Polyvinylpyridine-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki–Miyaura Coupling Reactions
Abstract
1. Introduction
2. Results and Discussion
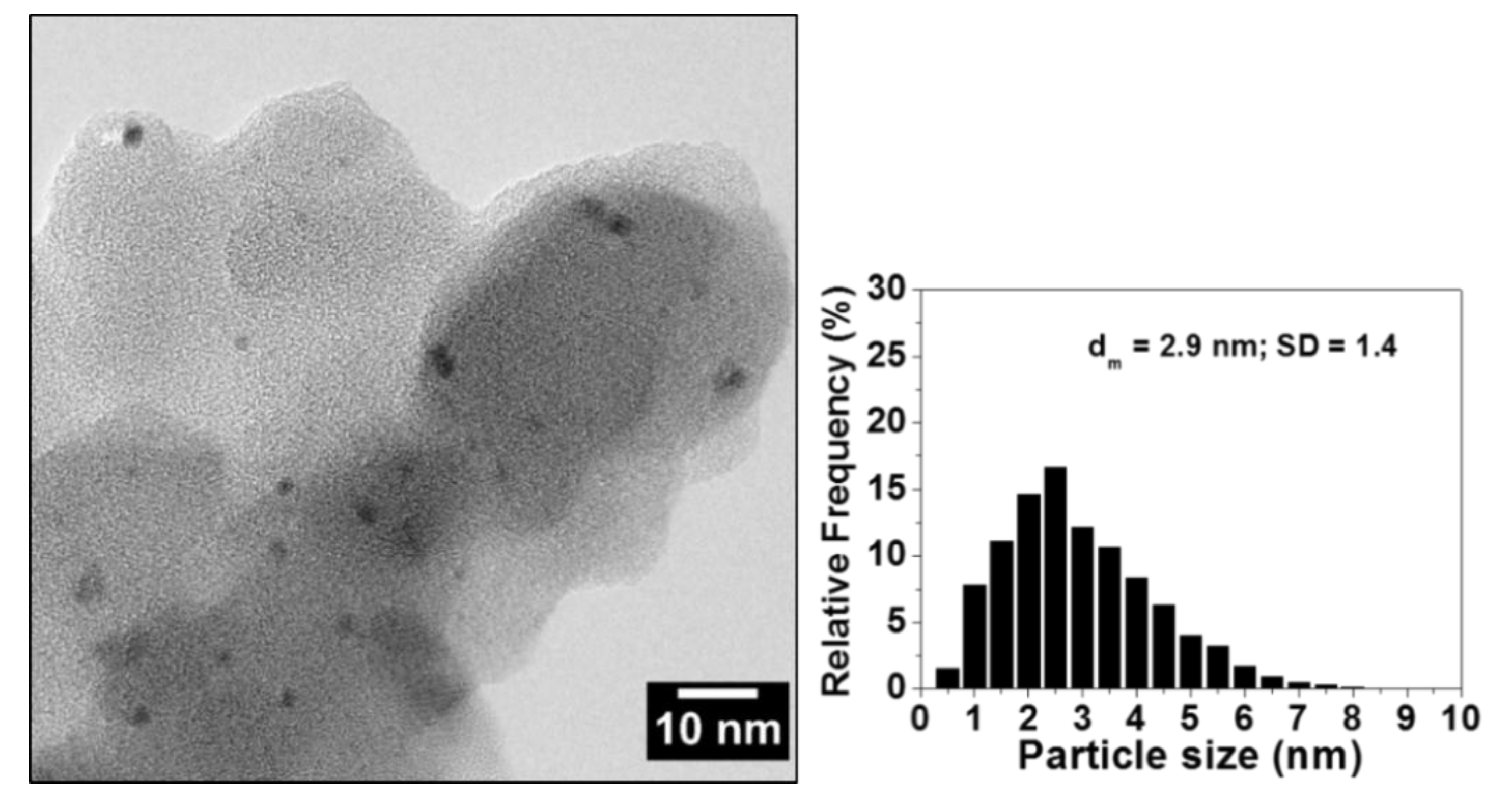
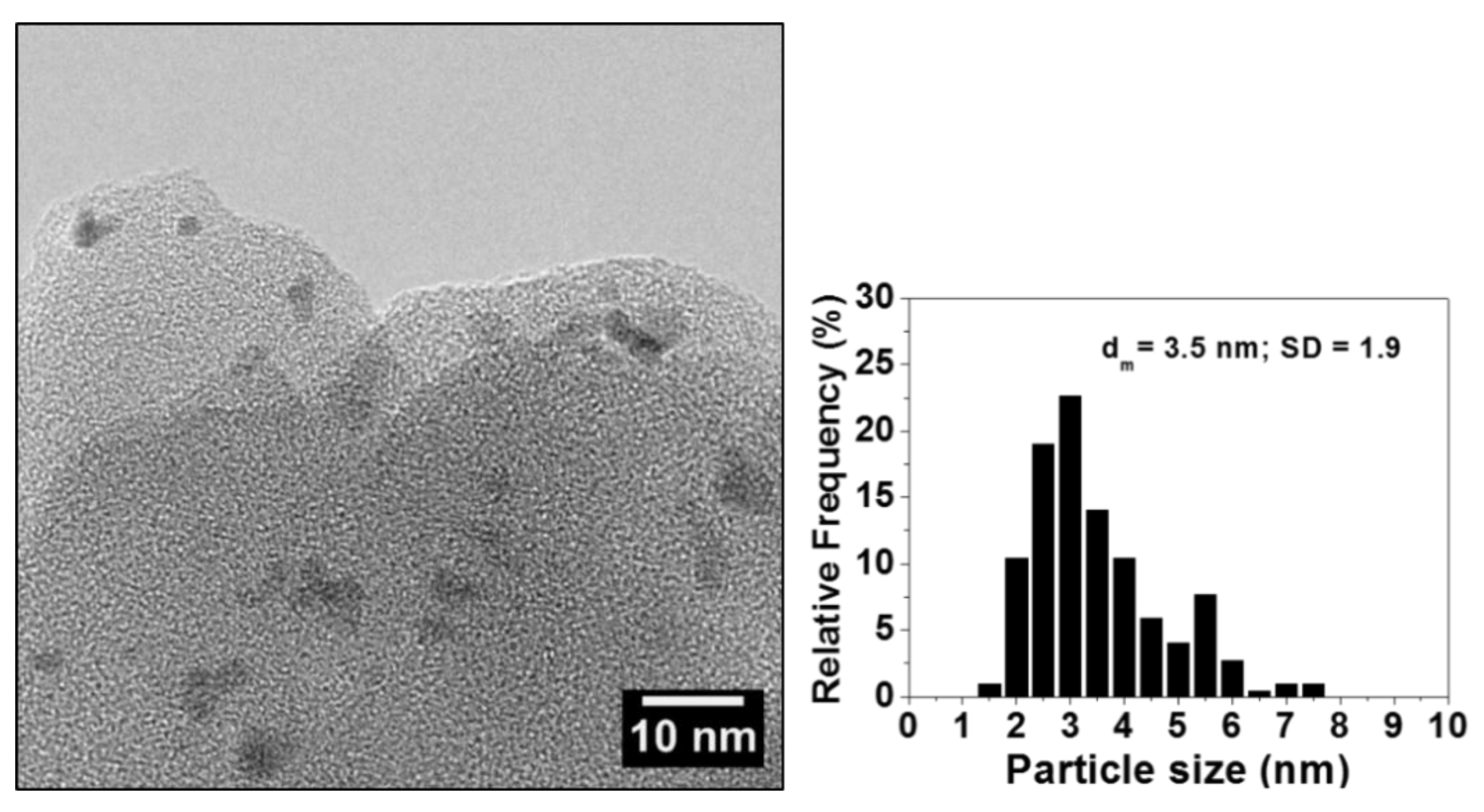
2.1. Characterization of Polyvinylpyridine-Supported Palladium (Pd/PVPy) Catalyst
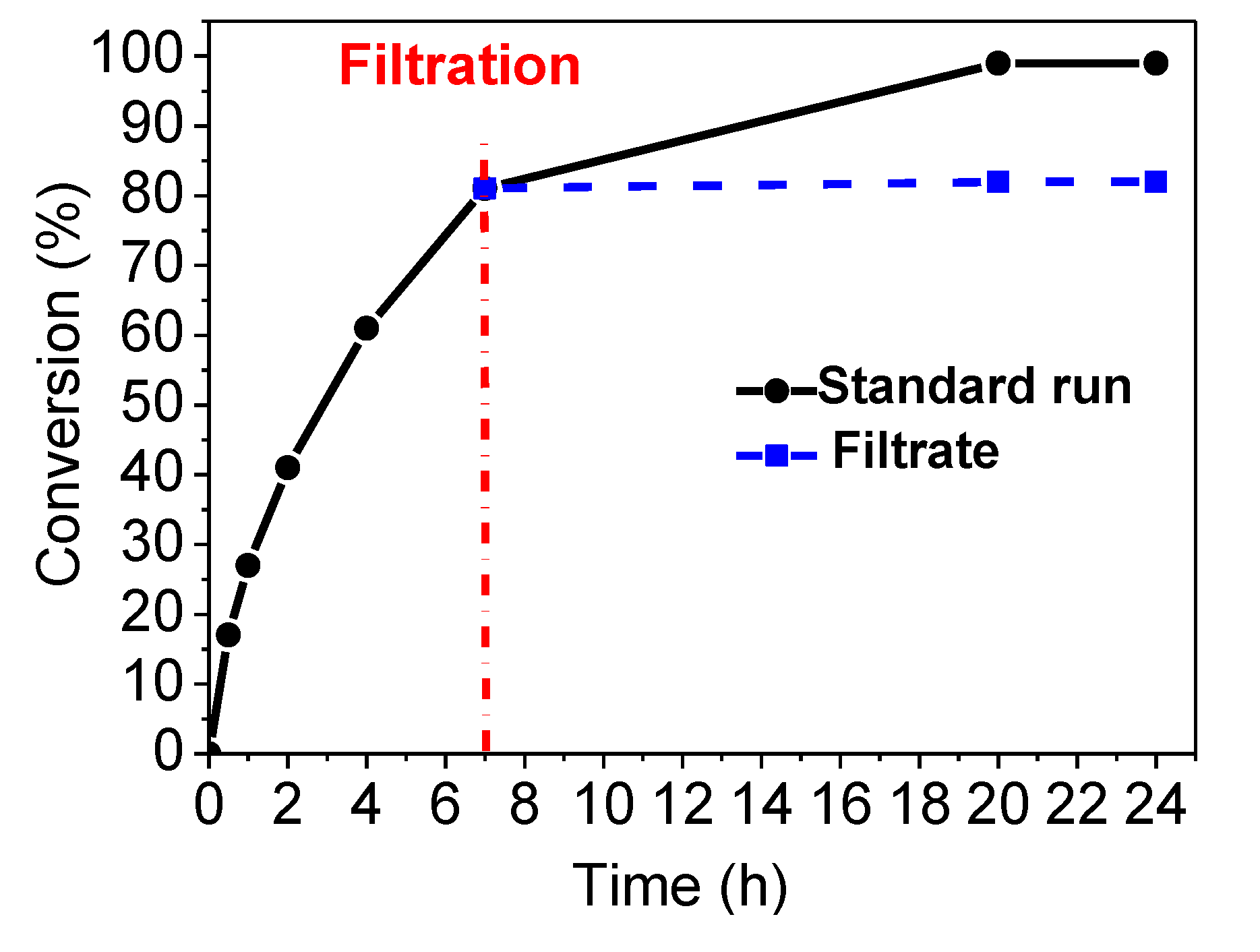
2.2. Catalytic Activity in Suzuki–Miyaura Reaction
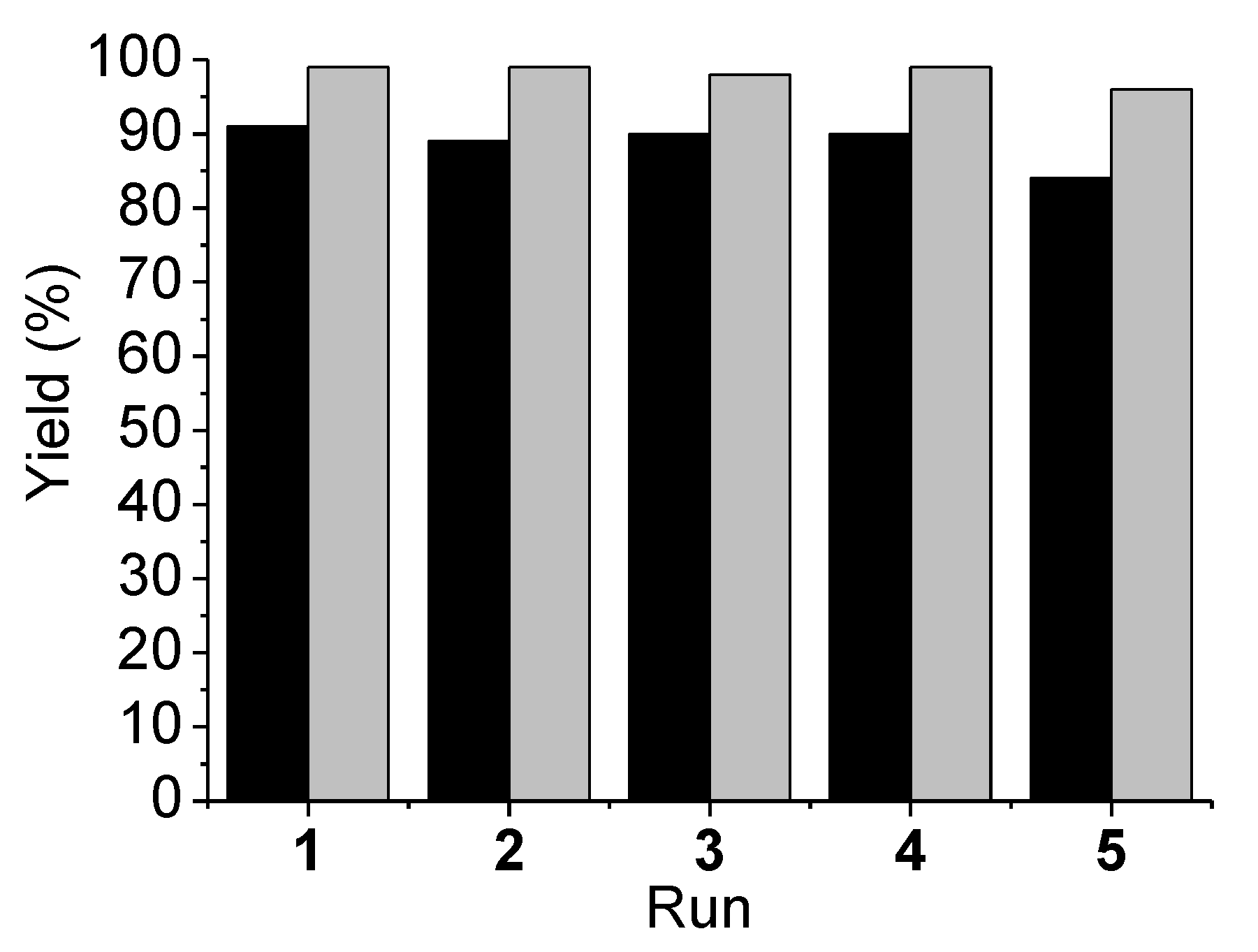
2.3. Catalyst Reusability
2.4. Comparison with Commercially Available Pd-Based Catalysts
3. Materials and Methods
3.1. Preparation of Pd/PVPy System
3.2. General Procedure for the Suzuki–Miyaura Reactions
3.2.1. Suzuki–Miyaura Reactions
3.2.2. Recycling Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Suzuki, A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C–C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Miyaura, N. Metal-Catalyzed Cross-Coupling Reactions of Organoboron Compounds with Organic Halides. In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Diederich, F., Stang, P.J., Eds.; Wiley-VCH: New York, NY, USA, 2004; pp. 41–123. [Google Scholar]
- Magano, J.; Dunetz, J.R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 3, 381–405. [Google Scholar] [CrossRef]
- Fliedel, C.; Ghisolfi, A.; Braunstein, P. Functional Short-Bite Ligands: Synthesis, Coordination Chemistry, and Applications of N-Functionalized Bis(diaryl/dialkylphosphino)amine-type Ligands. Chem. Rev. 2016, 116, 9237–9304. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Sluys, M.V.D.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Suzuki, A. Carbon–carbon bonding made easy. Chem. Commun. 2005, 4759–4763. [Google Scholar] [CrossRef]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium Catalysts for the Suzuki Cross-Coupling Reaction: An Overview of Recent Advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Fliedel, C.; Labande, A.; Manoury, E.; Poli, R. Chiral N-heterocyclic carbene ligands with additional chelating group(s) applied to homogeneous metal-mediated asymmetric catalysis. Coord. Chem. Rev. 2019, 394, 65–103. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes−Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Maluenda, I.; Navarro, O. Recent Developments in the Suzuki-Miyaura Reaction: 2010−2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura Reaction after the Nobel Prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Paul, S.; Islam, M.M.; Islam, S.M. Suzuki–Miyaura reaction by heterogeneously supported Pd in water: Recent studies. RSC Adv. 2015, 5, 42193–42221. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef]
- Barder, T.E.; Walker, S.D.; Martinelli, J.R.; Buchwald, S.L. Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005, 127, 4685–4696. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Poliakoff, M.; Fitzpatrick, J.M.; Farren, T.R.; Anastas, P.T. Green Chemistry: Science and Politics of Change. Science 2002, 297, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liebscher, J. Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Taladriz-Blanco, P.; Hervés, P.; Pérez-Juste, J. Supported Pd Nanoparticles for Carbon-Carbon Coupling Reactions. Top. Catal. 2013, 56, 1154–1170. [Google Scholar] [CrossRef]
- Islam, S.M.; Mondal, P.; Roy, A.S.; Mondal, S.; Hossain, D. Heterogeneous Suzuki and copper-free Sonogashira cross-coupling reactions catalyzed by a reusable palladium(II) complex in water medium. Tetrahedron Lett. 2010, 51, 2067–2070. [Google Scholar] [CrossRef]
- Pol, V.G.; Grisaru, H.; Gedanken, A. Coating Noble Metal Nanocrystals (Ag, Au, Pd, and Pt) on Polystyrene Spheres via Ultrasound Irradiation. Langmuir 2005, 21, 3635–3640. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. An Assembled Complex of Palladium and Non-Cross-linked Amphiphilic Polymer: A Highly Active and Recyclable Catalyst for the Suzuki−Miyaura Reaction. Org. Lett. 2002, 4, 3371–3374. [Google Scholar] [CrossRef]
- Ley, S.V.; Ramarao, C.; Gordon, R.S.; Holmes, A.B.; Morrison, A.J.; McConvey, I.F.; Shirley, I.M.; Smith, S.C.; Smith, M.D. Polyurea-encapsulated palladium(II) acetate: A robust and recyclable catalyst for use in conventional and supercritical media. Chem. Commun. 2002, 1134–1135. [Google Scholar] [CrossRef]
- Kunfi, A.; Mayc, Z.; Németh, P.; London, G. Polydopamine supported palladium nanoparticles: Highly efficient catalysts in Suzuki cross-coupling and tandem Suzuki cross-coupling/nitroarene reductions under green reaction conditions. J. Catal. 2018, 361, 84–93. [Google Scholar] [CrossRef]
- Chen, X.; He, J.; Yan, C.; Tang, H. Novel In Situ Fabrication of Chestnut-Like Carbon Nanotube Spheres from Polypropylene and Nickel Formate. J. Phys. Chem. B 2006, 110, 21684–21689. [Google Scholar] [CrossRef]
- Lu, A.-H.; Li, W.-C.; Hou, Z.; Schuth, F. Molecular level dispersed Pd clusters in the carbon walls of ordered mesoporous carbon as a highly selective alcohol oxidation catalyst. Chem. Commun. 2007, 1038–1040. [Google Scholar] [CrossRef]
- Mitsudome, T.; Nose, K.; Mori, K.; Mizugaki, T.; Ebitani, K.; Jitsukawa, K.; Kaneda, K. Montmorillonite-entrapped sub-nanoordered Pd clusters as a heterogeneous catalyst for allylic substitution reactions. Angew. Chem. Int. Ed. 2007, 46, 3288–3290. [Google Scholar] [CrossRef] [PubMed]
- Hoshiya, N.; Shimoda, M.; Yoshikawa, H.; Yamashita, Y.; Shuto, S.; Arisawa, M. Sulfur Modification of Au via Treatment with Piranha Solution Provides Low-Pd Releasing and Recyclable Pd Material, SAPd. J. Am. Chem. Soc. 2010, 132, 7270–7272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gao, Q. Heterogeneous Hydrogenation Catalyses over Recyclable Pd(0) Nanoparticle Catalysts Stabilized by PAMAM-SBA-15 Organic−Inorganic Hybrid Composites. J. Am. Chem. Soc. 2006, 128, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Dickshat, A.T.; Surmiak, S.; Studer, A. Pd Immobilized in Mesoporous Silica Particles as Recyclable Catalysts for Suzuki–Miyaura Coupling: Cooperative Effects Exerted by Co-Immobilized Amine Functionalities. Synlett 2013, 24, 1523–1528. [Google Scholar] [CrossRef]
- Pandarus, V.; Desplantier-Giscard, D.; Gingras, G.; Ciriminna, R.; Cara, P.D.; Beland, F.; Pagliaro, M. Enhanced heterogeneously catalyzed Suzuki–Miyaura reaction over SiliaCat Pd(0). Tetrahedron Lett. 2013, 54, 4712–4716. [Google Scholar] [CrossRef]
- Basudeb, B.; Susmita, P. An improved preparation of mesoporous silica-supported Pd as sustainable catalysts for phosphine-free Suzuki–Miyaura and Heck coupling reactions. Appl. Organomet. Chem. 2013, 27, 588–594. [Google Scholar]
- Webb, J.D.; MacQuarrie, S.; McEleney, K.; Crudden, C.M. Mesoporous silica-supported Pd catalysts: An investigation into structure, activity, leaching and heterogeneity. J. Catal. 2007, 252, 97–109. [Google Scholar] [CrossRef]
- Paul, S.; Clark, J.H. Structure-Activity Relationship between Some Novel Silica Supported Palladium Catalysts: A Study of the Suzuki Reaction. J. Mol. Catal. A Chem. 2004, 215, 107–111. [Google Scholar] [CrossRef]
- Baleizao, C.; Corma, A.; Garcia, H.; Leyva, A. An oxime–carbapalladacycle complex covalently anchored to silica as an active and reusable heterogeneous catalyst for Suzuki cross-coupling in water. Chem. Commun. 2003, 606–607. [Google Scholar] [CrossRef]
- Kirschning, A. (Ed.) Immobilized Catalysts. Solid Phases, Immobilization and Applications; Series: Topics in Current Chemistry; Springer: Berlin, Germany, 2004; Volume 242. [Google Scholar]
- Wang, F.; Mielby, J.; Herrmann Richter, F.; Wang, G.; Prieto, G.; Kasama, T.; Weidenthaler, C.; Bongard, H.-J.; Kegnæs, S.; Alois Fürstner, F.; et al. Polyphenylene Support for Pd Catalysts with Exceptional Catalytic Activity. Angew. Chem. Int. Ed. 2014, 53, 8645–8648. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, S.J.; Tyler McQuade, D. Investigating PdEnCat Catalysis. J. Org. Chem. 2006, 71, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Kwon, M.S.; Park, J. Palladium Nanoparticles in Polymers: Catalyst for Alkene Hydrogenation, Carbon-Carbon Cross-Coupling Reactions, and Aerobic Alcohol Oxidation. Synthesis 2006, 22, 3790–3794. [Google Scholar] [CrossRef]
- Evangelisti, C.; Panziera, N.; Pertici, P.; Vitulli, G.; Salvadori, P.; Battocchio, C.; Polzonetti, G. Palladium nanoparticles supported on polyvinylpyridine: Catalytic activity in Heck-type reactions and XPS structural studies. J. Catal. 2009, 262, 287–293. [Google Scholar] [CrossRef]
- Jumde, R.P.; Marelli, M.; Scotti, N.; Mandoli, A.; Psaro, R.; Evangelisti, C. Ultrafine palladium nanoparticles immobilized into poly(4-vinylpyridine)-based porous monolith for continuous-flow Mizoroki-Heck reaction. J. Mol. Catal. A Chem. 2016, 414, 55–61. [Google Scholar] [CrossRef]
- Oberhauser, W.; Evangelisti, C.; Jumde, R.P.; Petrucci, G.; Bartoli, M.; Frediani, M.; Mannini, M.; Capozzoli, L.; Passaglia, E.; Rosi, L. Palladium-nanoparticles on end-functionalized poly(lactic acid)-based stereocomplexes for the chemoselective cinnamaldehyde hydrogenation: Effect of the end-group. J. Catal. 2015, 330, 187–196. [Google Scholar] [CrossRef]
- Köhler, K.; Heidenreich, R.G.; Soomro, S.S.; Pröckl, S.S. Supported Palladium Catalysts for Suzuki Reactions: Structure-Property Relationships, Optimized Reaction Protocol and Control of Palladium Leaching. Adv. Synth. Catal. 2008, 350, 2930–2936. [Google Scholar] [CrossRef]
- Joucla, L.; Cusati, G.; Pinel, C.; Djakovitch, J. One-Pot Suzuki/Heck Sequence for the Synthesis of (E)-Stilbenes Featuring a Recyclable Silica-Supported Palladium Catalyst via a Multi-Component Reaction in 1,3-Propanediol. Adv. Synth. Catal. 2010, 352, 1993–2001. [Google Scholar] [CrossRef]
- Soomro, S.S.; Ansari, F.L.; Chatziapostolou, K.; Köhler, K. Palladium Leaching Dependent on Reaction Parameters in Suzuki−Miyaura Coupling Reactions Catalyzed by Palladium Supported on Alumina under Mild Reaction Conditions. J. Catal. 2010, 273, 138–146. [Google Scholar] [CrossRef]
- Collins, G.; Schmidt, M.; O’Dwyer, C.; Holmes, J.D.; McGlacken, G.P. The Origin of Shape Sensitivity in Palladium-Catalyzed Suzuki−Miyaura Cross Coupling Reactions. Angew. Chem. Int. Ed. 2014, 53, 4142–4145. [Google Scholar] [CrossRef]
- Ji, J.; Li, Y.; Fu, W.; Cui, Z.; Shen, J.; Ye, M. Self-assembled three-dimensional Pd/MoS2/reduced graphene oxide nanocatalyst: A case for homogeneous leaching mechanism. J. Coll. Interface Sci. 2017, 505, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöfer, S.; Heitz, W.; Greiner, A.; Oestreich, S.; Förster, S.; Antonietti, M. Preparation of Palladium Colloids in Block Copolymer Micelles and Their Use for the Catalysis of the Heck Reaction. J. Am. Chem. Soc. 1997, 119, 10116–10120. [Google Scholar] [CrossRef]
- Caporusso, A.M.; Innocenti, P.; Aronica, L.A.; Vitulli, G.; Gallina, R.; Biffis, A.; Zecca, M.; Corain, B. Functional resins in palladium catalysis: Promising materials for Heck reaction in aprotic polar solvents. J. Catal. 2005, 234, 1–13. [Google Scholar] [CrossRef]
| Entry | Atmosphere | Pd/PVPy (mol % of Pd) | Conversion b (%) b | Yield c (%) c | Pd Leaching (%, ppm) d |
|---|---|---|---|---|---|
| 1 | Ar | 0.5 | 64 | 61 | n.d. |
| 2 | Ar | 1.0 | 67 | 62 | n.d. |
| 3 | Ar | 0.15 | 64 | 55 | 1.2, 0.44 |
| 4 | Ar | 0.10 | 40 | 35 | n.d. |
| 5 | Air | 0.15 | 81 | 76 | 1.3, 0.48 |
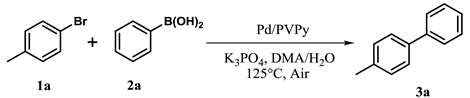
| Entry | (Het)Ar-X 1 | Ar’-B(OH)2 2 | Time (h) | Product 3 | Yield b (%) |
|---|---|---|---|---|---|
| 1 |  | 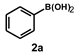 | 22 | 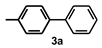 | 91 |
| 2 |  |  | 1.5 | 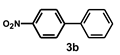 | 98 |
| 3 | 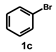 | 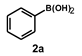 | 15 |  | 99 |
| 4 | 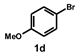 | 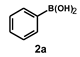 | 24 |  | 92 |
| 5 | 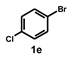 | 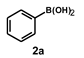 | 3.5 | 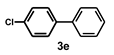 | 82 |
| 6 | 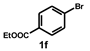 | 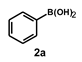 | 2.5 | 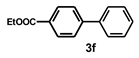 | 87 |
| 7 | 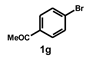 | 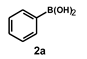 | 2.5 | 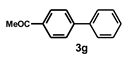 | 98 |
| 8 | 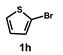 | 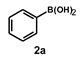 | 21 | 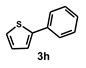 | 83 |
| 9 | 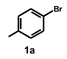 | 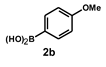 | 12 |  | 92 |
| 10 | 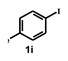 | 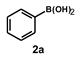 | 1.0 |  | 94 |
| 11 | 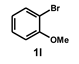 | 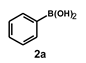 | 24 |  | 72 |
| 12 | 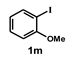 | 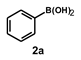 | 3.5 | 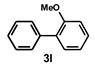 | 84 |
| 13 | 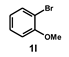 |  | 24 | 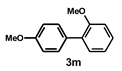 | 67 |
| 14 | 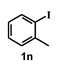 | 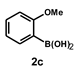 | 18 | 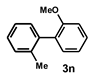 | 80 |
| 15 | 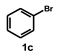 | 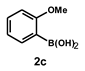 | 24 | 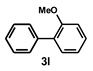 | 48 |
| 16 | 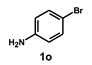 | 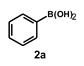 | 24 | 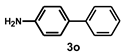 | 58 |
| 17 | 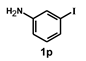 | 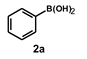 | 20 | 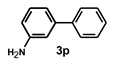 | 86 |
| 18 | 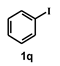 | 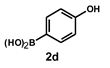 | 24 |  | 64 |
| 19 | 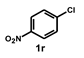 |  | 48 | 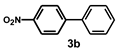 | 16 |
| Entry | Catalyst | Conversion b (%) | Selectivity b 3a/3b | Yield c (%) | Pd Leaching d (%, ppm) |
|---|---|---|---|---|---|
| 1 | Pd/PVPy | 81 | 99.9/0.1 | 76 | 1.3, 0.48 |
| 2 | Pd/C | 99 | 90/10 | 75 | 7.2, 2.67 |
| 3 | Pd EnCat® 40 | 97 | 98/2 | 62 | 2.4, 0.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusini, G.; Rizzo, F.; Angelici, G.; Pitzalis, E.; Evangelisti, C.; Carpita, A. Polyvinylpyridine-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki–Miyaura Coupling Reactions. Catalysts 2020, 10, 330. https://doi.org/10.3390/catal10030330
Fusini G, Rizzo F, Angelici G, Pitzalis E, Evangelisti C, Carpita A. Polyvinylpyridine-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki–Miyaura Coupling Reactions. Catalysts. 2020; 10(3):330. https://doi.org/10.3390/catal10030330
Chicago/Turabian StyleFusini, Graziano, Fabio Rizzo, Gaetano Angelici, Emanuela Pitzalis, Claudio Evangelisti, and Adriano Carpita. 2020. "Polyvinylpyridine-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki–Miyaura Coupling Reactions" Catalysts 10, no. 3: 330. https://doi.org/10.3390/catal10030330
APA StyleFusini, G., Rizzo, F., Angelici, G., Pitzalis, E., Evangelisti, C., & Carpita, A. (2020). Polyvinylpyridine-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki–Miyaura Coupling Reactions. Catalysts, 10(3), 330. https://doi.org/10.3390/catal10030330







