Volatile Hydrogen Intermediates of CO2 Methanation by Inelastic Neutron Scattering
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental boundary conditions of DRIFTS and INS
2.2. Reaction Mechanism of CO2 Methanation
2.3. Infrared Spectroscopy (DRIFTS)
2.4. Inelastic Neutron Scattering Spectroscopy (INS)
2.5. Complementarity of DRIFTS and INS
3. Experimental
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamadeh, I.; King, D.; Griffiths, P. Heatable-evacuable cell and optical system for diffuse reflectance FT-IR spectrometry of adsorbed species. J. Catal. 1984, 88, 264–272. [Google Scholar] [CrossRef]
- Ueckert, T.; Lamber, R.; Jaeger, N.I.; Schubert, U. Strong metal support interactions in a Ni/SiO2 catalyst prepared via sol-gel synthesis. Appl. Cat. A 1997, 155, 75–85. [Google Scholar] [CrossRef]
- Lamberti, C.; Zecchina, A.; Groppo, E.; Bordiga, S. Probing the surface of heterogeneous catalysts by in-situ IR spectroscopy. Chem. Soc. Rev. 2010, 39, 4951–5001. [Google Scholar] [CrossRef] [PubMed]
- Kubelka, P.; Munk, F.Z. Ein Beitrag Zur Optik der Farbanstriche. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Little, L.H. Infrared Spectra of Adsorbed Species; Academic Press: New York, NY, USA, 1966; pp. 1933–1941. [Google Scholar]
- Armaroli, T.; Bécue, T.; Gautier, S. Diffuse Reflection Infrared Spectroscopy (Drifts): Application to the in situ Analysis of Catalysts. Oil Gas Sci. Technol.-Rev. IFP 2004, 59, 215–237. [Google Scholar] [CrossRef]
- Venter, J.J.; Vannice, M.A. Applicability of "drifts" for the characterization of carbon-supported metal catalysts and carbon surfaces. Carbon 1988, 26, 889–902. [Google Scholar] [CrossRef]
- Primet, M.; Basset, J.; Mathieu, M.; Prettre, M. Infrared investigation of hydrogen adsorption on alumina-supported platinum. J. Catal. 1973, 28, 368–375. [Google Scholar] [CrossRef]
- Palecek, D.; Tek, G.; Lan, J.; Iannuzzi, M.; Hamm, P. Characterization of the Platinum-Hydrogen Bond by Surface-Sensitive Time-Resolved Infrared Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 1254–1259. [Google Scholar] [CrossRef]
- Parker, S.F.; Mukhopadhyay, S.; Jiménez-Ruiz, M.; Albers, P.W. Adsorbed States of Hydrogen on Platinum: A New Perspective. Chem. Eur. J. 2019, 25, 6496–6499. [Google Scholar] [CrossRef]
- Nakata, T. Infrared absorption of hydrogen and deuterium chemisorbed on nickel. J. Chem. Phys. 1976, 65, 487–488. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons with Applications in Chemistry, Biology, Materials Science and Catalysis; World Scientific Publishing: Singapore, 2005. [Google Scholar]
- Kandemir, T.; Friedrich, M.; Parker, S.F.; Studt, F.; Lennon, D.; Schlogl, R.; Behrens, M. Different routes to methanol: Inelastic neutron spectroscopy of adsorbates on supported copper catalysts. Phys. Chem. Chem. Phys. 2016, 18, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, W.; Sherwood, T. Conversion of parahydrogen to orthohydrogen using nickel/alumina catalysts. J. Catal. 1966, 6, 288–307. [Google Scholar] [CrossRef]
- Matsumoto, M.; Espenson, J.H. Kinetics of the Interconversion of Parahydrogen and Orthohydrogen Catalyzed by Paramagnetic Complex Ions. J. Am. Chem. Soc. 2005, 127, 11447–11453. [Google Scholar] [CrossRef] [PubMed]
- Donaubauer, P.J.; Cardella, U.; Decker, L.; Klein, H. Kinetics and Heat Exchanger Design for Catalytic Ortho-Para Hydrogen Conversion during Liquefaction. Chem. Eng. Technol. 2019, 42, 669–679. [Google Scholar] [CrossRef]
- Warringham, R.; Bellaire, D.; Parker, S.F.; Taylor, J.; Ewings, R.A.; Goodway, C.M.; Kibble, M.; Wakefield, S.R.; Jura, M.; Dudman, M.P.; et al. Sample environment issues relevant to the acquisition of inelastic neutron scattering measurements of heterogeneous catalyst samples. J. Phys. Conf. Ser. 2014, 554, 012005. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Sabatier, P. Chapter: The Method of Direct Hydrogenation by Catalysis, Nobel lecture 1912. In Nobel Lectures Chemistry 1901–1921; Elsevier Publishing Company: Amsterdam, The Netherlands, 1966. [Google Scholar]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanism of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation – From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Borgschulte, A.; Callini, E.; Stadie, N.; Arroyo, Y.; Rossell, M.D.; Erni, R.; Geerlings, H.; Züttel, A.; Ferri, D. Manipulating the reaction path of the CO2 hydrogenation reaction in molecular sieves. Catal. Sci. Technol. 2015, 5, 4613–4621. [Google Scholar] [CrossRef]
- van Herwijnen, T.; van Doesburg, H.; de Jong, W.A. Kinetics of the methanation of CO and CO2 on a nickel catalyst. J. Catal. 1973, 28, 391–402. [Google Scholar] [CrossRef]
- Fujita, S.I.; Nakamura, M.; Doi, T.; Takezawa, N. Mechanism of methanation of carbon dioxide and carbon monoxide over nickel/alumina catalysts. Appl. Catal. A 1993, 104, 87–100. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Bauch, C.G.; Ovalles, C. Mechanistic aspects of catalytic carbon dioxide methanation. Rev. Inorg. Chem. 1985, 7, 315–339. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Hvolbæk, B.; Abild-Pedersen, F.; Chorkendorff, I.; Christensen, C.H. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 2008, 37, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, T.; Trost, J.; Wintterlin, J.; Ertl, G. Diffusion and Atomic Hopping of N Atoms on Ru(0001) Studied by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1996, 76, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Conner, W.C.J.; Falconer, J.L. Spillover in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen Spillover. Facts and FictionChem. Rev. 2012, 112, 2714–2738. [Google Scholar]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Andersson, M.; Abild-Pedersen, F.; Remediakis, I.; Bligaard, T.; Jones, G.; Engbæk, J.; Lytken, O.; Horch, S.; Nielsen, J.; Sehested, J.; et al. Structure sensitivity of the methanation reaction: H2-induced CO dissociation on nickel surfaces. J. Catal. 2008, 255, 6–19. [Google Scholar] [CrossRef]
- Martin, N.M.; Hemmingsson, F.; Schaefer, A.; Ek, M.; Merte, L.R.; Hejral, U.; Gustafson, J.; Skoglundh, M.; Dippel, A.-C.; Gutowski, O.; et al. Structure-function relationship for CO2 methanation over ceria spported Rh and Ni catalysts under atmospheric pressure conditions. Catal. Sci. Technol. 2019, 9, 1644–1653. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef] [PubMed]
- Mutz, B.; Carvalho, H.W.P.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Czekaj, I.; Loviat, F.; Raimondi, F.; Wambach, J.; Biollaz, S.; Wokaun, A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS). Appl. Catal. A 2007, 329, 68–78. [Google Scholar] [CrossRef]
- Roiaz, M.; Monachino, E.; Dri, C.; Greiner, M.; Knop-Gericke, A.; Schl¨ogl, R.; Comelli, G.; Vesselli, E. Reverse Water-Gas Shift or Sabatier Methanation on Ni(110)? Stable Surface Species at Near-Ambient Pressure. J. Am. Chem. Soc. 2016, 138, 4146–4154. [Google Scholar] [CrossRef]
- Heine, C.; Lechner, B.A.J.; Bluhm, H.; Salmeron, M. Recycling of CO2: Probing the Chemical State of the Ni(111) Surface during the Methanation Reaction with Ambient-Pressure X-Ray Photoelectron Spectroscopy. J. Am. Chem. Soc. 2016, 138, 13246–13252. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gallandat, N.; Probst, B.; Suter, R.; Callini, E.; Ferri, D.; Arroyo, Y.; Erni, R.; Geerlings, H.; Züttel, A. Sorption enhanced CO2 methanation. Phys. Chem. Chem. Phys. 2013, 15, 9620–9625. [Google Scholar] [CrossRef]
- Ohya, H.; Fun, J.; Kawamura, I.; Itoh, K.; Ohashi, H.; Aihara, M.; Tanisho, S.; Negishi, Y. Methanation of carbon dioxide by using membrane reactor integrated with water vapor permselective membrane and its analysis. J. Membr. Sci. 1997, 131, 237–247. [Google Scholar] [CrossRef]
- Delmelle, R.; Terreni, J.; Remhof, A.; Heel, A.; Proost, J.; Borgschulte, A. Evolution of Water Diffusion in a Sorption-Enhanced Methanation Catalyst. Catalysts 2018, 8, 341. [Google Scholar] [CrossRef]
- Parker, S.F.; Bowron, D.T.; Imberti, S.; Soper, A.K.; Refson, K.; Lox, E.S.; Lopez, M.; Albers, P. Structure determination of adsorbed hydrogen on a real catalyst. Chem. Commun. 2010, 46, 2959–2961. [Google Scholar] [CrossRef]
- Zarfl, J.; Ferri, D.; Schildhauer, T.J.; Wambach, J.; Wokaun, A. DRIFTS study of a commercial Ni/γ-Al2O3 CO methanation catalyst. Appl. Catal. A Gen. 2015, 495, 104–114. [Google Scholar] [CrossRef]
- Mihaylov, M.; Hadjiivanov, K.; Knozinger, H. Formation of Ni(CO)4 during the Interaction between CO and Silica-Supported Nickel Catalysts: An FTIR Spectroscopic Study. Catal. Lett. 2001, 76, 59–63. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Y.; Zhang, L.; Hu, S.; Xiang, J.; Wang, Y.; Xu, L.; Liu, Q.; Zhang, S.; Hu, X. Impacts of nickel loading on properties, catalytic behaviors of Ni/γ-Al2O3 catalysts and the reaction intermediates formed in methanation of CO2. Int. J. Hydrog. Energy 2019, 44, 9291–9306. [Google Scholar] [CrossRef]
- Parker, S.F. The role of hydroxylgroups in low temperature carbon monoxide oxidation. Chem. Commun. 2011, 47, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, J.; Gonzalez, N.; Lualdi, M.; Boutonnet, S.; Jaras, M. The effect of catalyst pellet size on nickel carbonyl-induced particle sintering under low temperature CO methanation. Appl. Catal. A 2016, 514, 91–102. [Google Scholar] [CrossRef]
- Forney, D.; Jacox, M.E.; Thompson, W.E. Infrared spectra of trans-HOCO, HCOO+, and HCO2- trapped in solid neon. J. Chem. Phys. 2003, 119, 10814–10823. [Google Scholar] [CrossRef]
- Ho, W.; DiNardo, N.J.; Plummer, E.W. Angle-resolved and variable impact energy electron vibrational excitation spectroscopy of molecules adsorbed on surfaces. J. Vac. Sci. Technol. 1980, 17, 134–140. [Google Scholar] [CrossRef]
- Voigtlander, B.; Lehwald, S.; Ibach, H. Hydrogen adsorption and the adsorbate-induced Ni(110) reconstruction- an EELS study. Surf. Sci. 1989, 208, 113–135. [Google Scholar] [CrossRef]
- Birgeneau, R.J.; Cordes, J.; Dolling, G.; Woods, A.D.B. Normal Modes of Vibration in Nickel. Phys. Rev. 1964, 136, A1359–A1365. [Google Scholar] [CrossRef]
- Kresch, M.; Delaire, O.; Stevens, R.; Lin, J.Y.Y.; Fultz, B. Neutron scattering measurements of phonons in nickel at elevated temperatures. Phys. Rev. B 2007, 75, 104301. [Google Scholar] [CrossRef]
- Dietz, R.E.; Parisot, G.I.; Meixner, A.E. Infrared Absorption and Raman Scattering by Two-Magnon Processes in NiO. Phys. Rev. B 1971, 4, 2302–2310. [Google Scholar] [CrossRef]
- Parker, S.F.; Fernandez-Alonso, F.; Ramirez-Cuesta, A.J.; Tomkinson, J.; Rudic, S.; Pinna, R.S.; Gorini, G.; Castanon, J.F. Recent and future developments at TOSCA at ISIS. J. Phys. Conf. Ser. 2014, 554, 012003. [Google Scholar] [CrossRef]
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Škoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 896, 68–74. [Google Scholar] [CrossRef]
- Arnold, O.; Bilheux, J.C.; Borreguero, J.M.; Buts, A.; Campbell, S.I.; Chapon, L.; Doucet, M.; Draper, N.; Leal, R.F.; Gigg, M.A. Mantid—Data analysis and visualization package for neutron scattering and μ SR experiments. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 764, 156–166. [Google Scholar] [CrossRef]

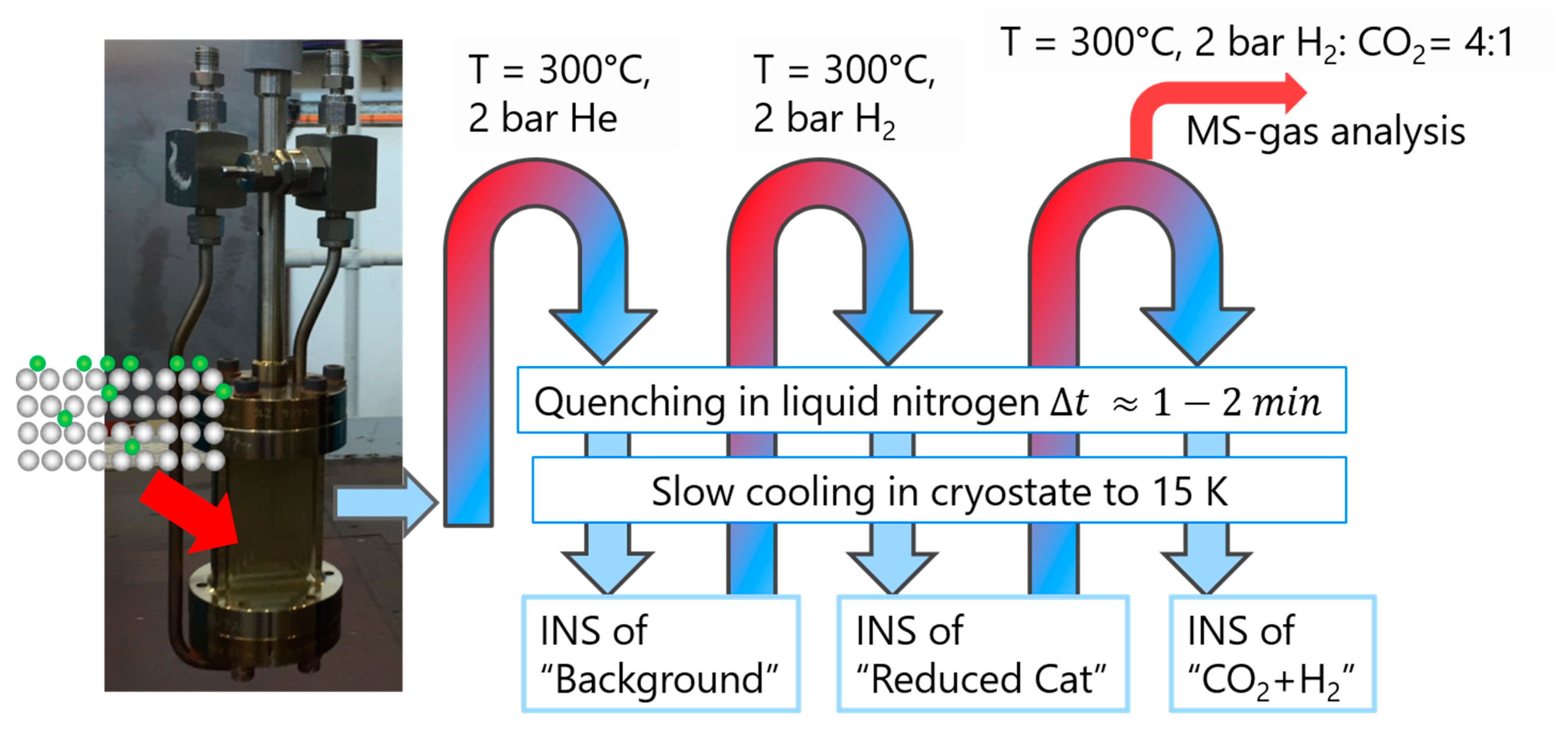


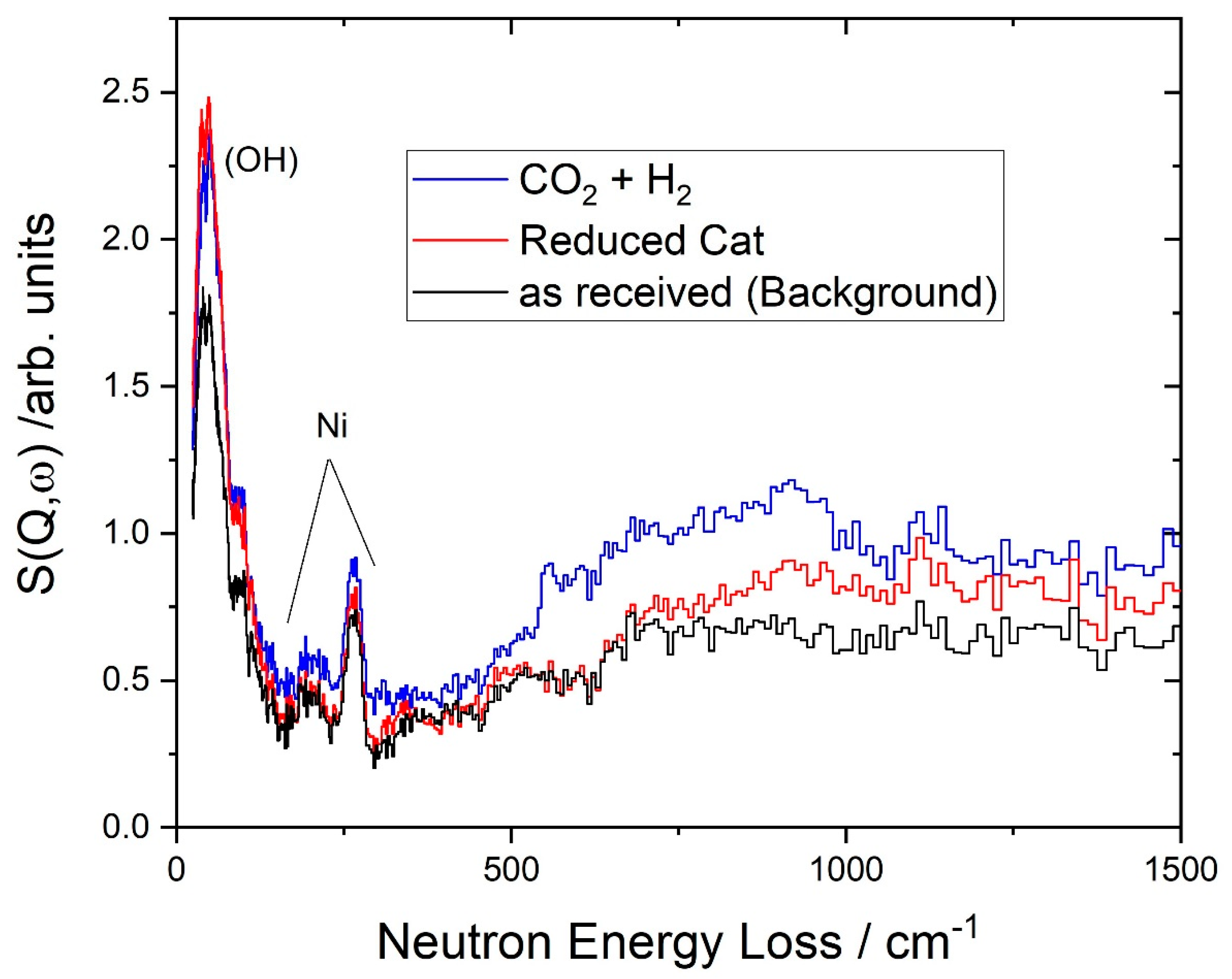

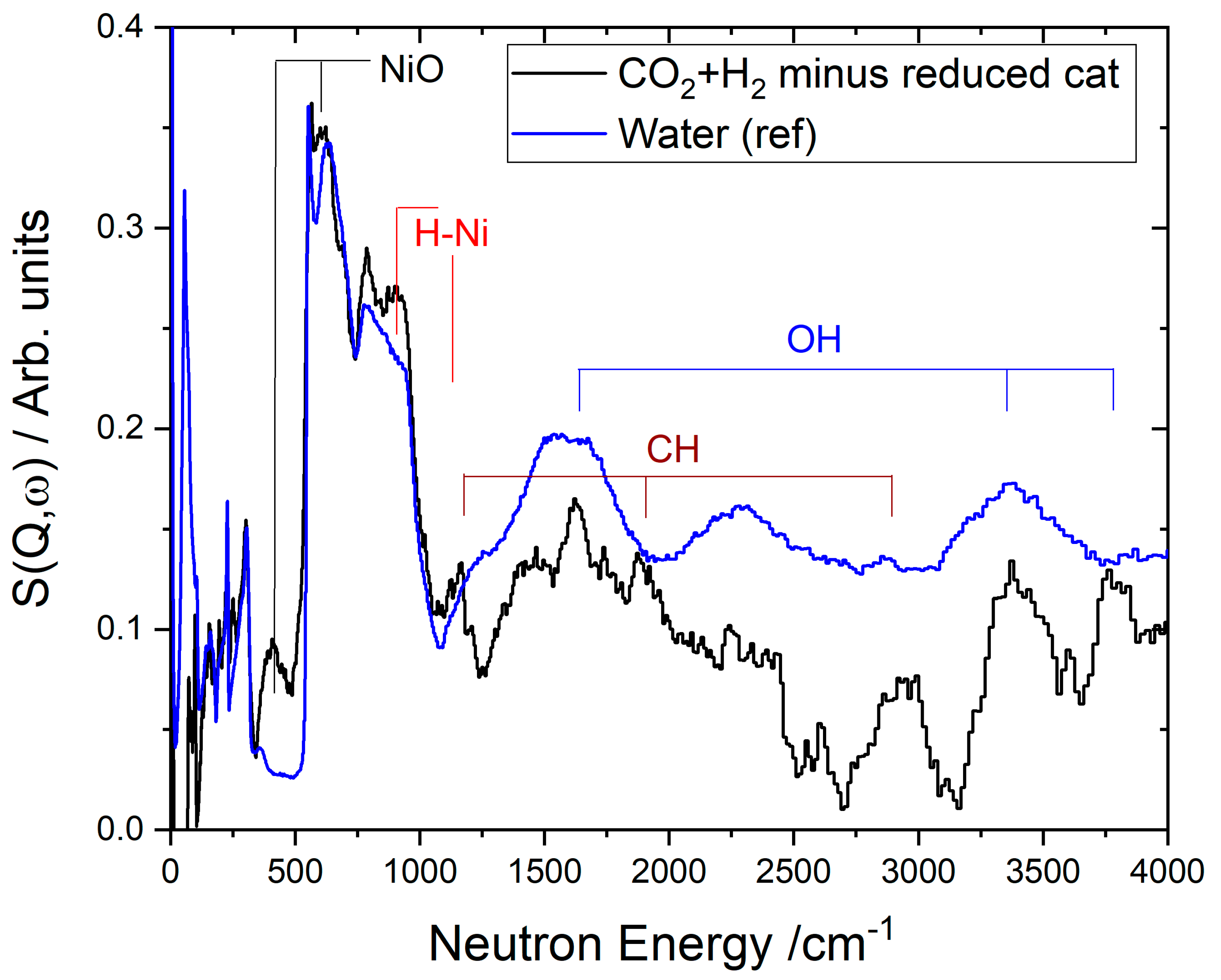
| Species | DRIFTS, This Work | DRIFTS, Ref | INS, This Work | INS, Ref |
|---|---|---|---|---|
| H on Ni | 760 | 1880 [12] | 940, 1140 | 920, 1093 [42]) |
| COtop on Ni | 2033 | 2070 [43] | - | - |
| CObridge on Ni | 1919 | 1944 [43] | - | - |
| COhollow on Ni | 1845 | 1880 [43] | - | - |
| Ni(CO)4 | 2045 | 2045 [44] | - | - |
| HCOO− | 1623 | - | - | - |
| C species | 1570 | [45] | - | - |
| OH | 1600, 3500 | 3500 | 1600, 3450, 3650 | 936, 3450 [13,46] |
| water | 1620, 3650 | 1595, 3657, 3756 [43] | 560, 634, 779, 912, | 1600, 2280, 3450 1600, 3450 [13] |
| C-H | see CH4 | see CH4 | 1154 1887, 2950 | [14] |
| CH4 | 1306, 3017 | 1305, 3017 [43] | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terreni, J.; Sambalova, O.; Borgschulte, A.; Rudić, S.; Parker, S.F.; Ramirez-Cuesta, A.J. Volatile Hydrogen Intermediates of CO2 Methanation by Inelastic Neutron Scattering. Catalysts 2020, 10, 433. https://doi.org/10.3390/catal10040433
Terreni J, Sambalova O, Borgschulte A, Rudić S, Parker SF, Ramirez-Cuesta AJ. Volatile Hydrogen Intermediates of CO2 Methanation by Inelastic Neutron Scattering. Catalysts. 2020; 10(4):433. https://doi.org/10.3390/catal10040433
Chicago/Turabian StyleTerreni, Jasmin, Olga Sambalova, Andreas Borgschulte, Svemir Rudić, Stewart F. Parker, and Anibal J. Ramirez-Cuesta. 2020. "Volatile Hydrogen Intermediates of CO2 Methanation by Inelastic Neutron Scattering" Catalysts 10, no. 4: 433. https://doi.org/10.3390/catal10040433
APA StyleTerreni, J., Sambalova, O., Borgschulte, A., Rudić, S., Parker, S. F., & Ramirez-Cuesta, A. J. (2020). Volatile Hydrogen Intermediates of CO2 Methanation by Inelastic Neutron Scattering. Catalysts, 10(4), 433. https://doi.org/10.3390/catal10040433





