TMSCl-Catalyzed Tandem Reaction of Dihydroisobenzofuran Acetals with Indoles
Abstract
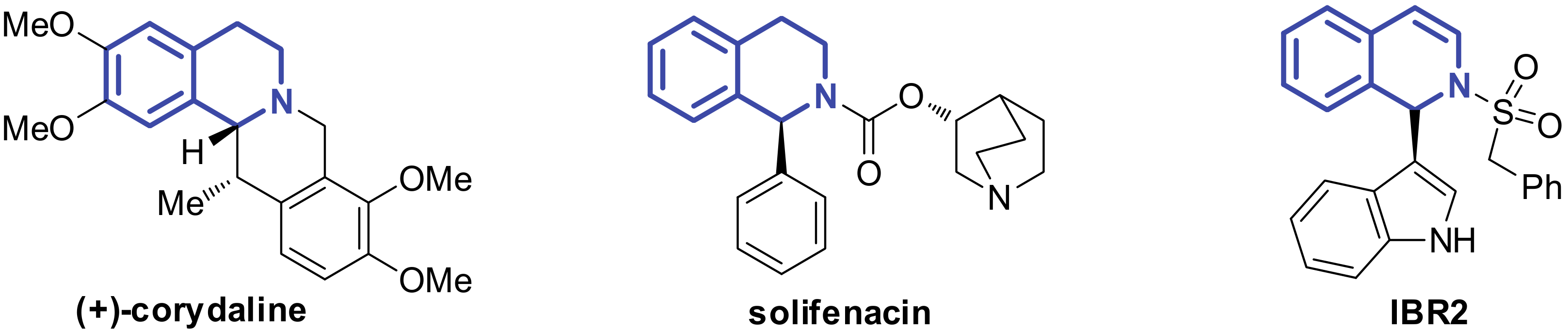
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2002, 19, 742–746. [Google Scholar] [CrossRef]
- Hansch, C.; Sammes, P.G.; Taylor, J.B. Comprehensive Medicinal Chemistry, 1st ed.; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Zhang, Q.; Tu, G.; Zhao, Y.; Cheng, T. Novel bioactive isoquinoline alkaloids from Carduus crispus. Tetrahedron 2002, 58, 6795–6798. [Google Scholar] [CrossRef]
- Reddy, N.S.S.; Reddy, B.J.M.; Reddy, B.S. A convergent and stereoselective total synthesis of (-)-crispine A, (-)-benzo[α]quinolizidine and (-)-salsolidine. Tetrahedron Lett. 2013, 54, 4228–4231. [Google Scholar] [CrossRef]
- Singh, H.; Singh, P.; Kumari, K.; Chandra, A.; K Dass, S.; Chandra, R. A review on noscapine, and its impact on heme metabolism. Curr. Drug Metab. 2013, 14, 351–360. [Google Scholar] [CrossRef]
- Ohtake, A.; Ukai, M.; Hatanaka, T.; Kobayashi, S.; Ikeda, K.; Sato, S.; Miyata, K.; Sasamata, M. In Vitro and In Vivo tissue selectivity profile of solifenacin succinate (YM905) for urinary bladder over salivary gland in rats. Eur. J. Pharmacol. 2004, 492, 243–250. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, L.; Wu, G.; Konig, H.; Lin, X.; Li, G.; Qiu, X.L.; Chen, C.F.; Hu, C.M.; Goldblatt, E. A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia. EMBO. Mol. Med. 2013, 5, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Chen, P.-L.; Zhu, J. Compositions and Methods for Disruption of BRCA2-RAD51 Interaction. PCT Int. Appl. WO 2006044933, 27 April 2006. Available online: https://worldwide.espacenet.com/patent/search/family/035695666/publication/WO2006044933A2?
- Chung, T.-W.; Hung, Y.-T.; Thikekar, T.; Paike, V.V.; Lo, F.Y.; Tsai, P.-H.; Liang, M.-C.; Sun, C.-M. Telescoped synthesis of 2-Acyl-1-aryl-1, 2-dihydroisoquinolines and their Inhibition of the transcription Factor NF-κB. ACS Comb. Sci. 2015, 17, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, H.; Guo, X.E.; Qiu, X.-L.; Hu, C.-M.; Chamberlin, A.R.; Lee, W.-H. Synthesis, molecular modeling, and biological evaluation of novel RAD51 inhibitors. Eur. J. Med. Chem. 2015, 96, 196–208. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.-J. CuBr-catalyzed direct indolation of tetrahydroisoquinolines via Cross-dehydrogenative coupling between sp3 C-H and sp2 C-H Bonds. J. Am. Chem. Soc. 2005, 127, 6968–6969. [Google Scholar] [CrossRef]
- Zhong, J.-J.; Meng, Q.-Y.; Liu, B.; Li, X.-B.; Gao, X.-W.; Lei, T.; Wu, C.-J.; Li, Z.-J.; Tung, C.-H.; Wu, L.-Z. Cross-Coupling hydrogen evolution reaction in homogeneous solution without noble metals. Org. Lett. 2014, 16, 1988–1991. [Google Scholar] [CrossRef]
- Patil, M.R.; Dedhia, N.P.; Kapdi, A.R.; Kumar, A.V. Cobalt (II)/N-Hydroxyphthalimide-catalyzed cross-dehydrogenative coupling reaction at room temperature under aerobic condition. J. Org. Chem. 2018, 83, 4477–4490. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Zhu, G.; Bao, G.; Zhang, B.; Hong, L.; Li, M.; Wang, R. Enantioselective dearomative arylation of Isoquinolines. ACS Catal. 2016, 6, 5290–5294. [Google Scholar] [CrossRef]
- Li, G.; Yao, Y.; Wang, Z.; Zhao, M.; Xu, J.; Huang, L.; Zhu, G.; Bao, G.; Sun, W.; Hong, L. Switchable skeletal rearrangement of dihydroisobenzofuran acetals with indoles. Org. Lett. 2019, 21, 4313–4317. [Google Scholar] [CrossRef]
- Davies, H.M.; Alford, J.S. Reactions of metallocarbenes derived from N-sulfonyl-1, 2, 3-triazoles. Chem. Soc. Rev. 2014, 43, 5151–5162. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, R.; Tang, X.Y.; Shi, M. Recent advances in the synthesis of heterocycles and related substances based on α-Imino Rhodium Carbene complexes derived from N-Sulfonyl-1, 2, 3-triazoles. Chem. Eur. J. 2016, 22, 17910–17924. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Zhai, H. The expanding utility of Rhodium-Iminocarbenes: Recent advances in the synthesis of natural products and related scaffolds. Chem. Eur. J. 2018, 24, 12757–12766. [Google Scholar] [CrossRef]
- Alford, J.S.; Davies, H.M. Mild aminoacylation of indoles and pyrroles through a three-component reaction with ynol ethers and sulfonyl azides. J. Am. Chem. Soc. 2014, 136, 10266–10269. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Z.Z.; Zhang, Y.S.; Tang, X.Y.; Shi, M. Rhodium (II)-Catalyzed Intramolecular Cycloisomerizations of Methylenecyclopropanes with N-Sulfonyl 1, 2, 3-Triazoles. Angew. Chem. Int. Ed. 2014, 53, 6645–6649. [Google Scholar] [CrossRef]
- Chuprakov, S.; Worrell, B.T.; Selander, N.; Sit, R.K.; Fokin, V.V. Stereoselective 1, 3-insertions of rhodium (II) azavinyl carbenes. J. Am. Chem. Soc. 2014, 136, 195–202. [Google Scholar] [CrossRef]
- He, J.; Shi, Y.; Cheng, W.; Man, Z.; Yang, D.; Li, C.Y. Rhodium-Catalyzed Synthesis of 4-Bromo-1, 2-dihydroisoquinolines: Access to Bromonium Ylides by the Intramolecular Reaction of a Benzyl Bromide and an α-Imino Carbene. Angew. Chem. Int. Ed. 2016, 55, 4557–4561. [Google Scholar] [CrossRef]
- Kwok, S.W.; Zhang, L.; Grimster, N.P.; Fokin, V.V. Catalytic Asymmetric Transannulation of NH-1, 2, 3-Triazoles with Olefins. Angew. Chem. Int. Ed. 2014, 53, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, V.N.; Viart, H.M.-F.; Sarpong, R. Stereodivergent intramolecular C (sp3)-H functionalization of azavinyl carbenes: Synthesis of saturated heterocycles and fused N-heterotricycles. J. Am. Chem. Soc. 2015, 137, 8368–8371. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, W.; Liu, Y.; Wei, Q.; Chen, J.; Wu, X.; Xia, F.; Hu, W. A Rh (II)-catalyzed multicomponent reaction by trapping an α-amino enol intermediate in a traditional two-component reaction pathway. Sci. Adv. 2017, 3, e1602467. [Google Scholar] [CrossRef]
- Mi, P.; Kiran Kumar, R.; Liao, P.; Bi, X. Tandem O-H Insertion/[1,3]-Alkyl Shift of Rhodium Azavinyl Carbenoids with Benzylic Alcohols: A Route To Convert C–OH Bonds into C–C Bonds. Org. Lett. 2016, 18, 4998–5001. [Google Scholar] [CrossRef]
- Miura, T.; Tanaka, T.; Biyajima, T.; Yada, A.; Murakami, M. One-Pot Procedure for the Introduction of Three Different Bonds onto Terminal Alkynes through N-Sulfonyl-1, 2, 3-Triazole Intermediates. Angew. Chem. Int. Ed. 2013, 52, 3883–3886. [Google Scholar] [CrossRef]
- Schultz, E.E.; Lindsay, V.N.; Sarpong, R. Expedient Synthesis of Fused Azepine Derivatives Using a Sequential Rhodium (II)-Catalyzed Cyclopropanation/1-Aza-Cope Rearrangement of Dienyltriazoles. Angew. Chem. Int. Ed. 2014, 53, 9904–9908. [Google Scholar] [CrossRef]
- Xu, Z.-F.; Dai, H.; Shan, L.; Li, C.-Y. Metal-Free Synthesis of (E)-Monofluoroenamine from 1-Sulfonyl-1, 2, 3-triazole and Et2O· BF3 via Stereospecific Fluorination of α-Diazoimine. Org. Lett. 2018, 20, 1054–1057. [Google Scholar] [CrossRef]
- Yang, J.M.; Zhu, C.Z.; Tang, X.Y.; Shi, M. Rhodium (II)-Catalyzed Intramolecular Annulation of 1-Sulfonyl-1, 2, 3-Triazoles with Pyrrole and Indole Rings: Facile Synthesis of N-Bridgehead Azepine Skeletons. Angew. Chem. Int. Ed. 2014, 53, 5142–5146. [Google Scholar]
- Bae, I.; Han, H.; Chang, S. Highly efficient one-pot synthesis of N-sulfonylamidines by Cu-catalyzed three-component coupling of sulfonyl azide, alkyne, and amine. J. Am. Chem. Soc. 2005, 127, 2038–2039. [Google Scholar] [CrossRef]
- Cho, S.H.; Chang, S. Rate-Accelerated Nonconventional Amide Synthesis in Water: A Practical Catalytic Aldol-Surrogate Reaction. Angew. Chem. Int. Ed. 2007, 46, 1897–1900. [Google Scholar] [CrossRef]
- Cho, S.H.; Chang, S. Room Temperature Copper-Catalyzed 2-Functionalization of Pyrrole Rings by a Three-Component Coupling Reaction. Angew. Chem. Int. Ed. 2008, 47, 2836–2839. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xie, J.; Yao, Y.; Mou, L.; Zhang, X.; Guo, X.; Sun, W.; Wang, Z.; Xu, J.; et al. Efficient synthesis of cyclic amidine-based fluorophores via 6p-electrocyclic ring closure. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Dodd, R.H.; Cariou, K. Ketenimines Generated from Ynamides: Versatile Building Blocks for Nitrogen-Containing Scaffolds. Chem. Eur. J. 2018, 24, 2297–2304. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, R.; Wang, Q.; Tang, X.-Y.; Shi, M. Cyclization of sulfide, ether or tertiary amine-tethered N-sulfonyl-1, 2, 3-triazoles: A facile synthetic protocol for 3-substituted isoquinolines or dihydroisoquinolines. Chem. Commun. 2015, 51, 16968–16971. [Google Scholar] [CrossRef]
- Shen, H.; Fu, J.; Gong, J.; Yang, Z. Tunable and Chemoselective Syntheses of Dihydroisobenzofurans and Indanones via Rhodium-Catalyzed Tandem Reactions of 2-Triazole-benzaldehydes and 2-Triazole-alkylaryl Ketones. Org. Lett. 2014, 16, 5588–5591. [Google Scholar] [CrossRef]
- Yuan, H.; Gong, J.; Yang, Z. A rhodium-catalyzed tandem reaction of N-sulfonyl triazoles with indoles: Access to indole-substituted indanones. Chem. Commun. 2017, 53, 9089–9092. [Google Scholar] [CrossRef]
- Li, G. CCDC 1832699: Experimental Crystal Structure Determination; Lanzhou University: Lanzhou, China, 2020. [Google Scholar] [CrossRef]




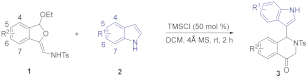
| Entry a | 1a (eq.) | 2a (eq.) | Acid (50 mol%) | T(°C) | Solvent | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | 1.0 | 1.2 | CF3CO2H | 0 | CHCl3 | 34 |
| 2 | 1.0 | 1.2 | AcOH | 0 | CHCl3 | - |
| 3 | 1.0 | 1.2 | ZnCl2 | 0 | CHCl3 | 39 |
| 4 | 1.0 | 1.2 | AlCl3 | 0 | CHCl3 | 58 |
| 5 | 1.0 | 1.2 | BF3.Et2O | 0 | CHCl3 | 24 |
| 6 | 1.0 | 1.2 | TMSCl | 0 | CHCl3 | 60 |
| 7 | 1.0 | 1.2 | TESOTf | 0 | CHCl3 | <10 |
| 8 | 1.2 | 1.0 | TMSCl | 0 | CHCl3 | 39 |
| 9 | 1.0 | 2.0 | TMSCl | 0 | CHCl3 | 76 |
| 10 | 1.0 | 2.0 | TMSCl | rt | CHCl3 | 84 |
| 11 | 1.0 | 2.0 | TMSCl | rt | CH2Cl2 | 92 |
| 12 | 1.0 | 2.0 | TMSCl | rt | DCE | 78 |
| 13 | 1.0 | 2.0 | TMSCl | rt | toluene | 77 |
| 14 | 1.0 | 2.0 | TMSCl | rt | Et2O | 51 |
| 15 | 1.0 | 2.0 | TMSCl | rt | MTBE | 64 |
| 16 | 1.0 | 2.0 | TMSCl | rt | THF | 50 |
| Entry | R1 | R2 | 3 | Yield (%) b |
|---|---|---|---|---|
| 1 | H | H | 3a | 92 |
| 2 | 4-Me | H | 3b | 68 |
| 3 | 5-Me | H | 3c | 83 |
| 4 | 5-OBn | H | 3d | 71 |
| 5 | 5-OMe | H | 3e | 77 |
| 6 | 5-F | H | 3f | 75 |
| 7 | 5-Cl | H | 3g | 82 |
| 8 | 6-Cl | H | 3h | 78 |
| 9 | 6-F | H | 3i | 77 |
| 10 | 6-Br | H | 3j | 86 |
| 11 | 6-Me | H | 3k | 78 |
| 12 | 7-OMe | H | 3l | 69 |
| 13 | 7-Me | H | 3m | 88 |
| 14 | 7-F | H | 3n | 77 |
| 15 | H | 4-F | 3o | 54 |
| 16 | H | 5-Cl | 3p | 57 |
| 17 | H | 5-F | 3q | 58 |
| 18 | H | 5-OMe | 3r | 72 |
| 19 | H | 6-Me | 3s | 63 |
| 20 | H | 6-F | 3t | 52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Lu, S.; Li, G.; Hong, L. TMSCl-Catalyzed Tandem Reaction of Dihydroisobenzofuran Acetals with Indoles. Catalysts 2020, 10, 392. https://doi.org/10.3390/catal10040392
Zhang M, Lu S, Li G, Hong L. TMSCl-Catalyzed Tandem Reaction of Dihydroisobenzofuran Acetals with Indoles. Catalysts. 2020; 10(4):392. https://doi.org/10.3390/catal10040392
Chicago/Turabian StyleZhang, Ming, Sicong Lu, Guofeng Li, and Liang Hong. 2020. "TMSCl-Catalyzed Tandem Reaction of Dihydroisobenzofuran Acetals with Indoles" Catalysts 10, no. 4: 392. https://doi.org/10.3390/catal10040392
APA StyleZhang, M., Lu, S., Li, G., & Hong, L. (2020). TMSCl-Catalyzed Tandem Reaction of Dihydroisobenzofuran Acetals with Indoles. Catalysts, 10(4), 392. https://doi.org/10.3390/catal10040392





