Abstract
There are active oxygen species that contribute to oxidative coupling or the partial oxidation during the oxidative dehydrogenation of methane when using solid oxide catalysts, and those species have not been definitively identified. In the present study, we clarify which of the active oxygen species affect the oxidative dehydrogenation of methane by employing photo-catalysts such as TiO2 or WO3, which generate active oxygen from UV-LED irradiation conditions under an oxygen flow. These photo-catalysts were studied in combination with Sm2O3, which is a methane oxidation coupling catalyst. For this purpose, we constructed a reaction system that could directly irradiate UV-LED to a solid catalyst via a normal fixed-bed continuous-flow reactor operated at atmospheric pressure. Binary catalysts prepared from TiO2 or WO3 were either supported on or kneaded with Sm2O3 in the present study. UV-LED irradiation clearly improved the partial oxidation from methane to CO and/or slightly improved the oxidative coupling route from methane to ethylene when binary catalysts consisting of Sm2O3 and TiO2 are used, while negligible UV-LED effects were detected when using Sm2O3 and WO3. These results indicate that with UV-LED irradiation the active oxygen of O2− from TiO2 certainly contributes to the activation of methane during the oxidative dehydrogenation of methane when using Sm2O3, while the active oxygen of H2O2 from WO3 under the same conditions afforded only negligible effects on the activation of methane.
1. Introduction
The conversion of methane to high value-added chemicals is an important issue in the field of catalyst research. In recent years, research on the catalytic reaction of methane has been actively conducted due to progress in the production technology of natural gas, which consists mainly of methane gas [1,2,3]. Although methane has the potential for conversion to a variety of important chemicals, its application as a raw material in catalytic reactions has been limited due to chemical stability. Therefore, methane is still used mainly as fuel.
To overcome the stability problem, many researchers are studying the direct conversion of methane to value-added chemicals such as methanol [4], carbon monoxide [5], ethylene [6], and aromatic compounds [7]. The direct conversion of methane is considered the most efficient way to use methane gas because the desired product requires only a one-step catalytic reaction. In particular, the oxidative coupling of methane (OCM) to ethylene and ethane has been the subject of much research over the past three decades since these C2 hydrocarbons are the most widely used petrochemicals in the world. In the OCM reaction, methane reacts with oxygen exothermically on a solid oxide catalyst to produce these C2 hydrocarbons together with water [8]. It is generally accepted that gaseous oxygen and active oxygen derived from a solid oxide catalyst could contribute to the oxidative conversion of methane [9,10,11]. Furthermore, the OCM is believed to consist of both heterogeneous and homogeneous reactions. First, in a heterogeneous reaction, active oxygen in the catalyst extracts hydrogen from methane to generate methyl radicals. The methyl radicals are then dimerized to C2 hydrocarbons by a homogeneous gas-phase reaction (Scheme 1) [12,13,14,15]. Contributions have been proposed from active oxygen species such as O2−, OH, H2O2, or 1O2 (singlet oxygen) together with gas-phase oxygen (O2) or catalytic lattice oxygen (O2−), but exactly what kind of active oxygen species contribute to the oxidative dehydrogenation of methane is yet to be clarified [16,17,18,19,20,21].
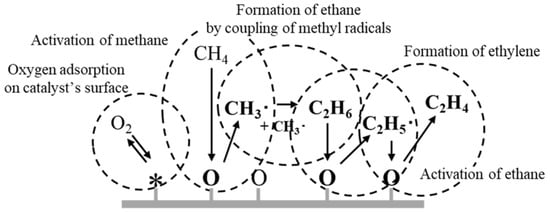
Scheme 1.
Mechanism for the oxidative coupling of methane.
In the present study, we focused on the characteristics of photo-catalysts. Photo-catalysts such as titanium oxide (TiO2) and tungsten oxide (WO3) activate oxygen when electrons (e−) are excited by irradiation from an excitation light (UV-LED in the present study) and holes (h+) are sequentially generated, which results in the formation of active oxygen (Scheme 2).
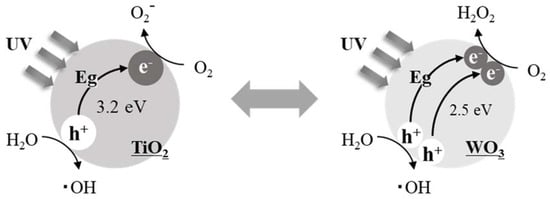
Scheme 2.
Production of active oxygen species via UV irradiation of TiO2 and WO3.
Based on Scheme 2, the oxidative dehydrogenation of methane was studied via contact with samarium oxide (Sm2O3; OCM-catalyst) and by examining the active oxygen species generated via irradiating UV-LED irradiation of either TiO2 or WO3 (photo-catalyst) under a gaseous O2 atmosphere. It is generally accepted that O2‒ is generated from a one-electron reduction of TiO2, and H2O2 is generated from a two-electron reduction of WO3 [22]. When the active oxygen species derived from either TiO2 or WO3 contacted Sm2O3 during the oxidative dehydrogenation of methane, the product distribution was expected to depend on the presence or absence of UV-LED irradiation. The purpose of this study was to confirm and clarify the contributions of each of the active oxygen species. It is noteworthy that titanium and tungsten have been used as the active species in various catalysts for the oxidative coupling of methane [23,24].
2. Results and Discussion
This study involved both mixed- and supported-catalysts that consisted of Sm2O3 together with TiO2 or WO3. Based on our preliminary experiments, the loading of photo-catalysts such as TiO2 and WO3 was fixed at 5 wt.%. First, the mixed-catalyst activity using 5 wt.% TiO2 + Sm2O3 was tested together with that of either Sm2O3 or TiO2. The specific surface areas of Sm2O3, TiO2, and 5 wt.% TiO2 + Sm2O3 were 7, 47, and 22 m2/g, respectively. Figure 1 shows the effect that UV-LED irradiation exerted on the oxidative dehydrogenation of methane at T = 898 K; P(CH4) = 28.7 kPa; and P(O2) = 2.03 kPa (P(CH4)/P(O2) = 14.2). Since stable catalytic activity was detected on all catalysts used to the point of 4.5 h on-stream, the activity at 0.75 h on-stream was discussed in the present study. As shown in Figure 1, UV-LED irradiation of Sm2O3 showed no advantageous effects on either C2 yield or on the conversions of O2 and CH4, while CO selectivity was slightly changed from 9.4% to 10.5% by the irradiation. A similar effect of UV-LED on TiO2 yielded CO selectivity of 83.8% to 85.2%. It should be noted that the conversions of CH4 and O2 were not influenced by the irradiation of UV-LED due to the oxygen-limiting conditions. When adding 5 wt.% TiO2 into Sm2O3 (5 wt.% TiO2 + Sm2O3), the unique nature of Sm2O3 that allows coupling with methane was mostly masked by the nature of TiO2 that allows the partial oxidation of methane, and this resulted in a slight formation of C2H6 on 5 wt.% TiO2 + Sm2O3. Furthermore, an evident improvement in CO selectivity of from 19.9% to 28.9% was detected followed by a suppression of CO2 selectivity of from 78.6% to 67.2% after UV-LED irradiation of the mixed-catalyst. It should be noted that C2H6 selectivity was also slightly improved from 1.5% to 3.9% via UV-LED irradiation using 5 wt.% TiO2 + Sm2O3. Therefore, O2− generated via the UV-LED irradiation of TiO2 under a gaseous O2 atmosphere seemed to contribute to the acceleration of the partial oxidation of CH4 to CO together with the oxidative dehydrogenation of CH4 to C2H6. No enhancement was detected from either the partial oxidation or the oxidative dehydrogenation of methane using 5 wt.% TiO2 + Sm2O3 via UV-LED at a P(O2) as high as 4.05 kPa, which indicated that the presence of large amounts of reactant oxygen may obliterate the effects of O2− due to the small amount of active oxygen.
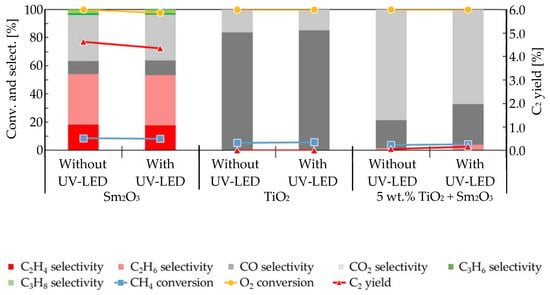
Figure 1.
Effect of UV-LED irradiation on the oxidative dehydrogenation of methane when using Sm2O3, TiO2, and 5 wt.% TiO2 + Sm2O3.
Figure 2 shows the effect of UV-LED irradiation on the oxidative dehydrogenation of methane over Sm2O3, WO3, and 5 wt.% WO3 + Sm2O3 as a mixed-catalyst under the same reaction conditions as those used for obtaining the results shown in Figure 1. The specific surface areas of WO3 and 5 wt.% WO3 + Sm2O3 were 5 and 6 m2/g, respectively. As shown in Figure 2, WO3 produced CO alone via partial oxidation of methane regardless of the use of UV-LED irradiation while O2 conversion was increased from 6% to 13%. In the present case, the addition of 5 wt.% WO3 into Sm2O3 did not completely mask the unique nature of Sm2O3 in the oxidative coupling of methane. The effects of UV-LED irradiation on the catalytic activity of 5 wt.% WO3 + Sm2O3 were rather small. Slight decreases were detected for CH4 conversion, C2 yield, C2H6 selectivity, CO selectivity, and CO2 selectivity together with slight increases in O2 conversion and C2H4 selectivity that ranged from 12.2% to 12.9%. Therefore, the effect of H2O2 generated by UV-LED irradiation on WO3 under a gaseous O2 atmosphere could have been negligible while those of H2O2 seemed to slightly contribute to an acceleration of the oxidative dehydrogenation of C2H6 to C2H4.
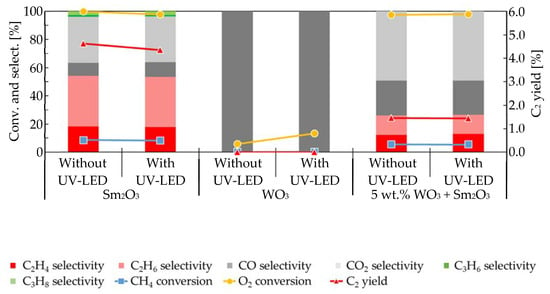
Figure 2.
Effects of UV-LED irradiation on the oxidative dehydrogenation of methane when using Sm2O3, WO3, and 5 wt.% WO3 + Sm2O3.
An effect from UV-LED irradiation was not evident when using mixed-catalysts. Therefore, supported-catalysts were used in the present study. In Figure 3, the use of UV-LED irradiation on the oxidative dehydrogenation of methane when using 5 wt.% TiO2 + Sm2O3 and 5 wt.% WO3 + Sm2O3 mixed-catalysts is compared with the results over 5 wt.% TiO2/Sm2O3 and 5 wt.% WO3/Sm2O3 supported-catalysts under the same reaction conditions as those used to obtain the results shown in Figure 1 and Figure 2. The specific surface areas of supported-catalysts 5 wt.% TiO2/Sm2O3 and 5 wt.% WO3/Sm2O3 were 9 and 6 m2/g, respectively. Figure 3 compares the effect of UV-LED irradiation using 5 wt.% TiO2 + Sm2O3 with that using 5 wt.% TiO2/Sm2O3, and the effect was more evident when using the supported catalyst. For example, CH4 conversion, C2 yield, C2H4 selectivity, C2H6 selectivity, and CO selectivity when using the supported catalyst all were enhanced by UV-LED irradiation from 3.6%, 0.3%, 0.0%, 9.0%, and 26.5% when using 5 wt.% TiO2 + Sm2O3 to 5.6%, 0.7%, 2.4%, 10.0%, and 47.7% when using 5 wt.% TiO2/Sm2O3. By contrast, during deep oxidation, CO2 selectivity was suppressed by UV-LED irradiation from 64.4% to 39.8%. It is noteworthy that the catalytic activity on the 5 wt.% TiO2 + Sm2O3 catalyst (Figure 3) was higher than that of TiO2 itself, because activities such as the methane conversion and C2 selectivity on Sm2O3 were higher than that on TiO2, as shown in Figure 1. As shown in Figure 3, the conversions of both CH4 and O2 were insensitive to the irradiation of UV-LED due to the oxygen-limiting conditions. It was evident that UV-LED irradiation enhanced the formation of C2 compounds and CO and suppressed the deep oxidation to CO2. A comparison of the activity when using 5 wt.% WO3 + Sm2O3 with the use of 5 wt.% WO3/Sm2O3 revealed a negligible effect from UV-LED. Additionally, an increase in C2H4 selectivity from 0.0% to 1.2% by UV-LED was detected when using 5 wt.% WO3/Sm2O3, which was similar to the use of 5 wt.% WO3 + Sm2O3, as shown in Figure 2.
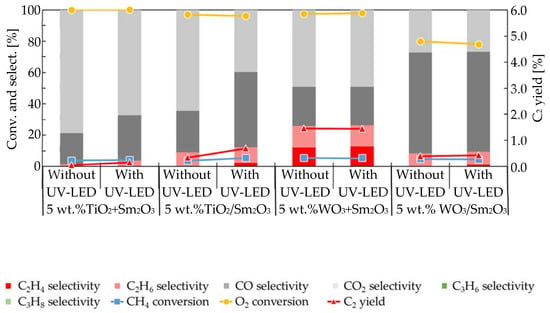
Figure 3.
Comparison of the effects of UV-LED irradiation of the oxidative dehydrogenation of methane when using 5 wt.% TiO2 + Sm2O3, 5 wt.% TiO2/Sm2O3, 5 wt.% WO3 + Sm2O3, and 5 wt.% WO3/Sm2O3.
Based on Figure 3, the effect of UV-LED irradiation was more evident when using the supported-catalysts than when the mixed-catalysts were used. Table 1 summarizes the effect of UV-LED irradiation on the selectivities for CO, CO2, C2H4, and C2H6 obtained from the oxidative dehydrogenation of methane over the mixed- and supported-catalysts using the data shown in Figure 3. The positive values in Table 1 indicate that the selectivity for each product was enhanced by UV-LED irradiation, while the negative values indicate that the selectivity was suppressed. Values less than 1.0 in Table 1 indicate that UV-LED irradiation had little effect on the corresponding selectivity.

Table 1.
Effect of UV-LED irradiation on the selectivity for each of the products when using the binary catalysts in the present study.
Although the effect of UV-LED irradiation was not evident for either 5 wt.% WO3 + Sm2O3 or 5 wt.% WO3/Sm2O3, Table 1 is used here to discuss the effects of UV-LED irradiation. Active oxygen such as O2− is generated when using both 5 wt.% TiO2 + Sm2O3 and 5 wt.% TiO2/Sm2O3 due to the presence of TiO2 in the binary catalysts [22]. When using these catalysts, the selectivities for CO, C2H6, and/or C2H4 were improved by UV-LED irradiation, while the selectivity for CO2 was suppressed. Therefore, the formation of O2− by UV-LED when using the binary catalysts seems to have contributed to an enhancement of the formation of partial oxidation products, while the deep oxidation production of CO2 was suppressed. When using 5 wt.% WO3 + Sm2O3 and 5 wt.% WO3/Sm2O3, active oxygen such as H2O2 is generated due to the presence of WO3 in the binary catalysts [22]. Although the effect of UV-LED irradiation was rather small or negligible when using these catalysts compared with that when using TiO2-loading catalysts, a small but rather negligible enhancement of the selectivity to C2H4 was detected with the use of 5 wt.% WO3 + Sm2O3 and 5 wt.% WO3/Sm2O3. Therefore, the formation of H2O2 from UV-LED when using these binary catalysts may slightly contribute to the oxidative dehydrogenation of C2H6 to C2H4. Based on these results, it is possible to summarize the influence that active oxygen species exert on the present catalyst system, as shown in Scheme 3.
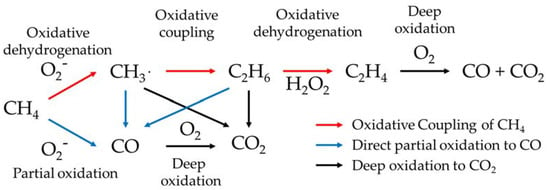
Scheme 3.
Proposed contribution of active oxygen in the present binary catalysts.
The active oxygen of O2‒ that formed when using TiO2 + Sm2O3 and TiO2/Sm2O3 contributed to the positive effect for the formations of CO, C2H6, and C2H4 together with a suppression of the deep oxidation of C2H4 to CO and CO2. Furthermore, as shown in the results for TiO2, the O2− formed on TiO2 alone directly contributed to the partial oxidation of CH4 to CO. The active oxygen of H2O2 that formed when using both 5 wt.% WO3 + Sm2O3 and 5 wt.% WO3/Sm2O3 showed a negligible contribution to the conversion of C2H6 to C2H4 via oxidative dehydrogenation. It should be noted that H2O2 is an active species for other partial oxidations such as the epoxidation of alkenes. Therefore, the WO3 system may be one of the most plausible candidates for the epoxidation of alkenes under UV-LED irradiation. Gaseous O2 is the main contributor to the deep oxidation to CO2.
Finally, the catalysts used in the present study were analyzed using XRD. XRD patterns of the single oxides of Sm2O3 and WO3 were matched to the reference patterns for the corresponding oxide (PDF 01-078-4055 and 01-083-0950, respectively; not shown). For 5 wt.% WO3 + Sm2O3 and 5 wt.% WO3/Sm2O3, the XRD peaks due to Sm2O3 were detected alone (not shown). As shown in Figure 4A, before the reaction, TiO2 was a mixture of anatase- and rutile-type TiO2 (PDF 00-064-0863 and 01-086-0148, respectively). The anatase-type remained after the reaction, regardless of the UV-LED irradiation. Furthermore, Figure 4B,C shows that 5 wt.% TiO2 + Sm2O3 and 5 wt.% TiO2/Sm2O3 contained a trace amount of anatase-type TiO2 together with Sm2O3 before the reaction. However, after the reaction with and without UV-LED irradiation, peaks due to Sm2O3 were detected together with a trace amount of anatase-type TiO2. Based on these XRD results, we concluded that anatase-type TiO2 remained during the reaction and the effect of UV-LED on the reaction came from the contribution of the anatase-type TiO2 [25].
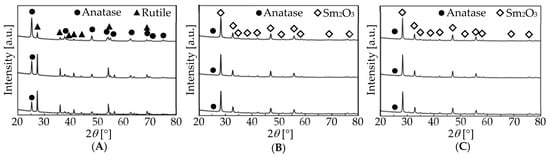
Figure 4.
XRD of (A) TiO2, (B) 5 wt.% TiO2 + Sm2O3, and (C) 5 wt.% WO3/Sm2O3. Upper—before the reaction. Middle and lower—after the reaction without and with UV-LED.
3. Materials and Methods
Mixed-catalysts (TiO2 + Sm2O3 and WO3 + Sm2O3) were prepared via the kneading of Sm2O3 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) with either TiO2 (JRC-TIO-15, a reference catalyst supplied from The Catalysis Society of Japan, Tokyo, Japan) or WO3 (Wako Pure Chemical Industries, Ltd.) for 30 min. For the preparation of 5 wt.% TiO2 + Sm2O3, 0.018 g of TiO2 was kneaded with 0.350 g of Sm2O3 for 30 min. Supported-catalysts (TiO2/Sm2O3 and WO3/Sm2O3) were prepared via impregnation. The preparation of 5 wt.% TiO2/Sm2O3 began with 20 mL of 2-propanol (Wako Pure Chemical Industries, Ltd.)) into which we dissolved 0.592 g of titanium tetraisopropoxide (Wako Pure Chemical Industries, Ltd.) and 3.00 g of Sm2O3, followed by the further addition of 35 mL of distilled water. The resultant suspension was then evaporated and dried at 333 K for 24 h. Finally, the resultant solid was calcined at 973 K for 3 h. The preparation of 5 wt.% WO3/Sm2O3 began with 20 mL of aqueous solution into which we dissolved 0.174 g of ammonium (para)tungstate hydrate (Sigma-Aldrich Japan Co. LLC, Tokyo, Japan) and 3.00 g of Sm2O3. The resultant suspension was treated in a manner similar to the preparation of TiO2/Sm2O3. In order to analyze those catalysts, X-ray diffraction (XRD) patterns were obtained using a SmartLab/R/INP/DX (Rigaku Co., Osaka Japan) with a Cu Kα radiation monochromator at 45 kV and 150 mA. In order to estimate the specific surface areas of those catalysts via BET, nitrogen adsorption isotherms of the catalysts pretreated at 473 K for 5 h were measured using a BELSORPmax12 (MicrotracBEL, Osaka, Japan) at 77 K.
The catalytic experiments were performed in a fixed-bed continuous-flow quartz reactor, which was placed in an electric furnace with an optical window, and operated at atmospheric pressure and 898 K (Scheme 4). As a light source for UV-LED irradiation, a Lightningcure LC-L1V3 (Hamamatsu Photonics K.K., Shizuoka, Japan) was used. This light source emits UV light at a wavelength of 365 nm for an average maximum irradiation intensity of 14,000 mW/cm2 and a maximum output of 450 mW, which is sufficient for the activation of O2 when using TiO2 and WO3 under the present reaction conditions.
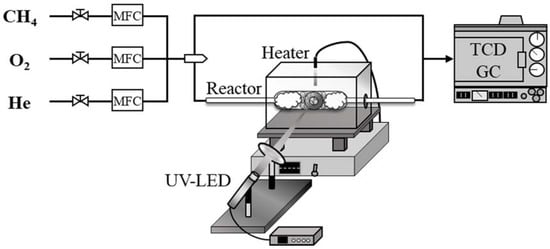
Scheme 4.
Fixed-bed continuous-flow quartz reactor with UV-LED.
The temperature of the catalyst (0.350 g and 0.368 g for single and binary oxide catalysts, respectively) was increased to 898 K under a flow of He. After the reaction temperature was stabilized, the catalyst was treated with a flow of O2 (15 mL/min) for 1 h. Activity tests were then carried out under 15 mL/min of a reactant gas flow that consisted of CH4 and O2 diluted with He. In the present study, partial-pressure ratios of 7.1 and 14.2 were employed for CH4/O2, and the partial pressures were then adjusted to P(CH4)/P(O2) = 28.7 kPa/4.05 kPa and 28.7 kPa/2.03 kPa. Under these conditions, homogeneous reactions were not detected. The reaction was monitored using an on-line gas chromatograph (GC-8APT, Shimadzu Corp., Kyoto, Japan) that involved the use of a thermal conductivity detector (TCD). The columns in the TCD-GC consisted of a Molecular Sieve 5A (0.3 m × Φ 3 mm) for the detection of O2, CO, and CH4 at 318 K and a Porapak Q (6 m × Φ 3 mm) for the detection of CO2, C2, and C3 species at the column temperatures between 318 and 493 K with a heating rate of 10 K/min. The conversion and the selectivity were estimated on a carbon basis.
4. Conclusions
In order to investigate the active oxygen effect that O2− and H2O2 exert on the catalytic oxidative dehydrogenation of methane, binary oxide consisting of Sm2O3, which is an oxidative coupling catalyst for methane, and TiO2 or WO3, which generate O2− or H2O2, respectively, when irradiated with UV-LED, were prepared using kneading and impregnation methods. Regardless of the preparation methods, O2− generated from TiO2 under UV-LED irradiation promoted the partial oxidation of methane to CO and oxidative conversion to C2 compounds, while it suppressed complete oxidation to CO2. By contrast, regardless of the preparation methods, H2O2 generated from WO3 under UV-LED irradiation had no evident effect on the oxidation of methane. It is noteworthy that the use of MgO instead of Sm2O3 had no effect on the results of UV-LED irradiation. Therefore, it is suggested that the use of any oxide catalyst with great redox properties equal to those of Sm2O3 would produce the above-mentioned advantageous effects via UV-LED irradiation.
Author Contributions
Conceptualization and methodology, S.S., A.F., Y.K., and W.N.; validation, Y.H., I.O., N.S., and M.K.; formal analysis and investigation, S.S., Y.Y., and I.O.; writing—original draft preparation, S.S.; writing—review and editing, S.S., N.S., M.K., A.F., Y.K., and W.N.; and, supervision, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Number JP17K19014 and by the Research Clusters Program of Tokushima University (1702001).
Acknowledgments
The authors gratefully acknowledge Toshihiro Okamoto of the Institute of Post-LED Photonics, Tokushima University for his valuable suggestions concerning photo-catalysts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tang, P.; Zhu, Q.; Wu, Z.; Ma, D. Methane activation: The past and future. Energy Environ. Sci. 2014, 7, 2580–2591. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: A review. J. Ind. Eng. Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Direct conversion technologies of methane to methanol: An overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- Sugiyama, S.; Minami, T.; Higaki, T.; Hayashi, H.; Moffat, J.B. High selective conversion of methane to carbon monoxide and the effects of chlorine additives in the gas- and solid-phases on the oxidation of methane on strontium hydroxyapatites. Ind. Eng. Chem. Res. 1997, 36, 328–334. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Wang, T.; Liu, Y.; Xu, T.; Zhang, Y. Efficient conversion of methane to aromatics by coupling methylation reaction. ACS Catal. 2016, 6, 5366–5370. [Google Scholar] [CrossRef]
- Gesser, H.D.; Hunter, N.R.; Prakash, C.B. The direct conversion of methane to methanol by controlled oxidation. Chem. Rev. 1985, 85, 235–244. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jeon, W.; Choi, J.W.; Suh, Y.W.; Ha, J.M.; Suh, D.J.; Park, Y.K. Scaled-up production of C-2 hydrocarbons by the oxidative coupling of methane over pelletized Na2WO4/Mn/SiO2 catalysts: Observing hot spots for the selective process. Fuel 2013, 106, 851–857. [Google Scholar] [CrossRef]
- Zhang, H.B.; Lin, G.D.; Wan, H.L.; Liu, Y.D.; Weng, W.Z.; Cai, J.X.; Shen, Y.F.; Tsai, K.R. Active-oxygen species on non-reducible rare-earth-oxide-based catalysts in oxidative coupling of methane. Catal. Lett. 2001, 73, 141–147. [Google Scholar] [CrossRef]
- Lunsford, J.H. The catalytic oxidative coupling of methane. Angew. Chem. Int. Ed. Engl. 1995, 34, 970–980. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Oxidative coupling of methane in Ba0.5Sr0.5Co0.8Fe0.2O3−δ tubular membrane reactors. Catal. Today 2005, 104, 160–167. [Google Scholar] [CrossRef]
- Lee, M.R.; Park, M.J.; Jeon, W.; Choi, J.W.; Suh, Y.W.; Suh, D.J. A kinetic model for the oxidative coupling of methane over Na2WO4/Mn/SiO2. Fuel Process. Technol. 2012, 96, 175–182. [Google Scholar] [CrossRef]
- Sun, J.; Thybaut, J.W.; Marin, G.B. Microkinetics of methane oxidative coupling. Catal. Today 2008, 137, 90–102. [Google Scholar] [CrossRef]
- Beck, B.; Fleischer, V.; Arndt, S.; Hevia, M.G.; Urakawa, A.; Hugo, P.; Schomäcker, R. Oxidative coupling of methane—A complex surface/gas phase mechanism with strong impact on the reaction engineering. Catal. Today 2014, 228, 212–218. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, Y. Direct oxidation of methyl radicals in OCM process deduced from correlation of product selectivities. J. Nat. Gas Chem. 2010, 19, 534–538. [Google Scholar] [CrossRef]
- Osada, Y.; Koike, S.; Fukushima, T.; Ogasawara, S.; Shikada, T.; Ikariya, T. Oxidative coupling of methane over Y,O,-CaO catalysts. Appl. Catal. 1990, 59, 59–74. [Google Scholar] [CrossRef]
- Yang, T.L.; Feng, L.B.; Shen, S.K. Oxygen species on the surface of La2O3/CaO and its role in the oxidative coupling of methane. J. Catal. 1994, 145, 384–389. [Google Scholar] [CrossRef]
- Spinicci, R.; Marini, P.; De Rossi, S.; Faticanti, M.; Porta, P. Oxidative coupling of methane on LaAlO3 perovskites partially substituted with alkali or alkali-earth ions. J. Mol. Catal. A 2001, 176, 253–265. [Google Scholar] [CrossRef]
- Jeon, W.; Lee, J.Y.; Lee, M.; Choi, J.; Ha, J.; Suh, D.J.; Kim, I.W. Oxidative coupling of methane to C2 hydrocarbons on the Mg–Ti mixed oxide-supported catalysts at the lower reaction temperature: Role of surface oxygen atoms. Appl. Catal. A 2013, 464–465, 68–77. [Google Scholar] [CrossRef]
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent Advances and Future Prospect in Catalysts for oxidative coupling of Methane to Ethylene: A Review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Yunarti, R.T.; Gu, S.; Choi, J.-W.; Jae, J.; Sun, D.J.; Ha, J.-M. Oxidative Coupling of Methane Using Mg/Ti-Doped SiO2-Spported Na2WO4/Mn Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 3667–3674. [Google Scholar] [CrossRef]
- Gu, S.; Oh, H.-S.; Choi, J.-W.; Suh, D.J.; Jae, J.; Choi, J.; Ha, J.-M. Effects of Metal or Metal Oxide Additives on Oxidative Coupling of Methane Using Na2WO4/SiO2 Catalysts: Reducibility of Metal Additives to Manipulate the Catalytic Activity. Appl. Catal. A 2018, 562, 114–119. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).