The Catalyst Loading Effects on the Feed Rate of NaBH4 Solution for the Hydrogen Production Rate and Conversion Efficiency
Abstract
:1. Introduction
2. Hydrogen Production from the Hydrolysis of Sodium Borohydride
3. Results and Discussion
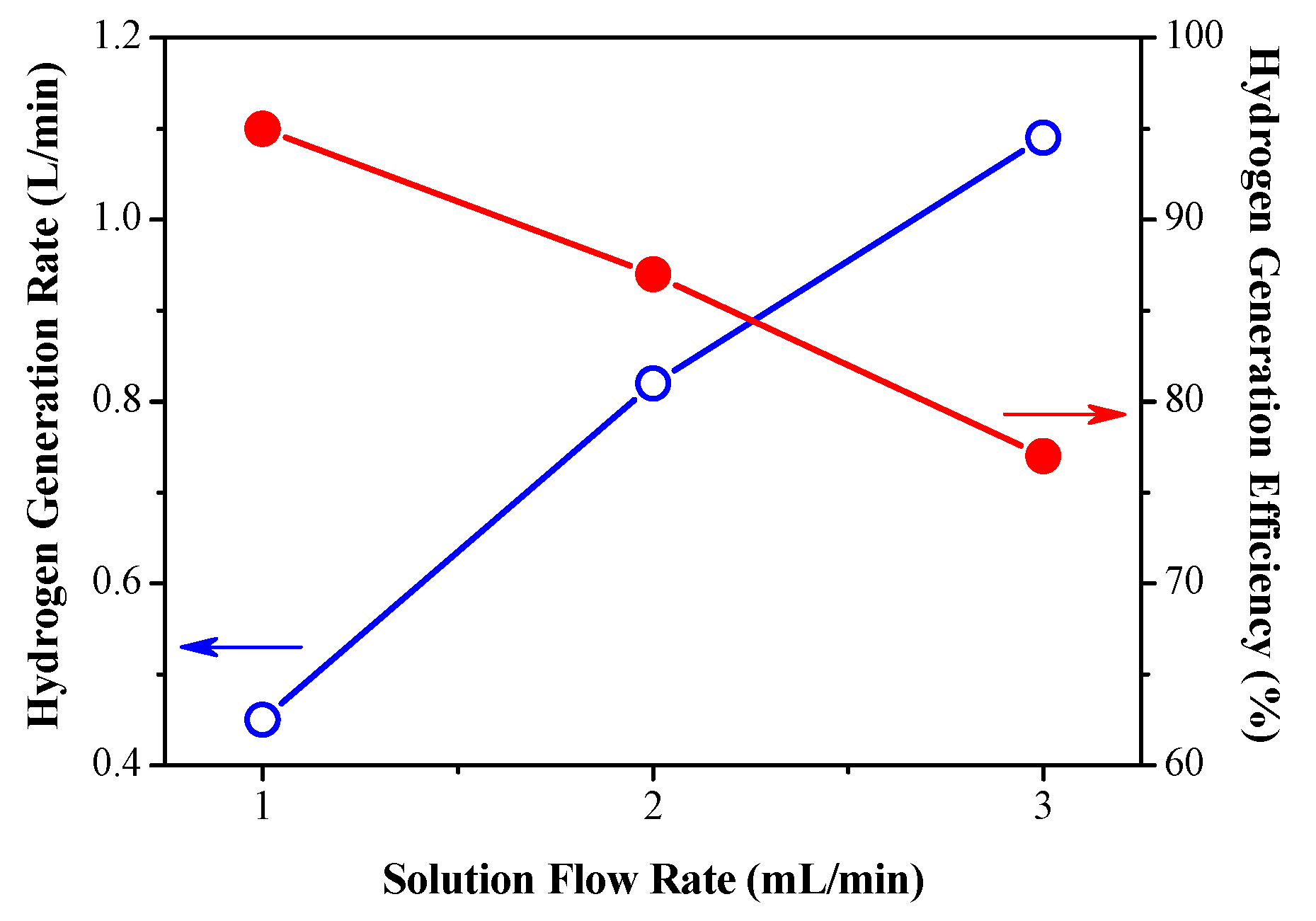
3.1. Effect of Fuel Feed Flow on Hydrogen Production Efficiency
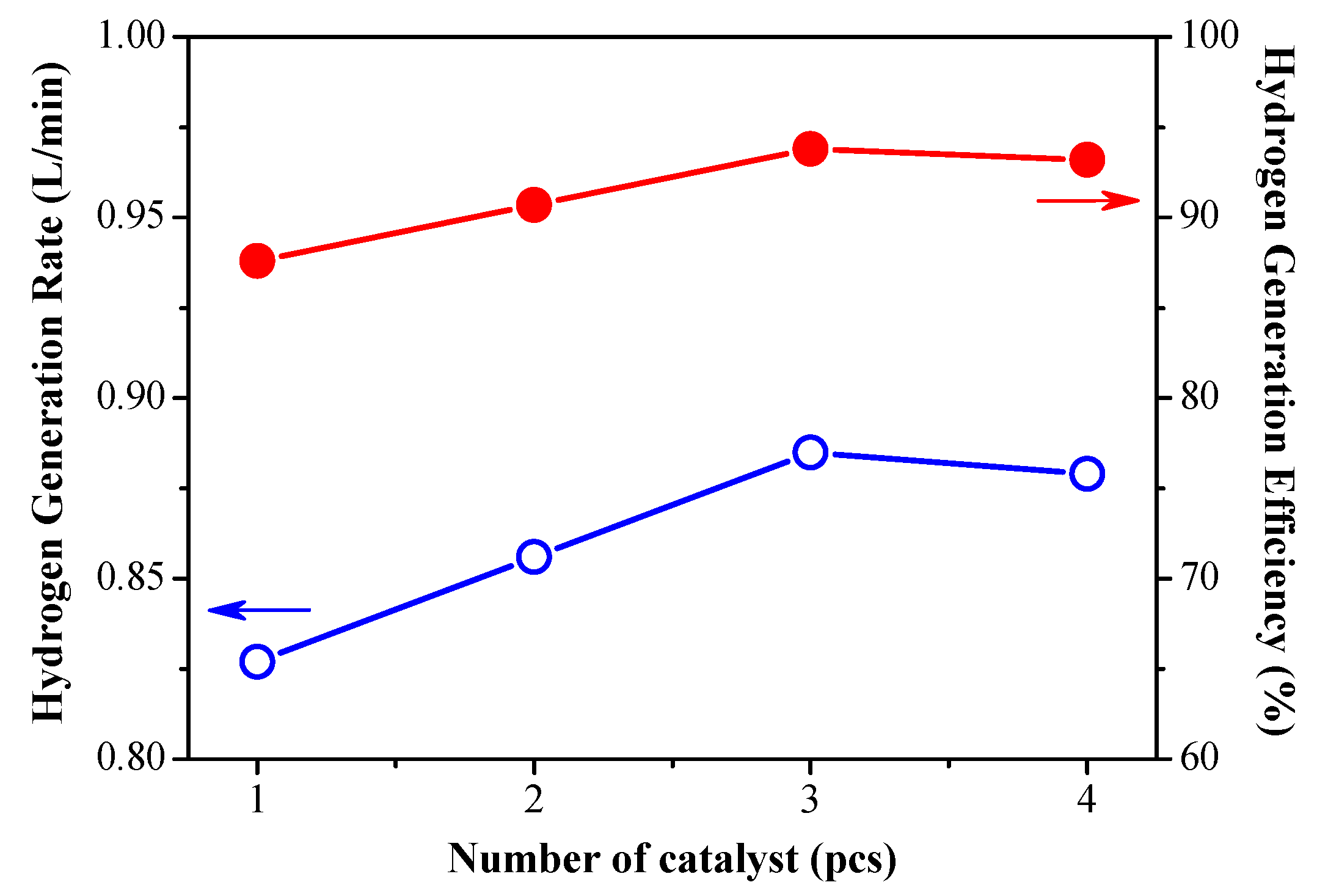
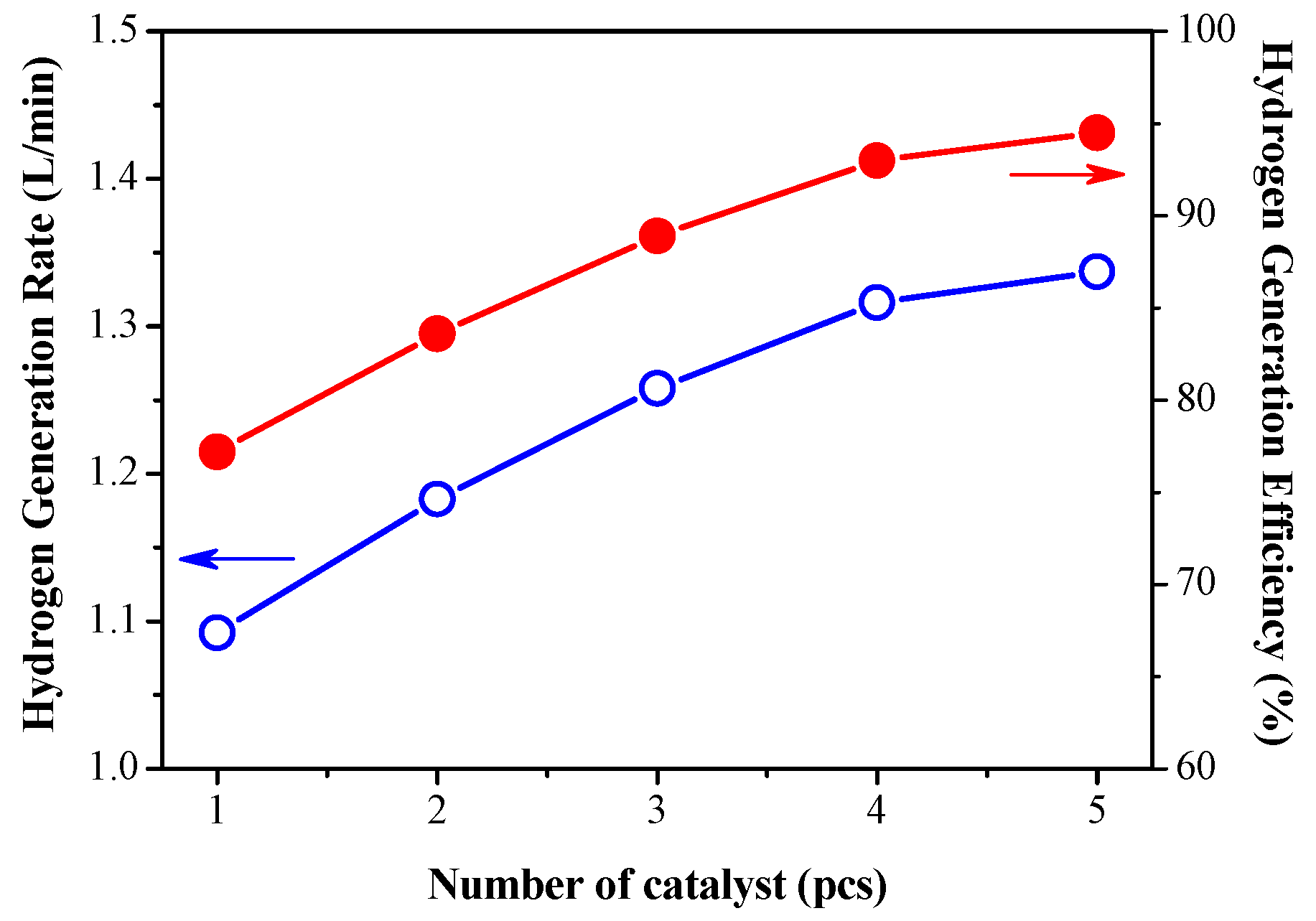
3.2. Effect of Catalyst Dosage on Hydrogen Production Efficiency
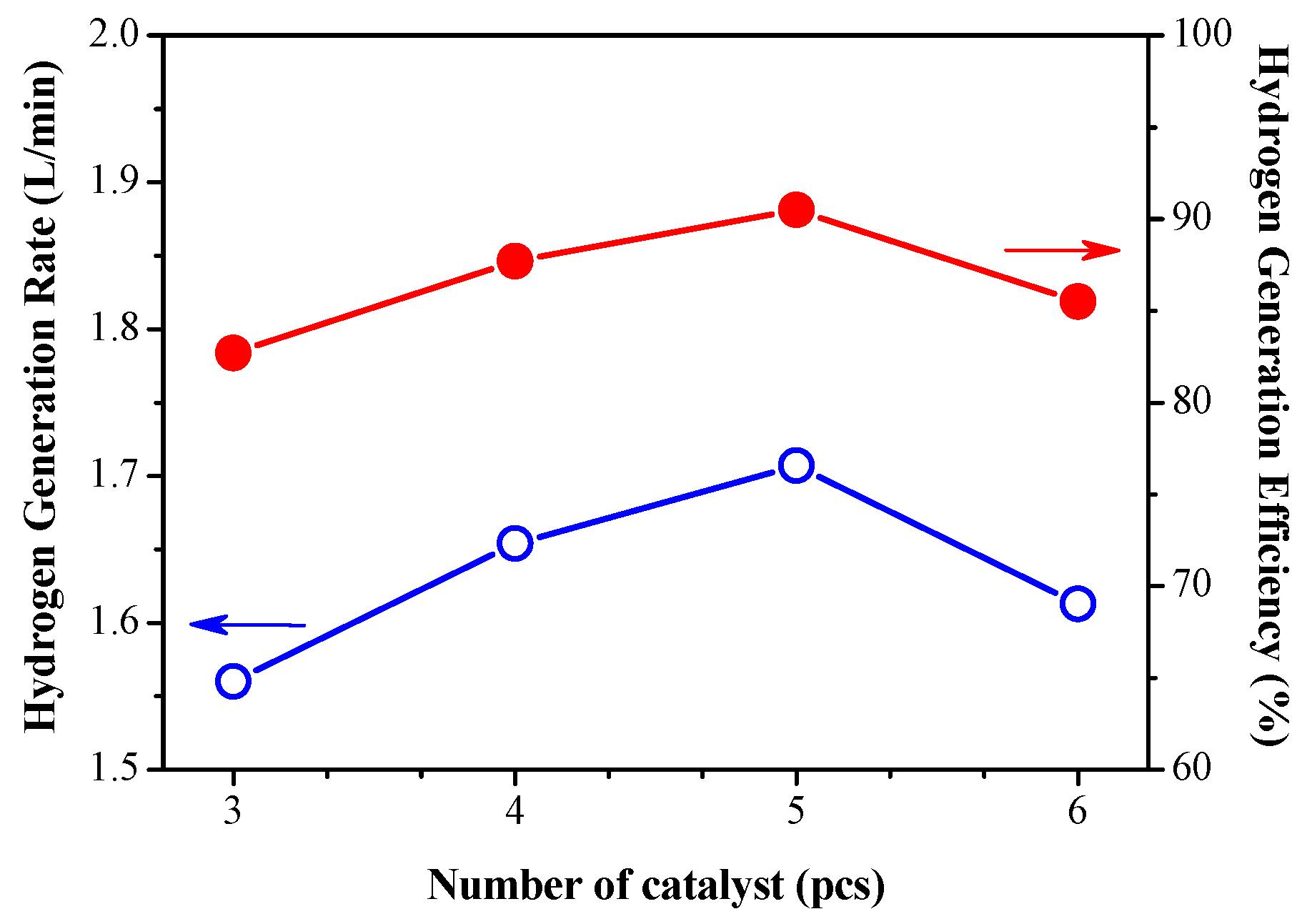
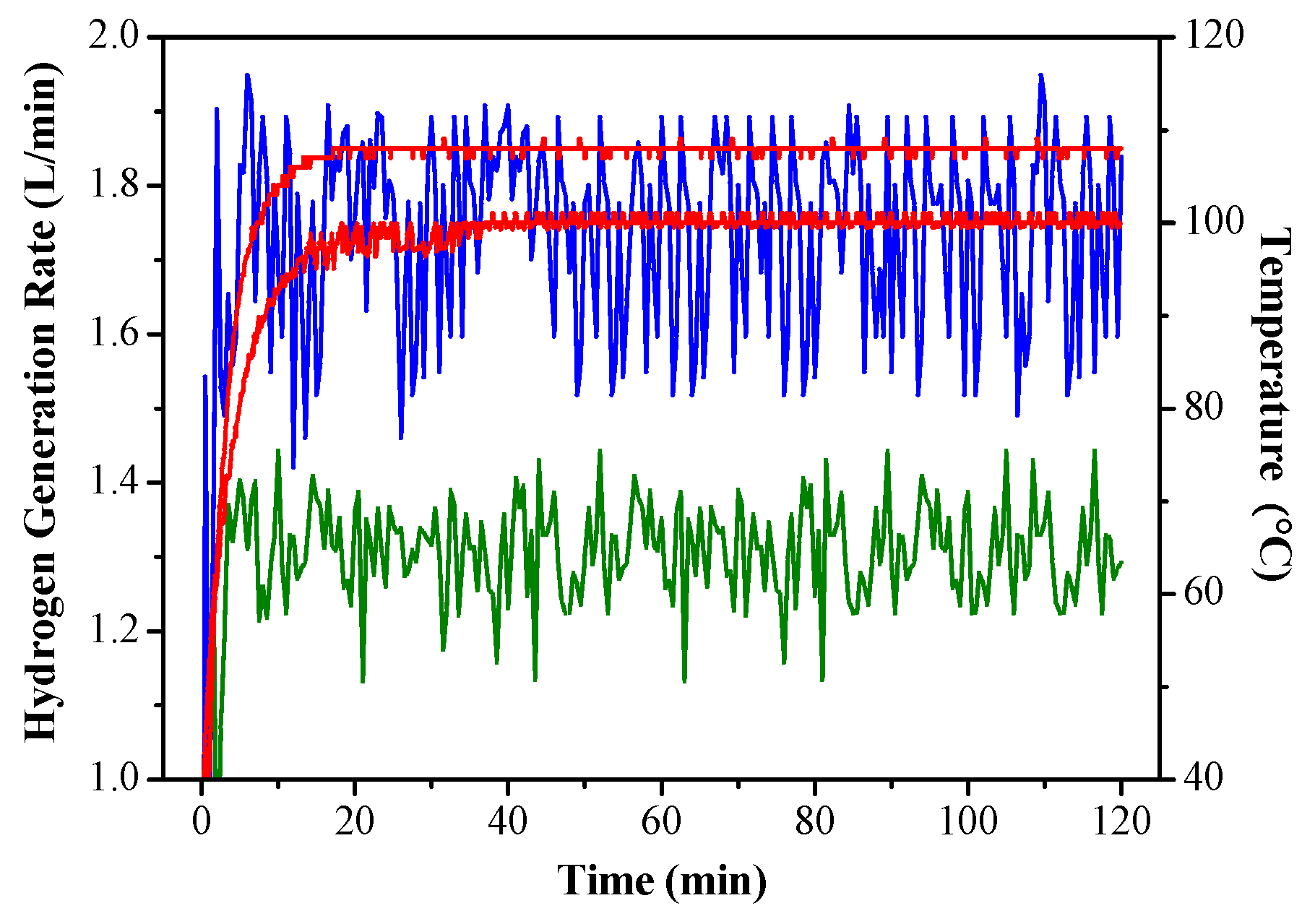
3.3. Long-Term Testing of Optimal Hydrogen Production Operating Conditions
4. Research Methods
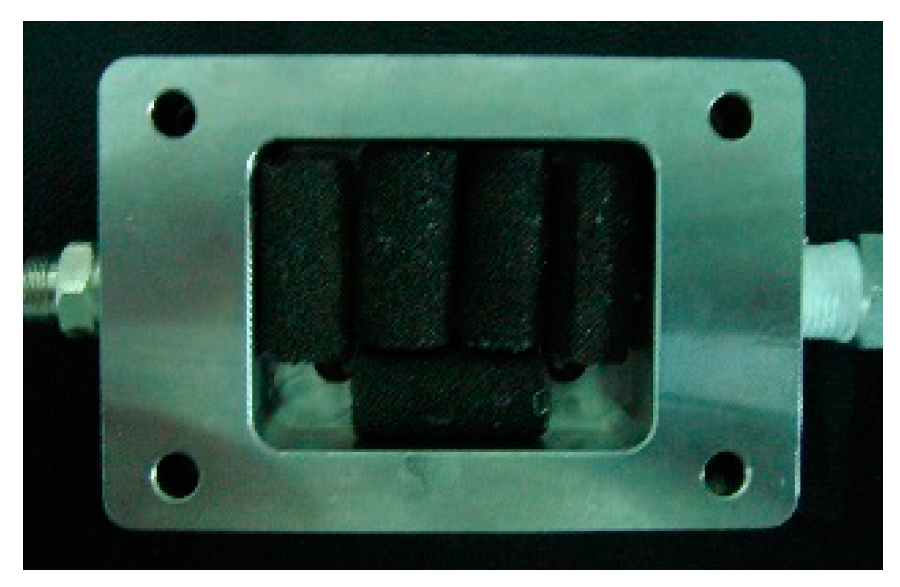
4.1. Catalyst Preparation
4.2. Hydrogen Reactor
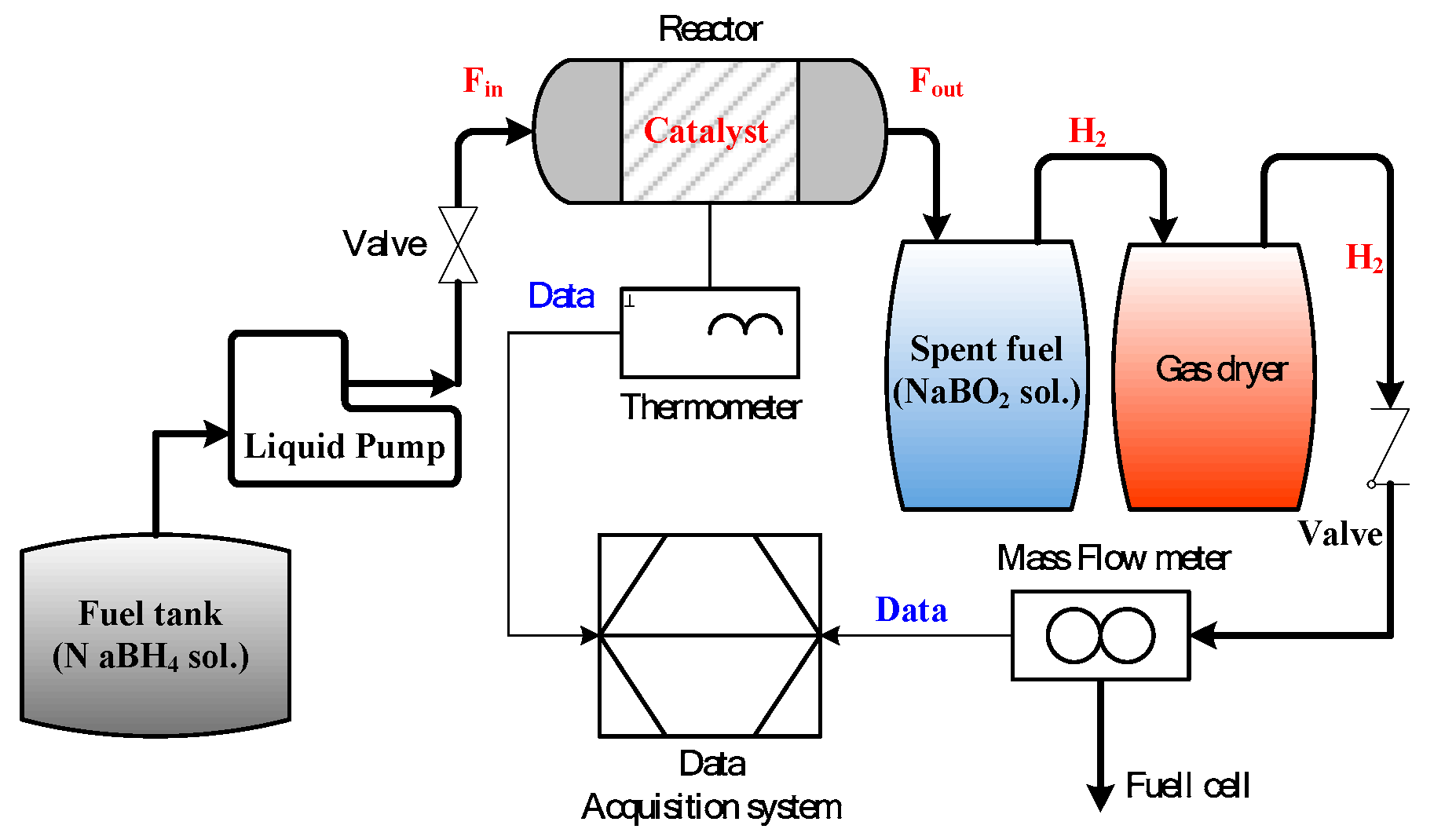
4.3. Chemical Hydrogen Production System
5. Conclusions
- (1)
- The hydrogen conversion efficiency using five catalysts was increased to 90.5%, and the increase was as high as 7.8%. After 2 h of testing, no solid sodium metaborate (NaBO2) was formed on the catalyst surface. The reason for this is that the reaction under the same matrix has been completed when multiple catalysts are used. So, the addition of reactionary raw materials is the main factor. That is to say, the optimal amount of catalyst used in this study should be a highly feasible.
- (2)
- Under the operating conditions of using five catalysts and a feed rate of 4 g/min, after a long-term hydrogen production test, the overall hydrogen production efficiency was as high as 91.2%, and the reaction temperature was maintained at 108 °C. The average hydrogen-yielding rate was 1.72 L per minute.
- (3)
- The catalyst is sufficient to release the hydrogen contained in the 1g/min solution completely, but it is not efficient enough for high-flow hydrogen production. The experimental results show that increasing the amount of catalysts has a significant effect on improving the reaction efficiency and promoting hydrogen gas production.
- (4)
- It is likely to be designed with a small volume, and therefore, the catalyst can evenly contact the solution for hydrogen production. Additionally, the length of time fuel spends in the reactor is prolonged to further aid the reaction with the catalyst. These are both advantageous and can be used as a reference for reactor design.
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.J.; Lee, J.; Kong, K.Y.; Jung, C.R.; Min, I.G.; Lee, S.Y.; Kim, H.J.; Nam, S.W.; Lim, T.H. Hydrogen generation system using sodium borohydride for operation of a 400W-scale polymer electrolyte fuel cell stack. J. Power Sources 2007, 170, 412–418. [Google Scholar] [CrossRef]
- Amendola, S.C.; Sharp-Goldman, S.L.; Janjua, M.S.; Kelly, M.T.; Petillo, P.J.; Binder, M. An ultrasafe hydrogen generator: Aqueous, alkaline borohydride solutions and Ru catalyst. J. Power Sources 2000, 85, 186–189. [Google Scholar] [CrossRef]
- Amendola, S.C.; Sharp-Goldman, S.L.; Janjua, M.S.; Spencer, N.C.; Kelly, M.T.; Petillo, P.J.; Binder, M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst. Int. J. Hydrogen Energy 2000, 25, 969–975. [Google Scholar] [CrossRef]
- Kojima, Y.; Suzuki, K.; Fukumoto, K.; Kawai, Y.; Kimbara, M.; Nakanishi, H.; Matsumoto, S. Development of 10 kW-scale hydrogen generator using chemical hydride. J. Power Sources 2004, 125, 22–26. [Google Scholar] [CrossRef]
- Mancier, V.; Willmann, P.; Metrot, A. A semi theoretical approach of the second plateau appearing during the discharge of aged nickel oxyhydroxide electrodes. J. Power Sources 2000, 85, 181–185. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Shanmin, G.; Narayan, S.H. Boron-enriched advanced energy materials. Inorg. Chim. Acta 2018, 471, 577–586. [Google Scholar]
- Zhang, Q.; Smith, G.M.; Wu, Y. Catalytic hydrolysis of sodium borohydride in an integrated reactor for hydrogen generation. Int. J. Hydrogen Energy 2007, 32, 4731–4735. [Google Scholar] [CrossRef]
- Gislon, P.; Monteleone, G.; Prosini, P.P. Hydrogen Production fromsolid sodium borohydride. Int. J. Hydrogen Energy 2008, 34, 929–937. [Google Scholar] [CrossRef]
- Galli, S.; De Francesco, M.; Monteleone, G.; Oronzio, R.; Pozio, A. Development of a compact hydrogen generator from sodium borohydride. Int. J. Hydrogen Energy 2010, 35, 7344–7349. [Google Scholar] [CrossRef]
- Kim, T. Hydrogen generation from sodium borohydride using microreactor for micro fuel cells. Int. J. Hydrogen Energy 2011, 36, 1404–1410. [Google Scholar] [CrossRef]
- Huang, Z.-M. Research on Hydrogen Production by Hydrolysis of Sodium Borohydride Catalyzed by Ruthenium/Foamite Nickel Catalyst. Master’s Thesis, Department of Mechanical Engineering, Yuan Ze University, Taoyuan Zhongli, Taiwan, 2010. [Google Scholar]

| Concentration | 20wt.%NaBH4 + 3wt.%NaOH | |
|---|---|---|
| Flow rate (mL/min) | 3 | 4 |
| Catalyst(pcs) | 4 | 5 |
| Theoretical (L/min) | 1.414 | 1.886 |
| Real (L/min) | 1.29 | 1.72 |
| Efficiency (%) | 91.5 | 91.2 |
| Concentration | 20wt.%NaBH4 + 3wt.%NaOH |
|---|---|
| Flow rate (mL/min) | 1 |
| Theoretical (L/min) | 0.471 |
| Real (L/min) | 0.45 |
| Efficiency (%) | 96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, J.-H.; Su, A.; Sun, J.-K.; Huang, Z.-M. The Catalyst Loading Effects on the Feed Rate of NaBH4 Solution for the Hydrogen Production Rate and Conversion Efficiency. Catalysts 2020, 10, 451. https://doi.org/10.3390/catal10040451
Leu J-H, Su A, Sun J-K, Huang Z-M. The Catalyst Loading Effects on the Feed Rate of NaBH4 Solution for the Hydrogen Production Rate and Conversion Efficiency. Catalysts. 2020; 10(4):451. https://doi.org/10.3390/catal10040451
Chicago/Turabian StyleLeu, Jai-Houng, Ay Su, Jung-Kang Sun, and Zhen-Ming Huang. 2020. "The Catalyst Loading Effects on the Feed Rate of NaBH4 Solution for the Hydrogen Production Rate and Conversion Efficiency" Catalysts 10, no. 4: 451. https://doi.org/10.3390/catal10040451
APA StyleLeu, J.-H., Su, A., Sun, J.-K., & Huang, Z.-M. (2020). The Catalyst Loading Effects on the Feed Rate of NaBH4 Solution for the Hydrogen Production Rate and Conversion Efficiency. Catalysts, 10(4), 451. https://doi.org/10.3390/catal10040451




