Abstract
Sulforaphane (SFN) is a health-promoting compound occurring in broccoli. It is formed by action of myrosinase in a two-step reaction that also yields undesirable compounds such as nitriles and isothionitriles. Different techniques affecting enzyme activity and tissue integrity were proposed to increase SFN content in the edible parts and discards of broccoli. Ultrasound processing is an emerging technology that produces these effects in foods, but has been poorly explored in broccoli so far. The aim of this work was to study the effect of ultrasound-assisted blanching on myrosinase activity and SFN content in broccoli florets. Myrosinase showed first-order inactivation kinetics in blanching at different temperatures with and without ultrasound processing. The inactivation rate was faster using ultrasound, with kinetic constants two orders of magnitude higher than without ultrasound. The activation energy (Ea) in traditional blanching (57.3 kJ mol−1) was higher than in ultrasound-assisted blanching (15.8 kJ mol−1). Accordingly, ultrasound accelerates myrosinase inactivation. The blanching time and temperature significantly affected myrosinase activity and SFN content. At 60 °C and 4 min of ultrasound-assisted blanching, myrosinase activity was minimum and SFN content was the highest. These findings may help to design SFN enrichment processes and will contribute to the valorization of agro-industrial wastes.
1. Introduction
Sulforaphane (SFN) is an isothiocyanate widely recognized as a powerful anti-cancer and health-promoting compound [1]. It occurs in cruciferous vegetables, mainly in broccoli. SFN formation takes place through the enzymatic hydrolysis of glucoraphanin, the main glucosinolate (GSL) found in broccoli, catalyzed by myrosinase (β-thioglucosidase glucohydrolase, EC 3.2.1.147). The conversion occurs through a two-step mechanism: hydrolysis of glucoraphanin by the action of myrosinase with the formation of an unstable intermediate (thiohydroxamate-O-sulfonate) and the release of an equimolar amount of glucose, followed by the spontaneous conversion of thiohydroxamate-O-sulfonate into sulforaphane or sulforaphane nitrile, depending on the chemical conditions. Acid pH together with the action of epithiospecifier protein (ESP) favor formation of sulforaphane nitrile, which is an undesirable product due to its potential toxicity. On the other hand, neutral pH favors the formation of sulforaphane. Figure 1 depicts a scheme of glucoraphanin conversion into sulforaphane mediated by myrosinase. The hydrolysis begins with the nucleophilic attack of Glu or Asp on the anomeric carbon of glucoraphanin with the release of an aglucone (thyohydroxamate-O-sulfonate), followed by Lossen rearrangement and the release of glucose [2]. The hydrolysis final product is sulforaphane at neutral pH, and sulforaphane nitrile at acid pH together with the action of epithiospecifier protein [3].
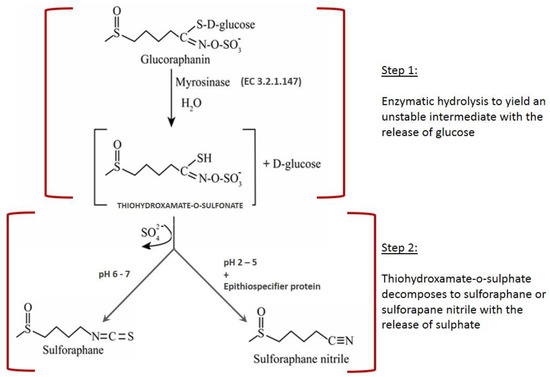
Figure 1.
Schematic representation of glucoraphanin hydrolysis by myrosinase.
The myrosinase-glucosinolate system constitutes a defense mechanism of the vegetal against biotic and abiotic stress. In the intact plant, myrosinase is compartmentalized in specialized myrosin cells, and GSL are ubiquitously distributed. As a result, the content of isothiocyanates in the intact vegetable tissue is relatively low. In order to increase the content of isothiocyanates, the plant tissue must be disrupted to allow that the enzyme gets in contact with GSL and the reaction to occur. This disruption can be achieved during mastication or by domestic or technological processing of the vegetable.
Several studies have sought to increase SFN content in the edible parts of broccoli by manipulating the post-harvest processing conditions, aiming at maximizing the functional properties of this vegetable. Pérez et al. [4] reported the blanching temperature and time that maximized SFN content in broccoli florets, obtaining a 4-fold increase with respect to fresh broccoli. Mahn and Pérez [5] optimized the incubation conditions, achieving an 8-fold increase in SFN content. Westphal et al. [6] reported that high-pressure treatment of broccoli sprouts significantly increased SFN content. Tabart et al. [7] investigated the effect of microwave processing of broccoli, reporting 4-fold increase of SFN content. Direct consumption of broccoli processed in such ways sometimes is not possible because of its unpleasant organoleptic characteristics and high perishability. Then, dehydration was proposed as a process to obtain a stable SFN-rich food ingredient that can be incorporated into further elaborated foods [8]. A drawback arises when incorporating this broccoli-based ingredient into elaborated foods. SFN is thermo labile, it starts degrading above 40 °C [9], and then any thermal process impairs SFN content in the final product. This limits the industrial application of the SFN-rich ingredient. An option to exploit the beneficial effects of sulforaphane and to give access to this compound to the consumers, is to deliver it as a food supplement or nutraceutical, avoiding the need of thermal processing. One way to obtain SFN from a natural source is to conduct glucoraphanin hydrolysis outside the vegetable, by extracting GSL from the vegetal tissues and adding exogenous myrosinase to perform the hydrolysis. In this way, SFN could be delivered directly to the consumer and not as part of a food matrix. In this sense, minimizing myrosinase activity during the glucoraphanin extraction process is desirable with the aim of obtaining the maximum conversion in SFN.
Ultrasound (US) processing is an emerging non-thermal technology recently adopted in the food industry with different purposes, such as tissue disruption and enzyme inactivation. It is considered a “green technology” that can assist traditional food processes [10]. Ultrasound corresponds to acoustic waves with frequencies beyond the human audible range. The effect of US on food matrixes respond to the cavitation phenomenon produced by the formation and collision of bubbles accompanied by high temperature and pressure [11]. This affects tissue structure by disrupting cell wall and membranes [12] and by reducing enzyme activity due to the modification of the structure by breaking hydrogen interactions and van der Waals forces [11]. Enzyme inactivation by US takes place at a higher rate than by thermal processing, thus reducing the processing time and temperature necessaries to achieve the desired denaturation [13], and making US attractive to the industry.
The use of US processing in broccoli is poorly documented so far. Briones-Labarca et al. [14] found that US-assisted extraction produced a 5-fold increase in SFN recovery from Chilean papaya seeds in a methanol solution. Pongmalai et al. [15] reported that US-assisted extraction increased extractability in methanol of glucoraphanin (1.8-fold) from steamed cabbage leaves in comparison with fresh cabbage. The authors used an ultrasound thermostatic bath, which does not ensure that ultrasound waves affected equally all the vegetal material. Aguilar-Camacho et al. [16] studied the effect of ultrasound treatment on the content of secondary metabolites in broccoli florets, and found an 8-fold increase in glucoraphanin content. The authors did not consider SFN or myrosinase.
Currently there are no studies about the effect of ultrasound processing on SFN content and myrosinase activity in broccoli florets. The hypothesis of this work was that US-assisted blanching reduces myrosinase activity without significant SFN leakage to the blanching water. In this way, an extract from US-blanched broccoli will be enriched in glucoraphanin that can be converted into SFN by adding exogenous myrosinase. If the basal SFN content in the vegetable is not affected by US-blanching, then this amount can be added to the final extract. Accordingly, the aim of this work was to investigate the effect of ultrasound-assisted blanching on myrosinase inactivation and SFN content in broccoli florets.
2. Results
The effect of time and temperature in ultrasound-assisted blanching on myrosinase activity and SFN content was studied through a multilevel factorial design. Table 1 shows the experimental conditions and the responses obtained in each experimental run. Statistically significant differences appeared on myrosinase activity and sulforaphane content. Myrosinase activity in fresh broccoli florets was 2450 ± 72 U, and in broccoli subjected to traditional blanching it was 1469 ± 17 U. In all runs, myrosinase activity was lower than the activity found in fresh broccoli. In runs 3, 4, 6, 9 and 14, myrosinase activity was lower than that found in traditionally blanched broccoli. The lowest activity corresponded to run 9 (508 ± 12 U). SFN content in fresh broccoli florets was 585.0 ± 9 µg g−1 and in traditionally blanched broccoli it was 863 ± 11 µg g−1. In runs 1, 2, 4, 7, 10, 11, 13 and 14, SFN content was significantly lower than that in the fresh vegetable. Runs 3, 6 and 9 showed SFN content significantly higher than in traditionally blanched broccoli. The lowest SFN content was obtained in run 13 (209 ± 21 µg/g), representing a 64% decrease in comparison with fresh broccoli. The highest SFN content was obtained in run 9, equal to 1185 ± 30 µg g−1, 2-fold the SFN content in fresh broccoli.

Table 1.
Experimental design and responses. Coded levels of experimental factors appear in parentheses.
The statistical analysis of the effect of each experimental factor on both responses is shown in Figure 2 as Pareto charts and response surfaces. The experimental factors significantly affected myrosinase activity and SFN content. Blanching time (B) had a significant positive effect on myrosinase activity; the other experimental factors did not affect significantly this response, as shown in Figure 2a. Temperature (A) and the interaction of temperature with itself (AA) had significant positive effect on SFN content, while blanching time had significant negative effect on this response (Figure 2b). Figure 2c shows the response surface for myrosinase activity. The minimum activity appears at 60 °C and 4 min of ultrasound-assisted blanching. Figure 2d shows that there is no maximum, i.e., the processing conditions that maximize SFN content could not be detected.
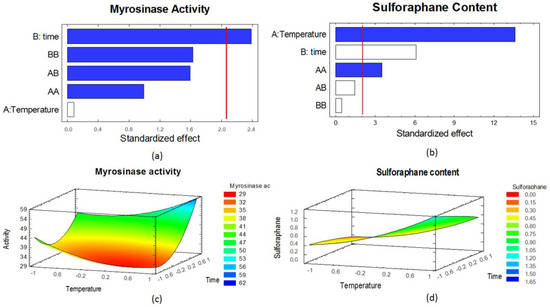
Figure 2.
Standardized statistical effects and response surface of the experimental factors on myrosinase activity and sulforaphane content. (a) Pareto chart for myrosinase activity; (b) Pareto chart for SFN content; (c) response surface for myrosinase activity; (d) response surface for SFN content. Blue bars indicate positive effect; white bars indicate negative effect. A: blanching temperature; B: blanching time; any combination of A and B: combined effect of the experimental factors.
Myrosinase inactivation was studied considering a first-order kinetics. The experimental data were adjusted to a first-order inactivation model, as suggested in the literature [13]. Figure 3 shows myrosinase inactivation kinetics during blanching at different temperatures with or without ultrasound processing. The determination coefficients (R2) were higher than 0.94, confirming that this kinetic model represents adequately myrosinase inactivation. The kinetic constants obtained in US-assisted blanching (Figure 3a) at the different temperatures were k25 °C = 0.1910 min−1, k42.5 °C = 0.2913 min−1 and k60 °C = 0.3716 min−1. The kinetic constants obtained in blanching without ultrasound (Figure 3b) at the different temperatures were k25 °C = 0.0022 min−1, k42.5 °C = 0.0031 min−1 and k60 °C = 0.0159 min−1.
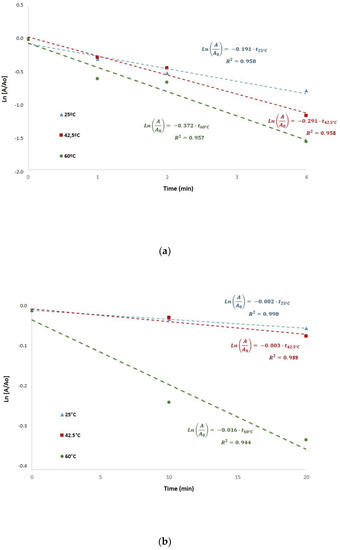
Figure 3.
First order inactivation kinetics of myrosinase during blanching with (a) and without (b) ultrasound assistance. A is enzyme activity, A0 is enzyme activity at time = 0, tT is time (min) of blanching at different temperatures, and R2 is the determination coefficient.
The activation energy (Ea) for myrosinase inactivation with and without ultrasound processing was calculated considering that the constants depend on temperature, and they can be expressed by the Arrhenius equation. In ultrasound-assisted blanching, the Ea for myrosinase inactivation was 15.8 kJ mol−1; in blanching without ultrasound, Ea was 57.3 kJ mol−1.
3. Discussion
Myrosinase activity in broccoli florets decreased after all treatments with respect to myrosinase activity in the fresh vegetable (2450 ± 72 U). This may obey to the inactivation of myrosinase by ultrasound-assisted blanching. The SFN content in broccoli florets before treatment was 585 ± 8 µg g−1. In runs 1, 2, 4, 7, 10, 11, 13 and 14, SFN content was significantly lower than that in the fresh vegetable, being the lowest content equal to 208.6 ± 20.9 µg g−1. This represents a 65% decrease (run 13). The highest SFN content was obtained in run 9, being equal to 1185 ± 30 µg g−1. This represents a 2-fold increase with respect to the fresh vegetable. This value is lower than those reported in literature [4], showing a 4-fold increase in SFN content after blanching at 57 °C for 13 min without using ultrasound. The lower SFN values obtained in the present work may be due to myrosinase inactivation that prevents the hydrolysis of glucoraphanin to yield SFN. SFN concentration in the blanching water was negligible (data not shown), probably because of the highly hydrophobic nature of this compound. Therefore, the modest increase in SFN content cannot be explained by lixiviation into the blanching water. Additionally, glucoraphanin, a water-soluble compound, was not detected in the blanching water, suggesting that US processing did not affect significantly the structure integrity of the vegetal tissue.
Response surface analyses suggest that the minimum activity appears at 60 °C and 4 min of ultrasound-assisted blanching. SFN content cannot be maximized by manipulating the experimental factors considered in this work since the response surface showed no maximum. However, higher temperature and shorter time produced a higher SFN content in ultrasound-assisted blanched broccoli. US-assisted blanching at 60 °C and 4 min resulted in the highest SFN content (see run 9 in Table 1). This behavior relates to the low leakage of SFN into the blanching water because of the low solubility of this hydrophobic compound, resulting in SFN accumulation in the vegetable tissue, in addition to the apparently slight tissue damage produced by ultrasound processing. SFN formation probably occurred during the temperature stabilization period at the beginning of blanching, before the significant inactivation of myrosinase.
Ultrasound processing resulted in significantly higher inactivation kinetic constants, being two orders of magnitude higher that the constants obtained in blanching without ultrasound processing (Figure 3). Accordingly, ultrasound processing significantly increases the inactivation rate of broccoli myrosinase. The kinetic constants obtained in blanching without ultrasound (k42.5 °C = 0.0031 min−1 and k60 °C = 0.0159 min−1) agree with the constants reported elsewhere [17] for thermal inactivation of myrosinase in rehydrated lyophilized broccoli: k40 °C = 0.0033 min−1 and k60 °C = 0.0079 min−1 at 40 and 60 °C, respectively.
In ultrasound-assisted blanching, the Ea for myrosinase inactivation was 15.8 kJ mol−1; in traditional blanching, Ea was 57.3 kJ mol−1. The latter value agrees with Olivero et al. [17], who reported an Ea in the same order of magnitude (44.5 ± 10.6 kJ mol−1). Accordingly, ultrasound processing reduces the activation energy required for myrosinase inactivation. This could represent significant energy savings in an industrial process, constituting a more efficient blanching process in comparison with traditional blanching.
Myrosinase inactivation may be useful to preserve glucoraphanin in the vegetable tissue if the objective is extracting the precursor of SFN, glucoraphanin, to conduct the hydrolysis exogenously under controlled conditions. This will prevent the formation of undesirable compounds such as nitriles and isothionitriles which compete with SFN formation, thus maximizing the conversion of glucoraphanin in SFN. Auspiciously, the minimum myrosinase activity agreed with the highest SFN content, and then ultrasound-assisted blanching at 60 °C for 4 min could constitute a step in an SFN production process. This process should include a size reduction operation, ultrasound-assisted blanching in the optimal conditions, and extraction of glucoraphanin to finally conduct the hydrolysis externally. These findings open the opportunity to valorize broccoli byproducts such as stalks, secondary inflorescences and leaves, all of them currently considered as discards.
4. Materials and Methods
4.1. Raw Material
Broccoli heads (Brassica oleracea var. italica) cv. Imperial (less than 48 h from harvest) were kindly provided by Agrocesar Ltd.a. (Curacaví, Región Metropolitana, Chile). Broccoli heads were washed immediately after purchasing, cut and sieved to particle size below 2.8 mm (mesh 7—ASTM E-11, Merck, Darmstadt, Germany). Broccoli pieces were stored at 4 °C in the dark, and they were processed before 36 h of storage.
4.2. Experimental Design
Myrosinase inactivation during ultrasound-assisted blanching was studied at 25, 42.5 and 60 °C during 1 to 12 min. Broccoli pieces (1 g) were immersed in 20 mL distilled water contained in a glass vessel (16 mm internal diameter and 130 mm height). The vessel was immersed in a thermostatic water bath (Stuart, UK) and the temperature inside the vessel was monitored with a digital thermometer (Checktemp 1, Hanna Instruments, Woonsocket, RI, USA). The ultrasound probe (3 mm diameter) of the processor (Misonix XL2000, Qsonica, Newtown, CT, USA) was introduced in the vessel 5 mm below the liquid surface. The ultrasound frequency was set at 23 kHz and the amplitude was set at 135 µm. Broccoli samples were taken out every minute and analyzed for myrosinase activity and sulforaphane content. Myrosinase activity and SFN content were also measured in the blanching water. Traditional blanching was conducted in the same system, at 57 °C for 13 min without ultrasound processing [4].
The effect of ultrasound-assisted blanching on myrosinase activity and SFN content was studied through a 31 × 51 multilevel factorial design with one replicate, whose factors (and levels) were temperature (25, 42.5 and 60 °C) and time (1, 2, 4, 8 and 12 min). Table 1 shows the experimental matrix in standard order. The experiments were conducted randomly.
4.3. Sulforaphane Content
Sulforaphane (SFN) was quantified by reverse phase HPLC, using the method proposed in the literature [18]. One gram of pulverized broccoli or lyophilized blanching solution was extracted twice with 10 mL of methylene-chloride (J.T. Baker, Center Valley, PA, USA) combined with 0.5 g anhydrous sodium sulfate (Sigma–Aldrich, Schnelldorf, Germany). The equipment was a HPLC-DAD (Shimadzu, Kyoto, Japan) equipped with a C18 column (5 μm particle size, 250 × 4.6 mm) (Agilent Technologies, Santa Clara, CA, USA). The solvent consisted of 20% acetonitrile (Merck, Darmstadt, Germany) in HPLC-grade water; changing linearly over 10 min to 60% acetonitrile and maintained at 100% acetonitrile for 5 min. The temperature was set at 30 °C, the flow rate was 1 mL min−1, and injection volume was 20 mL. Absorbance at 254 nm was recorded. Quantification was made by comparison with a sulforaphane standard curve. All analytical measurements were made in triplicate. Results are expressed as mean ± standard deviation.
4.4. Myrosinase Activity
Enzyme activity was quantified by the method described elsewhere [19]. An 800 μL aliquot of 33-mM sodium phosphate buffer (pH 7) and 100 μL of protein extract were pre incubated for 3 min at 37 °C, then 100 μL of sinigrin were added. The decline in absorbance at 227 nm was plotted and used to estimate enzyme activity as the absorbance decline within the linear phase of the graph. One activity unit was defined as the amount of myrosinase that catalyzes the hydrolysis of 1 μmol of sinigrin per minute, under the conditions described above. Analyses were made in triplicate.
4.5. Statistical Analyses
Statistical analyses were made with StatgraphicsTM Centurion XVII (Satgraphics Technologies, Inc., The Plains, Virginia, USA, 2013). The significance of the statistical effects was assessed by ANOVA. Significant differences between the responses of the experimental design were detected through the LSD multiple range test at 95% confidence interval. Significant differences between treated and fresh broccoli were detected by Student’s t-test at 95% confidence.
5. Conclusions
US-assisted blanching was effective in myrosinase inactivation while keeping SFN inside the vegetal tissue. US-blanching increased by two orders of magnitude the inactivation kinetic constants at different temperatures, and decreased the activation energy by 3.6-fold in comparison with traditional blanching. Then, the use of US could represent significant energy savings in an industrial blanching process. The minimum myrosinase activity agreed with the highest SFN content in broccoli florets when US-assisted blanching at 60 °C for 4 min was applied, constituting a more efficient step in an SFN production process based on conducting the hydrolysis outside the vegetable. These findings seem promising for valorization of broccoli byproducts such as stalks, secondary inflorescences and leaves, which are poorly exploited so far.
Author Contributions
Conceptualization, A.M. and J.Q.; methodology, A.M., N.C. and R.C.; formal analysis, A.M.; investigation, A.M., N.C. and R.C.; resources, A.M. and J.Q.; writing—original draft preparation, A.M.; writing—review and editing, A.M. and J.Q.; supervision, A.M.; project administration, A.M. and J.Q.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AGENCIA NACIONAL DE INVESTIGACIÓN Y DESARROLLO, grant number PAI No 77170008 and FONDECYT 1201418, and VICERRECTORÍA DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN, grants number DICYT 081711MO and 081911MO_PAP. The APC was funded by VICERRECTORÍA DE INVESTIGACIÓN, DESARROLLO E INNOVACIÓN, Universidad de Santiago de Chile.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ganai, S.A. Histone deacetylase inhibitor sulforaphane: The phytochemical with vibrant activity against prostate cancer. Biomed. Pharmacother. 2016, 81, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Román, J.; González, D.; Inostroza, M.; Mahn, A. Molecular modeling of epithiospecifier and nitrile-specifier proteins of broccoli and their interaction with aglycones. Molecules 2020, 25, 772. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Barrientos, H.; Roman, J.; Mahn, A. Optimization of a blanching step to maximize sulforaphane synthesis in broccoli florets. Food Chem. 2014, 145, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Pérez, C. Optimization of an incubation step to maximize sulforaphane content in pre-processed broccoli. J. Food Sci. Technol. 2016, 53, 4110–4115. [Google Scholar] [CrossRef] [PubMed]
- Westphal, A.; Riedl, K.M.; Cooperstone, J.L.; Kamat, S.; Balasubramaniam, V.M.; Schwartz, S.J.; Böhm, V. High-Pressure Processing of Broccoli Sprouts: Influence on Bioactivation of Glucosinolates to Isothiocyanates. J. Agric. Food Chem. 2017, 65, 8578–8585. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Pincemail, J.; Kevers, C.; Defraigne, J.O.; Dommes, J. Processing effects on antioxidant, glucosinolates, and sulforaphane contents in broccoli and red cabbage. Eur. Food Res. Technol. 2018, 244, 2085–2094. [Google Scholar] [CrossRef]
- Quintero, J.; Román, D.; Salazar, J.L.; Mahn, A. Economic assessment of a small-scale plant for production of sulforaphane-rich broccoli flour in Chile. Biofuels Bioprod. Biorefin. 2020, 14, 544–552. [Google Scholar] [CrossRef]
- Mahn, A.; Martins, C.; Reyes, A.; Saavedra, A. Evolution of sulforaphane content in sulforaphane-rich broccoli during tray drying. J. Food Eng. 2016, 186, 27–33. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process 2019, 117, 170–182. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Marques Silva, F.V.; Sulaiman, A. Advances in thermosonication for the inactivation of endogenous enzymes in foods. In Ultrasound: Advances in Food Processing and Preservation; Bermúdez-Aguirre, D., Ed.; Academic Press: New York, NY, USA, 2017; pp. 101–130. [Google Scholar]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea. pubescens.) seeds: Effects of extraction conditions and methods. LWT Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancing the recovery of cabbage glucoraphanin through the monitoring of sulforaphane content and myrosinase activity during extraction by different methods. Sep. Purif. Technol. 2017, 174, 338–344. [Google Scholar] [CrossRef]
- Aguilar-Camacho, M.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets. Ultrason. Sonochem. 2019, 50, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Verkerk, R.; Van Boekel, M.A.J.S.; Dekker, M. Effect of water content and temperature on inactivation kinetics of myrosinase in broccoli (Brassica. oleracea. var. italica.). Food Chem. 2014, 163, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yuan, Q.P.; Dong, H.R.; Liu, Y.M. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J. Food Comp. Anal. 2006, 19, 473–476. [Google Scholar] [CrossRef]
- Li, X.; Kushad, M.M. Purification and characterization of myrosinase from horseradish (Armoracia. rusticana.) roots. Plant Physiol. Biochem. 2005, 43, 503–511. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).