A Highly Efficient Monolayer Pt Nanoparticle Catalyst Prepared on a Glass Fiber Surface
Abstract
1. Introduction
2. Results and Discussion
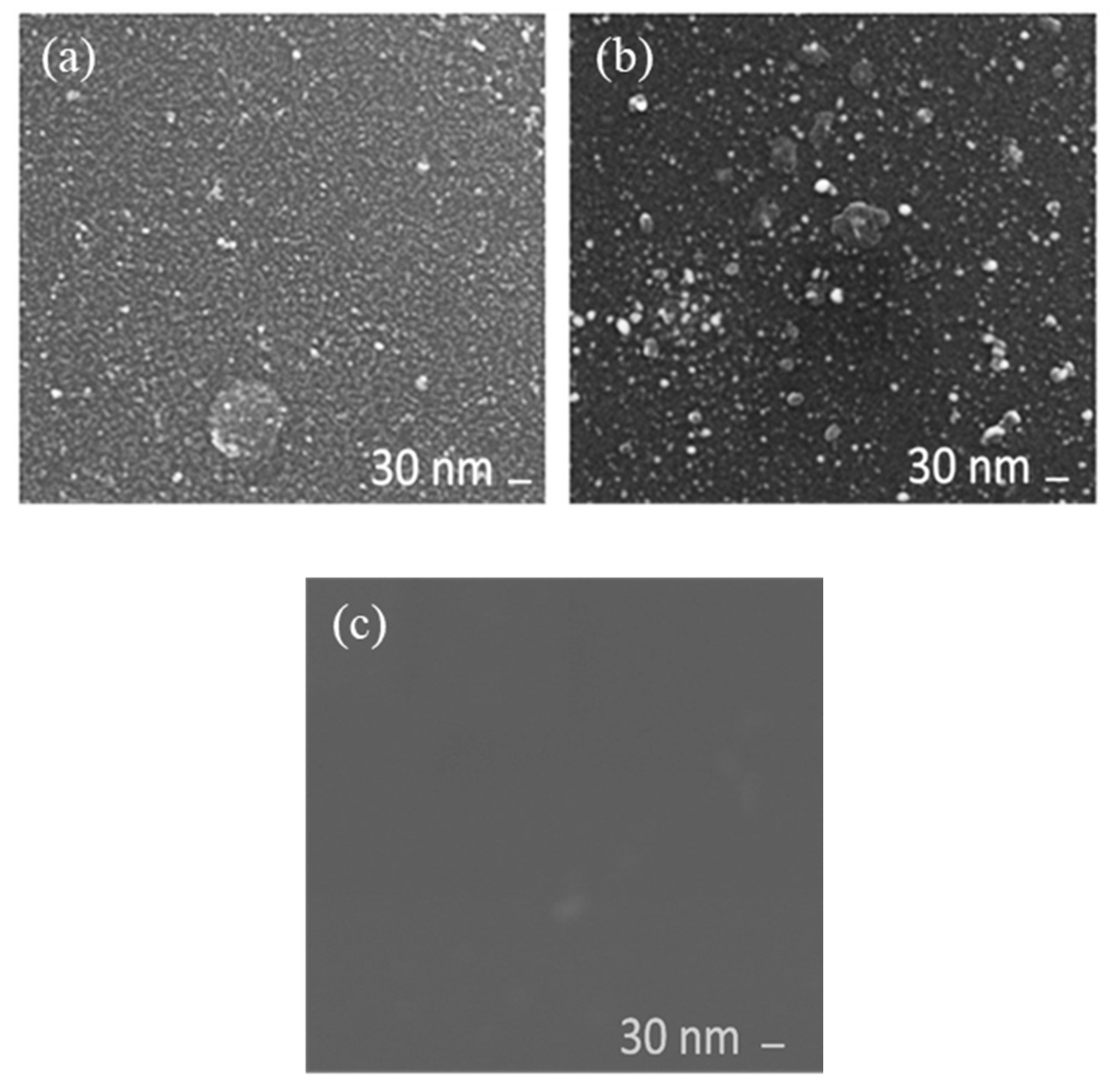
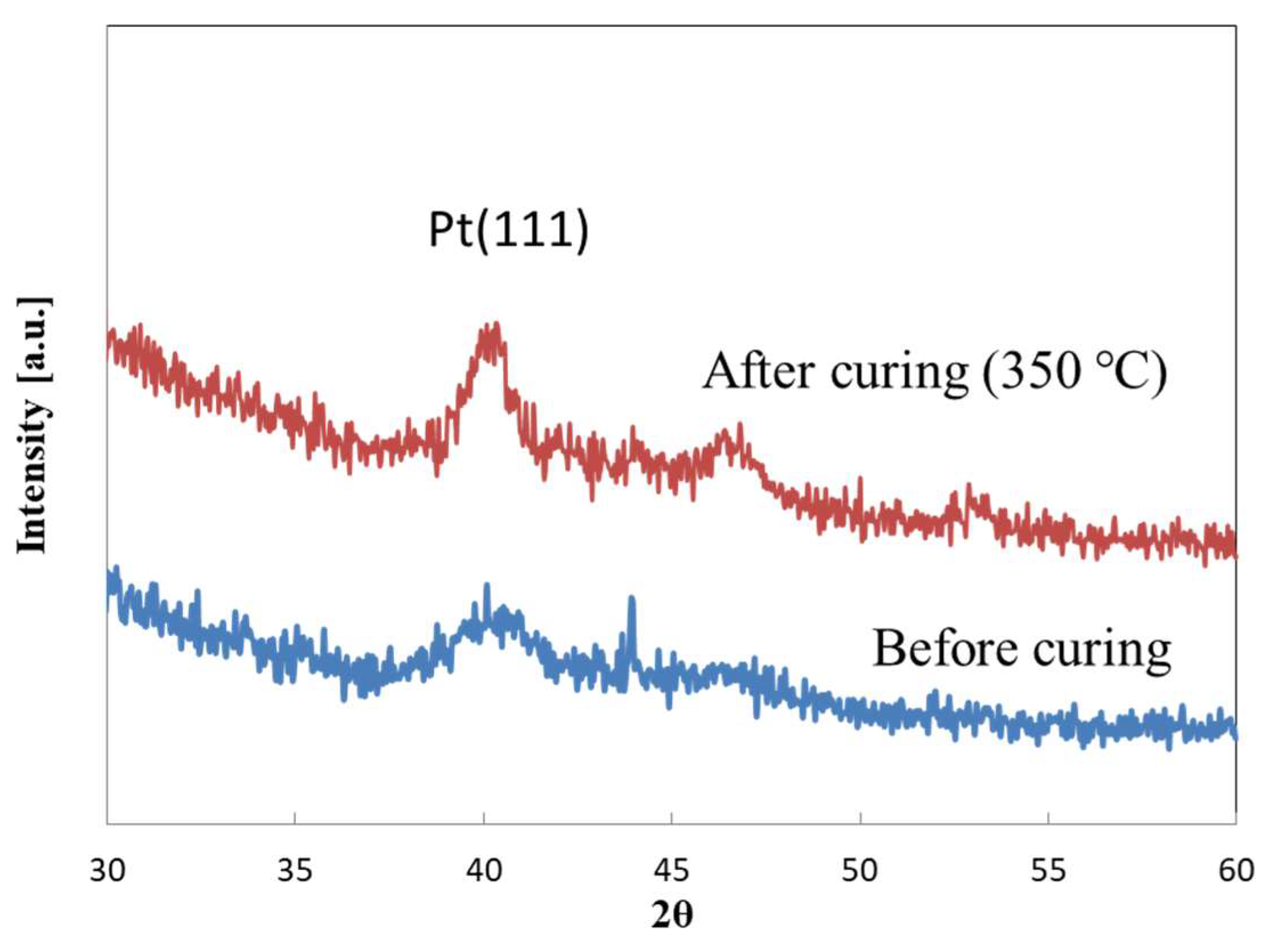
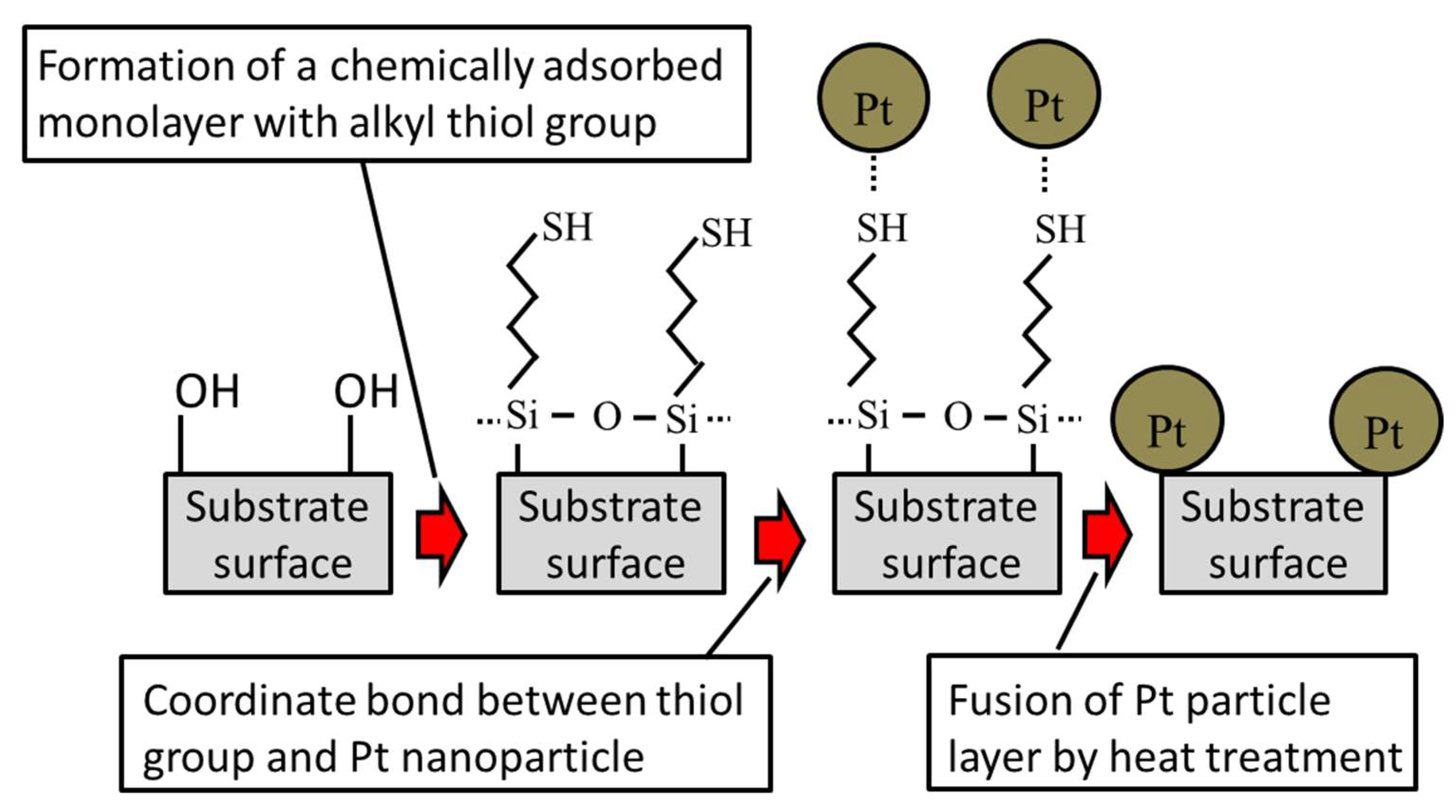
2.1. Observation of Pt Catalyst
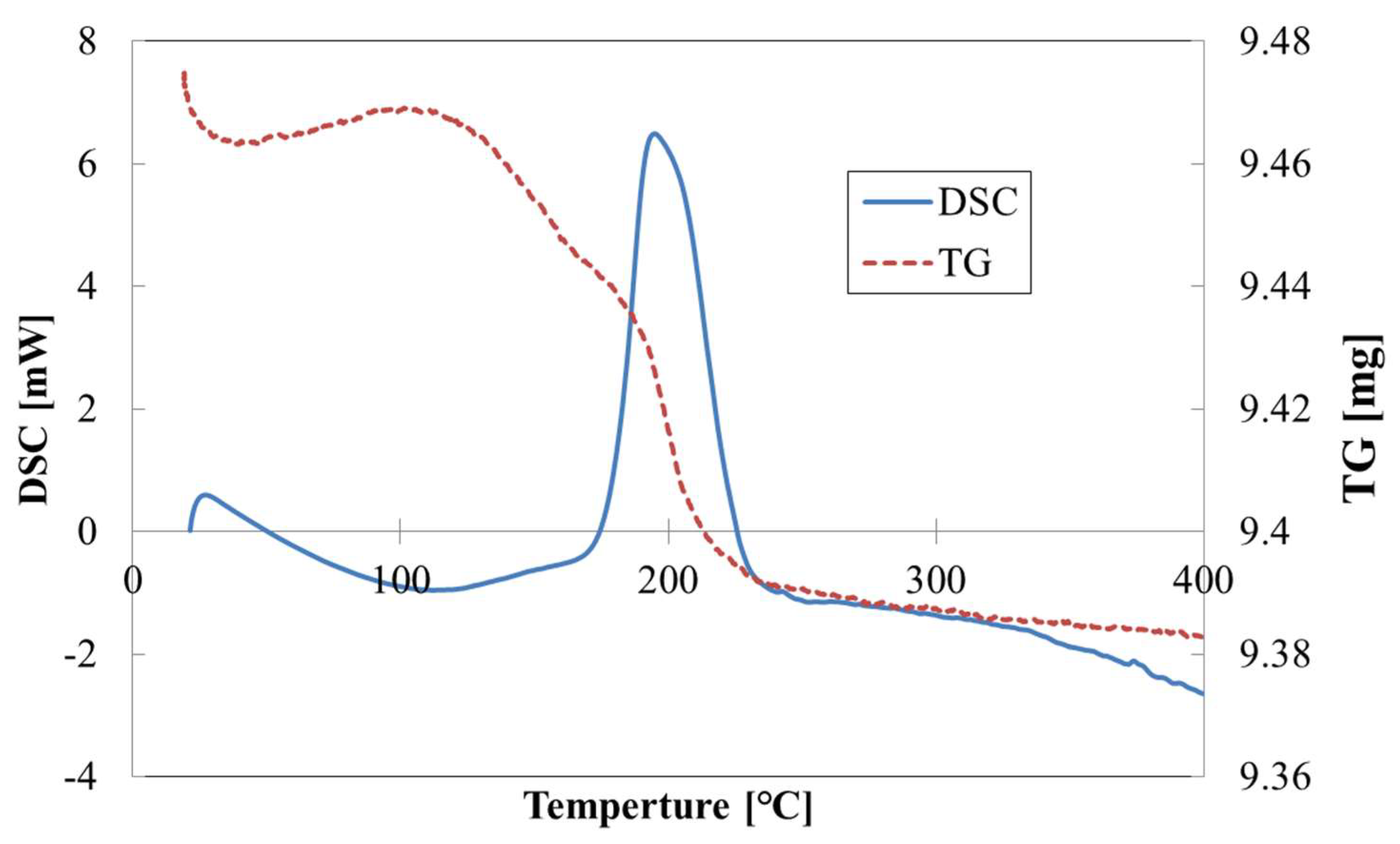
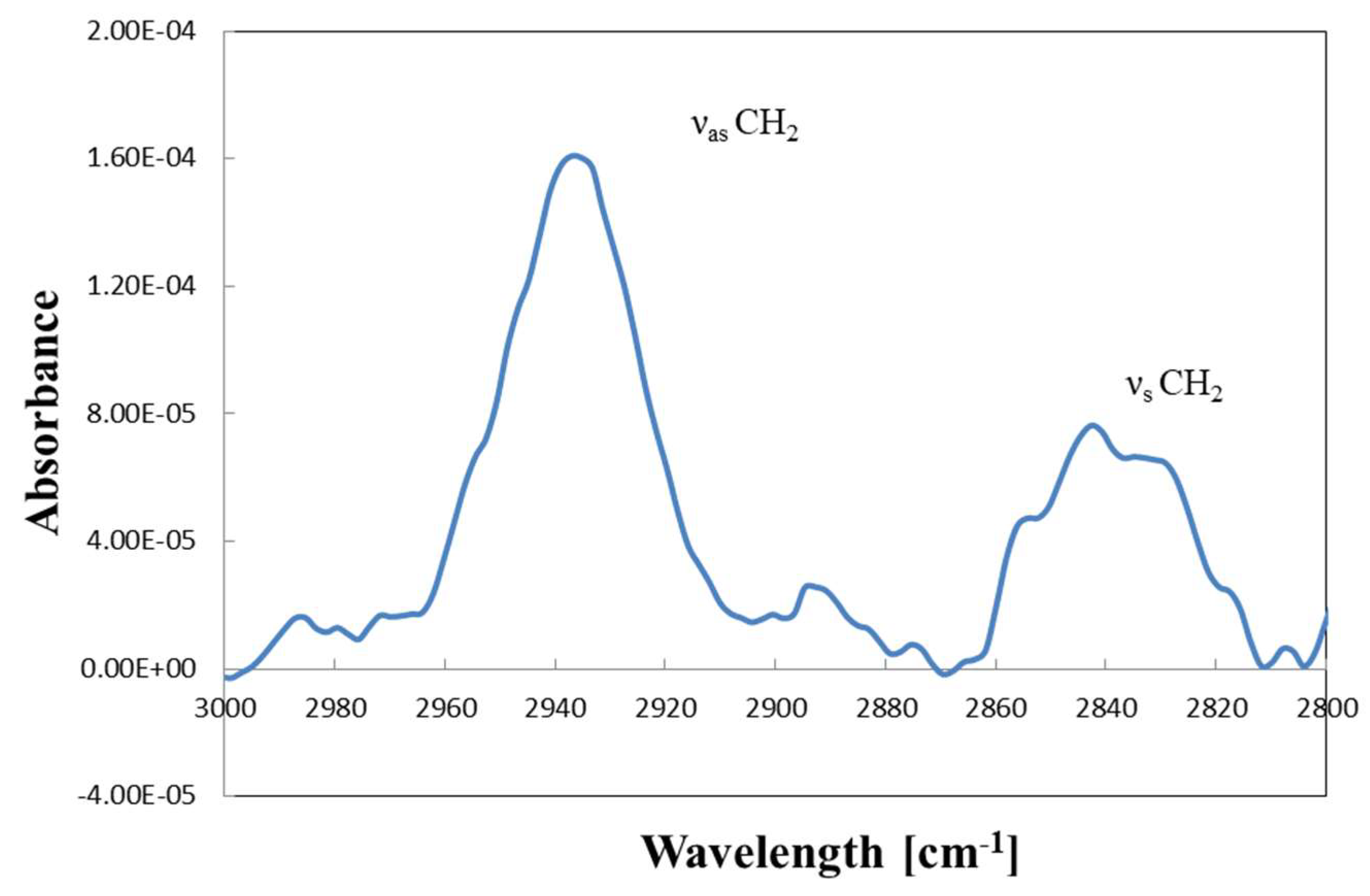
2.2. Thermal Characterization during Catalyst Preparation
2.3. Effective Surface Area of the Catalyst
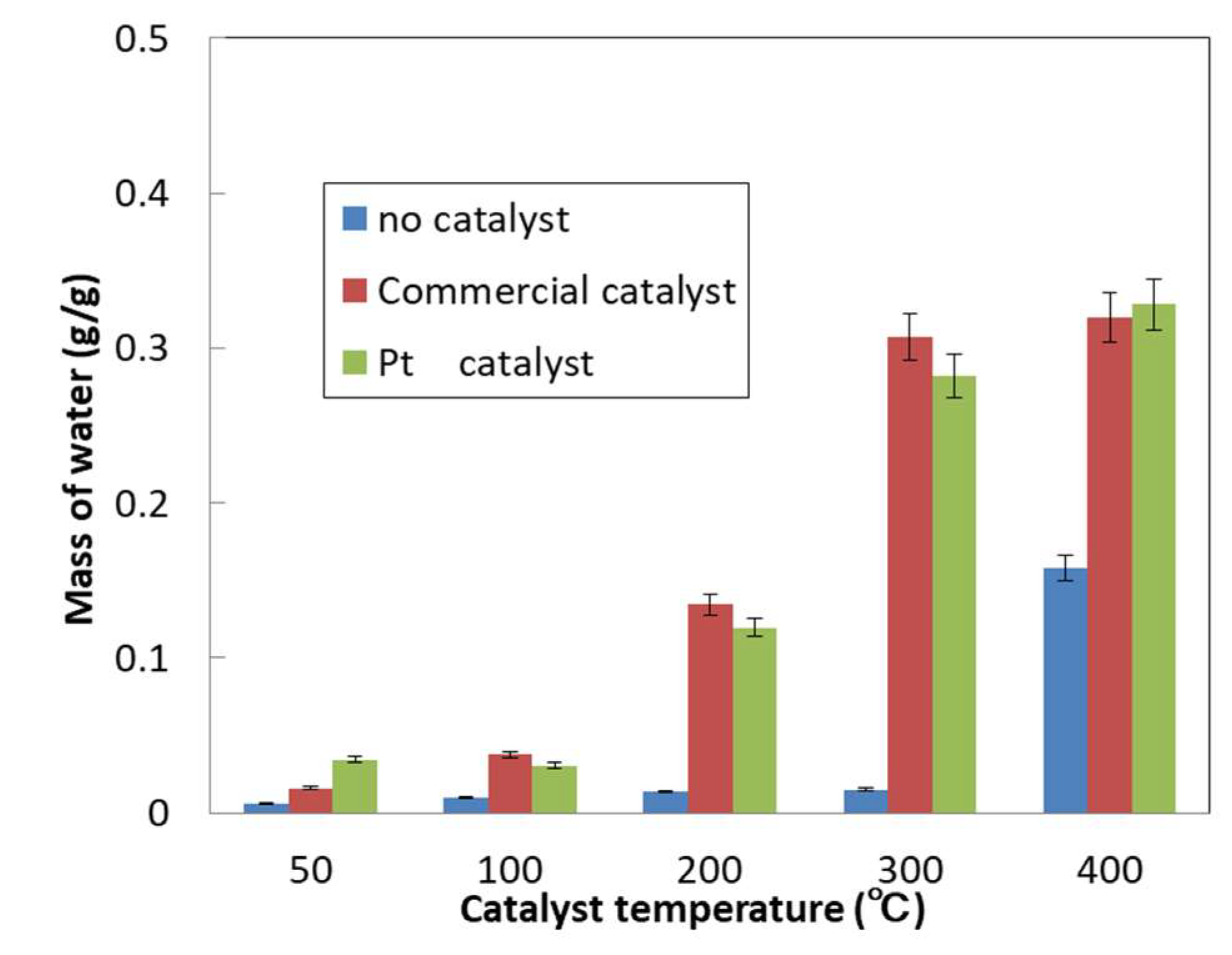
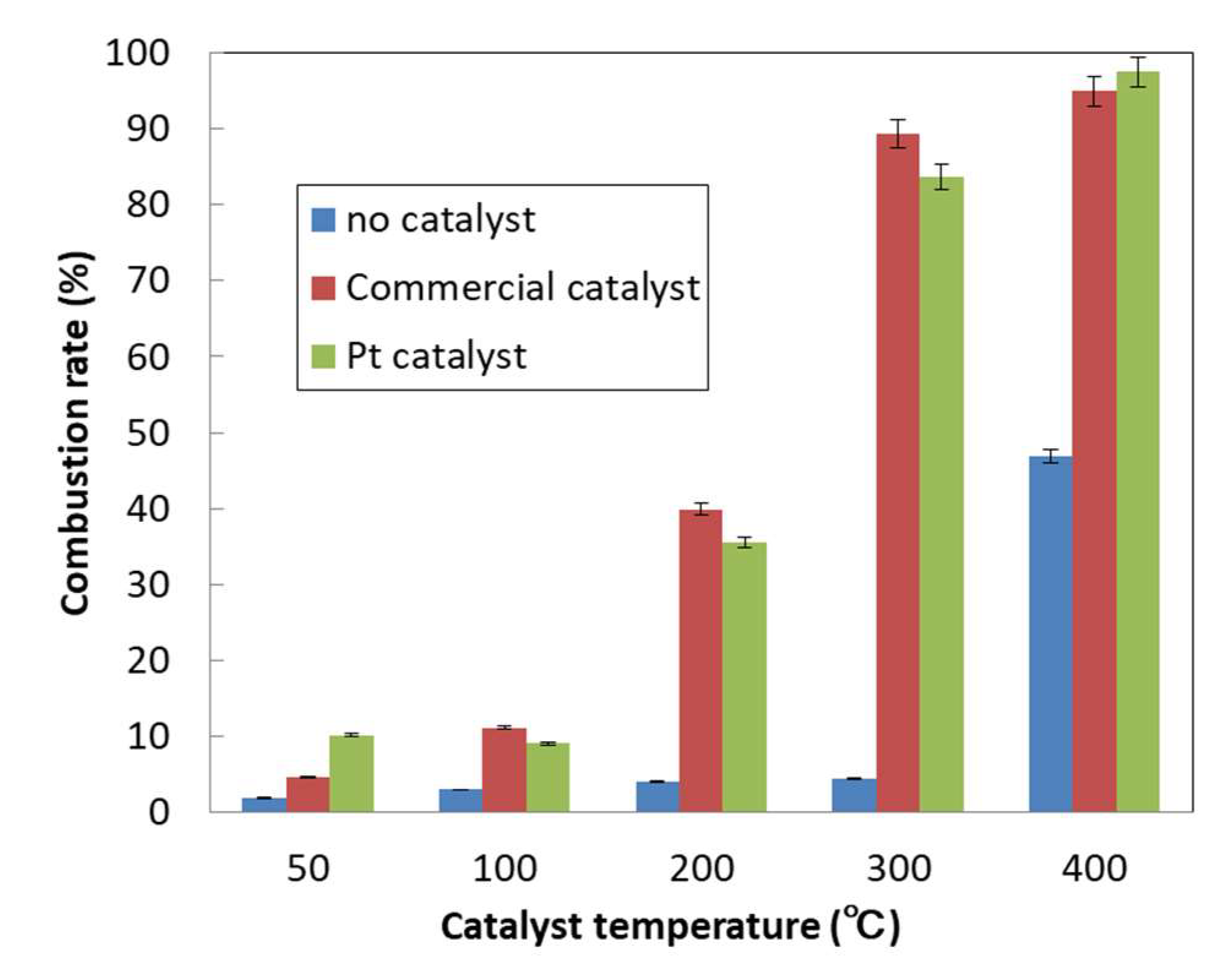
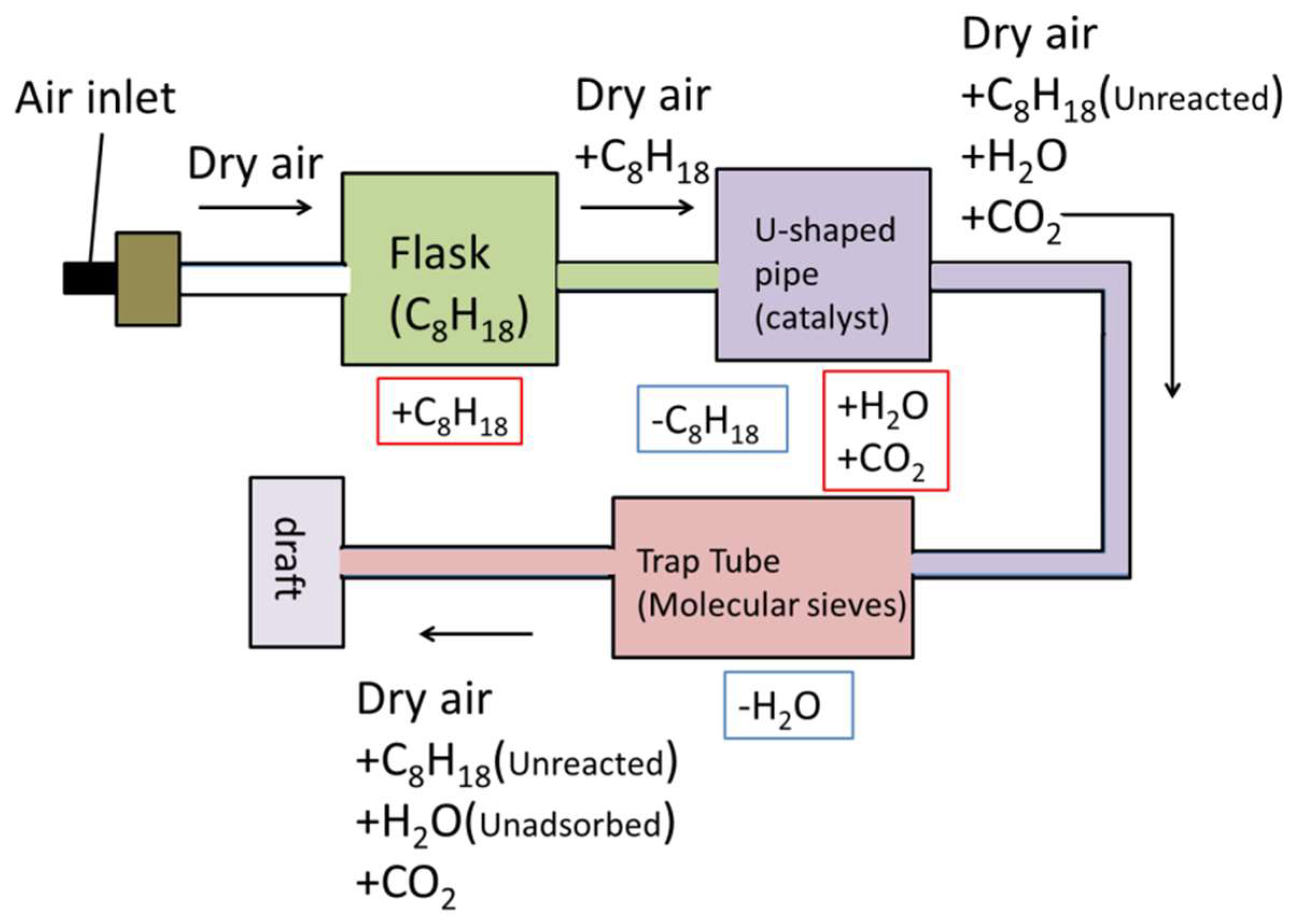
2.4. Catalyst Performance Evaluation
3. Materials and Methods
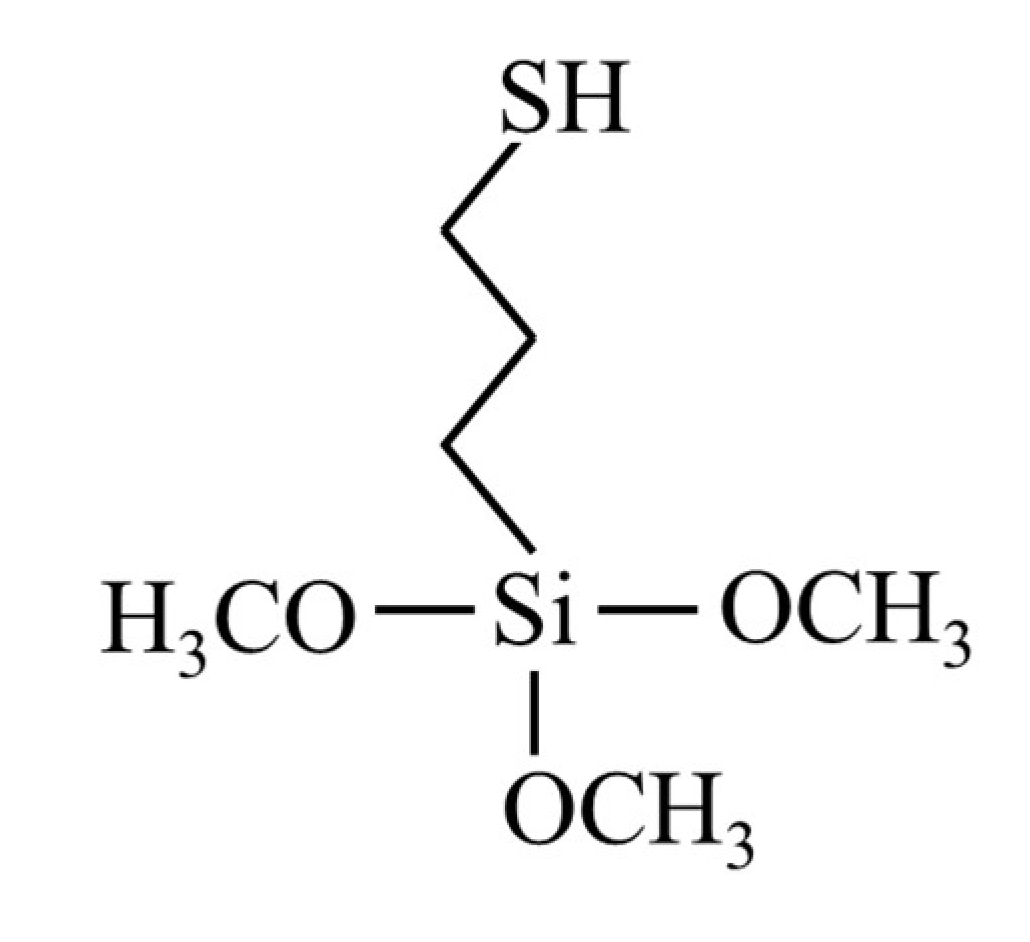
3.1. Preparation of Platinum Catalyst
3.2. Catalyst Performance Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toshima, N.; Wang, Y. Polymer-protected Cu/Pd bimetallic clusters. Adv. Mater. 1994, 6, 245–247. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Oshima, R.; Maeda, Y.; Nagata, Y. Preparation of Platinum Nanoparticles by Sonochemical Reduction of the Pt(II) Ion. Langmuir 1999, 15, 2733–2737. [Google Scholar] [CrossRef]
- Seino, S.; Kinoshita, T.; Nakagawa, T.; Kojima, T.; Taniguchi, R.; Okuda, S.; Yamamoto, T.A. Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam. J. Nanopart. Res. 2008, 10, 1071–1076. [Google Scholar] [CrossRef]
- Kageyama, S.; Sugano, Y.; Hamaguchi, Y.; Kugai, J.; Ohkubo, Y.; Seino, S.; Nakagawa, T.; Ichikawa, S.; Yamamoto, T.A. Pt/TiO2 composite nanoparticles synthesized by electron beam irradiation for preferential CO oxidation. Mater. Res. Bull. 2013, 48, 1347–1351. [Google Scholar] [CrossRef]
- Mohamed, R.M. Characterization and catalytic properties of nano-sized Pt metal catalyst on TiO2-SiO2 synthesized by photo-assisted deposition and impregnation methods. J. Mater. Process. Tech. 2009, 209, 577–583. [Google Scholar] [CrossRef]
- Ogawa, M.; Sato, R.; Kikuchi, S.; Iwachido, K. Effects of TWC’s PGM Loading Amount on the Exhaust-emission Purification Performance and on the PGM Sintering. Trans. Soc. Automot. Eng. Jpn. 2017, 48, 1015–1020. [Google Scholar] [CrossRef]
- Suda, A.; Sobukawa, H.; Suzuki, T.; Kandori, T.; Ukyo, Y.; Sugiura, M. Synthesis of Ceria Zirconia Solid Solution and Performance as Catalytic Promoter. Electron. J. R&D Rev. Toyota CRDL 1998, 3, 3–12. Available online: https://www.tytlabs.com/japanese/review/rev333j.html (accessed on 18 March 2018).
- Akama, H. Study on Effective Removal of Residual Methane in Wasted Gas originated from the Fuel Reforming Gas by Catalytic Combustion. J. Combust. Soc. Jpn. 2010, 52, 76–85. [Google Scholar] [CrossRef]
- Tsuji, I.; Ohkubo, Y.; Ogawa, K. Study on Super-Hydrophobic and Oleophobic Surfaces Prepared by Chemical Adsorption Technique. Jpn. J. Appl. Phys. 2008, 48, 040205. [Google Scholar] [CrossRef]
- Ogawa, K.; Mino, N.; Nakajima, K.; Azuma, Y.; Ohmura, T. Studies of molecular alignments of monolayers deposited by a chemical adsorption technique. Langmuir 1991, 7, 1473–1477. [Google Scholar] [CrossRef]
- Sawada, S.; Masuda, Y.; Zhu, P.; Koumoto, K. Micropatterning of copper on a poly(ethylene terephthalate) substrate modified with a self-assembled monolayer. Langmuir 2006, 22, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Sakakibara, S. Anhydrous hydrogen fluoride as solvent. J. Synthetic Org. Chem. Jpn. 1967, 25, 1176–1191. [Google Scholar] [CrossRef]
- Tanaka Kikinzoku Kogyo Co., Ltd. Latest Precious Metal Market Trends. Available online: https://pro.tanaka.co.jp/library/rate/ (accessed on 15 October 2019).

| - | Catalyst Weight (g) | Precious Metal Particle Weight (g) | Loading Rate (wt%) |
|---|---|---|---|
| Pt catalyst | 0.047 | 0.0060 | 12.7 |
| Commercial catalyst sample | 0.9722 | 0.0165 | 1.7 |
| Heating Temperature | Combustion Rate of Octane per g of Catalyst (%) | Octane Combustion Rate per g of Precious Metal Particles (%) | ||
|---|---|---|---|---|
| - | Commercial Catalyst | Pt Catalyst | Commercial Catalyst | Pt Catalyst |
| 50 | 2.2 | 56.2 | 97.8 | 466.3 |
| 100 | 5.1 | 49.9 | 232.4 | 414.2 |
| 200 | 18.3 | 195.3 | 825.5 | 1620.3 |
| 300 | 40.9 | 460.7 | 1848.5 | 3822.5 |
| 400 | 43.5 | 536.4 | 1964.7 | 4450.6 |
| - | Chemicals | Concentration | Amount |
|---|---|---|---|
| Solvent | Aqua solvent G-21 (Aqua Chemical Co., Ltd., Chuo-ku, Japan) | Undiluted | 50 mL |
| Adsorbent | 3-mercaptopropyltrimethoxysilane (MPTS) (dehydrated chloroform dilution) | 0.1 M | 76.5 μL |
| Catalyst | Tetra chlorosilane (TCS) (dehydrated chloroform dilution) | 0.01 M | 2.5 mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, T.; Horino, Y.; Ohtake, T.; Ogawa, K.; Suzaki, Y. A Highly Efficient Monolayer Pt Nanoparticle Catalyst Prepared on a Glass Fiber Surface. Catalysts 2020, 10, 472. https://doi.org/10.3390/catal10050472
Sasaki T, Horino Y, Ohtake T, Ogawa K, Suzaki Y. A Highly Efficient Monolayer Pt Nanoparticle Catalyst Prepared on a Glass Fiber Surface. Catalysts. 2020; 10(5):472. https://doi.org/10.3390/catal10050472
Chicago/Turabian StyleSasaki, Teruyoshi, Yusuke Horino, Tadashi Ohtake, Kazufumi Ogawa, and Yoshifumi Suzaki. 2020. "A Highly Efficient Monolayer Pt Nanoparticle Catalyst Prepared on a Glass Fiber Surface" Catalysts 10, no. 5: 472. https://doi.org/10.3390/catal10050472
APA StyleSasaki, T., Horino, Y., Ohtake, T., Ogawa, K., & Suzaki, Y. (2020). A Highly Efficient Monolayer Pt Nanoparticle Catalyst Prepared on a Glass Fiber Surface. Catalysts, 10(5), 472. https://doi.org/10.3390/catal10050472





