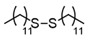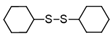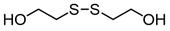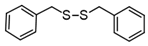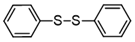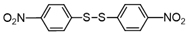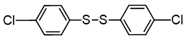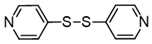Abstract
Organotellurium compounds are known to be useful oxidation reagents. For developing a recoverable and reusable reagent, this paper describes the use of an ionic liquid (IL) support for the organotellurium reagent and its application as a recyclable catalyst for thiol oxidation. We have successfully prepared a novel diphenyl telluride derivative 5 bearing an imidazolium hexafluorophosphate group in its structure. It is found that the IL-supported diphenyl telluride 5 efficiently catalyzed the aerobic oxidation of various thiols in [bmim]PF6 solution under photosensitized conditions to provide the corresponding disulfides in excellent yields. The product can be isolated by simple ether extraction. The IL-supported catalyst 5 remaining in the ionic liquid phase can be reused for five successive runs while retaining high catalytic activity (97% yield even in the fifth run).
1. Introduction
Recently, organotellurium oxides have been recognized as useful oxidation reagents [1]. In particular, diaryl telluroxides, tellurones, and aryltellurinic acid derivatives have been identified as versatile and effective oxidants for alcohols, phosphines, thiols, thiocarbonyl compounds, and so on [2,3,4,5,6,7,8,9,10,11]. Generally, these oxidation reactions require stoichiometric amounts of organotellurium reagents. From synthetic, economic, and environmental perspectives, the development of a catalytic process is desirable. Herein, we have developed organotelluride-catalyzed oxidation of phosphites to phosphates [12], silanes to silanols [13], and thiols to disulfides [14] while employing aerobic oxygen as a terminal oxidant under photosensitized conditions, where the in situ generation of tellurium oxide species by singlet oxygen oxidation is expected. However, the protocol is not without disadvantages, for instance, the product requires isolation by chromatographic purification, and the organotelluride catalyst is rarely reusable. To overcome these issues, we envisioned to immobilize the organotelluride catalyst on an ionic liquid (IL) support.
IL-supported organic synthesis and catalysis have been extensively studied in recent years [15]. Due to its high polarity, the IL support offers the advantages of easy product isolation and catalyst recycling via simple phase separation. We have previously reported the synthesis of IL-supported 18-crown-6 ether [16], ascorbate-based IL [17], and IL-supported benzyl chloride [18] for Huisgen click chemistry, IL-supported hypervalent iodine reagent [19] for alcohol oxidation, and IL-supported 1,3-dimethylimidazolidin-2-one for halogenation [20]. Herein, we describe the synthesis of IL-supported diphenyl telluride as a recoverable and reusable oxidation catalyst. The catalytic activity and recyclability of the reagent are evaluated via aerobic oxidation of thiols under photosensitized conditions. The transformation of thiols to disulfides is of interest from the viewpoint of organic and biological processes.
2. Results and Discussion
The IL-supported diphenyl telluride was prepared in the following manner (Scheme 1). Using (4-(hydroxymethyl)phenyl)boronic acid (1) as the starting material, (4-(phenyltellanyl)phenyl)methanol (2) was produced in 84% yield from the coupling reaction with diphenyl ditelluride. Next, (4-(chloromethyl)phenyl)(phenyl)tellane (3) was prepared in 94% yield via halogenation with thionyl chloride followed by basic hydrolysis. Then, 1-methyl-3-(4-(phenyltellanyl)benzyl)-1H-imidazol-3-ium chloride (4) was obtained in 75% yield by reacting the compound 3 with methyl imidazole. Since this IL-supported telluride 4 was extremely hygroscopic, the anion was subsequently converted to PF6− to produce hydrophobic IL-supported diphenyl telluride (5) in 96% yield, which was insoluble in low polarity organic solvents and water.
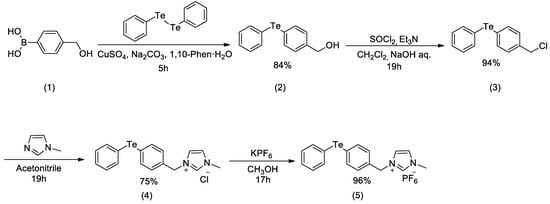
Scheme 1.
Synthesis of ionic liquid-supported diphenyl telluride.
Initially, we investigated the catalytic oxidation of thiol using IL-supported diphenyl telluride in various ILs employing thiophenol as the model substrate. An IL solution of the thiol, IL-supported catalyst, and rose bengal as a photosensitizer were stirred in an open flask and irradiated with a 500-W halogen lamp. After 3 h, the produced diphenyl disulfide was isolated by extracting with diethyl ether. The yields are compiled in Table 1. The catalytic activities of the IL-supported diphenyl tellurides 4 and 5 were similar to or higher than that of free diphenyl telluride (Entry 7), whereas the reaction was significantly retarded in the absence of the catalyst (Entry 8). The presence of oxygen is also essential for this transformation. In fact, the reaction under nitrogen atmosphere resulted in significant yield reduction (Entry 9). Although the reaction in [bmim](CF3SO2)2N and [bmim]MeSO4 reached completion, the isolated yields were slightly lowered owing to the phase separation problems (Entries 1 and 2). The best result was obtained using a hydrophobic IL, [bmim]PF6, as a solvent (Entry 3). Diphenyl disulfide was also isolated in quantitative yields in Entries 4 and 6, however, the IL [bmim]BF4 and the IL-supported catalyst 4 were unsuitable for reuse because of their hygroscopic nature.

Table 1.
Oxidation of thiophenol in the presence of organotellurium catalysts a.
Although the active species for this oxidation reaction could not be identified at the present stage, a possible catalytic cycle was proposed according to our previous paper (Figure 1) [14]. Namely, singlet oxygen oxidation of the telluride catalyst 5 gave the corresponding telluroxide (and/or tellurone), which underwent a nucleophilic attack by thiol to afford an adduct A. Then, the adduct A reacted with another thiol to give the disulfide along with regeneration of the catalyst 5.
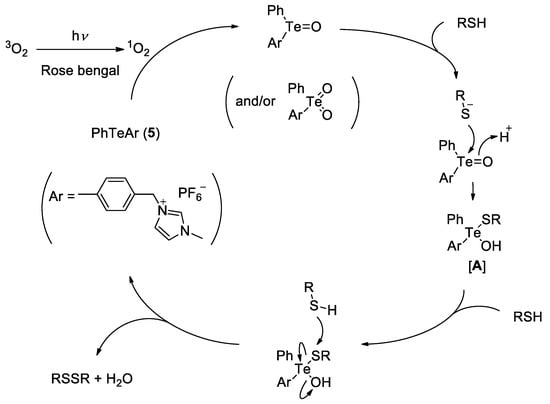
Figure 1.
Plausible reaction mechanism for the catalytic oxidation of thiols.
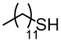
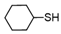
With the optimized conditions in hand, the substrate scope of the oxidation reaction using IL-supported catalyst 5 was evaluated with various thiols. The results are summarized in Table 2. Dodecanethiol (Entry 1), cyclohexanethiol (Entry 2), and benzyl mercaptane (Entry 6) were converted to their corresponding disulfides in high yields. However, the yield of the oxidation reaction of tert-butyl mercaptane decreased to 73% owing to the steric hindrance (Entry 3). Under the employed oxidation conditions, hydroxyl and ester groups remained untouched to produce their corresponding disulfides in excellent yields (Entries 4 and 5). The reaction also proceeded with the aromatic nitro and chloro functional groups intact (Entries 8 and 9). Furthermore, oxidation of heterocyclic 4-mercaptopyridine produced 4,4′-dipyridyl disulfide in quantitative yield without affecting the pyridine ring (Entry 10).

Table 2.
Catalytic oxidation of various thiols using IL-supported diphenyl telluride 5 a.
Finally, we investigated the reusability of the IL-supported diphenyl telluride 5 employing the conditions shown in Entry 7 of Table 2. After completing the thiophenol oxidation, the resulting diphenyl disulfide was isolated via extraction with diethyl ether, and the remaining [bmim]PF6 solution containing IL-supported catalyst 5 and rose bengal could be reused at least four times in the subsequent reactions with only a slight decrease in the product yield (97% after four times recycling, Figure 2).
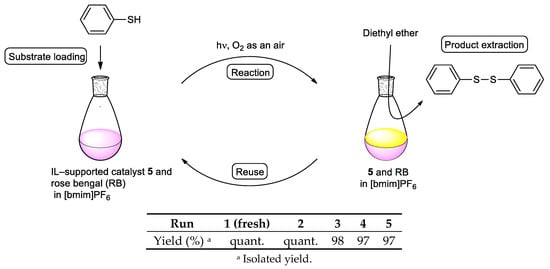
Figure 2.
Recycling experiment of IL-supported diphenyl telluride catalyst 5. Condition: thiol (1 mmol), tellurium catalyst (0.2 mmol), rose bengal (0.05 mmol), solvent (10 mL), irradiated with a 500-W halogen lamp under aerobic conditions, 3 h.
3. Materials and Methods
3.1. General
All reagents and solvents were commercially sourced and of reagent grade and were used without purification. The reactions were monitored using aluminum thin layer chromatography plates with silica gel 60 F254 (Merck, (Darmstadt, Germany)). Column chromatography was performed using silica gel 60 (Kanto Chemical, Japan, Tokyo). 1H, 13C, and 125Te NMR spectra were measured on a Bruker Advance DRX 500 (1H: 500 MHz, 13C: 125 MHz, 125Te: 159 MHz spectrometer. All chemical shifts are reported in parts per million (ppm) relative to TMS (0 ppm for 1H), CHCl3 (77 ppm, for 13C), DMSO (39 ppm for 13C), and PhTeTePh (419 ppm in CDCl3, 422ppm in DMSO for 125Te). Mass analyses were performed using a JEOL AccuTOF LC-plus JMS-T100LP spectrometer (Japan, Tokyo).
3.2. (4-(Hydroxymethyl)Phenyl)(Phenyl)Telluride (2)
A solution of diphenyl ditelluride (81.9 mg, 0.200 mmol), (4-hydroxymethyl)phenyl)boronic acid (1) (66.9 mg, 0.440 mmol), 1,10-phenthroline·H2O (2.20 mg, 12.0 mol), and CuSO4 (19.0 mg, 12.0 mol) in ethanol (0.6 mL) was stirred at room temperature for 1 min. Then, a 5% Na2CO3 aqueous solution (0.1 mL) was added to the solution and the mixture was stirred at room temperature for 5 h. The progress of the reaction was monitored by 1H NMR spectroscopy. After the reaction was completed, the mixture was dried over MgSO4, and the solvent was evaporated. The residue was purified via dry column chromatography to produce product 2 (0.104 g, 84%) as a colorless solid. Mp 62–63 °C; 1H-NMR (500 MHz, CDCl3): δ = 7.69–7,67 (m, 4H), J = 6, 4H), 7.21–7.29 (m, 5H), 4.66 (s, 2H), 1.80 (br, 1H); 13C-NMR (125 MHz, CDCl3): δ = 140.7, 138.3, 137.9, 129.5, 128.1, 127.9, 114.7, 113.7, 65.0; 125Te-NMR (159 MHz, CDCl3): δ = 690.7; HRMS (APCI): m/z [M–OH]+ calcd. for C13H11Te: 296.9917, found: 296.9874.
3.3. (4-(Chloromethyl)Phenyl)(Phenyl)Telluride (3)
To a solution of (4-(hydroxymethyl)phenyl)(phenyl)telluride (2) (2.32 g, 7.45 mmol) and triethylamine (1.50 mL, 10.4 mmol) in CH2Cl2 (4.6 mL), a solution of thionyl chloride (2.41 mL, 33.5 mmol) in CH2Cl2 (7.4 mL) was added dropwise at 0 °C. The resulting mixture was stirred at room temperature for 1 h. Then CH2Cl2 was removed by evaporation, THF (36.9 mL) and a 2 M NaOH solution (25.2 mL) were added, and the mixture was stirred at room temperature for 19 h. After the reaction was completed, the mixture was evaporated and extracted with ethyl acetate. The organic layer was washed with water, dried over MgSO4, and evaporated. The residue was purified via column chromatography to yield product 3 (2.32 g, 94%) as a yellow oil. 1H-NMR (500 MHz, CDCl3): δ = 7.72 (d, J = 8.0, 2H), 7.63 (d, J = 8.0, 2H), 7.33–7.21 (m, 5H), 4.55 (s, 2H); 13C-NMR (125 MHz, CDCl3): δ = 138.5, 137.8, 137.1, 129.6, 128.1 (overlapped), 115.3, 114.3, 45.9; 125Te-NMR (159 MHz, CDCl3): δ = 690.6; HRMS (APCI): m/z [M–Cl]+ calcd. for C13H11Te: 296.9917, found: 296.9951.
3.4. 1-Methyl-3-(4-(Phenyltellanyl)Benzyl)-1H-Imidazol-3-Ium Chloride (4)
A solution of phenyl(4-chloromethylphenyl)tellane (3) (2.32 g, 7.02 mmol) and N-methylimidazole (0.692 g, 8.43 mmol) in CH3CN (63.6 mL) was heated to reflux for 19 h. Then, the mixture was evaporated, and the residue was purified via column chromatography to yield product 4 (2.18 g, 75%) as a yellow oil. 1H-NMR (500 MHz, DMSO-d6): δ = 9.42 (s, 1H), 7.84 (s, 1H), 7.75 (s, 1H), 7.71 (d, J = 8.0, 2H), 7.66 (d, J = 8.0, 2H), 7.37–7.27 (m, 5H), 5.45 (s, 2H), 3.86 (s, 3H); 13C-NMR (125 MHz, DMSO-d6): δ = 138.7, 137.9, 137.24, 135.0, 130.3, 129.9, 128.7, 124.8, 122.8, 116.6, 115.0, 51.8, 36.3; 125Te-NMR (159 MHz, DMSO-d6): δ = 705.3; HRMS (APCI): m/z M+ calcd. for C17H17N2Te: 379.0448, found: 379.0462.
3.5. 1-Methyl-3-(4-(Phenyltellanyl)Benzyl)-1H-Imidazol-3-Ium Hexafluorophosphate (5)
A solution of 1-methyl-3-(4-(phenyltellanyl)benzyl)-1H-imidazol-3-ium chloride (4) (2.16 g, 5.24 mmol) and KPF6 (0.965 g, 5.24 mmol) in methanol (10 mL) was stirred at 30 °C for 17 h. Then, the mixture was filtered, and the solution was dried over MgSO4 and evaporated to yield product 5 (2.63 g, 96%) as a yellow oil. 1H-NMR (500 MHz, DMSO-d6): δ = 9.18 (s, 1H), 7.77–7.65 (m, 6H), 7.38–7.26 (m, 5H), 5.39 (s, 2H), 3.84 (s, 3H); 13C-NMR (125 MHz, DMSO-d6): δ = 138.7, 137.9, 137.2, 134.9, 130.3, 129.9, 128.7, 124.5, 122.8, 116.6, 115.0, 51.9, 36.3; 125Te-NMR (159 MHz, DMSO-d6): δ = 707.0; HRMS (APCI): m/z M+ calcd. for C17H17N2Te: 379.0448, found: 379.0491.
3.6. Oxidation of Thiols
Typically, [bmim]PF6 solution (10 mL) of thiophenol (0.110 g, 1.00 mmol), IL-supported diphenyl telluride 5 (0.104 g, 0.200 mmol), and rose bengal (50.9 mg, 0.0500 mmol) were vigorously stirred in an open flask and irradiated using a 500-W halogen lamp for 3 h. The resulting mixture was extracted with diethyl ether and evaporated to yield diphenyl disulfide (0.114 g, quant.). The catalytic oxidation of other thiols was similarly conducted and the products were identified by comparison of physical and spectral data with published values.
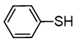
3.6.1. Diphenyl Disulfide
Pale pink solids, mp 55–56 °C (mp 59–61 °C [14]); 1H-NMR (500 MHz, CDCl3): δ = 7.50 (d, J = 8.5, 4H), 7.30 (t, J = 7.8, 4H), 7.26 (t, J = 8.5, 2H); 13C-NMR (125 MHz, CDCl3): δ = 137.0, 129.1, 127.5, 127.2.
3.6.2. Didodecyl Disulfide
Pale pink solids, mp 33–40 °C (mp 31–32 °C [14]); 1H-NMR (500 MHz, CDCl3): δ = 2.70 (t, J = 7.5, 4H), 1.68 (quin., J = 7.5, 4H), 1.39 (m, 4H), 1.26 (br, 32H), 0.88 (t, J = 6.8, 6H); 13C NMR (125 MHz, CDCl3): δ = 39.20, 31.90, 29.68, 29.66, 29.60 29.50, 29.40, 29.27, 29.25, 28.60, 22.70, 14.10.
3.6.3. Dicyclohexyl Disulfide
Orange oil; 1H-NMR (500 MHz, CDCl3): δ = 2.67–2.68 (m, 2H), 2.03–2.05 (m, 4H), 1.77–1.79 (m, 4H), 1.56–1.62 (m, 2H), 1.25–1.31 (m, 10H); 13C NMR (125 MHz, CDCl3): δ = 50.00, 32.90, 26.10, 25.70.
3.6.4. Di-Tert-Butyl Disulfide
Pink oil; 1H-NMR (500 MHz, CDCl3): δ = 1.31 (s, 18H); 13C NMR (125 MHz, CDCl3): δ = 46.20, 30.60.
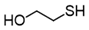
3.6.5. 2-Hydroxyethyl Disulfide
Pink oil; 1H-NMR (500 MHz, CDCl3): δ = 3.92 (t, J = 5.8, 4H), 2.89 (t, J = 5.8, 4H); 13C NMR (125 MHz, CDCl3): δ = 60.40, 41.20.
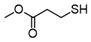
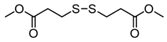
3.6.6. Dimethyl 3,3′-Dithiodipropionate
Pink oil; 1H-NMR (500 MHz, CDCl3): δ = 3.71 (s, 6H), 2.93 (t, J = 7.0, 4H), 2.76 (t, J = 7.3, 4H); 13C NMR (125 MHz, CDCl3): δ = 172.2, 51.90, 33.90, 33.10 [21].
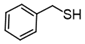
3.6.7. Dibenzyl Disulfide
Colorless solids, mp 65–70 °C (mp 70–71 °C [14]); 1H-NMR (500 MHz, CDCl3): δ = 7.29–7.33 (m, 4H), 7.26–7.28 (m, 4H), 7.22–7.24 (m, 2H), 3.59 (s, 1H); 13C NMR (125 MHz, CDCl3): δ = 137.40, 129.47, 128.53, 127.48, 43.300.
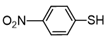
3.6.8. 1,2-Bis(4-Nitrophenyl)Disulfane
Yellow solids, mp 168-173 °C (mp 178-180 °C [22]); 1H-NMR (500 MHz, CDCl3): δ = 8.20 (d, J = 9 Hz, 4H), 7.61 (d, J = 9 Hz, 4H);13C NMR (125 MHz, CDCl3): δ = 147.30, 144.38, 126.70, 124.79.
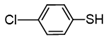
3.6.9. 1,2-Bis(4-Chlorophenyl)Disulfane
Colorless solids, mp 63–65 °C (mp 65–66 °C [14]); 1H-NMR (500 MHz, CDCl3): δ = 7.39 (d, J = 8.5 Hz, 4H), 7.27 (d, J = 8.5 Hz, 4H); 13C NMR (125 MHz, CDCl3): δ = 135.44, 133.94, 131.08.
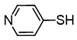
3.6.10. 4,4′-Dipyridyl Disulfide
Colorless solids, mp 59–64 °C (mp 72–74 °C [23]); 1H-NMR (500 MHz, CDCl3): δ = 8.56–8.50 (m, 4H), 7.46–7.24 (m, 4H); 13C NMR (125 MHz, CDCl3): δ = 150.4, 149.9, 121.8.
4. Conclusions
We demonstrated the synthesis of the IL-supported organotelluride reagent and its application as a recyclable catalyst for thiol oxidation. A novel diphenyl telluride catalyst 5 carrying an imidazolium hexafluorophosphate moiety as an IL support was successfully synthesized. The synthesis was very easy, requiring only four steps. The IL-supported diphenyl telluride 5 was found to be an efficient catalyst for aerobic oxidation of various thiols under photosensitized conditions, affording the corresponding disulfides in excellent yields. Although the oxidation proceeds smoothly in various IL solutions, [bmim]PF6 is best suited for efficient phase separation. The produced disulfides can be isolated and purified via simple extraction with diethyl ether. After removal of the product, the resulting IL solution containing IL-supported catalyst (5) and rose bengal can be reused at least four times without a considerable loss in catalytic activity (97% yield in the fifth run).
Author Contributions
Conceptualization, S.K., and M.O.; synthetic methodology, A.M., S.K., and M.O.; investigation, M.M., Y.S., and A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Yoshiki Oda and Yoshimi Kanie for helpful inputs related to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petragnani, N.; Stefani, H.A. Tellurium in Organic Synthesis, 2nd ed.; Academic Press: London, UK, 2007; pp. 162–183. [Google Scholar]
- Barton, D.H.R.; Ley, S.V.; Meerholz, C.A. Bis(p-methoxyphenyl) telluroxide: A new, mild oxidising agent. Chem. Commun. 1979, 755–756. [Google Scholar] [CrossRef]
- Ley, S.V.; Meerholz, C.A.; Barton, D.H.R. Catalytic oxidation of thiocarbonyl compounds involving the use of 1,2-dibromotetrachloroethane as a brominating reagent for diaryl TeII species. Tetrahedron Lett. 1980, 21, 1785–1788. [Google Scholar] [CrossRef]
- Ley, S.V.; Meerholz, C.A.; Barton, D.H.R. Diaryl telluroxides as new mild oxidising reagents. Tetrahedron 1981, 37, 213–223. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Finet, J.-P.; Thomas, M. Organotellurinic acid anhydrides as selective oxidants in organic synthesis. Tetrahedron 1986, 42, 2319–2324. [Google Scholar] [CrossRef]
- Hu, N.X.; Aso, Y.; Otsubo, T.; Ogura, F. Polymer-supported diaryl selenoxide and telluroxide as mild and selective oxidizing agents. Bull. Chem. Soc. Jpn. 1986, 59, 879–884. [Google Scholar] [CrossRef]
- Hu, N.X.; Aso, Y.; Otsubo, T.; Ogura, F. Novel oxidizing properties of p-methoxybenzenetellurinic acid anhydride. Tetrahedron Lett. 1986, 27, 6099–6102. [Google Scholar] [CrossRef]
- Fukumoto, T.; Matsuki, T.; Hu, N.X.; Aso, Y.; Otsubo, T.; Ogura, F. Benzenetellurinic mixed anhydrides as mild oxidizing agents. Chem. Lett. 1990, 19, 2269–2272. [Google Scholar] [CrossRef]
- Kambe, N.; Tsukamoto, T.; Miyoshi, N.; Murai, S.; Sonoda, N. Oxidation of olefins with benzenetellurinic anhydride. Chem. Lett. 1987, 16, 269–272. [Google Scholar] [CrossRef]
- Oba, M.; Endo, M.; Nishiyama, K.; Ouchi, A.; Ando, W. Photosensitized oxygenation of diaryl tellurides to telluroxides and their oxidizing properties. Chem. Commun. 2004, 14, 1672–1673. [Google Scholar] [CrossRef]
- Oba, M.; Okada, Y.; Nishiyama, K.; Shimada, S.; Ando, W. Synthesis, characterization and oxidizing properties of a diorgano tellurone carrying bulky aromatic substituents. Chem. Commun. 2008, 5378–5380. [Google Scholar] [CrossRef]
- Oba, M.; Okada, Y.; Nishiyama, K.; Ando, W. Aerobic photooxidation of phosphite esters using diorganotelluride catalysts. Org. Lett. 2009, 11, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Oba, M.; Arai, A.; Tanaka, K.; Nishiyama, K.; Ando, W. Diorganotelluride-catalyzed oxidation of silanes to silanols under atmospheric oxygen. Inorg. Chem. 2010, 49, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Tanaka, K.; Nishiyama, K.; Ando, W. Aerobic oxidation of thiols to disulfides catalyzed by diaryl tellurides under photosensitized conditions. J. Org. Chem. 2011, 76, 4173–4177. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Chan, T.H. Ionic–liquid–supported synthesis: A novel liquid–phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef]
- Koguchi, S. An ionic–liquid–supported 18-crown-6 ether: Recyclable catalyst for acetylation and fluorination in anionic liquid. Trans. Mater. Res. Soc. Jpn. 2013, 38, 35–36. [Google Scholar] [CrossRef][Green Version]
- Koguchi, S.; Nakamura, K. Ascorbic acid based ionic liquid: Recyclable and efficient catalytic systems for the Huisgen cycloaddition. Synlett 2013, 24, 2305–2309. [Google Scholar] [CrossRef]
- Koguchi, S.; Izawa, K. Ionic liquid-phase synthesis of 1,5-disubstituted 1,2,3-triazoles. ACS Comb. Sci. 2014, 16, 381–385. [Google Scholar] [CrossRef]
- Koguchi, S.; Mihoya, A.; Mimura, M. Alcohol oxidation via recyclable hydrophobic ionic liquid-supported IBX. Tetrahedron 2016, 72, 7633–7637. [Google Scholar] [CrossRef]
- Koguchi, S.; Shibuya, Y.; Igarashi, Y.; Takemura, H. Ionic liquid–supported 1,3-dimethylimidazolidin-2-one: Application as a reusable halogenation reagent. Synlett 2019, 30, 943–946. [Google Scholar] [CrossRef]
- Dethe, D.H.; Srivastava, A.; Dherange, B.D.; Kumar, B.V. Unsymmetrical disulfide synthesis through photoredox catalysis. Adv. Synth. Catal. 2018, 360, 3020–3025. [Google Scholar] [CrossRef]
- Kumar, V.; Kaushik, M.P. Efficient oxidative coupling of thiols into disulfides using N-tert-butyl-N-chlorocyanamide. Bull. Chem. Soc. Jpn. 2008, 81, 160–162. [Google Scholar] [CrossRef]
- Kosobokov, M.; Cui, B.; Balia, A.; Matsuzaki, K.; Tokunaga, E.; Saito, N.; Shibata, N. Importance of a fluorine substituent for the preparation of meta- and para-pentafluoro-λ6-sulfanyl-substituted pyridines. Angew. Chem. Int. Ed. 2016, 55, 10781–10785. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).