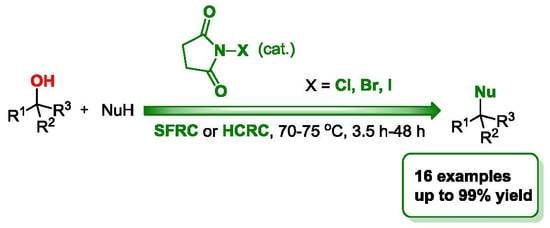
N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions
Abstract
1. Introduction
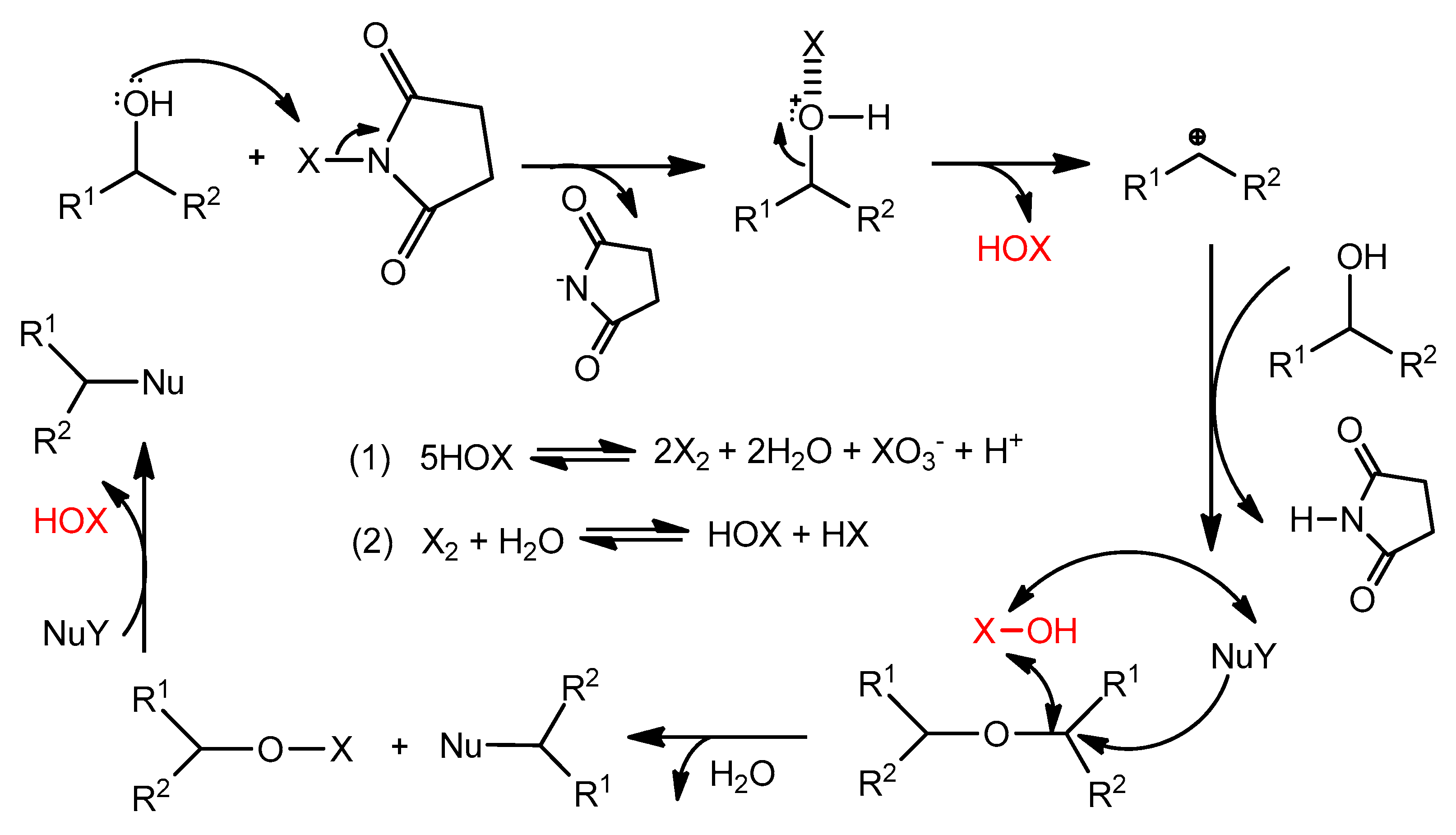
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Yasuda, M.; Saito, T.; Ueba, M.; Baba, A. Direct Substitution of the Hydroxy Group in Alcohols with Silyl Nucleophiles Catalyzed by Indium Trichloride. Angew. Chem. Int. Ed. 2004, 43, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Emer, E.; Sinisi, R.; Capdevila, M.G.; Petruzziello, D.; De Vincentiis, F.; Cozzi, P.G. Direct Nucleophilic SN1-Type Reactions of Alcohols. Eur. J. Org. Chem. 2011, 2011, 647–666. [Google Scholar] [CrossRef]
- Kumar, R.; Van der Eycken, E.V. Recent approaches for C-C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 2013, 42, 1121–1146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; He, T.; Ma, L.; Wang, Z. Recent developments in Ritter reaction. RSC Advances 2014, 4, 64936–64946. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.-P.; Wang, C.-H.; Zhou, J. Catalytic functionalization of tertiary alcohols to fully substituted carbon centres. Org. Biomol. Chem. 2014, 12, 6033–6048. [Google Scholar] [CrossRef]
- Uchuskin, M.G.; Makarov, A.S.; Butin, A.V. Catalytic Alkylation of Furans by π-Activated Alcohols (Review). Chem. Heterocycl. Compd. 2014, 50, 791–806. [Google Scholar] [CrossRef]
- Chaskar, A.; Murugan, K. Direct allylation of alcohols using allyltrimethylsilane: A move towards an economical and ecological protocol for C-C bond formation. Catal. Sci. Tech. 2014, 4, 1852–1868. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Z.-Q. Iron-Catalyzed Alkylation of Alkenes and Alkynes Using Alcohols as the Alkylating Reagent. Synthesis 2015, 47, 1706–1708. [Google Scholar] [CrossRef]
- Dryzhakov, M.; Richmond, E.; Moran, J. Recent Advances in Direct Catalytic Dehydrative Substitution of Alcohols. Synthesis 2016, 48, 935–959. [Google Scholar]
- Ajvazi, N.; Stavber, S. Alcohols in direct carbon-carbon and carbon-heteroatom bond-forming reactions: Recent advances. Arkivoc 2018, part ii, 288–329. [Google Scholar] [CrossRef]
- Guillena, G.; Ramón, D.J.; Yus, M. Alcohols as Electrophiles in C–C Bond-Forming Reactions: The Hydrogen Autotransfer Process. Angew. Chem. Int. Ed. 2007, 46, 2358–2364. [Google Scholar] [CrossRef]
- Dobereiner, G.E.; Crabtree, R.H. Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev. 2010, 110, 681–703. [Google Scholar] [CrossRef]
- Yang, G.-P.; Jiang, N.; Huang, X.-Q.; Yu, B.; Hu, C.-W. Non-corrosive heteropolyacid-based recyclable ionic liquid catalyzed direct dehydrative coupling of alcohols with alcohols or alkenes. Mol. Catal. 2019, 468, 80–85. [Google Scholar] [CrossRef]
- Chevella, D.; Macharla, A.K.; Kodumuri, S.; Banothu, R.; Gajula, K.S.; Amrutham, V.; Gennadievna, G.E.N.; Nama, N. Synthesis of internal olefins by direct coupling of alcohols and olefins over Moβ zeolite. Catal. Commun. 2019, 123, 114–118. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bora, U. Molecular Iodine-Catalyzed Selective C-3 Benzylation of Indoles with Benzylic Alcohols: A Greener Approach toward Benzylated Indoles. ACS Omega 2019, 4, 11770–11776. [Google Scholar] [CrossRef] [PubMed]
- Veenboer, R.M.P.; Nolan, S.P. Gold(i)-catalysed dehydrative formation of ethers from benzylic alcohols and phenols. Green Chem. 2015, 17, 3819–3825. [Google Scholar] [CrossRef]
- Böldl, M.; Fleischer, I. Dehydrative Coupling of Benzylic Alcohols Catalyzed by Brønsted Acid/Lewis Base. Eur. J. Org. Chem. 2019, 2019, 5856–5861. [Google Scholar] [CrossRef]
- Kolvari, E.; Ghorbani-Choghamarani, A.; Salehi, P.; Shirini, F.; Zolfigol, M.A. Application of N-halo reagents in organic synthesis. J. Iran Chem. Soc. 2007, 4, 126–174. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides. Molecules 2016, 21, 1325. [Google Scholar] [CrossRef]
- Karimi, B.; Ebrahimian, G.R.; Seradj, H. Efficient and Chemoselective Conversion of Carbonyl Compounds to 1,3-Dioxanes Catalyzed with N-Bromosuccinimide under Almost Neutral Reaction Conditions. Org. Lett. 1999, 1, 1737–1739. [Google Scholar] [CrossRef]
- Karimi, B.; Seradj, H. N-Bromosuccinimide (NBS), a Novel and Highly Effective Catalyst for Acetylation of Alcohols under Mild Reaction Conditions. Synlett 2001, 2001, 0519–0520. [Google Scholar] [CrossRef]
- Čebular, K.; Božić, B.Đ.; Stavber, S. Esterification of Aryl/Alkyl Acids Catalysed by N-bromosuccinimide under Mild Reaction Conditions. Molecules 2018, 23, 2235. [Google Scholar] [CrossRef] [PubMed]
- Wagh, Y.S.; Sawant, D.N.; Bhanage, B.M. Metal-free N-iodosuccinimide-catalyzed mild oxidative C–H bond amination of benzoxazoles. Tetrahedron Lett. 2012, 53, 3482–3485. [Google Scholar] [CrossRef]
- Kadam, S.T.; Kim, S.S. N-Iodosuccinimide (NIS) a novel and effective catalyst for the cyanosilylation of aldehydes under mild reaction conditions. Catal. Commun. 2008, 9, 1342–1345. [Google Scholar] [CrossRef]
- Guha, S.; Rajeshkumar, V.; Kotha, S.S.; Sekar, G. A Versatile and One-Pot Strategy to Synthesize α-Amino Ketones from Benzylic Secondary Alcohols Using N-Bromosuccinimide. Org. Lett. 2015, 17, 406–409. [Google Scholar] [CrossRef]
- Muneeswara, M.; Muthukumar, A.; Sekar, G. Dual Role of N-Bromosuccinimide as Oxidant and Succinimide Surrogate in Domino One-Pot Oxidative Amination of Benzyl Alcohols for the Synthesis of α–Imido Ketones. ChemistrySelect 2018, 3, 12524–12529. [Google Scholar] [CrossRef]
- Muneeswara, M.; Sundaravelu, N.; Sekar, G. NBS-mediated synthesis of β-keto sulfones from benzyl alcohols and sodium arenesulfinates. Tetrahedron 2019, 75, 3479–3484. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic Oxidative Iodination of Organic Compounds with Iodide Catalyzed by Sodium Nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite “on water” or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262–1267. [Google Scholar] [CrossRef]
- Stavber, G.; Stavber, S. Towards Greener Fluorine Organic Chemistry: Direct Electrophilic Fluorination of Carbonyl Compounds in Water and Under Solvent-Free Reaction Conditions. Adv. Synth. Catal. 2010, 352, 2838–2846. [Google Scholar] [CrossRef]
- Chun, S.; Chung, Y.K. Silver/NBS-Catalyzed Synthesis of α-Alkylated Aryl Ketones from Internal Alkynes and Benzyl Alcohols via Ether Intermediates. Org. Lett. 2018, 20, 5583–5586. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Breugst, M.; von der Heiden, D. Mechanisms in Iodine Catalysis. Chem. Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2006, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Čebular, K.; Božić, B.Đ.; Stavber, S. 1,3-Dibromo-5,5-dimethylhydantoin as a Precatalyst for Activation of Carbonyl Functionality. Molecules 2019, 24, 2608. [Google Scholar] [CrossRef]
- Beebe, T.R.; Adkins, R.L.; Bogardus, C.C.; Champney, B.; Hii, P.S.; Reinking, P.; Shadday, J.; Weatherford, W.D.; Webb, M.W.; Yates, S.W. Primary alcohol oxidation with N-iodosuccinimide. J. Org. Chem. 1983, 48, 3126–3128. [Google Scholar] [CrossRef]
- Anxionnat, B.; Guérinot, A.; Reymond, S.; Cossy, J. FeCl3-catalyzed Ritter reaction. Synthesis of amides. Tetrahedron Lett. 2009, 50, 3470–3473. [Google Scholar] [CrossRef]
- Theerthagiri, P.; Lalitha, A.; Arunachalam, P.N. Iodine-catalyzed one-pot synthesis of amides from nitriles via Ritter reaction. Tetrahedron Lett. 2010, 51, 2813–2819. [Google Scholar] [CrossRef]
- Ogata, O.; Nara, H.; Fujiwhara, M.; Matsumura, K.; Kayaki, Y. N-Monomethylation of Aromatic Amines with Methanol via PNHP-Pincer Ru Catalysts. Org. Lett. 2018, 20, 3866–3870. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Direct halogenation of alcohols with halosilanes under catalyst- and organic solvent-free reaction conditions. Tetrahedron Lett. 2016, 57, 2430–2433. [Google Scholar] [CrossRef]
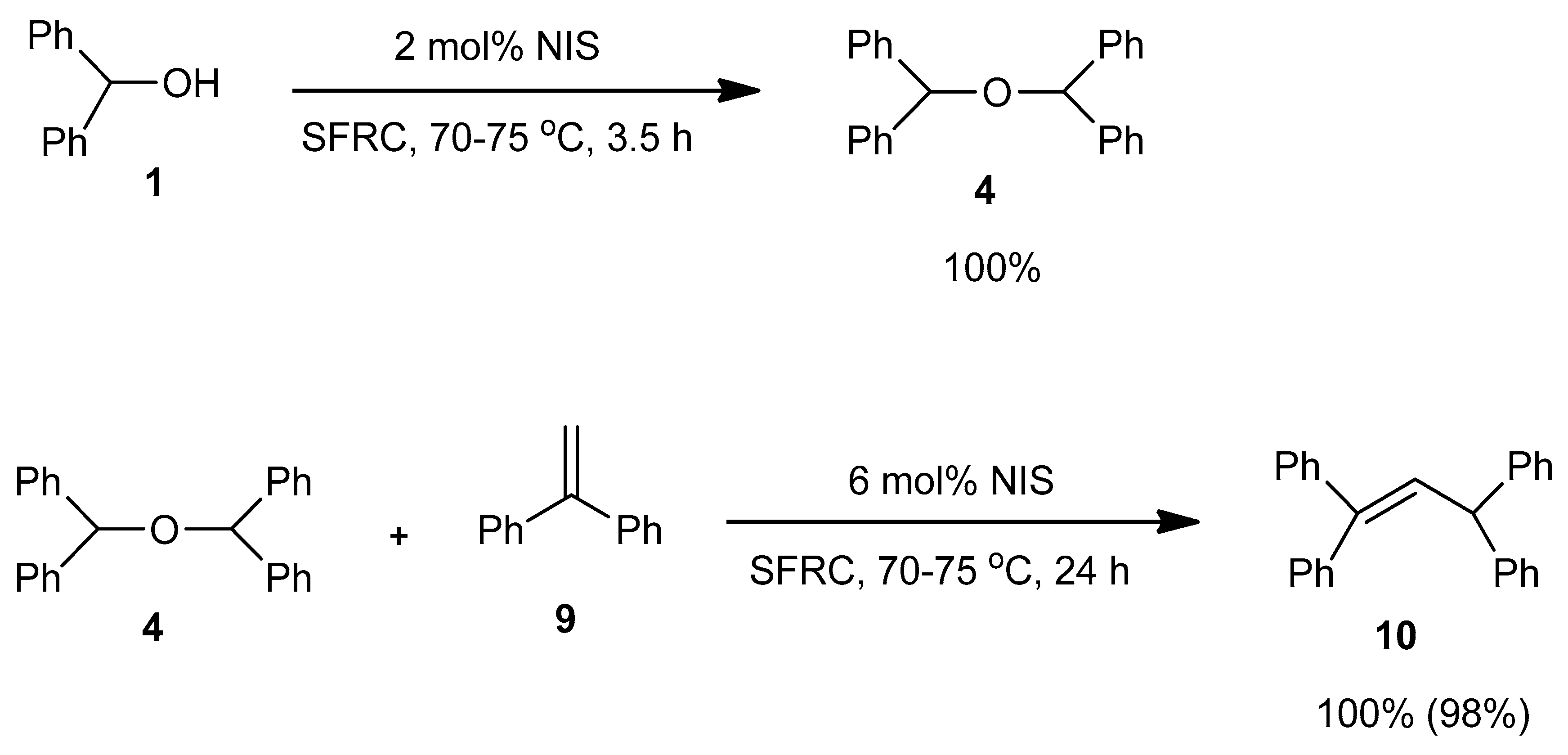


| Entry | NXS | Mol % | Reaction Conditions | Conversion b (%) of 1 | Relative Distribution b (%) | |
|---|---|---|---|---|---|---|
| 3 | 4 | |||||
| 1 | - | - | MeOH (1mmol), 70–75 °C, 6 h | - | - | - |
| 2 | NIS | 3 | MeOH (1mmol), 70–75 °C, 6 h | 100 | 100 | - |
| 3 | NCS | 3 | MeOH (1mmol), 70–75 °C, 6 h | 100 | 92 | 5 c |
| 4 | NBS | 3 | MeOH (1mmol), 70–75 °C, 6 h | 100 | 92 | 5 c |
| 5 | NIS | 2 | 70–75 °C, 3.5 h | 100 | - | 100 |
| 6 | NIS | 2 | In the dark, MeOH (1 mmol), 70–75 °C, 6 h | 100 | 100 | - |
| 7 | NIS | 2 | TEMPO (10 mol %), MeOH (1 mmol), 70–75 °C, 6 h | 100 | 100 | - |
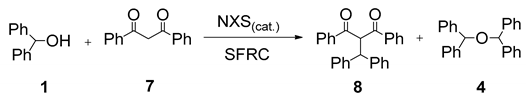
| Entry | NXS | Mol % | Conversion b (%) of 1 | Relative Distribution b (%) | |
|---|---|---|---|---|---|
| 8 | 4 | ||||
| 1 | - | - | 0 | - | - |
| 2 | NCS | 3 | - | - | - |
| 3 | NBS | 3 | 4 | - | 4 |
| 4 | NIS | 3 | 100 | 100 | - |
| 5 | NIS | 1 | 100 | 100 | - |
| 6 c | NIS | 1 | 100 | 100 | - |
| 7 d | NIS | 1 | 100 | 100 | |

| Entry | NXS | Mol % | Conversion b (%) of 14 | Relative Distribution b (%) | ||
|---|---|---|---|---|---|---|
| 16 | 17 | 18 | ||||
| 1 | - | - | - | - | - | - |
| 2 | NBS | 10 | 78 | 67 | - | 11 |
| 3 | NCS | 10 | 82 | 72 | - | 10 |
| 4 | NIS | 10 | 84 | 80 | 2 | 2 |
| 5 c | NIS | 10 | 84 | 80 | 2 | 2 |
| 6 d | NIS | 10 | 84 | 80 | 2 | 2 |

| Entry | Bond Formation | R1, R2, R3 | NuY | Product | Conversion.b (%) (Yield c (%)) |
|---|---|---|---|---|---|
| 1 | C–O | R1 = R2 = Me R3 = (CH2)2Ph 5 | MeOH 2 |  | 74 (64) |
| 2 | R1 = Ph, R1 = R2 = Me 33 | MeOH 2 | 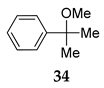 | 93 (90) | |
| 3 | R1 = Ph, R2 = H, R3 = Me 11 | MeOH 2 | 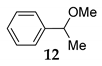 | 67 f (61) g | |
| 4 | R1 = Ph, R2 = R3 = H 27 | MeOH 2 | 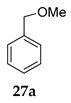 | 2 f - | |
| 5 | R1 = 4-MePh, R2 = H, R3 = Ph 30 | TMSOEt 23c | 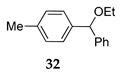 | 100 f (89) | |
| 6 | C–C | R1 = R2 = R3 = Ph 36 | 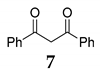 |  | - |
| 7 | R1 = Ph, R2 = R3 = H 27 |  | 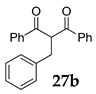 | - | |
| 8 | R1 = R2 = Ph, R3 = H 1 | 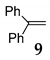 | 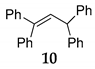 | 100 e (92) | |
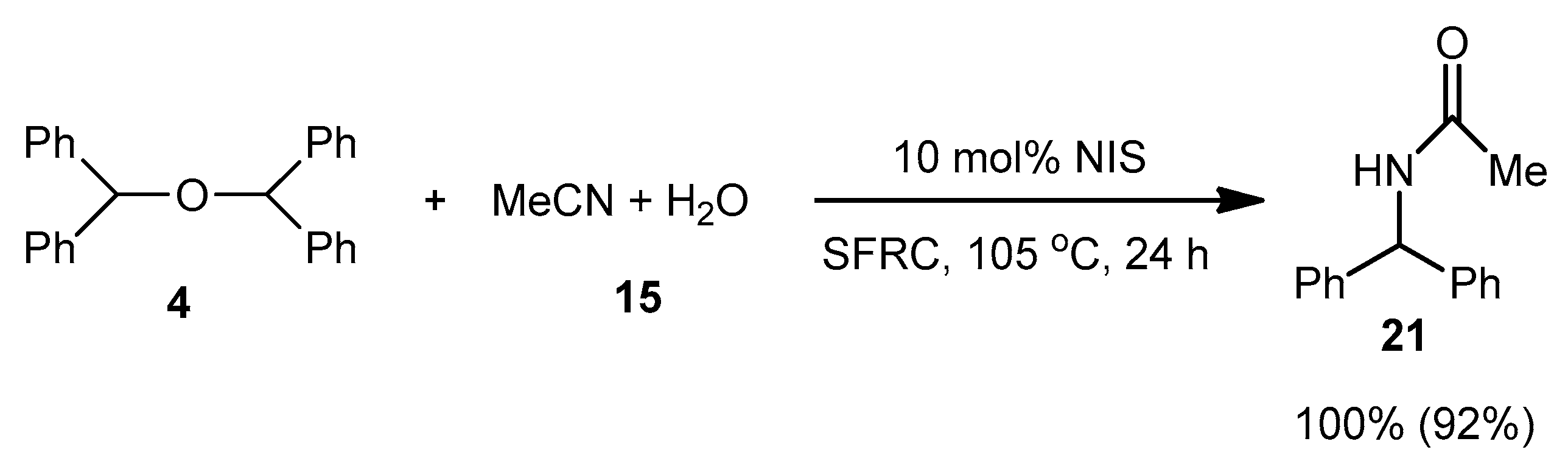
| 9 | C–N | R1 = Ph, R2 = R3 = H 27 | MeCN/H2O 15 |  | 8 f - |
| 10 | R1 = R2 = R3 = Ph 36 | MeCN/H2O 15 | 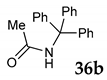 | - | |
| 11 | R1 = R2 = Ph, R3 = H 1 | 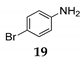 | 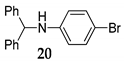 | 85 f (79) | |
| 12 | R1 = R2 = Ph, R3 = H 1 | MeCN/H2O 15 | 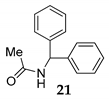 | 100 f (90) | |
| 13 | R1=Ph, R2=H, R3=Me 11 | MeCN/H2O 15 | 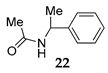 | 100 f (93) | |
| 14 | R1 = 4-MePh, R2 = H, R3 = Ph 30 | TMSNCS 23b | 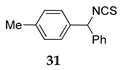 | 100 (97) | |
| 15h | C–Cl | R1 = 3-NO2Ph, R2 = R3 = H 24 | TMSCl 23a | 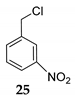 | [42] |
| 16 | R1 = 3-NO2Ph, R2 = R3 = H 24 | TMSCl 23a | 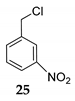 | 91 d (76) | |
| 17h | R1 = Ph, R2 = R3 = H 27 | TMSCl 23a | 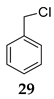 | 69 (65) [42] | |
| 18 | R1 = Ph, R2 = R3 = H 27 | TMSCl 23a | 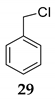 | 100 d (88) | |
| 19h | R1 = 4-MePh, R2 = H, R3 = Me 14 | TMSCl 23a | 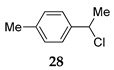 | 90 (86) [42] | |
| 20 | R1 = 4-MePh, R2 = H, R3 = Me 14 | TMSCl 23a | 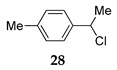 | 100 (98) | |
| 21h | R1=Ph, R1=R2=Me, 33 | TMSCl 23a | 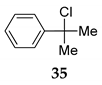 | 100 (98) [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajvazi, N.; Stavber, S. N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions. Catalysts 2020, 10, 460. https://doi.org/10.3390/catal10040460
Ajvazi N, Stavber S. N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions. Catalysts. 2020; 10(4):460. https://doi.org/10.3390/catal10040460
Chicago/Turabian StyleAjvazi, Njomza, and Stojan Stavber. 2020. "N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions" Catalysts 10, no. 4: 460. https://doi.org/10.3390/catal10040460
APA StyleAjvazi, N., & Stavber, S. (2020). N-Halosuccinimides as Precatalysts for C-, N-, O-, and X-Nucleophilic Substitution Reactions of Alcohols under Mild Reaction Conditions. Catalysts, 10(4), 460. https://doi.org/10.3390/catal10040460