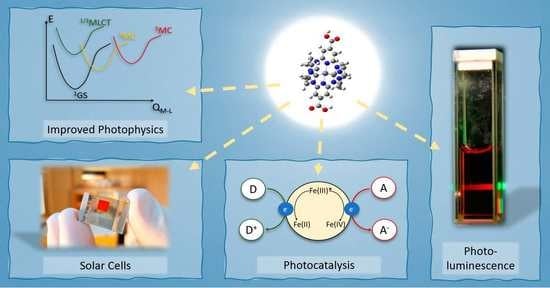
Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis
Abstract
:1. Introduction
2. Photophysics of FeNHC Complexes
3. Fe(II)NHC Complexes
3.1. Homoleptic FeII(CNC)2 Complexes
3.2. Heteroleptic FeII-Bistridentate Complexes with Extended Absorption
3.3. Heteroleptic FeII-Trisbidentate Complexes
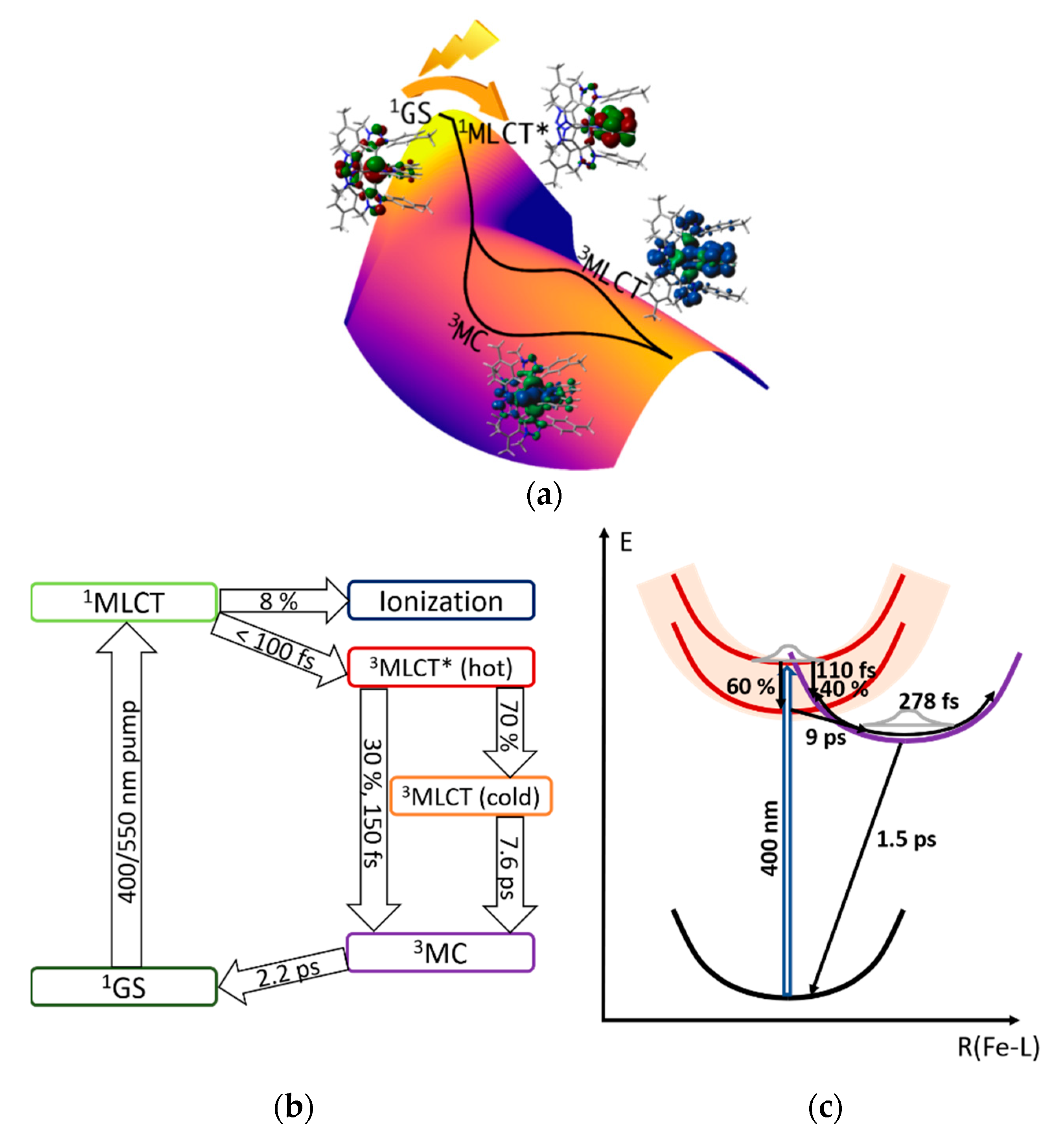
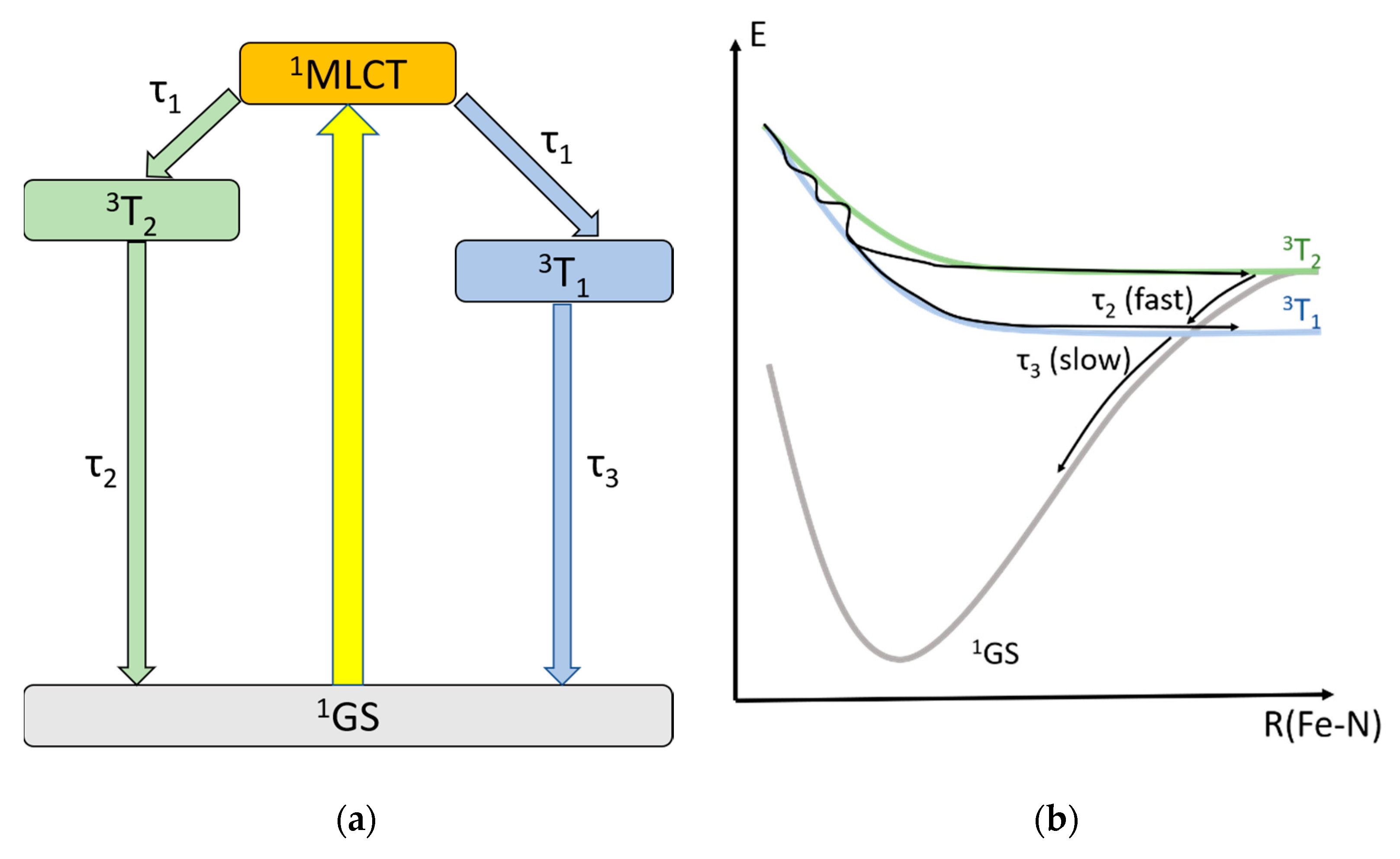
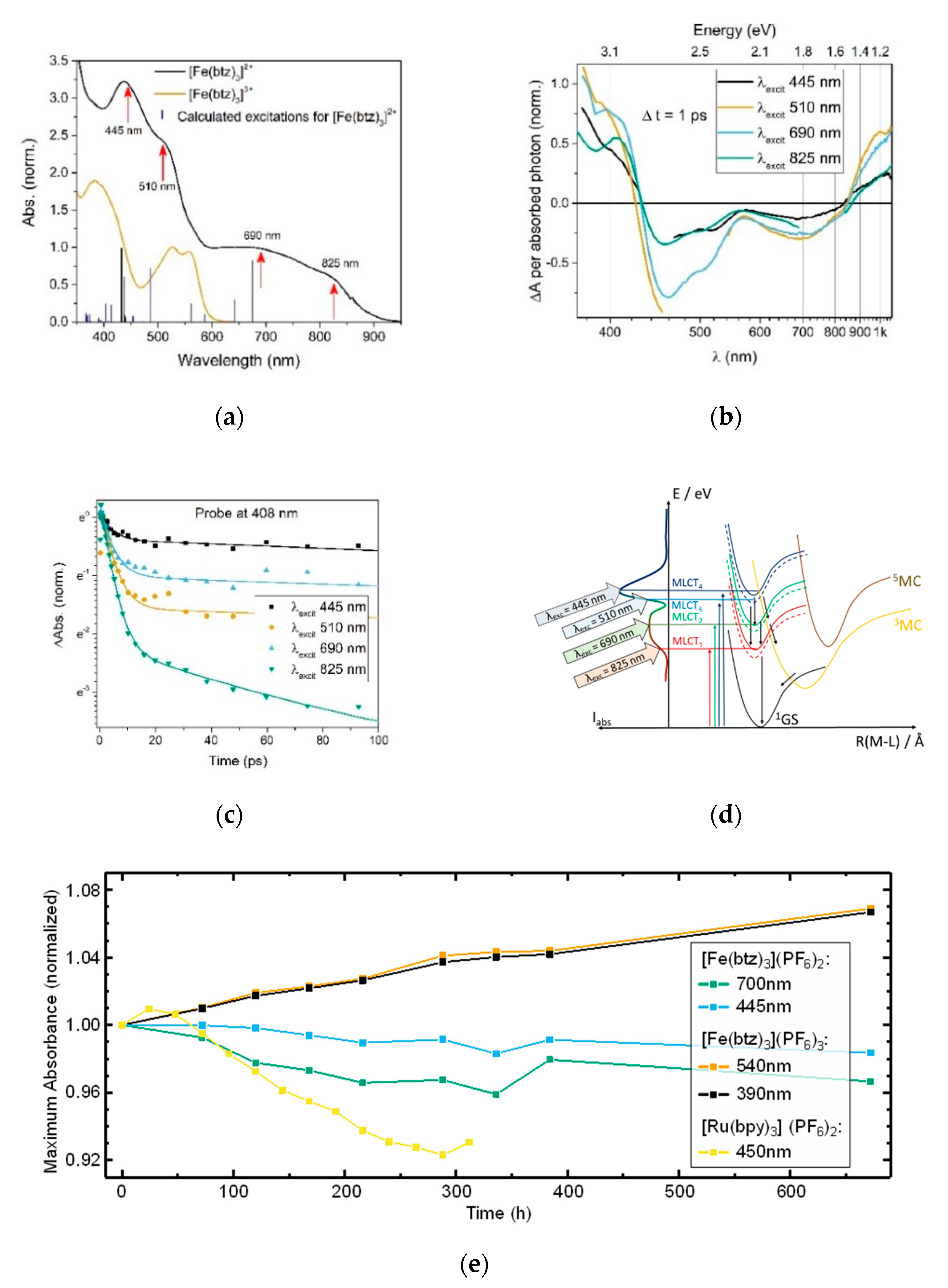
3.4. Hot Branching and Wave Packet Dynamics
3.5. Ligand Modifications
3.6. Coordination Arrangement- Isomerism and Carbene Count
3.7. Hexa-Carbene Complexes
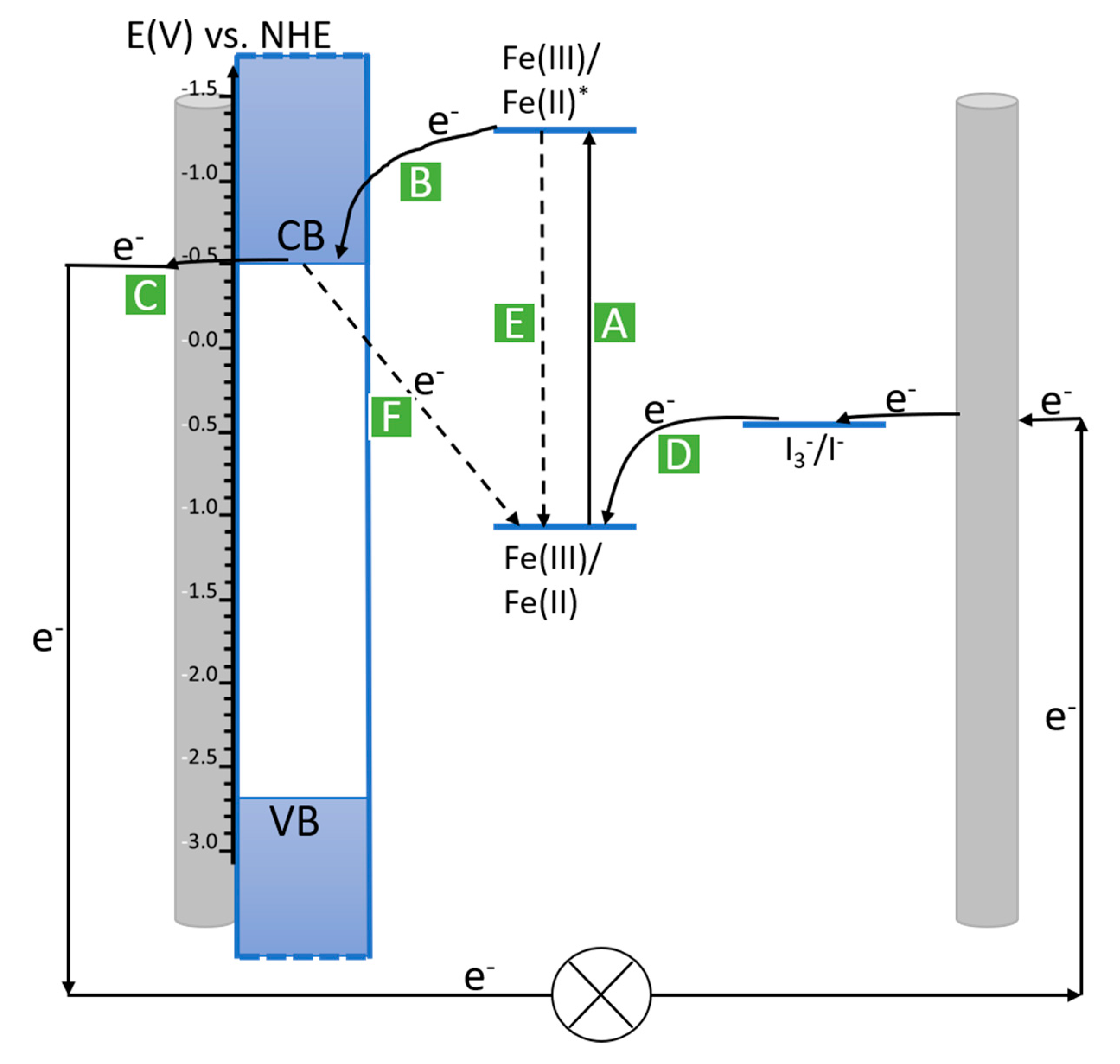
3.8. Molecular Photovoltaics
4. Fe(III) Complexes
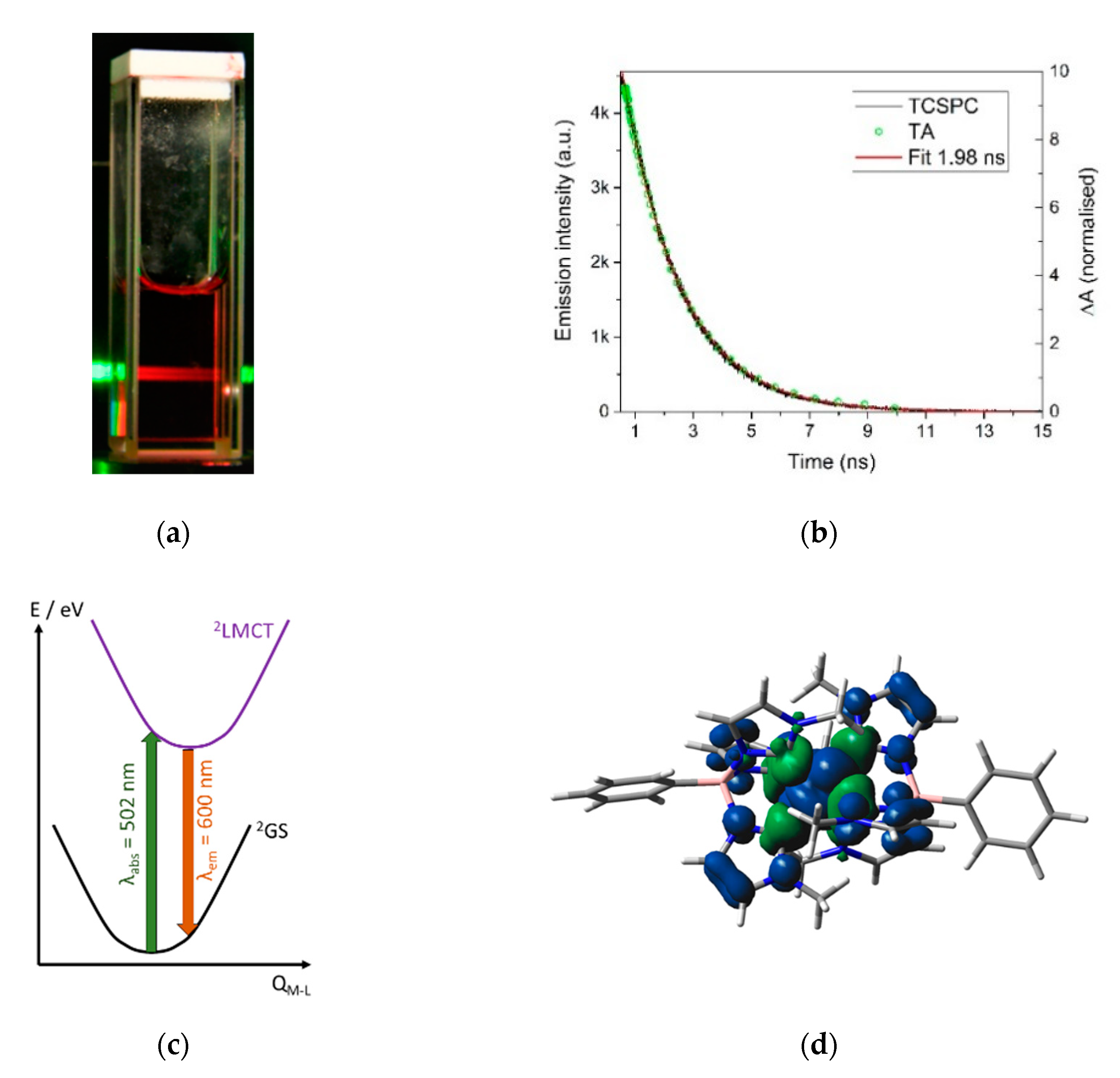
Photoluminescent Fe(III) Hexa-Carbene Complexes
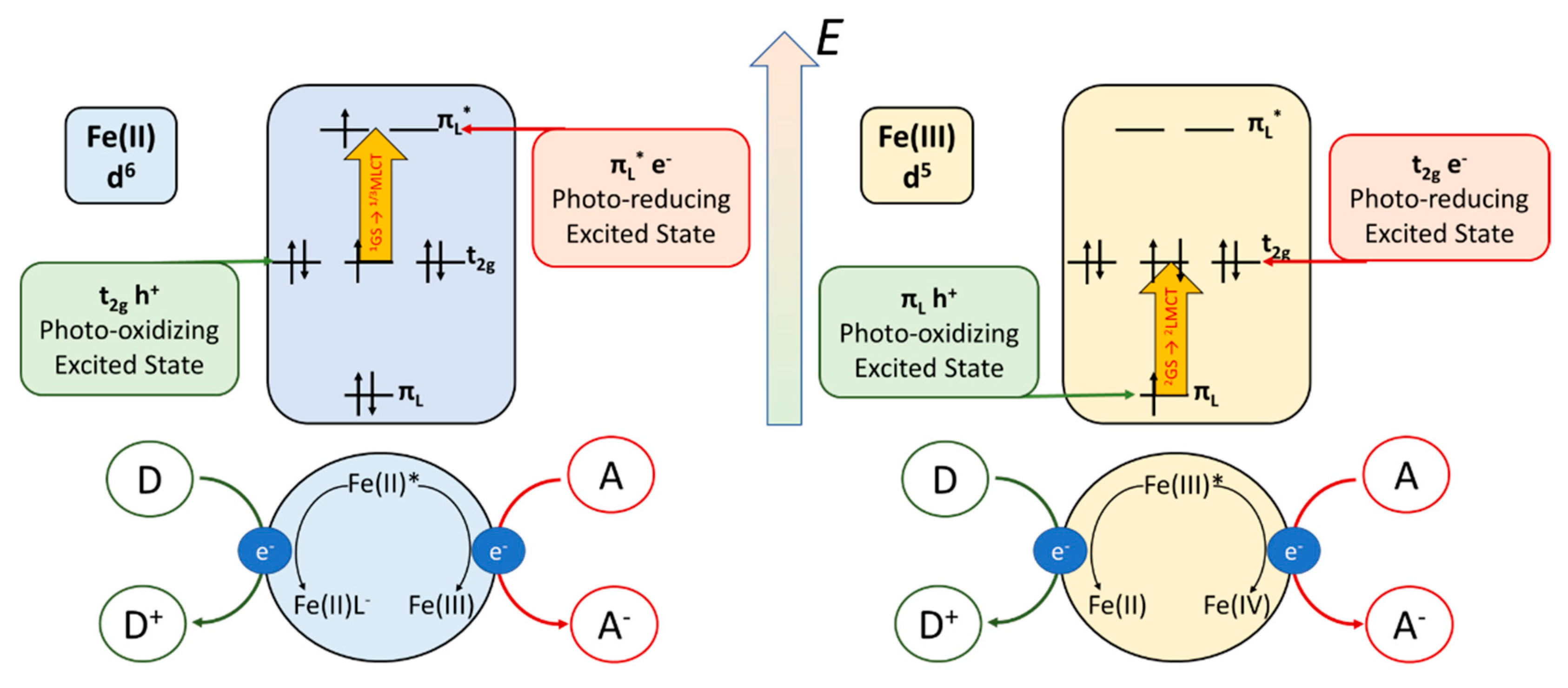
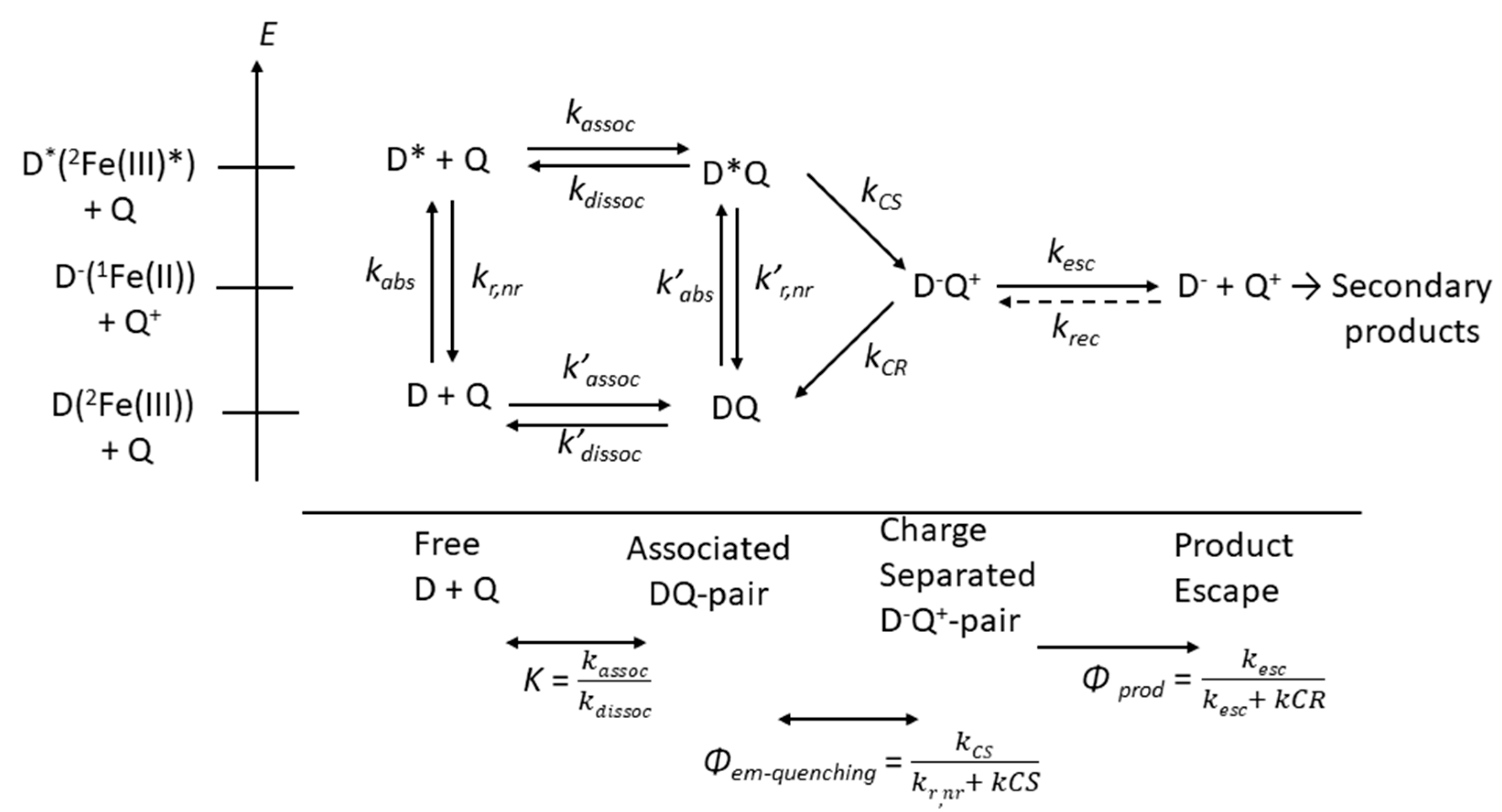
5. Photocatalysis
6. Outlook and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gray, H.B.; Maverick, A.W. Solar Chemistry of Metal Complexes. Science 1981, 214, 1201–1205. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Light harvesting with Earth abundant d-block metals: Development of sensitizers in dye-sensitized solar cells (DSCs). Coord. Chem. Rev. 2013, 257, 3089–3106. [Google Scholar] [CrossRef] [Green Version]
- Wenger, O.S. Photoactive Complexes with Earth-Abundant Metals. J. Am. Chem. Soc. 2018, 140, 13522–13533. [Google Scholar] [CrossRef] [Green Version]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef]
- Glaser, F.; Wenger, O.S. Recent progress in the development of transition-metal based photoredox catalysts. Coord. Chem. Rev. 2020, 405. [Google Scholar] [CrossRef]
- Förster, C.; Heinze, K. Photophysics and photochemistry with Earth-abundant metals – fundamentals and concepts. Chem. Soc. Rev. 2020, 49, 1057–1070. [Google Scholar] [CrossRef]
- Balzani, V.; Bergamini, G.; Campagna, S.; Puntoriero, F. Photochemistry and photophysics of coordination compounds: Overview and general concepts. Top. Curr. Chem. 2007, 280, 1–36. [Google Scholar] [CrossRef]
- Campagna, S.; Puntoriero, F.; Nastasi, F.; Bergamini, G.; Balzani, V. Photochemistry and photophysics of coordination compounds: Ruthenium. Top. Curr. Chem. 2007, 280, 117–214. [Google Scholar] [CrossRef]
- Liu, Y.; Persson, P.; Sundström, V.; Wärnmark, K. Fe N-Heterocyclic Carbene Complexes as Promising Photosensitizers. Acc. Chem. Res. 2016, 49, 1477–1485. [Google Scholar] [CrossRef]
- Abrahamsson, M. Solar energy conversion using iron polypyridyl type photosensitizers—A viable route for the future? In Photochemistry; Albini, A., Fasani, E., Eds.; RSC: London, UK, 2017; pp. 285–295. [Google Scholar] [CrossRef]
- Wenger, O.S. Is Iron the New Ruthenium? Chem. A Eur. J. 2019, 25, 6043–6052. [Google Scholar] [CrossRef] [Green Version]
- Motley, T.C.; Meyer, G.J. Are we on a path to solar cells that utilize iron? NPG Asia Mater. 2016, 8, e261. [Google Scholar] [CrossRef] [Green Version]
- Galoppini, E. Light harvesting: Strike while the iron is cold. Nat. Chem. 2015, 7, 861–862. [Google Scholar] [CrossRef]
- Boillot, M.L.; Weber, B. Mononuclear ferrous and ferric complexes. Comptes Rendus Chim. 2018, 21, 1196–1208. [Google Scholar] [CrossRef]
- McCusker, J.K. Electronic structure in the transition metal block and its implications for light harvesting. Science 2019, 363, 484–488. [Google Scholar] [CrossRef]
- Mccusker, J.K.; Vlcek, J. Antonin, Ultrafast Excited-State Processes in Inorganic Systems. Acc. Chem. Res. 2015, 48, 1207–1208. [Google Scholar] [CrossRef]
- Zhang, E.; Gaffney, K.J. Mechanistic studies of photoinduced spin crossover and electron transfer in inorganic complexes. Acc. Chem. Res. 2015, 48, 1140–1148. [Google Scholar] [CrossRef]
- Auböck, G.; Chergui, M. Sub-50-fs photoinduced spin crossover in [Fe(bpy)3]2+. Nat. Chem. 2015, 7, 629–633. [Google Scholar] [CrossRef]
- Zhang, W.; Alonso-Mori, R.; Bergmann, U.; Bressler, C.; Chollet, M.; Galler, A.; Gawelda, W.; Hadt, R.G.; Hartsock, R.W.; Kroll, T.; et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 2014, 509, 345–348. [Google Scholar] [CrossRef]
- Zhang, W.; Kjær, K.S.; Alonso-Mori, R.; Bergmann, U.; Chollet, M.; Fredin, L.A.; Hadt, R.G.; Hartsock, R.W.; Harlang, T.; Kroll, T.; et al. Manipulating charge transfer excited state relaxation and spin crossover in iron coordination complexes with ligand substitution. Chem. Sci. 2017, 8, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.; de Graaf, C.; Rudavskyi, A.; Broer, R.; Tatchen, J.; Etinski, M.; Marian, C.M. Ultrafast deactivation mechanism of the excited singlet in the light-induced spin crossover of [Fe(2,2-bipyridine)3]2+. Chem. A Eur. J. 2013, 19, 17541–17551. [Google Scholar] [CrossRef]
- Monat, J.E.; McCusker, J.K. Femtosecond Excited-State Dynamics of an Iron(II) Polypyridyl Solar Cell Sensitizer Model. J. Am. Chem. Soc. 2000, 122, 4092–4097. [Google Scholar] [CrossRef]
- Engel, N.; Bokarev, S.I.; Moguilevski, A.; Raheem, A.A.; Al-Obaidi, R.; Möhle, T.; Grell, G.; Siefermann, K.R.; Abel, B.; Aziz, S.G.; et al. Light-induced relaxation dynamics of the ferricyanide ion revisited by ultrafast XUV photoelectron spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 14248–14255. [Google Scholar] [CrossRef] [Green Version]
- Penfold, T.J.; Gindensperger, E.; Daniel, C.; Marian, C.M. Spin-Vibronic Mechanism for Intersystem Crossing. Chem. Rev. 2018, 118, 6975–7025. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.J.; Gray, H.B. Electronic Structures of Hexacyanometalate Complexes. J. Am. Chem. Soc. 1968, 90, 4260–4271. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 100th ed.; Rumble, J.R. (Ed.) CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019. [Google Scholar]
- Ponseca, C.S.; Chábera, P.; Uhlig, J.; Persson, P.; Sundström, V. Ultrafast Electron Dynamics in Solar Energy Conversion. Chem. Rev. 2017, 117, 10940–11024. [Google Scholar] [CrossRef]
- Chen, J.; Browne, W.R. Photochemistry of Iron Complexes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Vöhringer, P. Vibrations tell the tale. A time-resolved mid-infrared perspective of the photochemistry of iron complexes. Dalt. Trans. 2019, 49, 256–266. [Google Scholar] [CrossRef]
- Scattergood, P.A.; Sinopoli, A.; Elliott, P.I.P. Photophysics and photochemistry of 1,2,3-triazole-based complexes. Coord. Chem. Rev. 2017, 350, 136–154. [Google Scholar] [CrossRef]
- Duchanois, T.; Liu, L.; Pastore, M.; Monari, A.; Cebrián, C.; Trolez, Y.; Darari, M.; Magra, K.; Francés-Monerris, A.; Domenichini, E.; et al. NHC-Based Iron Sensitizers for DSSCs. Inorganics 2018, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Pastore, M.; Duchanois, T.; Liu, L.; Monari, A.; Assfeld, X.; Haacke, S.; Gros, P.C. Interfacial charge separation and photovoltaic efficiency in Fe(II)-carbene sensitized solar cells. Phys. Chem. Chem. Phys. 2016, 18, 28069–28081. [Google Scholar] [CrossRef] [Green Version]
- Chabera, P.; Lindh, L.; Rosemann, N.W.; Prakash, O.; Uhlig, J.; Yartsev, A.; Wärnmark, K.; Sundström, V.; Persson, P. Photofunctionality of Iron N-Heterocyclic Carbenes and Related d5 Complexes. Coord. Chem. Rev. 2020. submitted. [Google Scholar]
- Kaufhold, S.; Wärnmark, K. Design and synthesis of photoactive iron n-heterocyclic carbene complexes. Catalysts 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Hagfeldt, A.; Graetzel, M. Light-Induced Redox Reactions in Nanocrystalline Systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Meyer, G.J. Molecular approaches to solar energy conversion with coordination compounds anchored to semiconductor surfaces. Inorg. Chem. 2005, 44, 6852–6864. [Google Scholar] [CrossRef]
- Mccusker, J.K. Femtosecond Absorption Spectroscopy of Transition Metal Charge-Transfer Complexes. Acc. Chem. Res. 2003, 36, 876–887. [Google Scholar] [CrossRef]
- Reinhard, M.; Penfold, T.J.; Lima, F.A.; Rittmann, J.; Rittmann-Frank, M.H.; Abela, R.; Tavernelli, I.; Rothlisberger, U.; Milne, C.J.; Chergui, M. Photooxidation and photoaquation of iron hexacyanide in aqueous solution: A picosecond X-ray absorption study. Struct. Dyn. 2014, 1. [Google Scholar] [CrossRef]
- Chergui, M.; Collet, E. Photoinduced Structural Dynamics of Molecular Systems Mapped by Time-Resolved X-ray Methods. Chem. Rev. 2017, 117, 11025–11065. [Google Scholar] [CrossRef] [Green Version]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Decurtins, S.; Gutlich, P.; Hasselbach, K.M.; Hauser, A.; Spiering, H. Light-induced excited-spin-state trapping in iron(II) spin-crossover systems. Optical spectroscopic and magnetic susceptibility study. Inorg. Chem. 1985, 24, 2174–2178. [Google Scholar] [CrossRef]
- Cannizzo, A.; Milne, C.J.; Consani, C.; Gawelda, W.; Bressler, C.; van Mourik, F.; Chergui, M. Light-induced spin crossover in Fe(II)-based complexes: The full photocycle unraveled by ultrafast optical and X-ray spectroscopies. Coord. Chem. Rev. 2009, 254, 2677–2686. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Torres, D.E.; Jakubikova, E. HOMO inversion as a strategy for improving the light-absorption properties of Fe(II) chromophores. Chem. Sci. 2017, 8, 8115–8126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredin, L.A.; Pápai, M.; Rozsályi, E.; Vankó, G.; Wärnmark, K.; Sundström, V.; Persson, P. Exceptional Excited State Lifetime of an Iron (II) N-heterocyclic Carbene Complex Explained. J. Phys. Chem. Lett. 2014, 5, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Dixon, I.M.; Alary, F.; Boggio-Pasqua, M.; Heully, J.L. The (N4C2)2- donor set as promising motif for bis(tridentate) iron(II) photoactive compounds. Inorg. Chem. 2013, 52, 13369–13374. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.N.; Bondarev, A.; Mukherjee, S.; Jakubikova, E. Tuning the Electronic Structure of Fe(II) Polypyridines via Donor Atom and Ligand Scaffold Modifications: A Computational Study. Inorg. Chem. 2015, 54, 8786–8793. [Google Scholar] [CrossRef] [PubMed]
- Nance, J.; Bowman, D.N.; Mukherjee, S.; Kelley, C.T.; Jakubikova, E. Insights into the Spin-State Transitions in [Fe(tpy)2]2+: Importance of the Terpyridine Rocking Motion. Inorg. Chem. 2015, 54, 11259–11268. [Google Scholar] [CrossRef]
- Canton, S.E.; Zhang, X.; Daku, M.L.; Smeigh, A.L.; Zhang, J.; Liu, Y.; Wallentin, C.-J.; Attenkofer, K.; Jennings, G.; Kurtz, C.A.; et al. Probing the Anisotropic Distortion of Photoexcited Spin Crossover Complexes with Picosecond X-ray Absorption Spectroscopy. J. Phys. Chem. C 2014, 118, 4536–4545. [Google Scholar] [CrossRef]
- Leshchev, D.; Harlang, T.C.B.; Fredin, L.A.; Khakhulin, D.; Liu, Y.; Biasin, E.; Laursen, M.G.; Newby, G.E.; Haldrup, K.; Nielsen, M.M.; et al. Tracking the picosecond deactivation dynamics of a photoexcited iron carbene complex by time-resolved X-ray scattering. Chem. Sci. 2018, 9, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Ashley, D.C.; Jakubikova, E. Ironing out the photochemical and spin-crossover behavior of Fe(II) coordination compounds with computational chemistry. Coord. Chem. Rev. 2017, 337, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Ingleson, M.J.; Layfield, R.A. N-Heterocyclic carbene chemistry of iron: Fundamentals and applications. Chem. Commun. 2012, 48, 3579–3589. [Google Scholar] [CrossRef]
- Chábera, P.; Liu, Y.; Prakash, O.; Thyrhaug, E.; el Nahhas, A.; Honarfar, A.; Essén, S.; Fredin, L.A.; Harlang, T.C.B.; Kjær, K.S.; et al. A low-spin Fe(III) complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 2017, 543, 695–699. [Google Scholar] [CrossRef]
- Kjær, K.S.; Kaul, N.; Prakash, O.; Chábera, P.; Rosemann, N.W.; Honarfar, A.; Gordivska, O.; Fredin, L.A.; Bergquist, K.-E.E.; Häggström, L.; et al. Luminescence and reactivity of a charge-transfer excited iron complex with nanosecond lifetime. Science 2019, 363, 249–253. [Google Scholar] [CrossRef]
- Liu, Y.; Harlang, T.; Canton, S.E.; Chábera, P.; Suárez-Alcántara, K.; Fleckhaus, A.; Vithanage, D.A.; Göransson, E.; Corani, A.; Lomoth, R.; et al. Towards longer-lived metal-to-ligand charge transfer states of iron(II) complexes: An N-heterocyclic carbene approach. Chem. Commun. 2013, 49, 6412–6414. [Google Scholar] [CrossRef] [Green Version]
- Pavlishchuk, V.V.; Addison, A.W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorganica Chim. Acta. 2000, 298, 97–102. Available online: www.elsevier.nl/locate/ica (accessed on 31 July 2017).
- Zimmer, P.; Burkhardt, L.; Schepper, R.; Zheng, K.; Gosztola, D.; Neuba, A.; Flörke, U.; Wölper, C.; Schoch, R.; Gawelda, W.; et al. Towards Noble-Metal-Free Dyads: Ground and Excited State Tuning by a Cobalt Dimethylglyoxime Motif Connected to an Iron N-Heterocyclic Carbene Photosensitizer. Eur. J. Inorg. Chem. 2018, 2018, 5203–5214. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, P.; Müller, P.; Burkhardt, L.; Schepper, R.; Neuba, A.; Steube, J.; Dietrich, F.; Flörke, U.; Mangold, S.; Gerhards, M.; et al. N-Heterocyclic Carbene Complexes of Iron as Photosensitizers for Light-Induced Water Reduction. Eur. J. Inorg. Chem. 2017, 2017, 1504–1509. [Google Scholar] [CrossRef]
- Duchanois, T.; Etienne, T.; Beley, M.; Assfeld, X.; Perpète, E.A.; Monari, A.; Gros, P.C. Heteroleptic pyridyl-carbene iron complexes with tuneable electronic properties. Eur. J. Inorg. Chem. 2014, 2014, 3747–3753. [Google Scholar] [CrossRef]
- Duchanois, T.; Etienne, T.; Cebrián, C.; Liu, L.; Monari, A.; Beley, M.; Assfeld, X.; Haacke, S.; Gros, P.C. An iron-based photosensitizer with extended excited-state lifetime: Photophysical and photovoltaic properties. Eur. J. Inorg. Chem. 2015, 2015, 2469–2477. [Google Scholar] [CrossRef]
- Zimmer, P.; Burkhardt, L.; Friedrich, A.; Steube, J.; Neuba, A.; Schepper, R.; Müller, P.; Flörke, U.; Huber, M.; Lochbrunner, S.; et al. The Connection between NHC Ligand Count and Photophysical Properties in Fe(II) Photosensitizers: An Experimental Study. Inorg. Chem. 2018, 57, 360–373. [Google Scholar] [CrossRef]
- Liu, Y.; Kjaer, K.S.; Fredin, L.A.; Chabera, P.; Harlang, T.; Canton, S.E.; Lidin, S.; Zhang, J.; Lomoth, R.; Bergquist, K.E.; et al. A heteroleptic ferrous complex with mesoionic bis(1,2,3-triazol-5-ylidene) ligands: Taming the MLCT excited state of iron(II). Chem. A Eur. J. 2015, 21, 3628–3639. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Duchanois, T.; Etienne, T.; Monari, A.; Beley, M.; Assfeld, X.; Haacke, S.; Gros, P.C. A new record excited state 3MLCT lifetime for metalorganic iron(II) complexes. Phys. Chem. Chem. Phys. 2016, 18, 12550–12556. [Google Scholar] [CrossRef]
- Darari, M.; Domenichini, E.; Francés-Monerris, A.; Cebrián, C.; Magra, K.; Beley, M.; Pastore, M.; Monari, A.; Assfeld, X.; Haacke, S.; et al. Iron(ii) complexes with diazinyl-NHC ligands: Impact of π-deficiency of the azine core on photophysical properties. Dalt. Trans. 2019, 48, 10915–10926. [Google Scholar] [CrossRef]
- Francés-Monerris, A.; Magra, K.; Darari, M.; Cebrián, C.; Beley, M.; Domenichini, E.; Haacke, S.; Pastore, M.; Assfeld, X.; Gros, P.C.; et al. Synthesis and Computational Study of a Pyridylcarbene Fe(II) Complex: Unexpected Effects of fac/ mer Isomerism in Metal-to-Ligand Triplet Potential Energy Surfaces. Inorg. Chem. 2018, 57, 10431–10441. [Google Scholar] [CrossRef]
- Magra, K.; Domenichini, E.; Francés-Monerris, A.; Cebrián, C.; Beley, M.; Darari, M.; Pastore, M.; Monari, A.; Assfeld, X.; Haacke, S.; et al. Impact of the fac/ mer Isomerism on the Excited-State Dynamics of Pyridyl-carbene Fe(II) Complexes. Inorg. Chem. 2019, 58, 5069–5081. [Google Scholar] [CrossRef] [Green Version]
- Chábera, P.; Kjaer, K.S.; Prakash, O.; Honarfar, A.; Liu, Y.; Fredin, L.A.; Harlang, T.C.B.; Lidin, S.; Uhlig, J.; Sundström, V.; et al. FeII Hexa N-Heterocyclic Carbene Complex with a 528 ps Metal-To-Ligand Charge-Transfer Excited-State Lifetime. J. Phys. Chem. Lett. 2018, 9, 459–463. [Google Scholar] [CrossRef]
- Tatsuno, H.; Kjær, K.S.; Kunnus, K.; Harlang, T.C.B.; Timm, C.; Guo, M.; Chàbera, P.; Fredin, L.A.; Hartsock, R.W.; Reinhard, M.E.; et al. Hot Branching Dynamics in a Light-Harvesting Iron Carbene Complex Revealed by Ultrafast X-ray Emission Spectroscopy. Angew. Chem. 2020, 132, 372–380. [Google Scholar] [CrossRef]
- Kunnus, K.; Vacher, M.; Harlang, T.C.B.; Kjær, K.S.; Haldrup, K.; Biasin, E.; van Driel, T.B.; Pápa, M.; Chabera, P.; Liu, Y.; et al. Origin of vibrational wavepacket dynamics in Fe carbene photosensitizer determined with femtosecond X-ray emission and scattering. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pápai, M.; Penfold, T.J.; Møller, K.B. Effect of tert-Butyl Functionalization on the Photoexcited Decay of a Fe(II)-N-Heterocyclic Carbene Complex. J. Phys. Chem. C 2016, 120, 17234–17241. [Google Scholar] [CrossRef] [Green Version]
- Penfold, T.J.; Pápai, M.; Rozgonyi, T.; Møller, K.B.; Vankó, G. Probing spin-vibronic dynamics using femtosecond X-ray spectroscopy. In Faraday Discuss; The Royal Society of Chemistry: London, UK, 2016; pp. 731–746. [Google Scholar] [CrossRef] [Green Version]
- Pápai, M.; Vankó, G.; Rozgonyi, T.; Penfold, T.J. High-Efficiency Iron Photosensitizer Explained with Quantum Wavepacket Dynamics. J. Phys. Chem. Lett. 2016, 7, 2009–2014. [Google Scholar] [CrossRef] [Green Version]
- Pápai, M.; Simmermacher, M.; Penfold, T.J.; Møller, K.B.; Rozgonyi, T. How to Excite Nuclear Wavepackets into Electronically Degenerate States in Spin-Vibronic Quantum Dynamics Simulations. J. Chem. Theory Comput. 2018, 14, 3967–3974. [Google Scholar] [CrossRef]
- Pápai, M.; Abedi, M.; Levi, G.; Biasin, E.; Nielsen, M.M.; Møller, K.B. Theoretical Evidence of Solvent-Mediated Excited-State Dynamics in a Functionalized Iron Sensitizer. J. Phys. Chem. C 2019, 123, 2056–2065. [Google Scholar] [CrossRef] [Green Version]
- Pápai, M.; Rozgonyi, T.; Penfold, T.J.; Nielsen, M.M.; Møller, K.B. Simulation of ultrafast excited-state dynamics and elastic x-ray scattering by quantum wavepacket dynamics. J. Chem. Phys. 2019, 151, 104307. [Google Scholar] [CrossRef]
- Canton, S.E.; Zhang, X.; Daku, M.L.L.; Liu, Y.; Zhang, J.; Alvarez, S. Mapping the ultrafast changes of continuous shape measures in photoexcited spin crossover complexes without long-range order. J. Phys. Chem. C 2015, 119, 3322–3330. [Google Scholar] [CrossRef]
- Chábera, P.; Fredin, L.A.; Kjær, K.S.; Rosemann, N.W.; Lindh, L.; Prakash, O.; Liu, Y.; Wärnmark, K.; Uhlig, J.; Sundström, V.; et al. Band-selective dynamics in charge-transfer excited iron carbene complexes. Faraday Discuss. 2019, 216, 191–210. [Google Scholar] [CrossRef] [Green Version]
- Hagfeldt, A.; Grätzel, M. Molecular photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Ardo, S.; Meyer, G.J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 2009, 38, 115–164. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Galoppini, E. Linkers for anchoring sensitizers to semiconductor nanoparticles. Coord. Chem. Rev. 2004, 248, 1283–1297. [Google Scholar] [CrossRef]
- Ferrere, S.; Gregg, B.A. Photosensitization of TiO 2 by [Fe II (2,2′-bipyridine-4,4′-dicarboxylic acid) 2 (CN)2]: Band Selective Electron Injection from Ultra-Short-Lived Excited States. 1993. Available online: https://pubs.acs.org/sharingguidelines (accessed on 17 January 2019).
- Ferrere, S. New photosensitizers based upon [FeII(L)2(CN)2] and [FeIIL3], where L is substituted 2,2′-bipyridine. Inorg. Chim. Acta 2002, 329, 79–92. [Google Scholar] [CrossRef]
- Yang, M.; Thompson, D.W.; Meyer, G.J. Dual Pathways for TiO2 Sensitization by Na2[Fe(bpy)(CN)4]. Inorg. Chem. 2000, 39, 3738–3739. [Google Scholar] [CrossRef]
- Yang, M.; Thompson, D.W.; Meyer, G.J. Charge-transfer studies of iron cyano compounds bound to nanocrystalline TiO2 surfaces. Inorg. Chem. 2002, 41, 1254–1262. [Google Scholar] [CrossRef]
- Harlang, T.C.B.; Liu, Y.; Gordivska, O.; Fredin, L.A.; Ponseca, C.S.; Huang, P.; Chábera, P.; Kjaer, K.S.; Mateos, H.; Uhlig, J.; et al. Iron sensitizer converts light to electrons with 92% yield. Nat. Chem. 2015, 7, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Fredin, L.A.; Wärnmark, K.; Sundström, V.; Persson, P. Molecular and Interfacial Calculations of Iron(II) Light Harvesters. ChemSusChem 2016, 9, 667–675. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liu, C.; Jakubikova, E. Comparison of interfacial electron transfer efficiency in [Fe(ctpy)2]2+-TiO2 and [Fe(cCNC)2]2+-TiO2 Assemblies: Importance of conformational sampling. J. Phys. Chem. A 2018, 122, 1821–1830. [Google Scholar] [CrossRef]
- Bowman, D.N.; Mukherjee, S.; Barnes, L.J.; Jakubikova, E. Linker dependence of interfacial electron transfer rates in Fe(II)-polypyridine sensitized solar cells. J. Phys. Condens. Matter. 2015, 27, 134205. [Google Scholar] [CrossRef]
- Jakubikova, E.; Bowman, D.N. Fe(II)-Polypyridines as Chromophores in Dye-Sensitized Solar Cells: A Computational Perspective. Acc. Chem. Res. 2015, 48, 1441–1449. [Google Scholar] [CrossRef]
- Karpacheva, M.; Housecroft, C.E.; Constable, E.C. Electrolyte tuning in dye-sensitized solar cells with N-heterocyclic carbene (NHC) iron(II) sensitizers. Beilstein J. Nanotechnol. 2018, 9, 3069–3078. [Google Scholar] [CrossRef] [Green Version]
- Karpacheva, M.; Wyss, V.; Housecroft, C.E.; Constable, E.C. There Is a Future for N-Heterocyclic Carbene Iron(II) Dyes in Dye-Sensitized Solar Cells: Improving Performance through Changes in the Electrolyte. Materials 2019, 12, 4181. [Google Scholar] [CrossRef] [Green Version]
- Marchini, E.; Darari, M.; Lazzarin, L.; Boaretto, R.; Argazzi, R.; Bignozzi, C.A.; Gros, P.C.; Caramori, S. Recombination and regeneration dynamics in FeNHC(II)-sensitized solar cells. Chem. Commun. 2020, 56, 543–546. [Google Scholar] [CrossRef]
- Vogler, A.; Kunkely, H. Charge transfer excitation of organometallic compounds. Coord. Chem. Rev. 2004, 248, 273–278. [Google Scholar] [CrossRef]
- Fränkel, R.; Kernbach, U.; Bakola-Christianopoulou, M.; Plaia, U.; Suter, M.; Ponikwar, W.; Nöth, H.; Moinet, C.; Fehlhammer, W.P. Homoleptic carbene complexes: Part VIII. Hexacarbene complexes. J. Organomet. Chem. 2001. [Google Scholar] [CrossRef]
- Ericson, F.; Honarfar, A.; Prakash, O.; Tatsuno, H.; Fredin, L.A.; Handrup, K.; Chabera, P.; Gordivska, O.; Kjær, K.S.; Liu, Y.; et al. Electronic structure and excited state properties of iron carbene photosensitizers – A combined X-ray absorption and quantum chemical investigation. Chem. Phys. Lett. 2017, 683, 559–566. [Google Scholar] [CrossRef]
- Temperton, R.H.; Rosemann, N.W.; Guo, M.; Johansson, N.; Fredin, L.A.; Prakash, O.; Wärnmark, K.; Handrup, K.; Uhlig, J.; Schnadt, J.; et al. Site-Selective Orbital Interactions in an Ultrathin Iron Carbene Photosensitizer Film. J. Phys. Chem. A 2020, 124, 1603–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, B.M.; Gray, H.B.; Müller, A.M. Earth-Abundant Heterogeneous Water Oxidation Catalysts. Chem. Rev. 2016, 116, 14120–14136. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Cao, Z.C.; Shi, Z.J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Crabtree, R.H.; Brudvig, G.W. Molecular Catalysts for Water Oxidation. Chem. Rev. 2015, 115, 12974–13005. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [Green Version]
- Nocera, D.G. The artificial leaf. Acc. Chem. Res. 2012, 45, 767–776. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Verho, O.; Johnston, E.V.; Åkermark, B. Artificial photosynthesis: Molecular systems for catalytic water oxidation. Chem. Rev. 2014, 114, 11863–12001. [Google Scholar] [CrossRef]
- Magnusson, A.; Anderlund, M.; Johansson, O.; Lindblad, P.; Lomoth, R.; Polivka, T.; Ott, S.; Stensjo, K.; Styring, S.; Sundstrom, V.; et al. Biomimetic and Microbial Approaches to Solar Fuel Generation. Acc. Chem. Res. 2009, 42, 1899. [Google Scholar] [CrossRef]
- Chen, J.; Browne, W.R. Photochemistry of iron complexes. Coord. Chem. Rev. 2018, 374, 15–35. [Google Scholar] [CrossRef]
- Danforth, R.A.; Kohler, B. Ultrafast photochemical dynamics of the hexaaquairon(III) ion. Chem. Phys. Lett. 2017, 683, 315–321. [Google Scholar] [CrossRef]
- Päpcke, A.; Friedrich, A.; Lochbrunner, S. Revealing the initial steps in homogeneous photocatalysis by time-resolved spectroscopy. J. Phys. Condens. Matter. 2020, 32, 153001. [Google Scholar] [CrossRef]
- Smith, J.M. Strongly donating scorpionate ligands. Comments Inorg. Chem. 2008, 29, 189–233. [Google Scholar] [CrossRef]
- Baslon, V.; Harris, J.P.; Reber, C.; Colmer, H.E.; Jackson, T.A.; Forshaw, A.P.; Smith, J.M.; Kinney, R.A.; Telser, J. Near-infrared 2Eg → 4A2g and visible LMCT luminescence from a molecular bis -(tris(carbene)borate) manganese(IV) complex. Can. J. Chem. 2017, 95, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Young, E.R.; Oldacre, A. Iron hits the mark. Science 2019, 363, 225–226. [Google Scholar] [CrossRef]
- Lee, Y.F.; Kirchhoff, J.R. Absorption and Luminescence Spectroelectrochemical Characterization of a Highly Luminescent Rhenium(I1) Complex. J. Am. Chem. Soc. 1994, 116, 3599–3600. [Google Scholar] [CrossRef]
- Adams, J.J.; Arulsamy, N.; Sullivan, B.P.; Roddick, D.M.; Neuberger, A.; Schmehl, R.H. Homoleptic Tris-Diphosphine Re(I) and Re(II) Complexes and Re(II) Photophysics and Photochemistry. Inorg. Chem. 2015, 54, 11136–11149. [Google Scholar] [CrossRef]
- Del Negro, A.S.; Seliskar, C.J.; Heineman, W.R.; Hightower, S.E.; Bryan, S.A.; Sullivan, B.P. Highly oxidizing excited states of Re and Tc complexes. J. Am. Chem. Soc. 2006, 128, 16494–16495. [Google Scholar] [CrossRef]
- Francés-Monerris, A.; Gros, P.C.; Pastore, M.; Assfeld, X.; Monari, A. Photophysical properties of bichromophoric Fe(II) complexes bearing an aromatic electron acceptor. Theor. Chem. Acc. 2019, 138, 86. [Google Scholar] [CrossRef]
- Vogler, A.; Kunkely, H. Luminescent Metal Complexes: Diversity of Excited States; Yersin, H., Ed.; Top. Curr. Chem. 2013—Transition Metal and Rare Earth Compounds; Springer: Berlin/Heidelberg, Germany, 2001; pp. 143–182. [Google Scholar]
- Braun, J.D.; Lozada, I.B.; Kolodziej, C.; Burda, C.; Newman, K.M.E.; van Lierop, J.; Davis, R.L.; Herbert, D.E. Iron(ii) coordination complexes with panchromatic absorption and nanosecond charge-transfer excited state lifetimes. Nat. Chem. 2019, 11, 1144–1150. [Google Scholar] [CrossRef]
- Steube, J.; Burkhardt, L.; Päpcke, A.; Moll, J.; Zimmer, P.; Schoch, R.; Wölper, C.; Heinze, K.; Lochbrunner, S.; Bauer, M. Excited-State Kinetics of an Air-Stable Cyclometalated Iron(II) Complex. Chem. A Eur. J. 2019, 25, 11826–11830. [Google Scholar] [CrossRef]
- Hockin, B.M.; Li, C.; Robertson, N.; Zysman-Colman, E. Photoredox catalysts based on earth-abundant metal complexes. Catal. Sci. Technol. 2019, 9, 889–915. [Google Scholar] [CrossRef] [Green Version]
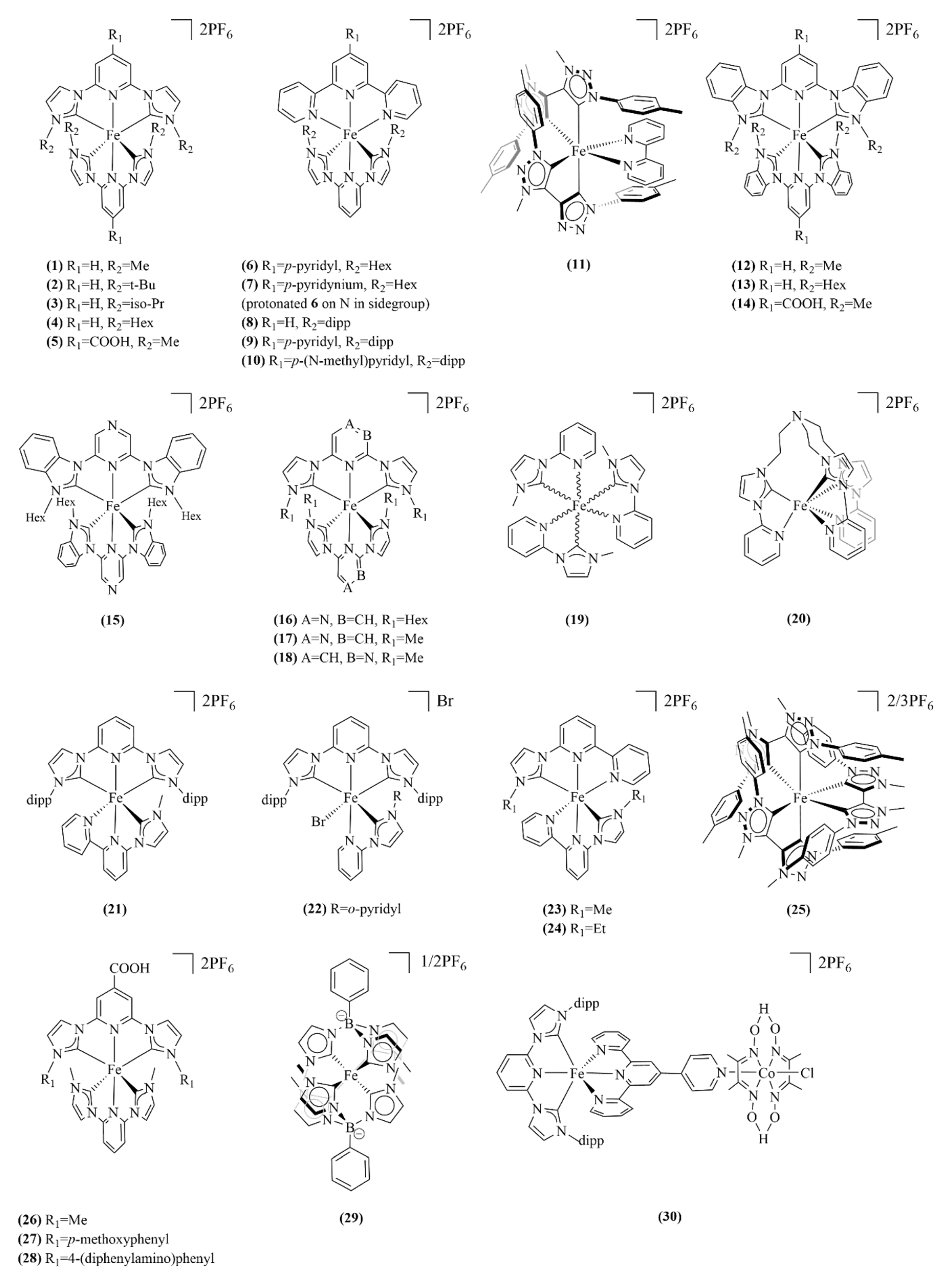
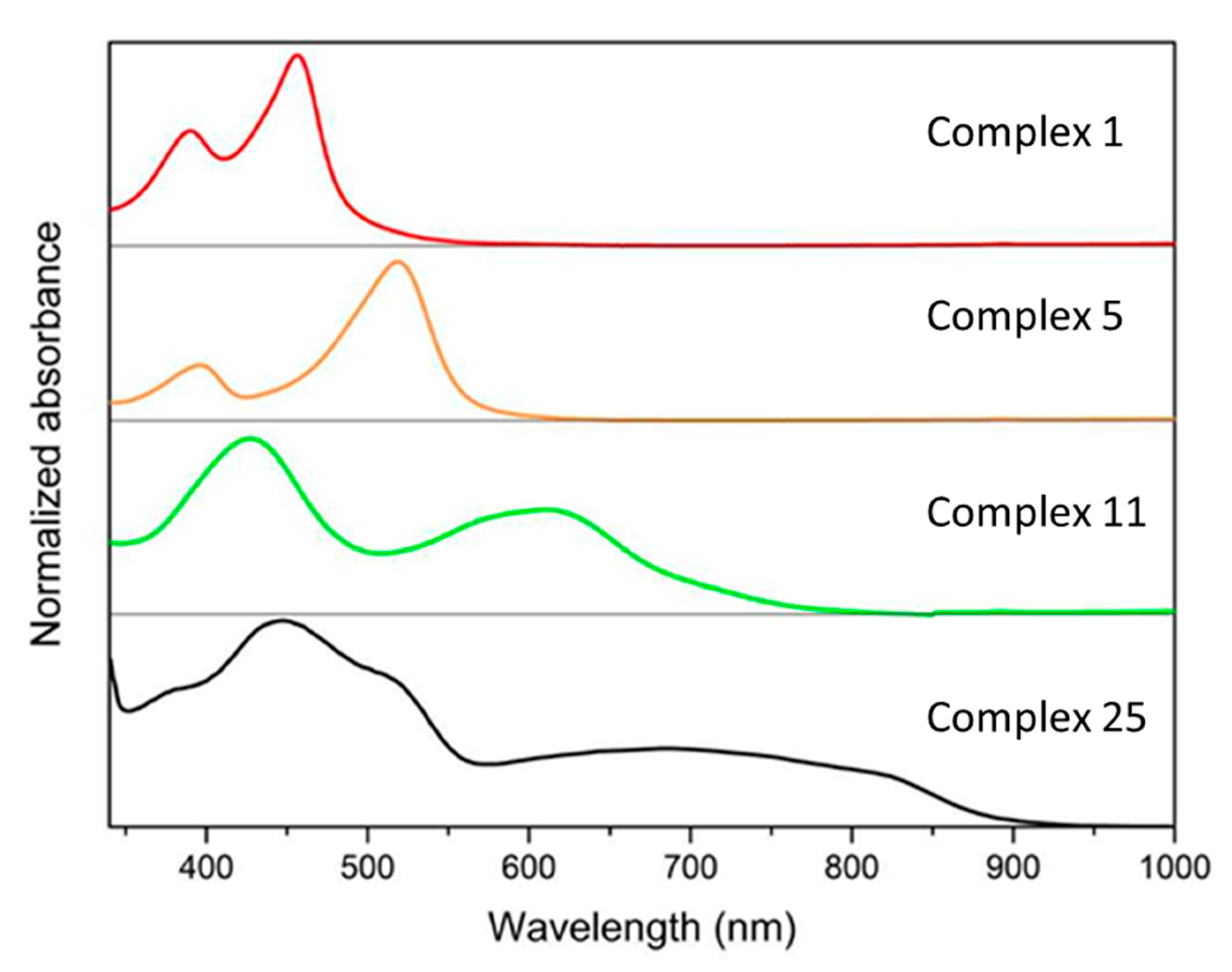


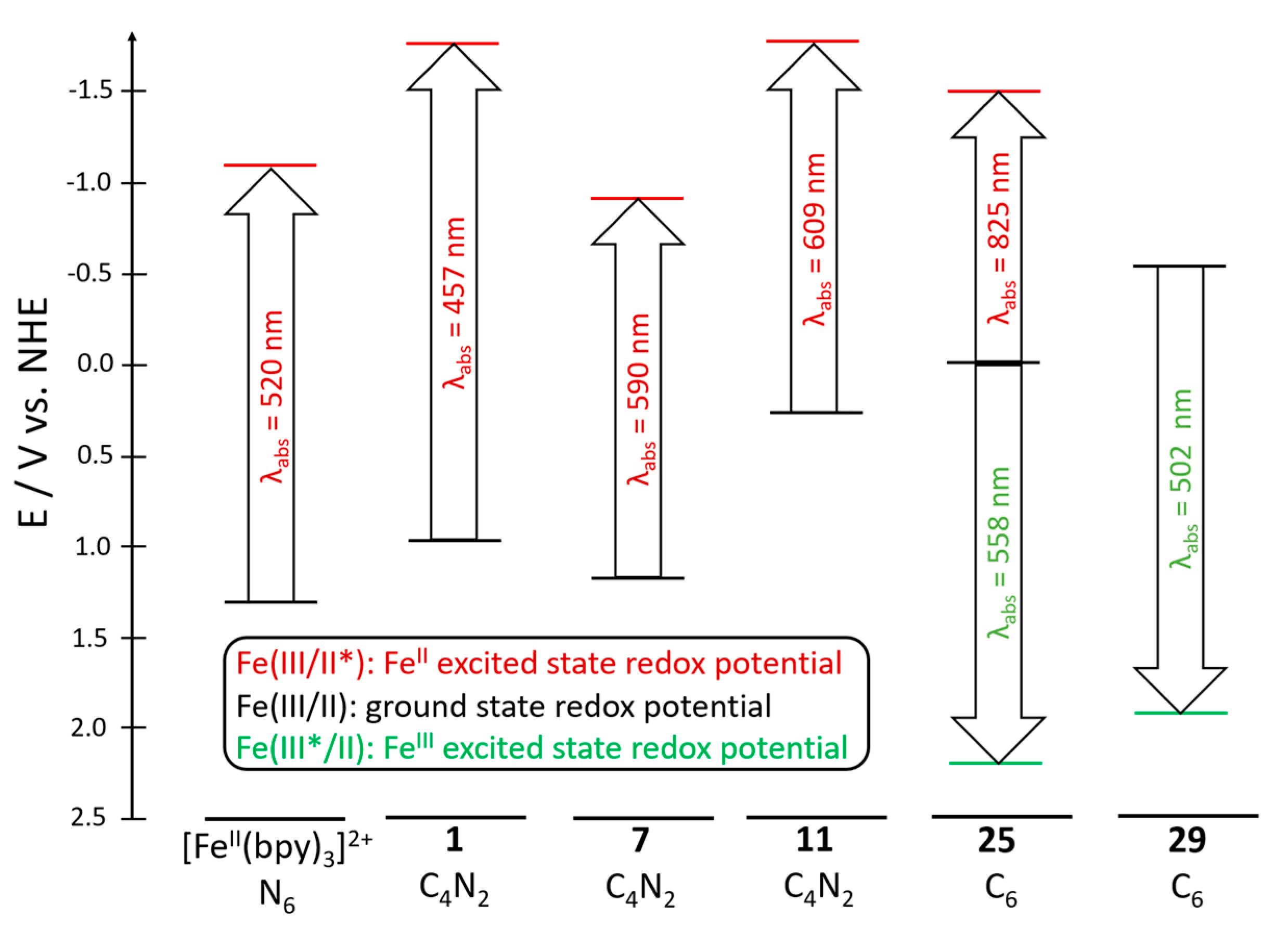

| Complex | λabsmax a /nm | ε /M−1cm−1 | τMCLT/ps | E1/2 (FeIII/II) /V vs. NHE | E1/2 (L-/0) b /V vs. NHE | Ref |
|---|---|---|---|---|---|---|
| 1 | 457 | 15200 | 9 | 0.94 | −1.76 | [54] |
| 2 | 478 | 14800 | 0.3 | 1.03 | −1.72 | [54] |
| 3 | 458 c | 14500 c | 8.1 | 1.06 | −1.68 d | [56,57] |
| 4 | 460 | 15900 | 12 | 1.04 | −1.71 d | [31,58] |
| 5 | 520 | 16200 | 16.5 | 1.09 | −1.11 d | [59] |
| 6 | 522 (560) | 9500 | 2.7 e | 1.17 | −1.18 | [31,58] |
| 7 | 590 | 11500 | - | 1.19 | −1.02 | [58] |
| 8 | 503 (538) ((630)) c | 9800 c | < 0.1 | 1.19 | −1.26 | [57,60] |
| 9 | 515 (553) ((630)) | 10000 | 1.1 | 1.23 | −1.13 | [56] |
| 10 | 586 | 12700 | - | 1.29 | −0.56 | [56] |
| 11 | 432 (609) ((720)) | 5100 | 13 | 0.28 | −1.65 d | [61] |
| 12 | 440 | 12500 | 16.4 | 1.28 | −1.56 d | [62] |
| 13 | 445 | - | 19.5 | - | - | [31] |
| 14 | 501 | 12800 | 26 | 1.37 | −0.99 d | [62] |
| 15 | 479 | 16200 | 32 f | 1.46 | −0.97 | [63] |
| 16 | 493 | 17300 | 22 f | 1.33 | −1.08 | [63] |
| 17 | 487 | 16100 | 23 f | 1.22 | −1.15 | [63] |
| 18 | 477 | 12600 | 12 f | 1.17 | −1.46 d | [63] |
| 19 | 430 | 12000 | g | 0.89 | −1.73 d | [64,65] |
| 20 | 438 | 8000 | g | 0.85 | −1.70 d | [65] |
| 21 | 409 (466, 506, 538) | 6400 | 3.6 | 1.09 | −1.30 | [60] |
| 22 | - | - | - | - | - | [60] |
| 23 | 557 ((640)) | 7000 | <0.1 | 1.07 | −1.11 | [60] |
| 24 | 560 ((620)) | 6000 | 0.12 | 1.08 | −1.11 | [31] |
| 25 | 440 (510, 690, 825) | 5600 | 528 | 0.05 | −1.75 | [66] |
| 26 | 506 | 12700 | 14 | 1.06 | −1.11 d | [32] |
| 27 | 509 | 17700 | 10 | 1.05 | −1.09 d | [32] |
| 28 | 509 | 9500 | 12 | 1.06 | −1.10 d | [32] |
| 30 | 379 (520, 558) ((630)) | 10300 | 1.4 | 1.26 | −0.27 d,h | [56] |
| Photosensitizer | Jsc /mAcm−2 | Voc /mV | Fill Factor | Conversion Efficiency/% | Ref |
|---|---|---|---|---|---|
| 5 | 3.3 | 440 | 0.63 | 0.92 | [92] |
| 14 | 0.12 | 368 | 0.71 | 0.03 | [32] |
| 26 | 0.33 | 400 | 0.73 | 0.10 | [32] |
| 27 | 0.36 | 440 | 0.73 | 0.11 | [32] |
| 28 | 0.36 | 390 | 0.71 | 0.10 | [32] |
| Complex | λabs,max a /nm | ε /M−1cm−1 | τLMCT /ps | λem,LMCT b /nm | QYPL /% | E1/2 Fe(III/II) /V vs. NHC | E1/2 Fe(IV/III) /V vs. NHC | Ref |
|---|---|---|---|---|---|---|---|---|
| 25 | 384 (528, 558) | 2400 | 100 | 600 | 0.03 | 0.05 | c | [53] |
| 29 | 502 | 3000 | 1960 | 655 | 2.1 | −0.53 | 0.88 | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindh, L.; Chábera, P.; Rosemann, N.W.; Uhlig, J.; Wärnmark, K.; Yartsev, A.; Sundström, V.; Persson, P. Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis. Catalysts 2020, 10, 315. https://doi.org/10.3390/catal10030315
Lindh L, Chábera P, Rosemann NW, Uhlig J, Wärnmark K, Yartsev A, Sundström V, Persson P. Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis. Catalysts. 2020; 10(3):315. https://doi.org/10.3390/catal10030315
Chicago/Turabian StyleLindh, Linnea, Pavel Chábera, Nils W. Rosemann, Jens Uhlig, Kenneth Wärnmark, Arkady Yartsev, Villy Sundström, and Petter Persson. 2020. "Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis" Catalysts 10, no. 3: 315. https://doi.org/10.3390/catal10030315
APA StyleLindh, L., Chábera, P., Rosemann, N. W., Uhlig, J., Wärnmark, K., Yartsev, A., Sundström, V., & Persson, P. (2020). Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis. Catalysts, 10(3), 315. https://doi.org/10.3390/catal10030315