pressure CO2 Capture and Hydrogenation To Form Methanol. ACS Catal. 2015, 5, 2416–2422. [Google Scholar] [CrossRef]
- Wu, X.; Ji, L.; Ji, Y.; Elageed, E.H.; Gao, G. Hydrogenation of Ethylene Carbonate Catalyzed by Lutidine-Bridged N-Heterocyclic Carbene Ligands and Ruthenium Precursors. Catal. Commun. 2016, 85, 57–60. [Google Scholar] [CrossRef]
- Balaraman, E.; Ben-David, Y.; Milstein, D. Unprecedented Catalytic Hydrogenation of Urea Derivatives to Amines and Methanol. Angew. Chem. Int. Ed. 2011, 50, 11702–11705. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, P.; Ben-David, Y.; Milstein, D. A Reversible Liquid Organic Hydrogen Carrier System Based on Methanol-Ethylenediamine and Ethylene Urea. Angew. Chem. Int. Ed. 2019, 58, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2017, 118, 372–433. [Google Scholar] [CrossRef]
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Technological Challenges. ACS Energy Lett. 2018, 3, 1938–1966. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)−Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef]
- Langer, R.; Diskin-Posner, Y.; Leitus, G.; Shimon, L.J.W.; Ben-David, Y.; Milstein, D. Low-Pressure Hydrogenation of Carbon Dioxide Catalyzed by an Iron Pincer Complex Exhibiting Noble Metal Activity. Angew. Chem. Int. Ed. 2011, 50, 9948–9952. [Google Scholar] [CrossRef]
- Wheelaghan, O.R.; Dauth, A.; Leitus, G.; Diskin-Posner, Y.; Milstein, D. Synthesis and Reactivity of Iron Complexes with a New Pyrazine-Based Pincer Ligand, and Application in Catalytic Low-Pressure Hydrogenation of Carbon Dioxide. Inorg. Chem. 2015, 54, 4526–4538. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, G.; van Putten, R.; Schulpen, E.N.; Hensen, E.J.; Pidko, E. Highly Efficient Reversible Hydrogenation of Carbon Dioxide to Formates Using a Ruthenium PNP-Pincer Catalyst. ChemCatChem 2014, 6, 1526–1530. [Google Scholar] [CrossRef]
- Filonenko, G.; Smykowski, D.; Szyja, B.M.; Li, G.; Szczygiel, J.; Hensen, E.J.; Pidko, E. Catalytic Hydrogenation of CO2 to Formates by a Lutidine-Derived Ru–CNC Pincer Complex: Theoretical Insight into the Unrealized Potential. ACS Catal. 2015, 5, 1145–1154. [Google Scholar] [CrossRef]
- Bertini, F.; Glatz, M.; Gorgas, N.; Stöger, B.; Peruzzini, M.; Veiros, L.F.; Kirchner, K.; Gonsalvi, L. Carbon Dioxide Hydrogenation Catalyzed by Well-Defined Mn(I) PNP Pincer Hydride Complexes. Chem. Sci. 2017, 8, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Daw, P.; Espinosa-Jalapa, N.A.; Leitus, G.; Shimon, L.J.W.; Ben-David, Y.; Milstein, D. CO2 Activation by Manganese Pincer Complexes through Different Modes of Metal-Ligand Cooperation. Dalton Trans. 2019, 48, 14580–14584. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Srimani, D.; Ben-David, Y.; Milstein, D. Low-Pressure Hydrogenation of Nitriles to Primary Amines Catalyzed by Ruthenium Pincer Complexes. Scope and Mechanism. ChemCatChem 2017, 9, 559–563. [Google Scholar] [CrossRef]
- Hernández-Juarez, M.; Vaquero, M.; Álvarez, E.; Salazar, V.; Suárez, A.; González, E.A. Hydrogenation of Imines Catalysed by Ruthenium(ii) Complexes Based on Lutidine-Derived CNC Pincer Ligands. Dalton Trans. 2013, 42, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Feller, M.; Ben-David, Y.; Milstein, D. Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex. J. Am. Chem. Soc. 2017, 139, 5720–5723. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Applications of Acceptorless Dehydrogenation and Related Transformations in Chemical Synthesis. Science 2013, 341, 1229712. [Google Scholar] [CrossRef]
- Siddiki, S.M.A.H.; Toyao, T.; Shimizu, K. Acceptorless Dehydrogenative Coupling Reactions with Alcohols over Heterogeneous Catalysts. Green Chem. 2018, 20, 2933–2952. [Google Scholar] [CrossRef]
- Friedrich, A.; Schneider, S. Acceptorless Dehydrogenation of Alcohols: Perspectives for Synthesis and H2 Storage. ChemCatChem 2009, 1, 72–73. [Google Scholar] [CrossRef]
- Trincado, M.; Banerjee, D.; Grützmacher, H. Molecular Catalysts for Hydrogen Production from Alcohols. Energy Environ. Sci. 2014, 7, 2464–2503. [Google Scholar] [CrossRef]
- Crabtree, R.H. Homogeneous Transition Metal Catalysis of Acceptorless Dehydrogenative Alcohol Oxidation: Applications in Hydrogen Storage and to Heterocycle Synthesis. Chem. Rev. 2017, 117, 9228–9246. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatti, T.M.; Goldman, A. Dehydrogenation of Alkanes and Aliphatic Groups by Pincer-Ligated Metal Complexes. Chem. Rev. 2017, 117, 12357–12384. [Google Scholar] [CrossRef]
- Charman, H.B. Hydride Transfer Reactions Catalyzed by Rhodium–Tin Complexes. J. Chem. Soc. B 1970, 584–587. [Google Scholar] [CrossRef]
- Dobscn, A.; Robinson, S.D. Catalytic Dehydrogenation of Primary and Secondary Alcohols by Ru(OCOCF3)2(CO)(PPh3)2. J. Organomet. Chem. 1975, 87, C52–C53. [Google Scholar] [CrossRef]
- Morton, D.; Cole-Hamilton, D.J. Rapid thermal hydrogen production from alcohols catalyzed by [Rh(2,2’-bipyridyl)2]Cl. J. Chem. Soc. Chem. Commun. 1987, 248–249. [Google Scholar] [CrossRef]
- Zhang, J.; Gandelman, M.; Shimon, L.J.W.; Rozenberg, H.; Milstein, D. Electron-Rich, Bulky Ruthenium PNP-Type Complexes. Acceptorless Catalytic Alcohol Dehydrogenation. Organometallics 2004, 23, 4026–4033. [Google Scholar] [CrossRef]
- Fujita, K.; Tanino, N.; Yamaguchi, R. Ligand-Promoted Dehydrogenation of Alcohols Catalyzed by Cp*Ir Complexes. A New Catalytic System for Oxidant-Free Oxidation of Alcohols. Org. Lett. 2007, 9, 109–111. [Google Scholar] [CrossRef]
- Zhang, J.; Leitus, G.; Ben-David, Y.; Milstein, D. Facile Conversion of Alcohols into Esters and Dihydrogen Catalyzed by New Ruthenium Complexes. J. Am. Chem. Soc. 2005, 127, 10840–10841. [Google Scholar] [CrossRef]
- He, L.-P.; Chen, T.; Gong, D.; Lai, Z.; Huang, K.-W. Enhanced Reactivities toward Amines by Introducing an Imine Arm to the Pincer Ligand: Direct Coupling of Two Amines To Form an Imine Without Oxidant. Organometallics 2012, 31, 5208–5211. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, T.; He, L.; Pinnau, I.; Lai, Z.; Huang, K.-W. A Green Approach to Ethyl Acetate: Quantitative Conversion of Ethanol through Direct Dehydrogenation in a Pd-Ag Membrane Reactor. Chem. Eur. J. 2012, 18, 15940–15943. [Google Scholar] [CrossRef]
- Cheisson, T.; Mazaud, L.; Auffrant, A. Ruthenium Complexes Featuring Cooperative Phosphine–Pyridine–Iminophophorane (PNN) ligands: Synthesis, reactivity and catalytic activity. Dalton Trans. 2018, 47, 14521–14530. [Google Scholar] [CrossRef]
- Langer, R.; Fuchs, I.; Vogt, M.; Balaraman, E.; Diskin-Posner, Y.; Shimon, L.J.W.; Ben-David, Y.; Milstein, D. Stepwise Metal-Ligand Cooperation by a Reversible Aromatization/Deconjugation Sequence in Ruthenium Complexes with a Tetradentate Phenanthroline-Based Ligand. Chem. Eur. J. 2013, 19, 3407–3414. [Google Scholar] [CrossRef]
- Hunsicker, D.M.; Dauphinais, B.C.; Mc Ilrath, S.P.; Robertson, N.J. Synthesis of High Molecular Weight Polyesters via In Vacuo Dehydrogenation Polymerization of Diols. Macromol. Rapid Commun. 2011, 33, 232–236. [Google Scholar] [CrossRef]
- Srimani, D.; Gnanaprakasam, B.; Milstein, D.; Ben-David, Y.; Balaraman, E. Ruthenium Pincer-Catalyzed Cross-Dehydrogenative Coupling of Primary Alcohols with Secondary Alcohols under Neutral Conditions. Adv. Synth. Catal. 2012, 354, 2403–2406. [Google Scholar] [CrossRef]
- Gnanaprakasam, B.; Milstein, D.; Ben-David, Y. Ruthenium Pincer-Catalyzed Acylation of Alcohols Using Esters with Liberation of Hydrogen under Neutral Conditions. Adv. Synth. Catal. 2010, 352, 3169–3173. [Google Scholar] [CrossRef]
- Gunanathan, C.; Ben-David, Y.; Milstein, D. Direct Synthesis of Amides from Alcohols and Amines with Liberation of H2. Science 2007, 317, 790–792. [Google Scholar] [CrossRef]
- Gnanaprakasam, B.; Balaraman, E.; Ben-David, Y.; Milstein, D. Synthesis of Peptides and Pyrazines from β-Amino Alcohols through Extrusion of H2 Catalyzed by Ruthenium Pincer Complexes: Ligand-Controlled Selectivity. Angew. Chem. Int. Ed. 2011, 50, 12240–12244. [Google Scholar] [CrossRef]
- Zeng, H.; Guan, Z.N. Direct Synthesis of Polyamides via Catalytic Dehydrogenation of Diols and Diamines. J. Am. Chem. Soc. 2011, 133, 1159–1161. [Google Scholar] [CrossRef]
- Gnanaprakasam, B.; Balaraman, E.; Gunanathan, C.; Milstein, D. Synthesis of Polyamides from Diols and Diamines with Liberation of H2. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1755–1765. [Google Scholar] [CrossRef]
- Gnanaprakasam, B.; Milstein, D. Synthesis of Amides from Esters and Amines with Liberation of H2 under Neutral Conditions. J. Am. Chem. Soc. 2011, 133, 1682–1685. [Google Scholar] [CrossRef]
- Gnanaprakasam, B.; Zhang, J.; Milstein, D. Direct Synthesis of Imines from Alcohols and Amines with Liberation of H2. Angew. Chem. Int. Ed. 2010, 49, 1468–1471. [Google Scholar] [CrossRef]
- Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon, L.J.W.; Ben-David, Y.; Jalapa, N.A.E.; Milstein, D. Manganese-Catalyzed Environmentally Benign Dehydrogenative Coupling of Alcohols and Amines to form Aldimines and H2: A Catalytic and Mechanistic Study. J. Am. Chem. Soc. 2016, 138, 4298–4301. [Google Scholar] [CrossRef]
- Mastalir, M.; Glatz, M.; Gorgas, N.; Stöger, B.; Pittenauer, E.; Allmaier, G.; Veiros, L.F.; Kirchner, K. Divergent Coupling of Alcohols and Amines Catalyzed by Isoelectronic Hydride MnI and FeII PNP Pincer Complexes. Chem. Eur. J. 2016, 22, 12316–12320. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Tanigawa, I.; Taguchi, H.-O.; Takeuchi, K.; Ozawa, F. Iridium(I) Complexes Bearing a Noninnocent PNP-Pincer-Type Phosphaalkene Ligand: Catalytic Application in the Base-Free N-Alkylation of Amines with Alcohols. Eur. J. Inorg. Chem. 2016, 754–760. [Google Scholar] [CrossRef]
- Bauer, J.O.; Leitus, G.; Ben-David, Y.; Milstein, D. Direct Synthesis of Symmetrical Azines from Alcohols and Hydrazine Catalyzed by a Ruthenium Pincer Complex: Effect of Hydrogen Bonding. ACS Catal. 2016, 6, 8415–8419. [Google Scholar] [CrossRef]
- Das, U.K.; Ben-David, Y.; Diskin-Posner, Y.; Milstein, D. N-Substituted Hydrazones by Manganese-Catalyzed Coupling of Alcohols with Hydrazine: Borrowing Hydrogen and Acceptorless Dehydrogenation in One System. Angew. Chem. Int. Ed. 2018, 57, 2179–2182. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Handbook of Heterocyclic Chemistry, 3rd ed.; Elsevier: Amsterdam, The Netherland, 2010; ISBN 978-0-080-95844-6. [Google Scholar]
- Hille, T.; Irrgang, T.; Kempe, R. The Synthesis of Benzimidazoles and Quinoxalines from Aromatic Diamines and Alcohols by Iridium-Catalyzed Acceptorless Dehydrogenative Alkylation. Chem. Eur. J. 2014, 20, 5569–5572. [Google Scholar] [CrossRef]
- Deibl, N.; Kempe, R. Manganese-Catalyzed Multicomponent Synthesis of Pyrimidines from Alcohols and Amidines. Angew. Chem. Int. Ed. 2017, 56, 1663–1666. [Google Scholar] [CrossRef]
- Khaghaninejad, S.; Heravi, M.M. Paal–Knorr Reaction in the Synthesis of Heterocyclic Compounds. Adv. Heterocycl. Chem. 2014, 111, 95–146. [Google Scholar]
- Michlik, S.; Kempe, R. A Sustainable Catalytic Pyrrole Synthesis. Nat. Chem. 2013, 5, 140–144. [Google Scholar] [CrossRef]
- Srimani, D.; Ben-David, Y.; Milstein, D. Direct Synthesis of Pyrroles by Dehydrogenative Coupling of β-Aminoalcohol with Secondary Alcohols Catalyzed by Ruthenium Pincer Complexes. Angew. Chem. Int. Ed. 2013, 52, 4012–4015. [Google Scholar] [CrossRef]
- Kallmeier, F.; Dudziec, B.; Irrgang, T.; Kempe, R. Manganese-Catalyzed Sustainable Synthesis of Pyrroles from Alcohols and Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 7261–7265. [Google Scholar] [CrossRef]
- Daw, P.; Chakraborty, S.; Garg, J.A.; Ben-David, Y.; Milstein, D. Direct Synthesis of Pyrroles by Dehydrogenative Coupling of Diols and Amines Catalyzed by Cobalt Pincer Complexes. Angew. Chem. Int. Ed. 2016, 55, 14373–14377. [Google Scholar] [CrossRef]
- Borghs, J.C.; Azofra, L.M.; Biberger, T.; Linnenberg, O.; Cavallo, L.; Rueping, M.; El-Sepelgy, O. Manganese-Catalyzed Multicomponent Synthesis of Pyrroles through Acceptorless Dehydrogenation Hydrogen Autotransfer Catalysis: Experiment and Computation. ChemSusChem 2019, 12, 3083–3088. [Google Scholar] [CrossRef]
- Additions to C–X Π-Bonds, Part 2. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherland, 2014; Volume 2, ISBN 978-0-080-97743-0. [Google Scholar]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. 3d-Metal Catalyzed N- and C-Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2018, 119, 2524–2549. [Google Scholar] [CrossRef]
- Obora, Y. Recent Advances in α-Alkylation Reactions using Alcohols with Hydrogen Borrowing Methodologies. ACS Catal. 2014, 4, 3972–3981. [Google Scholar] [CrossRef]
- Shimizu, K. Heterogeneous Catalysis for the Direct Synthesis of Chemicals by Borrowing Hydrogen Methodology. Catal. Sci. Technol. 2015, 5, 1412–1427. [Google Scholar] [CrossRef]
- Zhang, G.; Irrgang, T.; Dietel, T.; Kallmeier, F.; Kempe, R. Manganese-Catalyzed Dehydrogenative Alkylation or α-Olefination of Alkyl-Substituted N-Heteroarenes with Alcohols. Angew. Chem. Int. Ed. 2018, 57, 9131–9135. [Google Scholar] [CrossRef]
- Gawali, S.S.; Pandia, B.K.; Gunanathan, C. Manganese(I)-Catalyzed α-Alkenylation of Ketones Using Primary Alcohols. Org. Lett. 2019, 21, 3842–3847. [Google Scholar] [CrossRef]
- Gawali, S.S.; Pandia, B.K.; Pal, S.; Gunanathan, C. Manganese(I)-Catalyzed Cross-Coupling of Ketones and Secondary Alcohols with Primary Alcohols. ACS Omega 2019, 4, 10741–10754. [Google Scholar] [CrossRef]
- Balaraman, E.; Khaskin, E.; Leitus, G.; Milstein, D. Catalytic Transformation of Alcohols to Carboxylic Acid Salts and H2 Using Water as the Oxygen Atom Source. Nat. Chem. 2013, 5, 122–125. [Google Scholar] [CrossRef]
- Hu, P.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Reusable Homogeneous Catalytic System for Hydrogen Production from Methanol and Water. ACS Catal. 2014, 4, 2649–2652. [Google Scholar] [CrossRef]
- Vogt, M.; Nerush, A.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Reversible CO2 Binding Triggered by Metal–Ligand Cooperation in a Rhenium(I) PNP Pincer-Type Complex and the Reaction with Dihydrogen. Chem. Sci. 2014, 5, 2043–2051. [Google Scholar] [CrossRef]
- Luconi, L.; Demirci, U.B.; Peruzzini, M.; Giambastiani, G.; Rossin, A. Ammonia Borane and Hydrazine Bis(borane) Dehydrogenation Mediated by an Unsymmetrical (PNN) Ruthenium Pincer Hydride: Metal–Ligand Cooperation for Hydrogen Production. Sustain. Energy Fuels 2019, 3, 2583–2596. [Google Scholar] [CrossRef]
- Bruffaerts, J.; Von Wolff, N.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Formamides as Isocyanate Surrogates: A Mechanistically Driven Approach to the Development of Atom-Efficient, Selective Catalytic Syntheses of Ureas, Carbamates, and Heterocycles. J. Am. Chem. Soc. 2019, 141, 16486–16493. [Google Scholar] [CrossRef]
- Štefane, B.; Požgan, F. Advances in Catalyst Systems for the Asymmetric Hydrogenation and Transfer Hydrogenation of Ketones. Catal. Rev. 2014, 56, 82–174. [Google Scholar] [CrossRef]
- Ito, J.; Nishiyama, H. Recent Topics of Transfer Hydrogenation. Tetrahedron Lett. 2014, 55, 3133–3146. [Google Scholar] [CrossRef]
- Wang, N.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, A.; Kayaki, Y. Upgrading and Expanding the Scope of Homogeneous Transfer Hydrogenation. Tetrahedron Lett. 2018, 59, 504–513. [Google Scholar] [CrossRef]
- Warner, M.C.; Casey, C.P.; Bäckvall, J.-E. Shvo’s Catalyst in Hydrogen Transfer Reactions. In Organometallics in Process Chemistry; Springer Science and Business Media LLC: Berlin, Germany, 2011; Volume 37, pp. 85–125. ISBN 978-3-642-20731-0. [Google Scholar]
- Fujii, A.; Hashiguchi, S.; Uematsu, N.; Ikariya, T.; Noyori, R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid−Triethylamine Mixture. J. Am. Chem. Soc. 1996, 118, 2521–2522. [Google Scholar] [CrossRef]
- He, L.-P.; Chen, T.; Xue, D.-X.; Eddaoudi, M.; Huang, K.-W. Efficient Transfer Hydrogenation Reaction Catalyzed by a Dearomatized PN3P ruthenium pincer complex under base-free Conditions. J. Organomet. Chem. 2012, 700, 202–206. [Google Scholar] [CrossRef]
- Chen, T.; He, L.-P.; Gong, D.; Yang, L.; Miao, X.; Eppinger, J.; Huang, K.-W. Ruthenium(II) Pincer Complexes with Oxazoline Arms for Efficient Transfer Hydrogenation Reactions. Tetrahedron Lett. 2012, 53, 4409–4412. [Google Scholar] [CrossRef]
- Guillena, G.; Ramón, D.J.; Yus, M. Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds using Alcohols and Amines as Electrophiles. Chem. Rev. 2009, 110, 1611–1641. [Google Scholar] [CrossRef]
- Bähn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. The Catalytic Amination of Alcohols. ChemCatChem 2011, 3, 1853–1864. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Yu, Z. Substitution of Alcohols by N-Nucleophiles via Transition Metal-Catalyzed Dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Yao, W.; Leng, X.; Huang, Z. Iridium-Catalyzed Selective α-Alkylation of Unactivated Amides with Primary Alcohols. Org. Lett. 2013, 15, 1144–1147. [Google Scholar] [CrossRef]
- Montag, M.; Zhang, J.; Milstein, D. Aldehyde Binding through Reversible C–C Coupling with the Pincer Ligand upon Alcohol Dehydrogenation by a PNP–Ruthenium Catalyst. J. Am. Chem. Soc. 2012, 134, 10325–10328. [Google Scholar] [CrossRef]
- Huff, C.A.; Kampf, J.W.; Sanford, M.S. Role of a Noninnocent Pincer Ligand in the Activation of CO2 at (PNN)Ru(H)(CO). Organometallics 2012, 31, 4643–4645. [Google Scholar] [CrossRef]
- Vogt, M.; Wheelaghan, O.R.; Iron, M.A.; Leitus, G.; Diskin-Posner, Y.; Shimon, L.J.W.; Ben-David, Y.; Milstein, D. Anionic Nickel(II) Complexes with Doubly Deprotonated PNP Pincer-Type Ligands and Their Reactivity toward CO2. Organometallics 2012, 32, 300–308. [Google Scholar] [CrossRef]
- Vogt, M.; Nerush, A.; Iron, M.A.; Leitus, G.; Diskin-Posner, Y.; Shimon, L.J.W.; Ben-David, Y.; Milstein, D. Activation of Nitriles by Metal Ligand Cooperation. Reversible Formation of Ketimido- and Enamido-Rhenium PNP Pincer Complexes and Relevance to Catalytic Design. J. Am. Chem. Soc. 2013, 135, 17004–17018. [Google Scholar] [CrossRef] [PubMed]
- Nerush, A.; Vogt, M.; Gellrich, U.; Leitus, G.; Ben-David, Y.; Milstein, D. Template Catalysis by Metal–Ligand Cooperation. C–C Bond Formation via Conjugate Addition of Non-Activated Nitriles under Mild, Base-Free Conditions Catalyzed by a Manganese Pincer Complex. J. Am. Chem. Soc. 2016, 138, 6985–6997. [Google Scholar] [CrossRef]
- Nising, C.F.; Bräse, S. The Oxa-Michael Reaction: From Recent Developments to Applications in Natural Product Synthesis. Chem. Soc. Rev. 2008, 37, 1218–1228. [Google Scholar] [CrossRef]
- Nising, C.F.; Bräse, S.; Bräse, S. Recent Developments in the Field of Oxa-Michael Reactions. Chem. Soc. Rev. 2012, 41, 988–999. [Google Scholar] [CrossRef]
- Perdriau, S.; Zijlstra, D.S.; Heeres, H.J.; de Vries, J.G.; Otten, E. A Metal-Ligand Cooperative Pathway for Intermolecular Oxa-Michael Additions to Unsaturated Nitriles. Angew. Chem. Int. Ed. 2015, 54, 4236–4240. [Google Scholar] [CrossRef]
- Guo, B.; Zijlstra, D.S.; de Vries, J.G.; Otten, E. Oxa-Michael Addition to α,β-Unsaturated Nitriles: An Expedient Route to γ-Amino Alcohols and Derivatives. ChemCatChem 2018, 10, 2868–2872. [Google Scholar] [CrossRef]
- Tang, S.; Milstein, D. Template Catalysis by Manganese Pincer Complexes: Oxa- and Aza-Michael Additions to Unsaturated Nitriles. Chem. Sci. 2019, 10, 8990–8994. [Google Scholar] [CrossRef]
- Guo, B.; de Vries, J.G.; Otten, E. Hydration of Nitriles Using a Metal-Ligand Cooperative Ruthenium Pincer Catalyst. Chem. Sci. 2019, 10, 10647–10652. [Google Scholar] [CrossRef]
- Anaby, A.; Butschke, B.; Ben-David, Y.; Shimon, L.J.W.; Leitus, G.; Feller, M.; Milstein, D. B–H Bond Cleavage via Metal–Ligand Cooperation by Dearomatized Ruthenium Pincer Complexes. Organometallics 2014, 33, 3716–3726. [Google Scholar] [CrossRef]
- Scharf, A.; Goldberg, I.; Vigalok, A. Evidence for Metal–Ligand Cooperation in a Pd–PNF Pincer-Catalyzed Cross-Coupling. J. Am. Chem. Soc. 2012, 135, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Katritzky, A.R. Tautomeric pyridines. Part XV. Pyridone-Hydroxypyridine Equilibria in Solvents of Differing Polarity. J. Chem. Soc. Perkin Trans. 1976, 2, 1428–1431. [Google Scholar] [CrossRef]
- Hejazi, S.A.; Osman, O.I.; Alyoubi, A.O.; Aziz, S.G.; Hilal, A.R. The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. Int. J. Mol. Sci. 2016, 17, 1893. [Google Scholar] [CrossRef] [PubMed]
- Royer, A.M.; Rauchfuss, T.B.; Gray, D.L. Organoiridium Pyridonates and Their Role in the Dehydrogenation of Alcohols. Organometallics 2010, 29, 6763–6768. [Google Scholar] [CrossRef]
- Tard, C.; Pickett, C.J. Structural and Functional Analogues of the Active Sites of the [Fe]-, [NiFe]-, and [FeFe]-Hydrogenases. Chem. Rev. 2009, 109, 2245–2274. [Google Scholar] [CrossRef]
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E.; Rüdiger, O. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef]
- Moore, C.M.; Dahl, E.; Szymczak, N.K. Beyond H2: Exploiting 2-Hydroxypyridine as a Design Element from [Fe]-Hydrogenase for Energy-Relevant Catalysis. Curr. Opin. Chem. Boil. 2015, 25, 9–17. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Cooperative Catalysis by Iridium Complexes with a Bipyridonate Ligand: Versatile Dehydrogenative Oxidation of Alcohols and Reversible Dehydrogenation-Hydrogenation between 2-Propanol and Acetone. Angew. Chem. Int. Ed. 2012, 51, 12790–12794. [Google Scholar] [CrossRef]
- Onoda, M.; Nagano, Y.; Fujita, K. Iridium-Catalyzed Dehydrogenative Lactonization of 1,4-Butanediol and Reversal Hydrogenation: New Hydrogen Storage System Using Cheap Organic Resources. Int. J. Hydrogen Energy 2019, 44, 28514–28520. [Google Scholar] [CrossRef]
- Hull, J.F.; Himeda, Y.; Wang, W.-H.; Hashiguchi, B.; Periana, R.; Szalda, D.J.; Muckerman, J.T.; Fujita, E. Reversible Hydrogen Storage Using CO2 and a Proton-Switchable Iridium Catalyst in Aqueous Media under Mild Temperatures and Pressures. Nat. Chem. 2012, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Mechanistic Insight through Factors Controlling Effective Hydrogenation of CO2 Catalyzed by Bioinspired Proton-Responsive Iridium(III) Complexes. ACS Catal. 2013, 3, 856–860. [Google Scholar] [CrossRef]
- Wang, L.; Onishi, N.; Murata, K.; Hirose, T.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Inside Cover: Efficient Hydrogen Storage and Production Using a Catalyst with an Imidazoline-Based, Proton-Responsive Ligand (ChemSusChem 6/2017). ChemSusChem 2017, 10, 1035. [Google Scholar] [CrossRef]
- Wang, W.-H.; Hull, J.F.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Second-Coordination-Sphere and Electronic Effects Enhance Iridium(iii)-Catalyzed Homogeneous Hydrogenation of Carbon Dioxide in Water Near Ambient Temperature and Pressure. Energy Environ. Sci. 2012, 5, 7923. [Google Scholar] [CrossRef]
- Suna, Y.; Ertem, M.Z.; Wang, W.-H.; Kambayashi, H.; Manaka, Y.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Positional Effects of Hydroxy Groups on Catalytic Activity of Proton-Responsive Half-Sandwich Cp*Iridium(III) Complexes. Organometallics 2014, 33, 6519–6530. [Google Scholar] [CrossRef]
- Siek, S.; Burks, D.B.; Gerlach, D.L.; Liang, G.; Tesh, J.M.; Thompson, C.R.; Qu, F.; Shankwitz, J.E.; Vasquez, R.M.; Chambers, N.; et al. Iridium and Ruthenium Complexes of N-Heterocyclic Carbene- and Pyridinol-Derived Chelates as Catalysts for Aqueous Carbon Dioxide Hydrogenation and Formic Acid Dehydrogenation: The Role of the Alkali Metal. Organometallics 2017, 36, 1091–1106. [Google Scholar] [CrossRef]
- Sahoo, A.R.; Jiang, F.; Bruneau, C.; Sharma, G.V.M.; Suresh, S.; Roisnel, T.; Dorcet, V.; Achard, M.J. Phosphine-Pyridonate Ligands Containing Octahedral Ruthenium Complexes: Access to Esters and Formic Acid. Catal. Sci. Technol. 2017, 7, 3492–3498. [Google Scholar] [CrossRef]
- Dubey, A.; Nencini, L.; Fayzullin, R.R.; Nervi, C.; Khusnutdinova, J.R. Bio-Inspired Mn(I) Complexes for the Hydrogenation of CO2 to Formate and Formamide. ACS Catal. 2017, 7, 3864–3868. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hydrogen Storage in Liquid Organic Heterocycles. Energy Environ. Sci. 2008, 1, 134. [Google Scholar] [CrossRef]
- Shimbayashi, T.; Fujita, K. Metal-Catalyzed Hydrogenation and Dehydrogenation Reactions for Efficient Hydrogen Storage. Tetrahedron 2020, 76, 130946. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Ikeda, C.; Takahashi, Y.; Fujita, K. Homogeneous Catalytic System for Reversible Dehydrogenation–Hydrogenation Reactions of Nitrogen Heterocycles with Reversible Interconversion of catalytic species. J. Am. Chem. Soc. 2009, 131, 8410–8412. [Google Scholar] [CrossRef]
- Fujita, K.; Tanaka, Y.; Kobayashi, M.; Yamaguchi, R. Homogeneous Perdehydrogenation and Perhydrogenation of Fused Bicyclic N-Heterocycles Catalyzed by Iridium Complexes Bearing a Functional Bipyridonate Ligand. J. Am. Chem. Soc. 2014, 136, 4829–4832. [Google Scholar] [CrossRef]
- Fujita, K.; Wada, T.; Shiraishi, T. Reversible Interconversion between 2,5-Dimethylpyrazine and 2,5-Dimethylpiperazine by Iridium-Catalyzed Hydrogenation/Dehydrogenation for Efficient Hydrogen Storage. Angew. Chem. Int. Ed. 2017, 56, 10886–10889. [Google Scholar] [CrossRef]
- Maji, B.; Choudhury, J. Switchable Hydrogenation with a Betaine-Derived Bifunctional Ir-NHC Catalyst. Chem. Commun. 2019, 55, 4574–4577. [Google Scholar] [CrossRef]
- Fujita, K. Development and Application of New Iridium Catalysts for Efficient Dehydrogenative Reactions of Organic Molecules. Bull. Chem. Soc. Jpn. 2019, 92, 344–351. [Google Scholar] [CrossRef]
- Fujita, K.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Dehydrogenative Oxidation of Primary and Secondary Alcohols Catalyzed by a Cp*Ir Complex Having a Functional C,N-Chelate Ligand. Org. Lett. 2011, 13, 2278–2281. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Using Water-Soluble and Reusable Cp*Ir Catalysts Bearing a Functional Bipyridine Ligand. J. Am. Chem. Soc. 2012, 134, 3643–3646. [Google Scholar] [CrossRef]
- Toyomura, K.; Fujita, K. Synthesis of Coordinatively Unsaturated Iridium Complexes Having Functional 8-Quinolinolato Ligands: New Catalysts for Dehydrogenative Oxidation of Alcohols in Aqueous Media. Chem. Lett. 2017, 46, 808–810. [Google Scholar] [CrossRef]
- Fujita, K.; Tamura, R.; Tanaka, Y.; Yoshida, M.; Onoda, M.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Catalyzed by a Water-Soluble Dicationic Iridium Complex Bearing a Functional N-Heterocyclic Carbene Ligand without Using Base. ACS Catal. 2017, 7, 7226–7230. [Google Scholar] [CrossRef]
- Yoshida, M.; Wang, H.; Shimbayashi, T.; Fujita, K. Dehydrogenative Transformation of Alcoholic Substrates in Aqueous Media Catalyzed by an Iridium Complex Having a Functional Ligand with α-Hydroxypyridine and 4,5-Dihydro-1H-imidazol-2-yl Moieties. Catalysts 2018, 8, 312. [Google Scholar] [CrossRef]
- Zeng, G.; Sakaki, S.; Fujita, K.; Sano, H.; Yamaguchi, R. Efficient Catalyst for Acceptorless Alcohol Dehydrogenation: Interplay of Theoretical and Experimental Studies. ACS Catal. 2014, 4, 1010–1020. [Google Scholar] [CrossRef]
- Kuwahara, M.; Nishioka, M.; Yoshida, M.; Fujita, K. A Sustainable Method for the Synthesis of Acetic Acid Based on Dehydrogenation of an Ethanol-Water Solution Catalyzed by an Iridium Complex Bearing a Functional Bipyridonate Ligand. ChemCatChem 2018, 10, 3636–3640. [Google Scholar] [CrossRef]
- Dahl, E.; Louis-Goff, T.; Szymczak, N.K. Second Sphere Ligand Modifications Enable a Recyclable Catalyst for Oxidant-Free Alcohol Oxidation to Carboxylates. Chem. Commun. 2017, 53, 2287–2289. [Google Scholar] [CrossRef]
- Fujita, K.; Kawahara, R.; Aikawa, T.; Yamaguchi, R. Hydrogen Production from a Methanol-Water Solution Catalyzed by an Anionic Iridium Complex Bearing a Functional Bipyridonate Ligand under Weakly Basic Conditions. Angew. Chem. Int. Ed. 2015, 54, 9057–9060. [Google Scholar] [CrossRef]
- de Boer, S.Y.; Korstanje, T.J.; La Rooij, S.R.; Kox, R.; Reek, J.N.H.; van der Vlugt, J.I. Ruthenium PNN(O) Complexes: Cooperative Reactivity and Application as Catalysts for Acceptorless Dehydrogenative Coupling Reactions. Organometallics 2017, 36, 1541–1549. [Google Scholar] [CrossRef]
- Fujita, K.; Ito, W.; Yamaguchi, R. Dehydrogenative Lactonization of Diols in Aqueous Media Catalyzed by a Water-Soluble Iridium Complex Bearing a Functional Bipyridine Ligand. ChemCatChem 2014, 45, 109–112. [Google Scholar] [CrossRef]
- Wang, R.; Fan, H.; Zhao, W.; Li, F. Acceptorless Dehydrogenative Cyclization of o-Aminobenzyl Alcohols with Ketones to Quinolines in Water Catalyzed by Water-Soluble Metal–Ligand Bifucntional Catalyst [Cp*(6,6‘-(OH)2bpy)(H2O)][OTf]2. Org. Lett. 2016, 18, 3558–3561. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, B.; Chen, D.; Xia, H. Manganese(I)-Catalyzed Transfer Hydrogenation and Acceptorless Dehydrogenative Condensation: Promotional Influence of the Uncoordinated N-Heterocycle. Organometallics 2019, 38, 3218–3226. [Google Scholar] [CrossRef]
- Maji, M.; Chakrabarti, K.; Panja, D.; Kundu, S. Sustainable Synthesis of N-Heterocycles in Water Using Alcohols Following the Double Dehydrogenation Strategy. J. Catal. 2019, 373, 93–102. [Google Scholar] [CrossRef]
- Li, F.; Lu, L.; Liu, P. Acceptorless Dehydrogenative Coupling of o-Aminobenzamides with the Activation of Methanol as a C1 Source for the Construction of Quinazolinones. Org. Lett. 2016, 18, 2580–2583. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Maji, M.; Kundu, S. Cooperative Iridium Complex-Catalyzed Synthesis of Quinoxalines, Benzimidazoles and Quinazolines in Water. Green Chem. 2019, 21, 1999–2004. [Google Scholar] [CrossRef]
- Maji, M.; Kundu, S. Cooperative Ruthenium Complex Catalyzed Multicomponent Synthesis of Pyrimidines. Dalton Trans. 2019, 48, 17479–17487. [Google Scholar] [CrossRef]
- Wang, W.-H.; Ertem, M.Z.; Xu, S.; Onishi, N.; Manaka, Y.; Suna, Y.; Kambayashi, H.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Highly Robust Hydrogen Generation by Bioinspired Ir Complexes for Dehydrogenation of Formic Acid in Water: Experimental and Theoretical Mechanistic Investigations at Different pH. ACS Catal. 2015, 5, 5496–5504. [Google Scholar] [CrossRef]
- Nieto, I.; Livings, M.S.; Sacci, J.B.; Reuther, L.E.; Zeller, M.; Papish, E.T. Transfer Hydrogenation in Water via a Ruthenium Catalyst with OH Groups near the Metal Center on a bipy Scaffold. Organometallics 2011, 30, 6339–6342. [Google Scholar] [CrossRef]
- Moore, C.M.; Szymczak, N.K. 6,6’-Dihydroxy Terpyridine: A Proton-Responsive Bifunctional Ligand and Its Application in Catalytic Transfer Hydrogenation of Ketones. Chem. Commun. 2013, 49, 400–402. [Google Scholar] [CrossRef]
- Shi, J.; Hu, B.; Gong, D.; Shang, S.; Hou, G.; Chen, D. Ruthenium Complexes Bearing an Unsymmetrical Pincer Ligand with a 2-Hydroxypyridylmethylene Fragment: Active Catalysts for Transfer Hydrogenation of Ketones. Dalton Trans. 2016, 45, 4828–4834. [Google Scholar] [CrossRef]
- Shi, J.; Hu, B.; Chen, X.; Shang, S.; Deng, D.; Sun, Y.; Shi, W.; Yang, X.; Chen, D. Synthesis, Reactivity, and Catalytic Transfer Hydrogenation Activity of Ruthenium Complexes Bearing NNN Tridentate Ligands: Influence of the Secondary Coordination Sphere. ACS Omega 2017, 2, 3406–3416. [Google Scholar] [CrossRef]
- Shi, J.; Shang, S.; Hu, B.; Chen, D. Ruthenium NNN Complexes with a 2-Hydroxypyridylmethylene Fragment for Transfer Hydrogenation of Ketones. Appl. Organomet. Chem. 2017, 32, e4100. [Google Scholar] [CrossRef]
- Paul, B.; Chakrabarti, K.; Kundu, S. Optimum Bifunctionality in a 2-(2-Pyridyl-2-ol)-1,10-phenanthroline Based Ruthenium Complex for Transfer Hydrogenation of Ketones and Nitriles: Impact of the Number of 2-Hydroxypyridine Fragments. Dalton Trans. 2016, 45, 11162–11171. [Google Scholar] [CrossRef]
- Wang, R.; Tang, Y.; Xu, M.; Meng, C.; Li, F. Transfer Hydrogenation of Aldehydes and Ketones with Isopropanol under Neutral Conditions Catalyzed by a Metal–Ligand Bifunctional Catalyst [Cp*Ir(2,2′-bpyO)(H2O)]. J. Org. Chem. 2018, 83, 2274–2281. [Google Scholar] [CrossRef]
- Yoshida, M.; Hirahata, R.; Inoue, T.; Shimbayashi, T.; Fujita, K. Iridium-Catalyzed Transfer Hydrogenation of Ketones and Aldehydes Using Glucose as a Sustainable Hydrogen Donor. Catalysts 2019, 9, 503. [Google Scholar] [CrossRef]
- Wang, R.; Han, X.; Xu, J.; Liu, P.; Li, F. Transfer Hydrogenation of Ketones and Imines with Methanol under Base-Free Conditions Catalyzed by an Anionic Metal–Ligand Bifunctional Iridium Catalyst. J. Org. Chem. 2020, 85, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Uejima, T.; Yamaguchi, R. Hydrogen-Transfer Oxidation of Primary Alcohols Catalyzed by Iridium Complexes Bearing a Functional Pyridonate Ligand Using Isopropenyl Acetate as a Hydrogen Acceptor. Chem. Lett. 2013, 42, 1496–1498. [Google Scholar] [CrossRef]
- Li, F.; Ma, J.; Wang, N. α-Alkylation of Ketones with Primary Alcohols Catalyzed by a Cp*Ir Complex Bearing a Functional Bipyridonate Ligand. J. Org. Chem. 2014, 79, 10447–10455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.-P.; Hu, B.; Shi, J.; Chen, D. Ruthenium-Catalyzed β-Alkylation of Secondary Alcohols and α-Alkylation of Ketones via Borrowing Hydrogen: Dramatic Influence of the Pendant N-Heterocycle. Organometallics 2019, 38, 654–664. [Google Scholar] [CrossRef]
- Roy, B.C.; Chakrabarti, K.; Shee, S.; Paul, S.; Kundu, S. Bifunctional RuII-Complex-Catalysed Tandem C−C Bond Formation: Efficient and Atom Economical Strategy for the Utilisation of Alcohols as Alkylating Agents. Chem. Eur. J. 2016, 22, 18147–18155. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, K.; Paul, B.; Maji, M.; Roy, B.C.; Shee, S.; Kundu, S. Bifunctional Ru(II) Complex Catalyzed Carbon–Carbon Bond Formation: An Eco-Friendly Hydrogen Borrowing Strategy. Org. Biomol. Chem. 2016, 14, 10988–10997. [Google Scholar] [CrossRef]
- Shi, J.; Hu, B.; Ren, P.; Shang, S.; Yang, X.; Chen, D. Synthesis and Reactivity of Metal–Ligand Cooperative Bifunctional Ruthenium Hydride Complexes: Active Catalysts for β-Alkylation of Secondary Alcohols with Primary Alcohols. Organometallics 2018, 37, 2795–2806. [Google Scholar] [CrossRef]
- Salamanca, V.; Toledo, A.; Albeniz, A.C. [2,2′-Bipyridin]-6(1H)-one, A Truly Cooperating Ligand in the Palladium-Mediated C–H Activation Step: Experimental Evidence in the Direct C-3 Arylation of Pyridine. J. Am. Chem. Soc. 2018, 140, 17851–17856. [Google Scholar] [CrossRef]

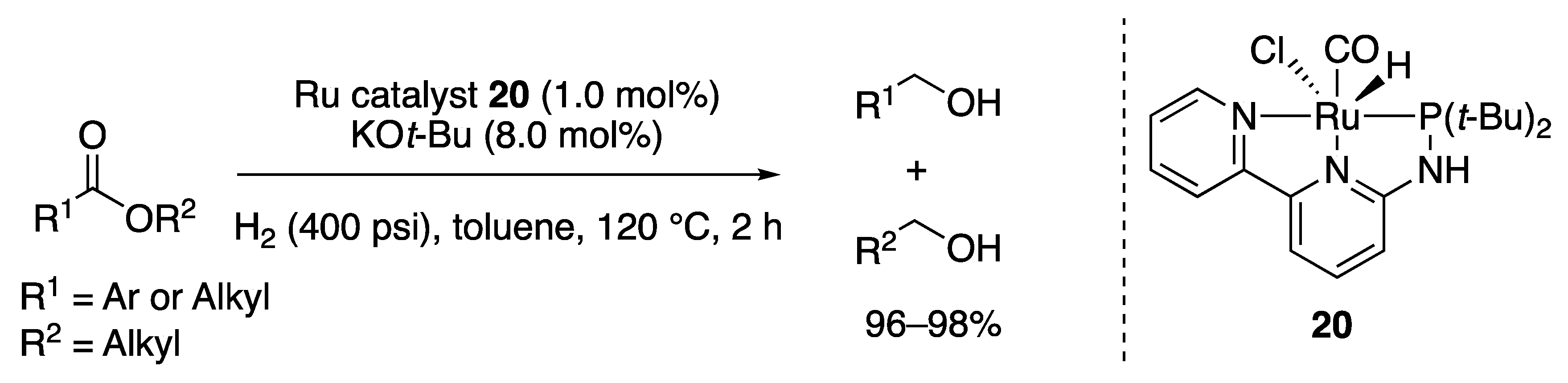















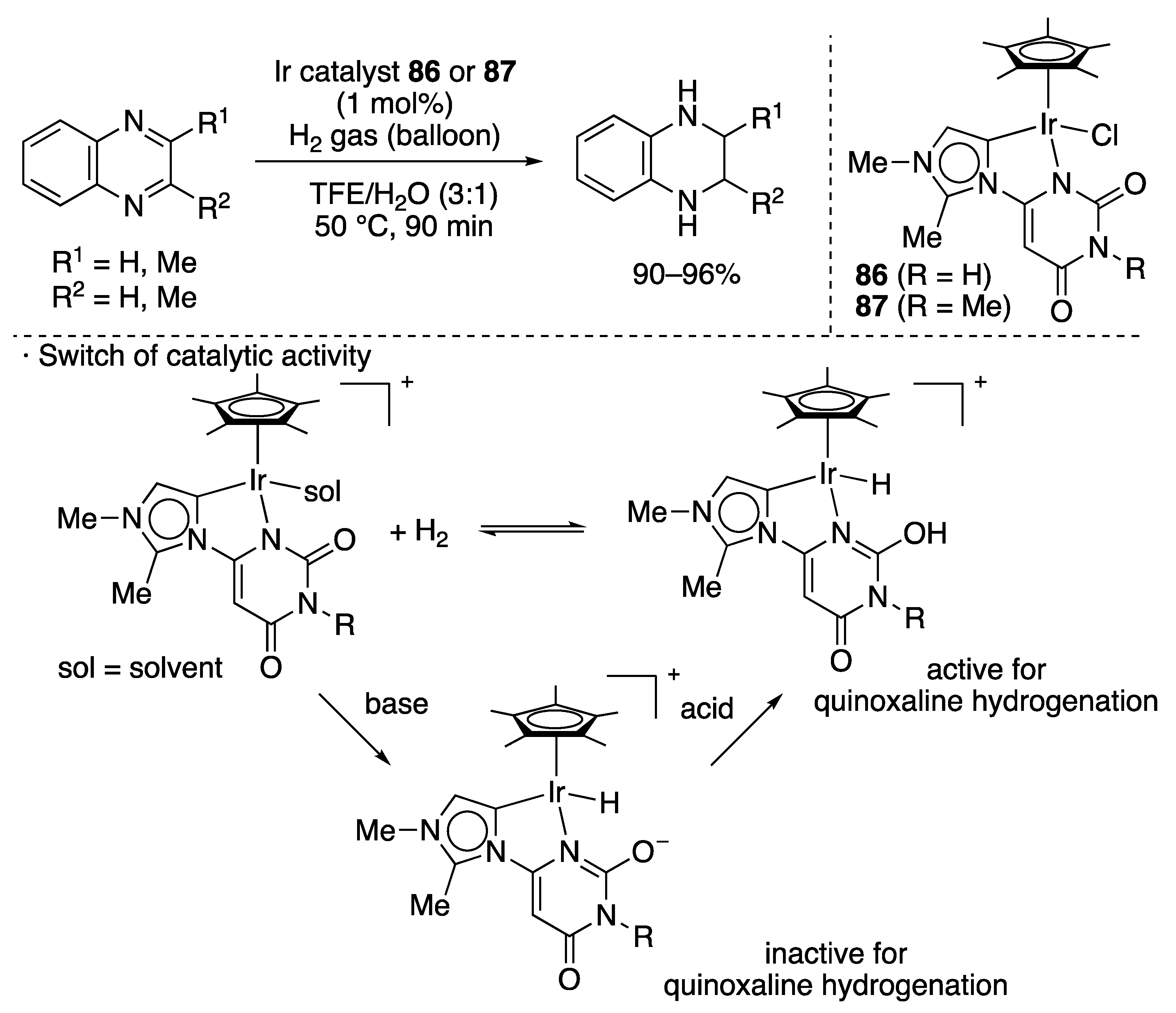
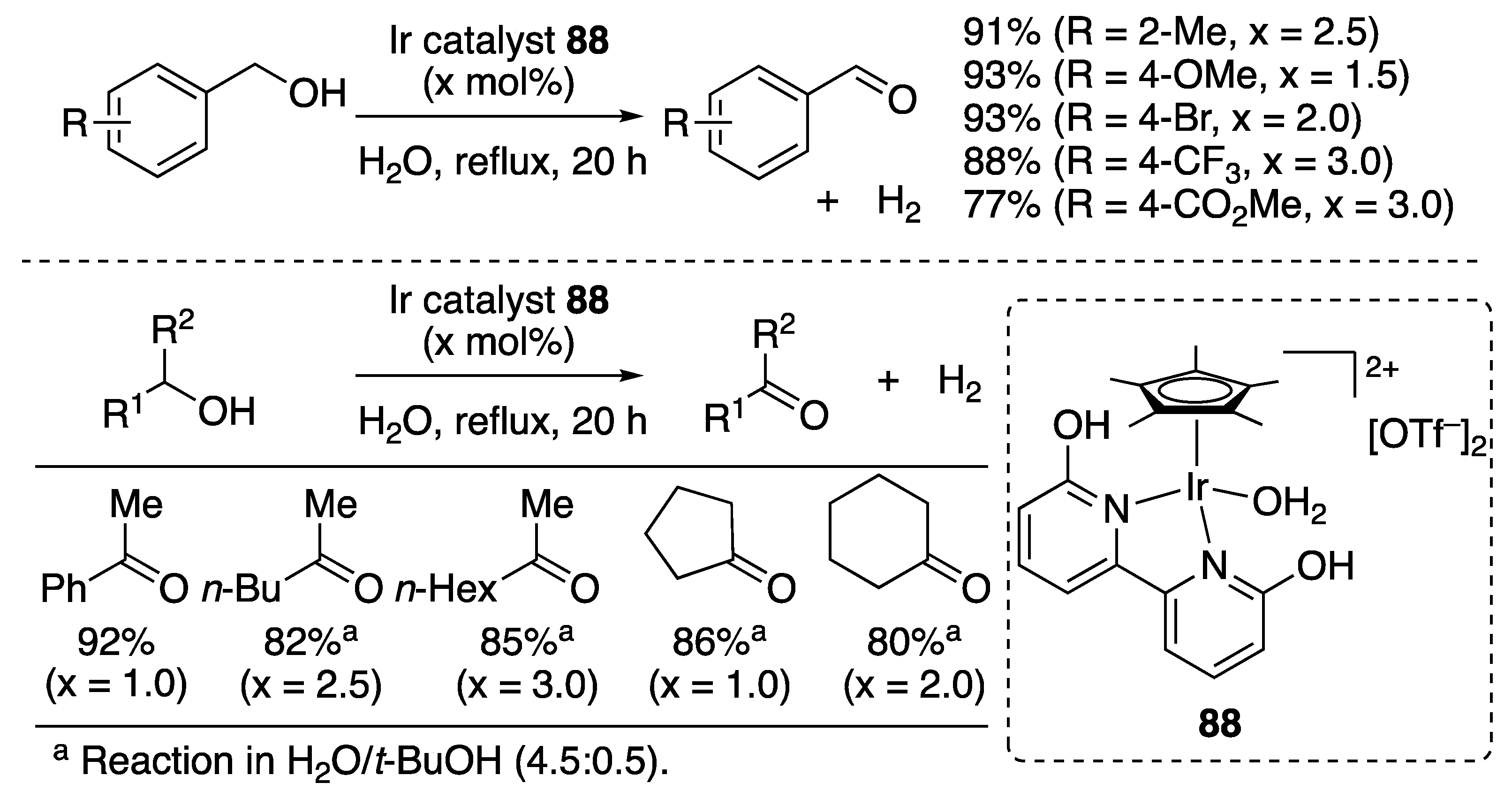
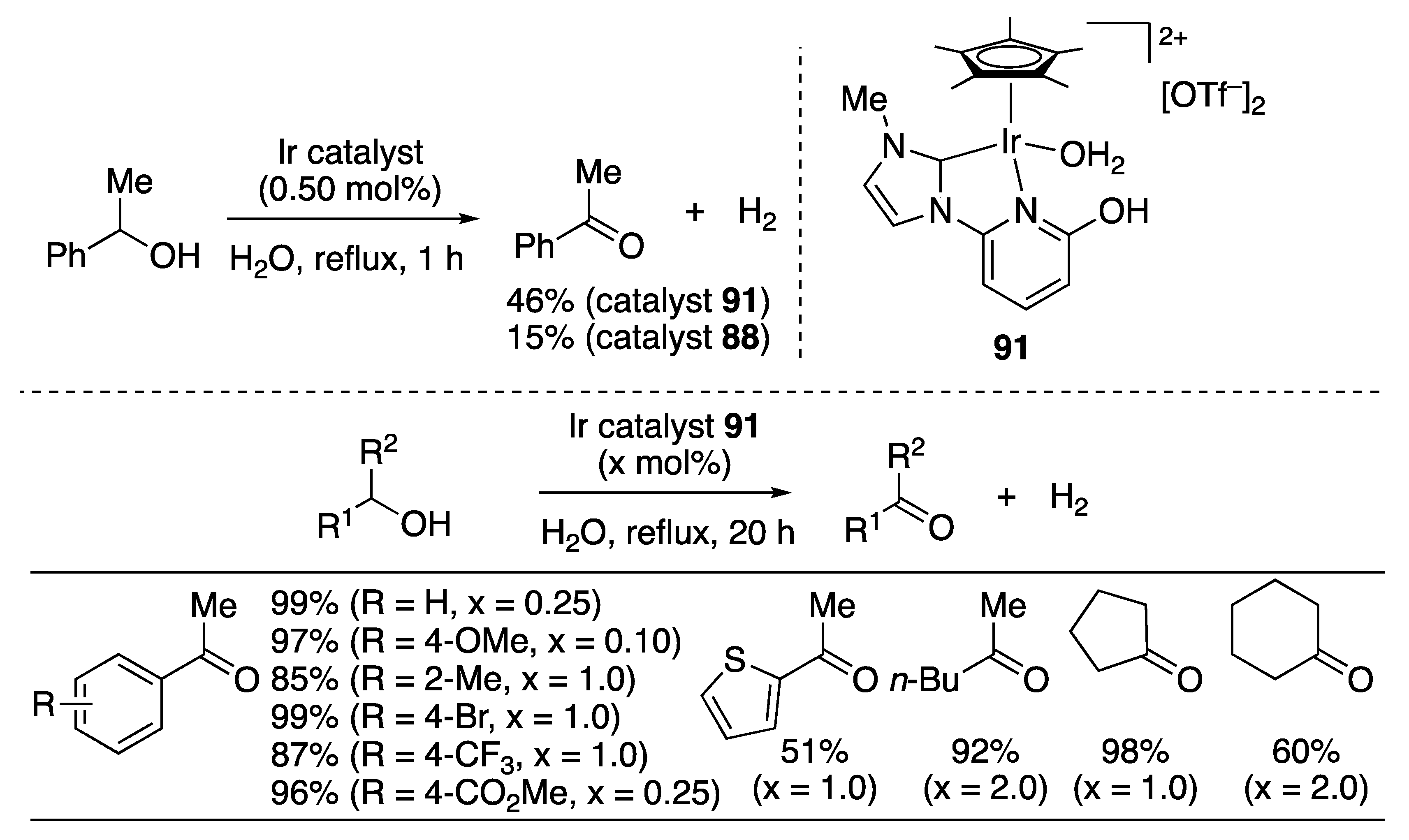
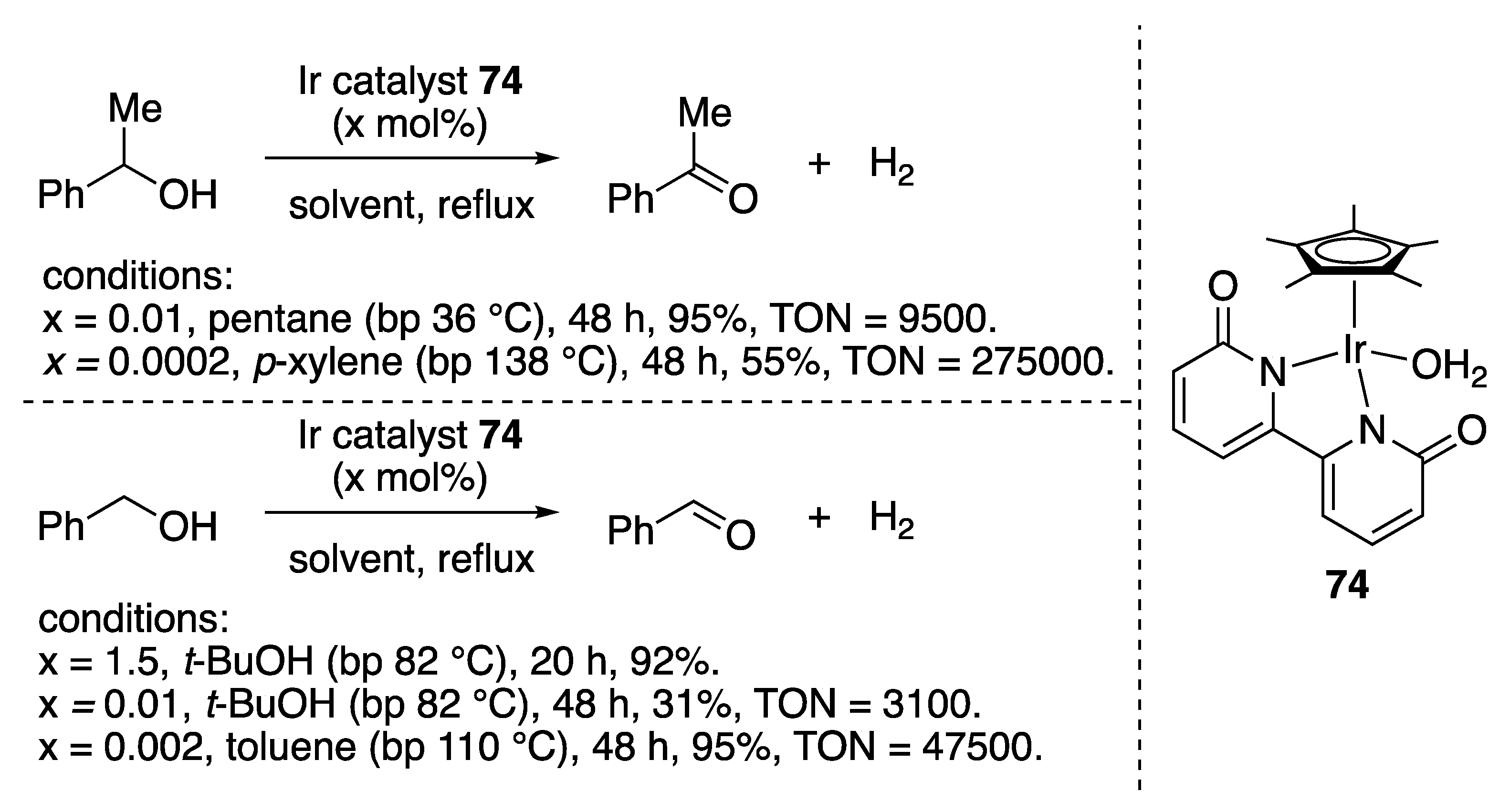
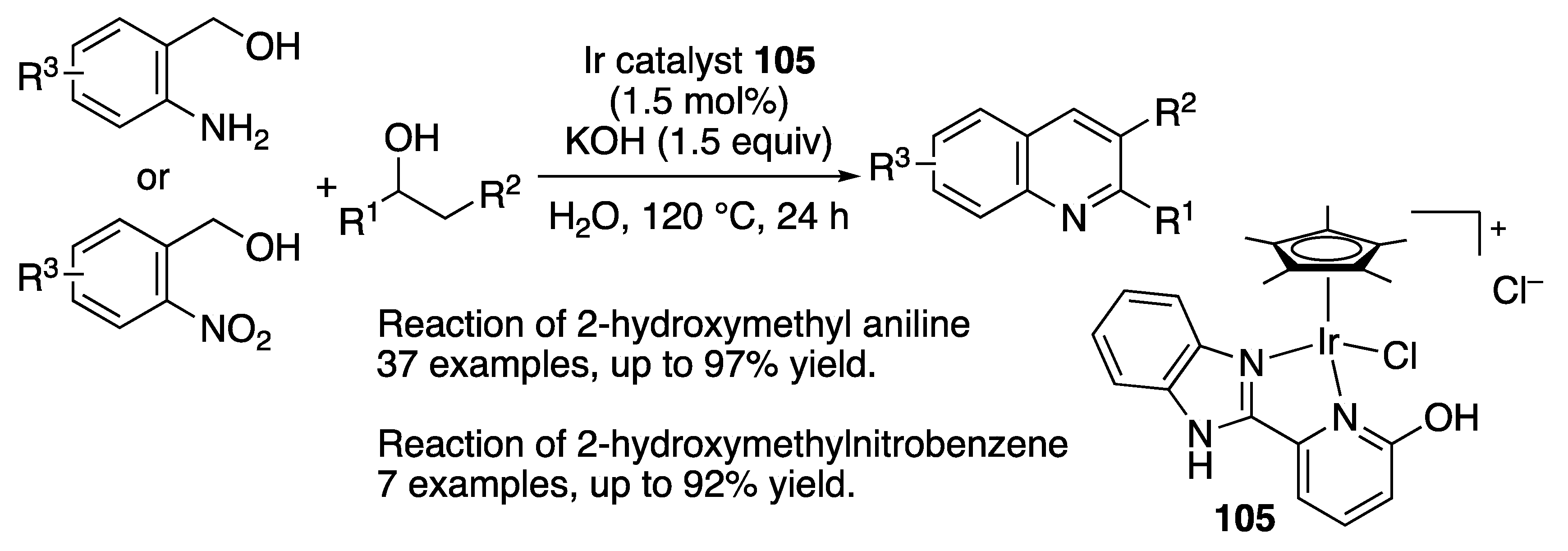
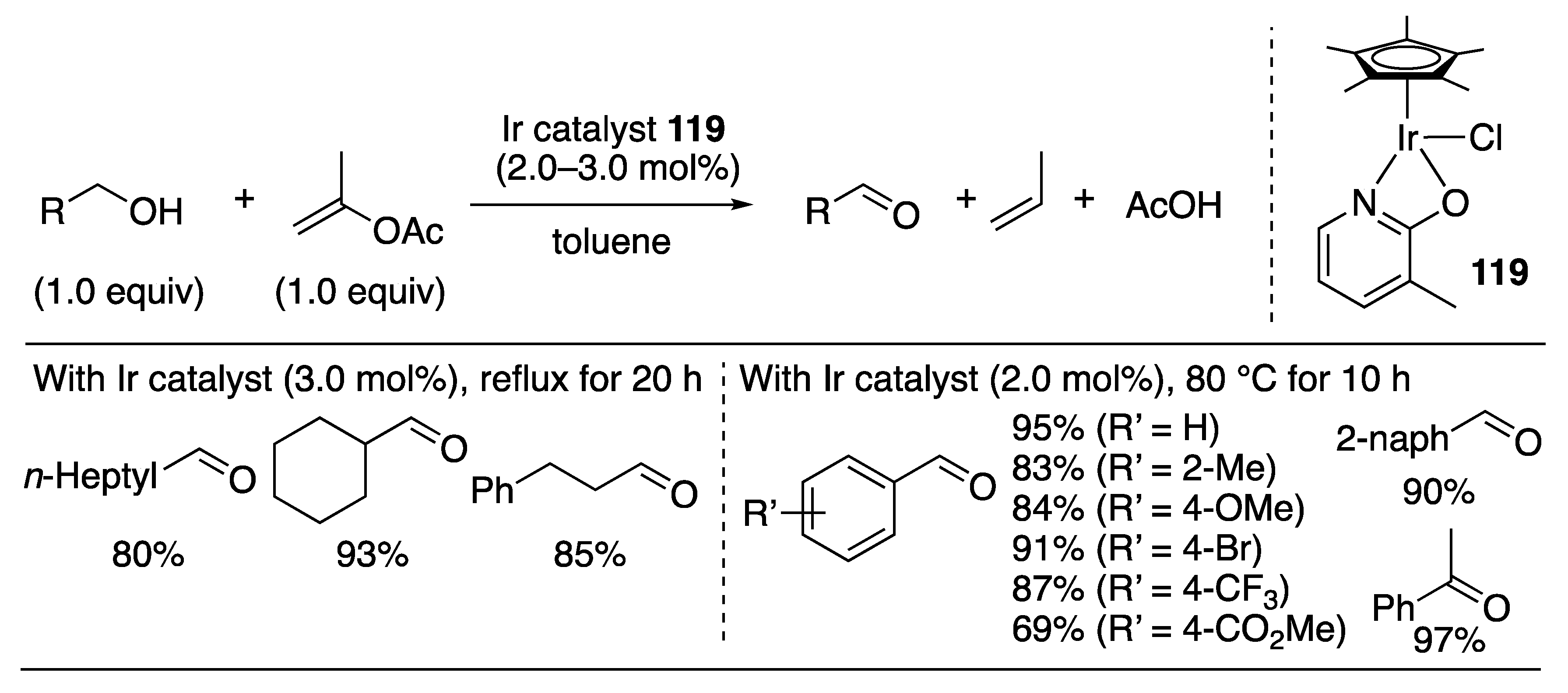

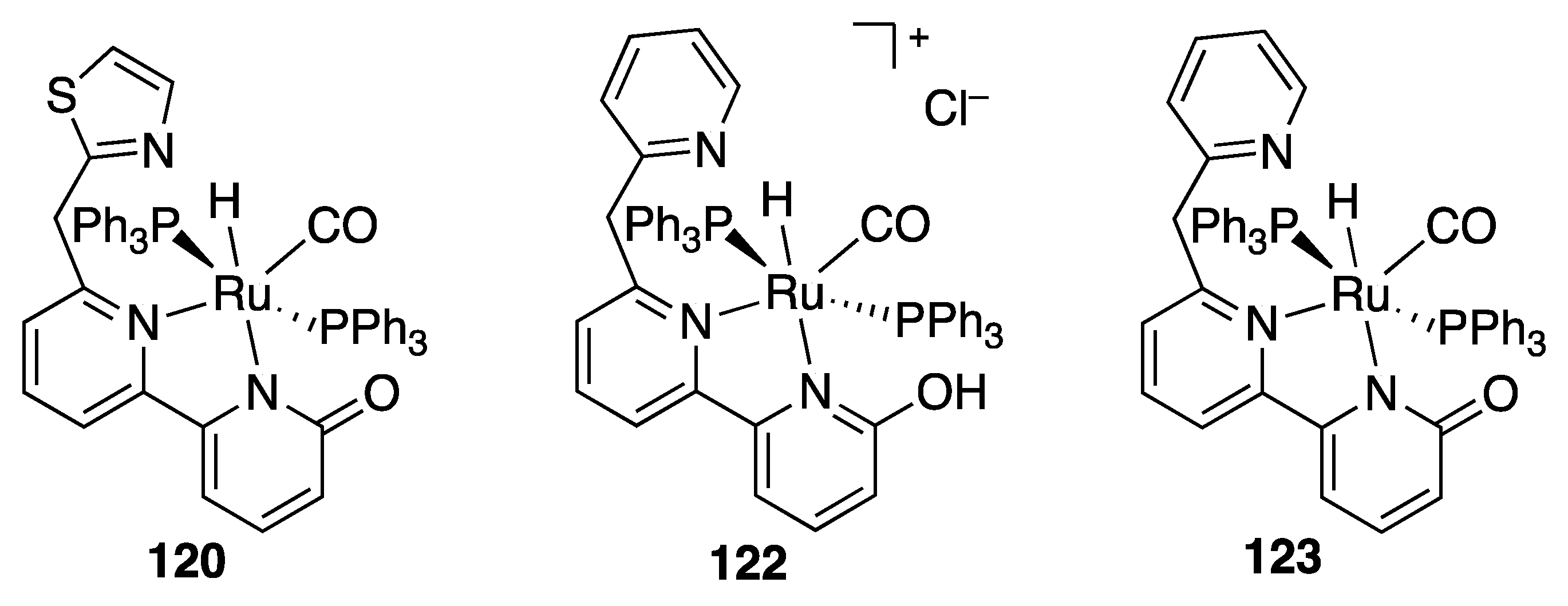
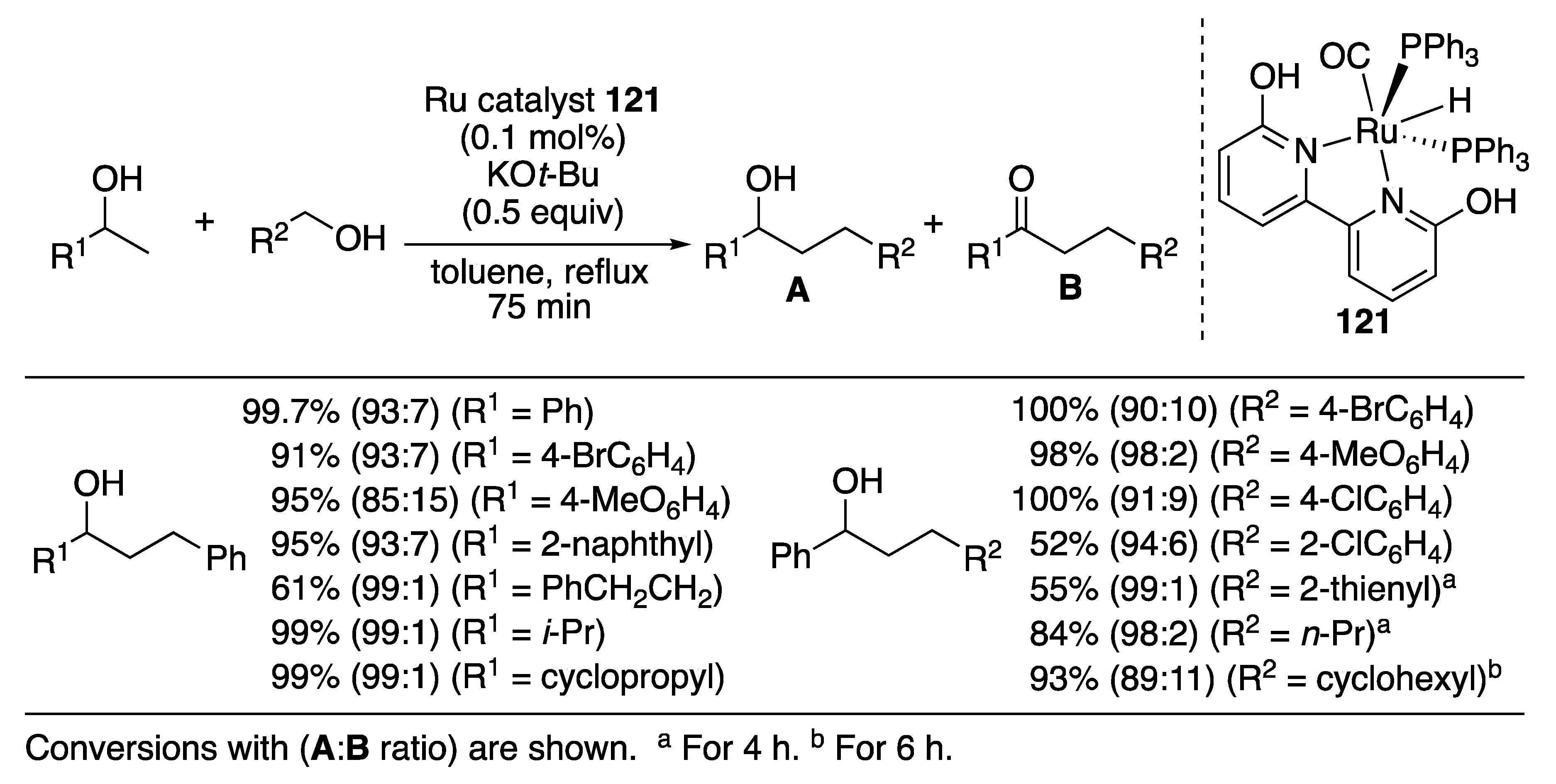
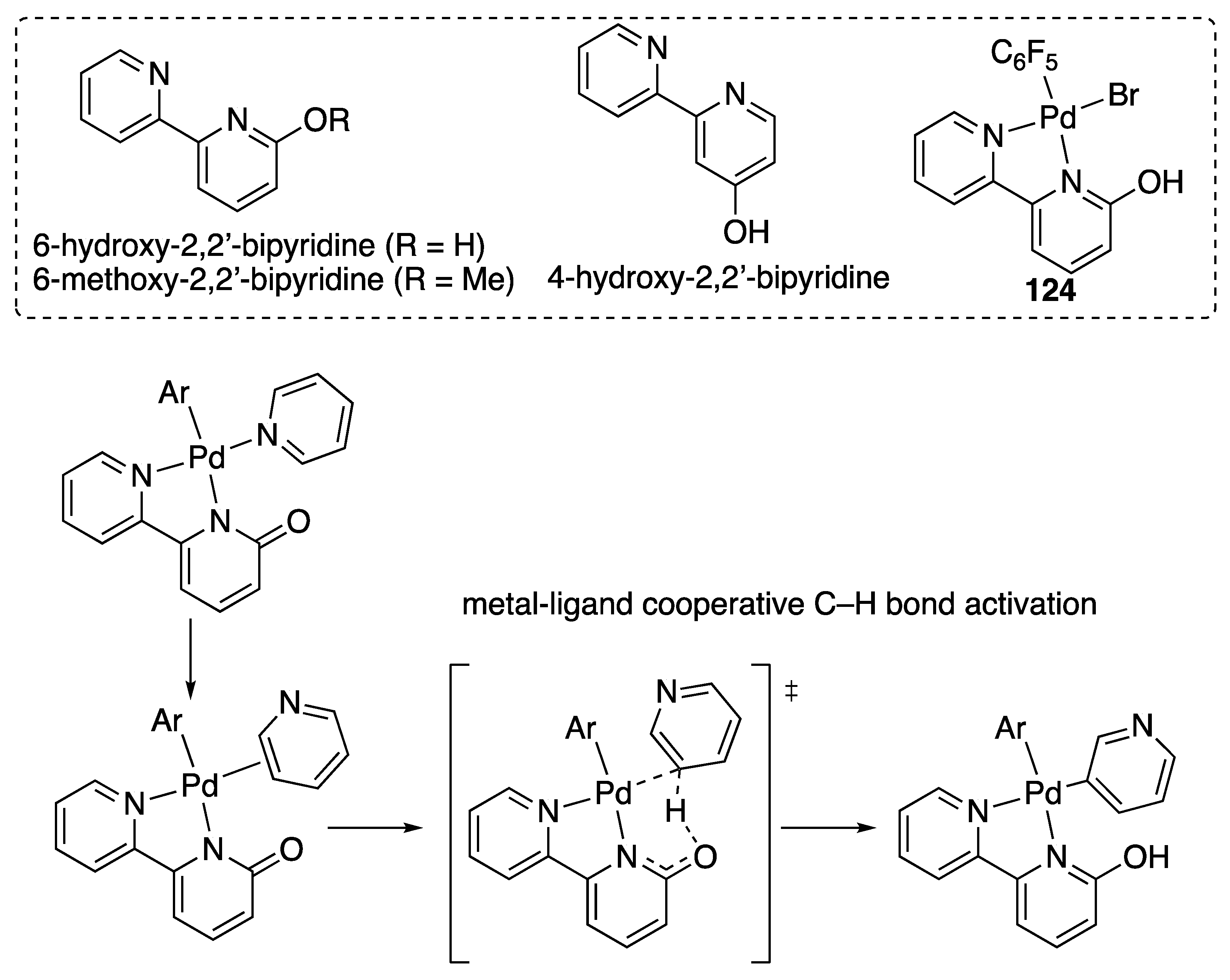
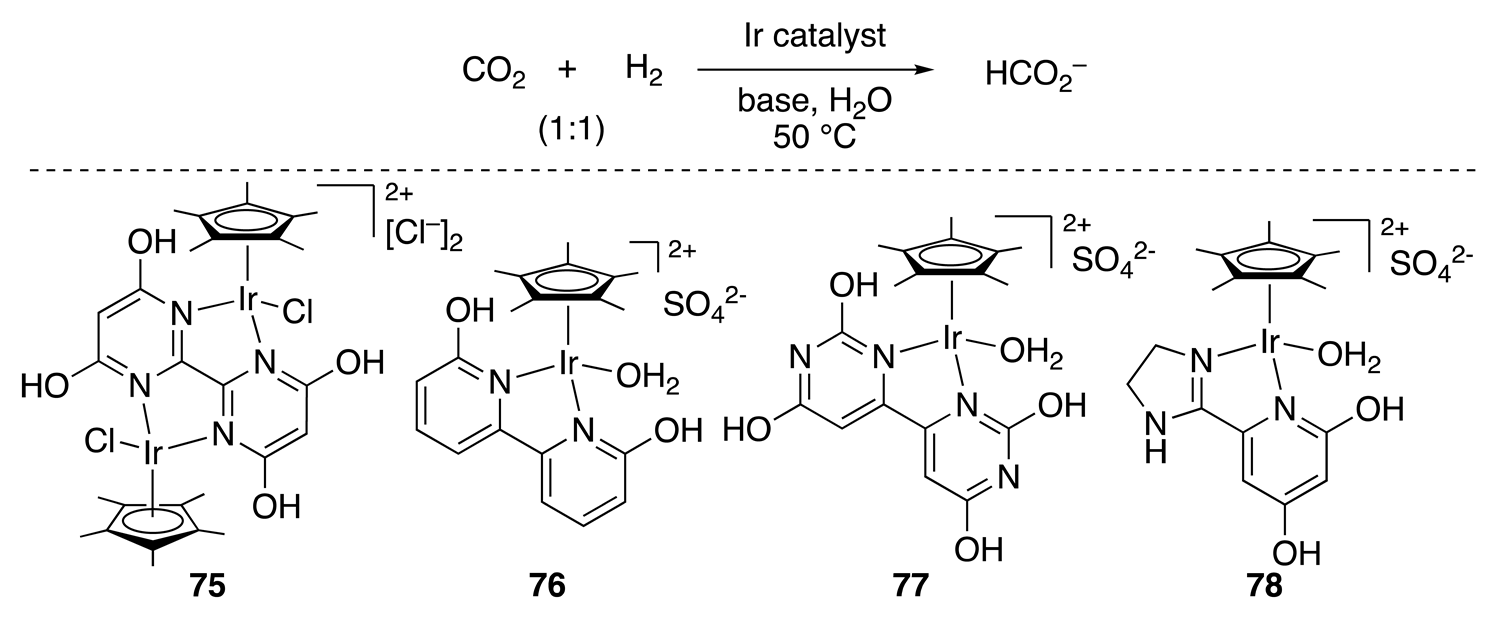
| Entry | Catalyst | Pressure (MPa) | Base | TON |
|---|---|---|---|---|
| 1 | 75 | 1 | 1 M NaHCO3 | 6100 |
| 2 | 75 | 4 | 2 M KHCO3 | 153,000 |
| 3 | 76 | 1.0 | 1 M NaHCO3 | 5150 |
| 4 | 77 | 1.0 | 1 M NaHCO3 | 28,000 |
| 5 | 77 | 3.8 | 1 M NaHCO3 | 38,400 |
| 6 a | 78 | 0.1 | 1 M NaHCO3 | 7280 |
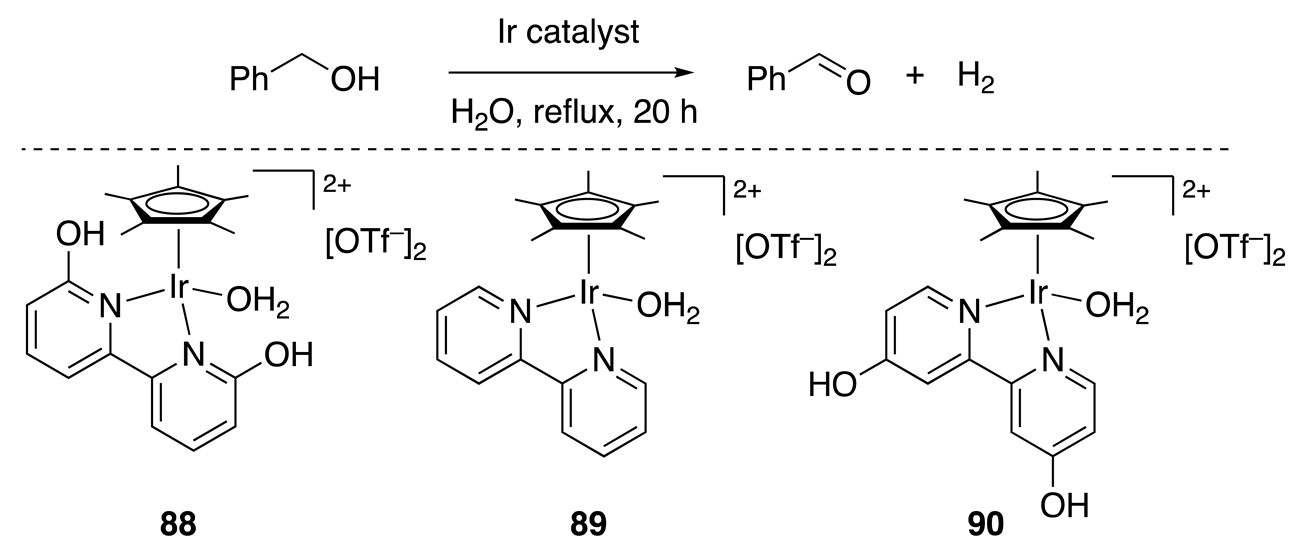
| Entry | Catalyst (mol%) | Conv (%) | Yield (%) |
|---|---|---|---|
| 1 | 88 (0.5) | 63 | 62 |
| 2 | 88 (1.5) | 92 | 92 |
| 3 | 89 (0.5) | 25 | 25 |
| 4 | 90 (0.5) | 23 | 22 |
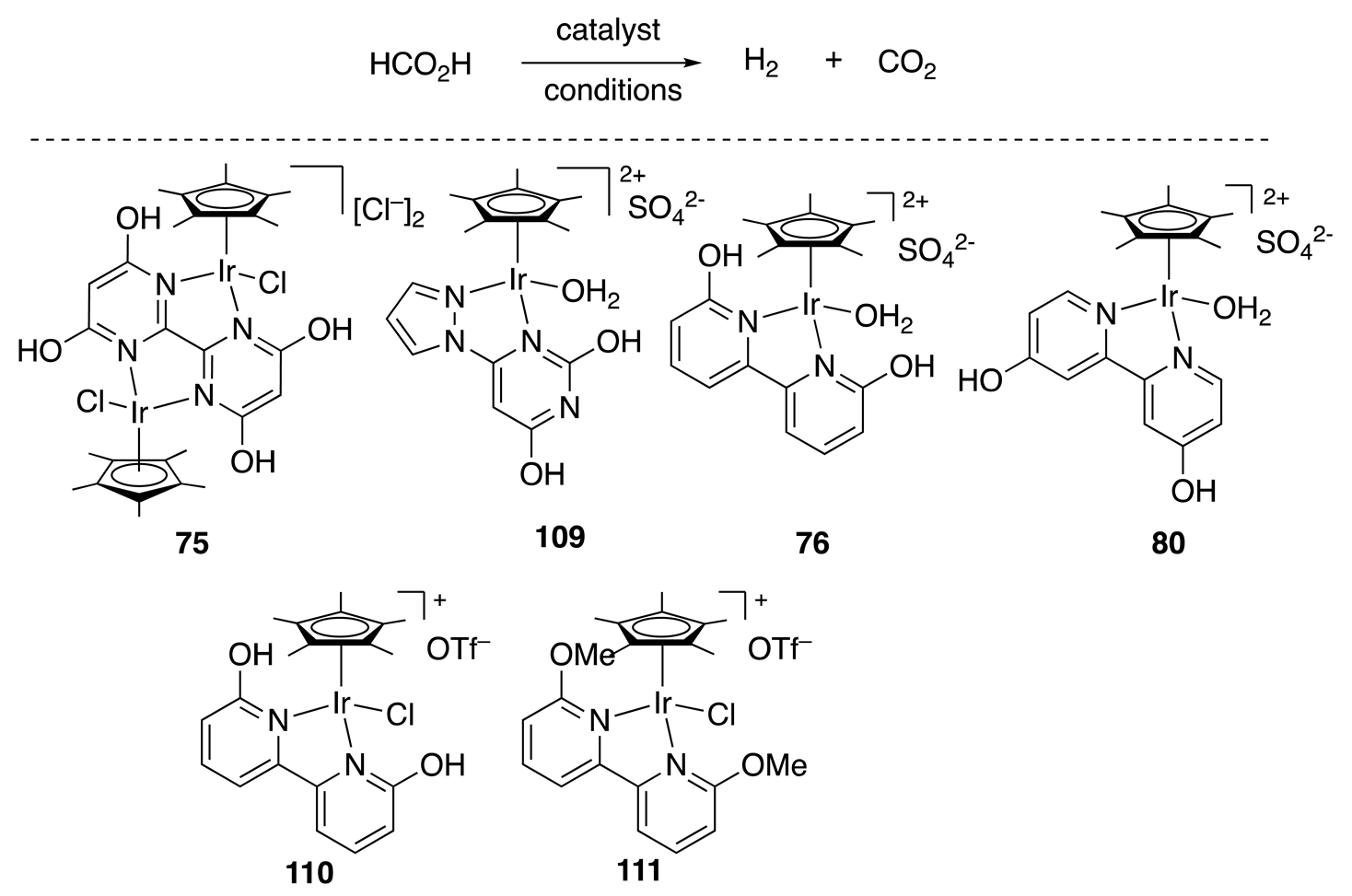
| Entry | Catalyst | Conditions | T (°C) | Time (h) | TON | Initial TOF (h−1) |
|---|---|---|---|---|---|---|
| 1 | 75 | 1 M HCO2H/HCO2Na (1:1, pH 3.5) | 80 | 12 | 308,000 | 158,000 |
| 2 | 109 | 8 M HCO2H | 100 | 4 | 400,000 | 173,000 |
| 3 | 109 | 6 M HCO2H | 60 | 580 | 2,050,000 | N.A. |
| 4 | 76 | 1.0 M HCO2H (pH 1.8) | 60 | 3 | 5000 | 2200 |
| 5 | 80 | 1.0 M HCO2H (pH 1.8) | 60 | 4 | 5000 | 2400 |
| 6 | 110 | 1.02 M HCO2H | 60 | 3 | >3500 | 1200 |
| 7 | 111 | 1.02 M HCO2H | 60 | 3 | >3500 | 1200 |
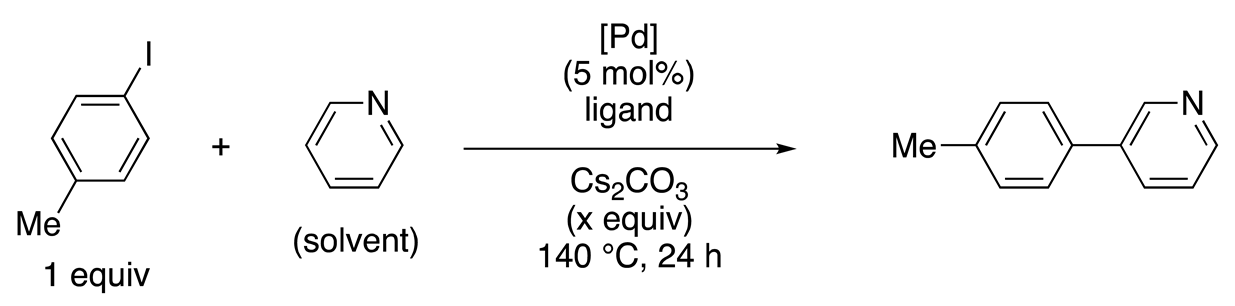
| Entry | (Pd) | Ligand (mol%) | x | Yield (%) (conv. (%)) a |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | 2,2′-bipyridine (15) | 3 | 6 (7) |
| 2 | Pd(OAc)2 | 6-hydroxy-2,2′-bipyridine (15) | 3 | 90 (100) |
| 3 | Pd(OAc)2 | 6-methoxy-2,2′-bipyridine (15) | 3 | 15 (24) |
| 4 | Pd(OAc)2 | 4-hydroxy-2,2′-bipyridine (15) | 3 | 0 (0) |
| 5 | Pd(OAc)2 | 6-hydroxy-2,2′-bipyridine (6) | 1 | 90 (100) |
| 6 b | 124 | none | 1 | 80 (100) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimbayashi, T.; Fujita, K.-i. Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization. Catalysts 2020, 10, 635. https://doi.org/10.3390/catal10060635
Shimbayashi T, Fujita K-i. Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization. Catalysts. 2020; 10(6):635. https://doi.org/10.3390/catal10060635
Chicago/Turabian StyleShimbayashi, Takuya, and Ken-ichi Fujita. 2020. "Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization" Catalysts 10, no. 6: 635. https://doi.org/10.3390/catal10060635
APA StyleShimbayashi, T., & Fujita, K.-i. (2020). Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization. Catalysts, 10(6), 635. https://doi.org/10.3390/catal10060635






