Porous Polymer-Titanium Dioxide/Copper Composite with Improved Photocatalytic Activity toward Degradation of Organic Pollutants in Wastewater: Fabrication and Characterization as Well as Photocatalytic Activity Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Formation of PP and PPTC
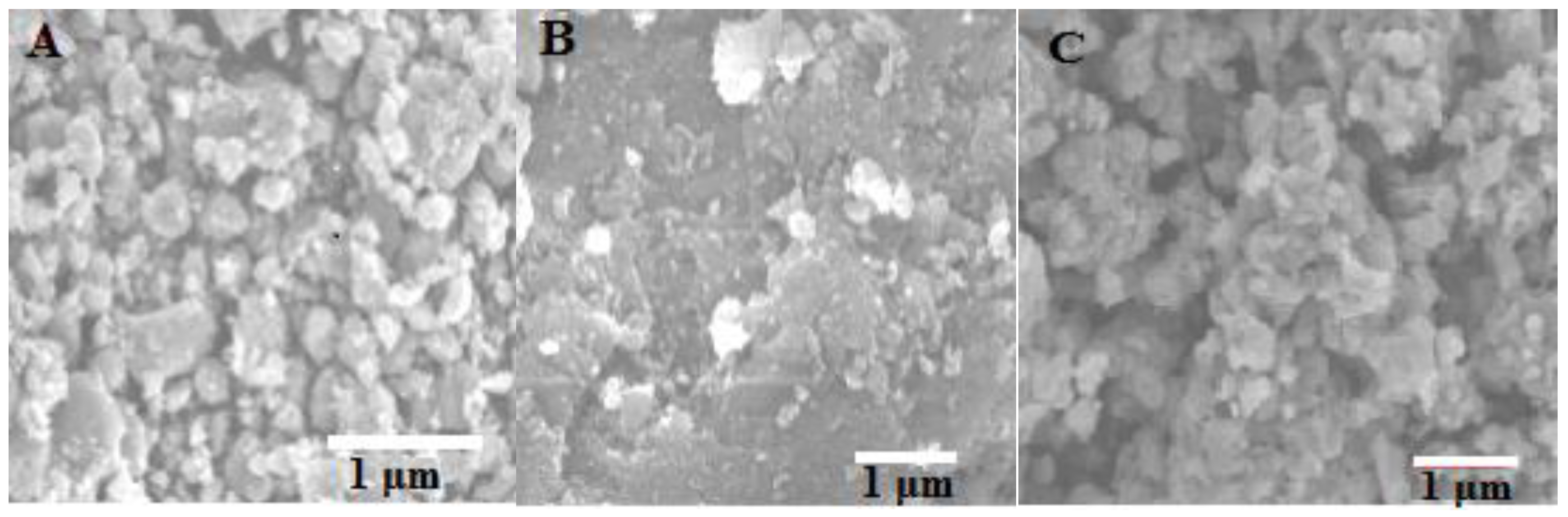
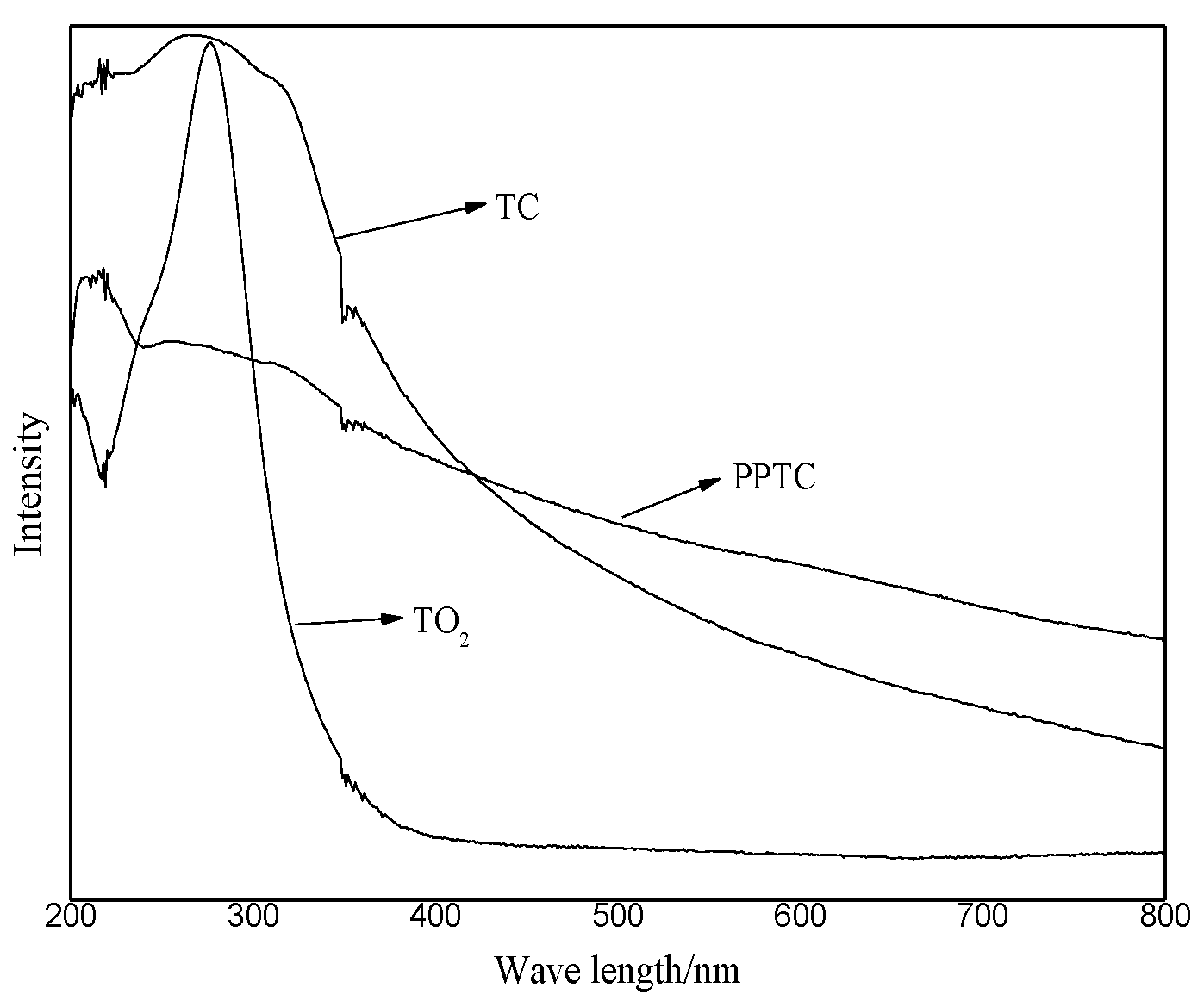
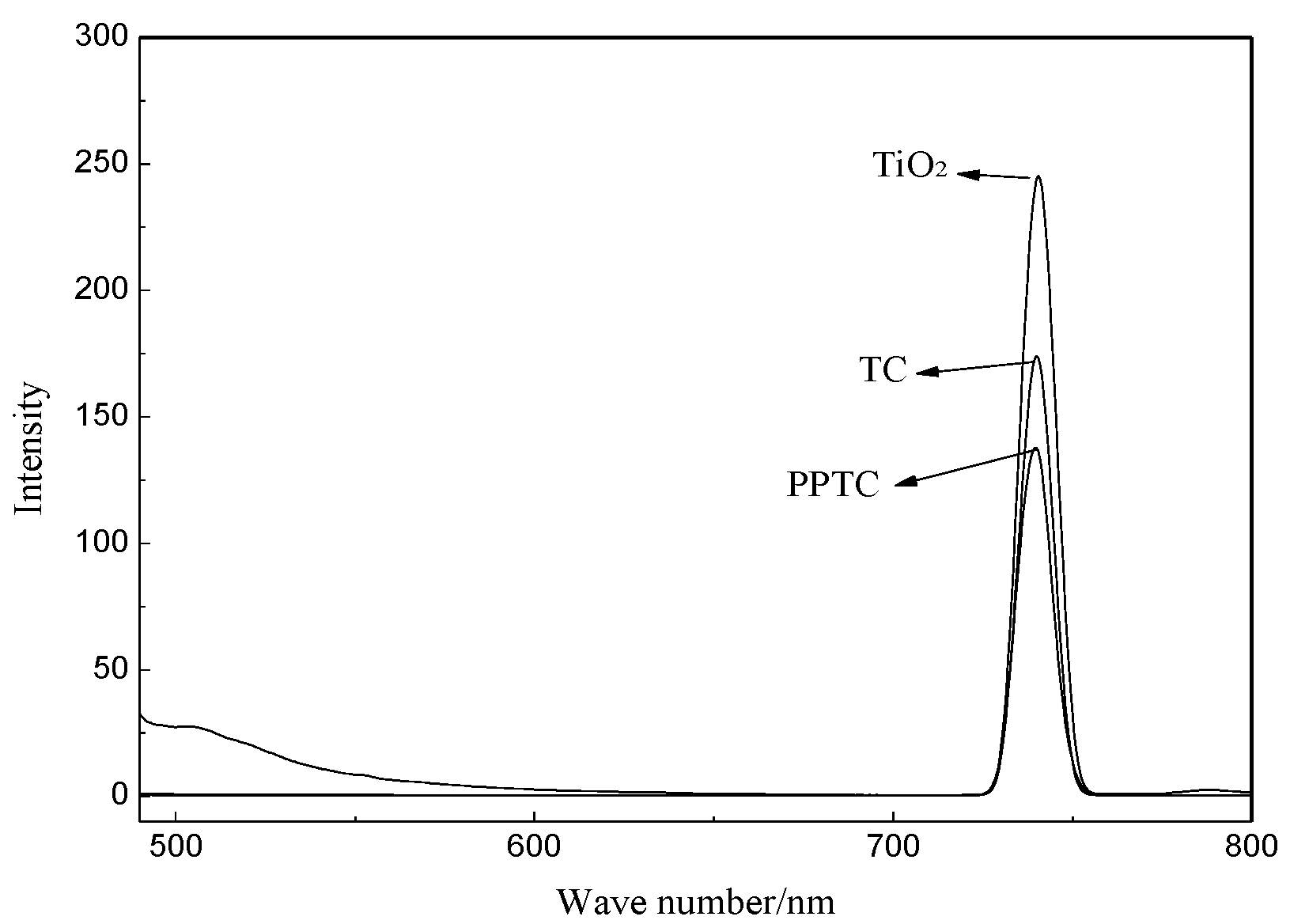
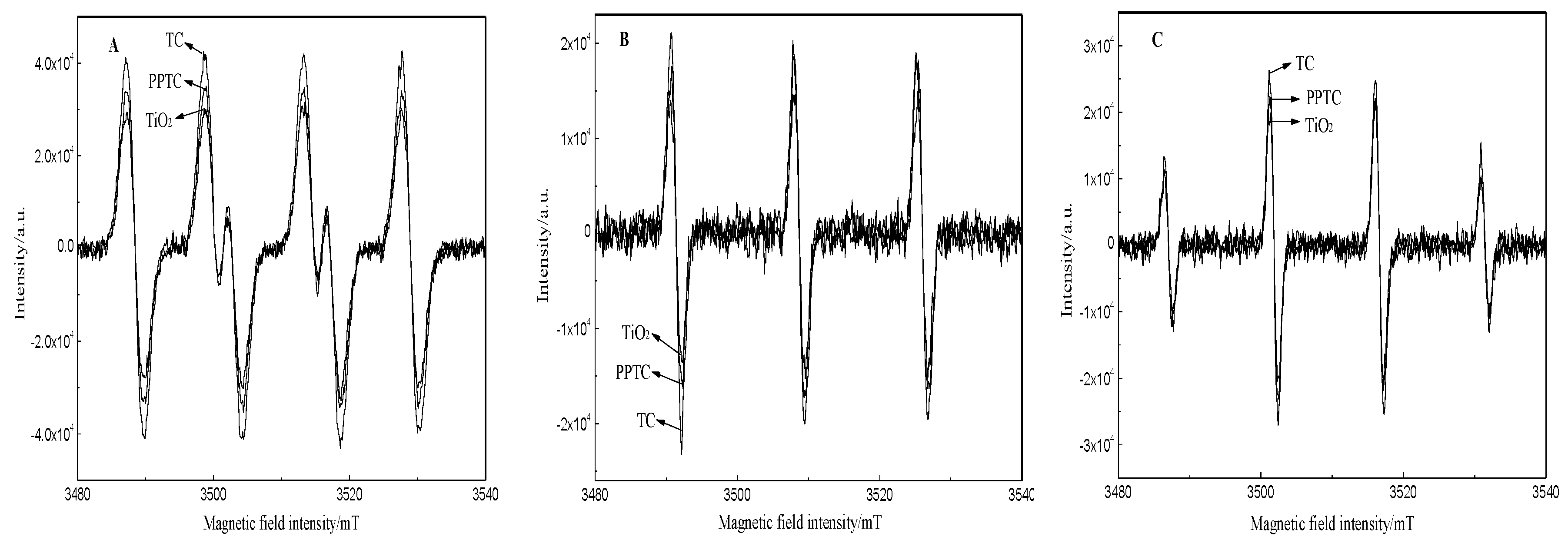
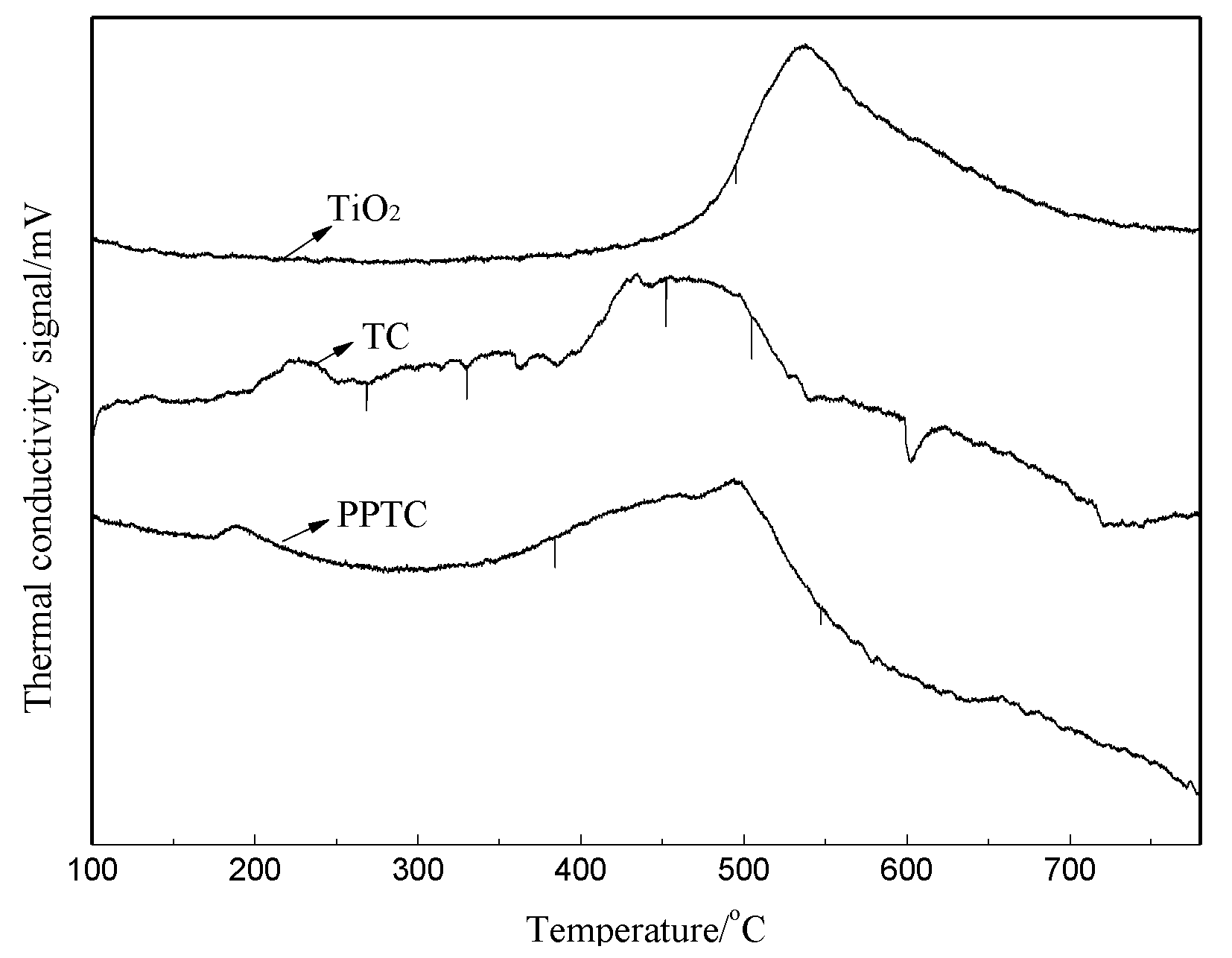
2.2. Characterization of TiO2, TC, and PPTC
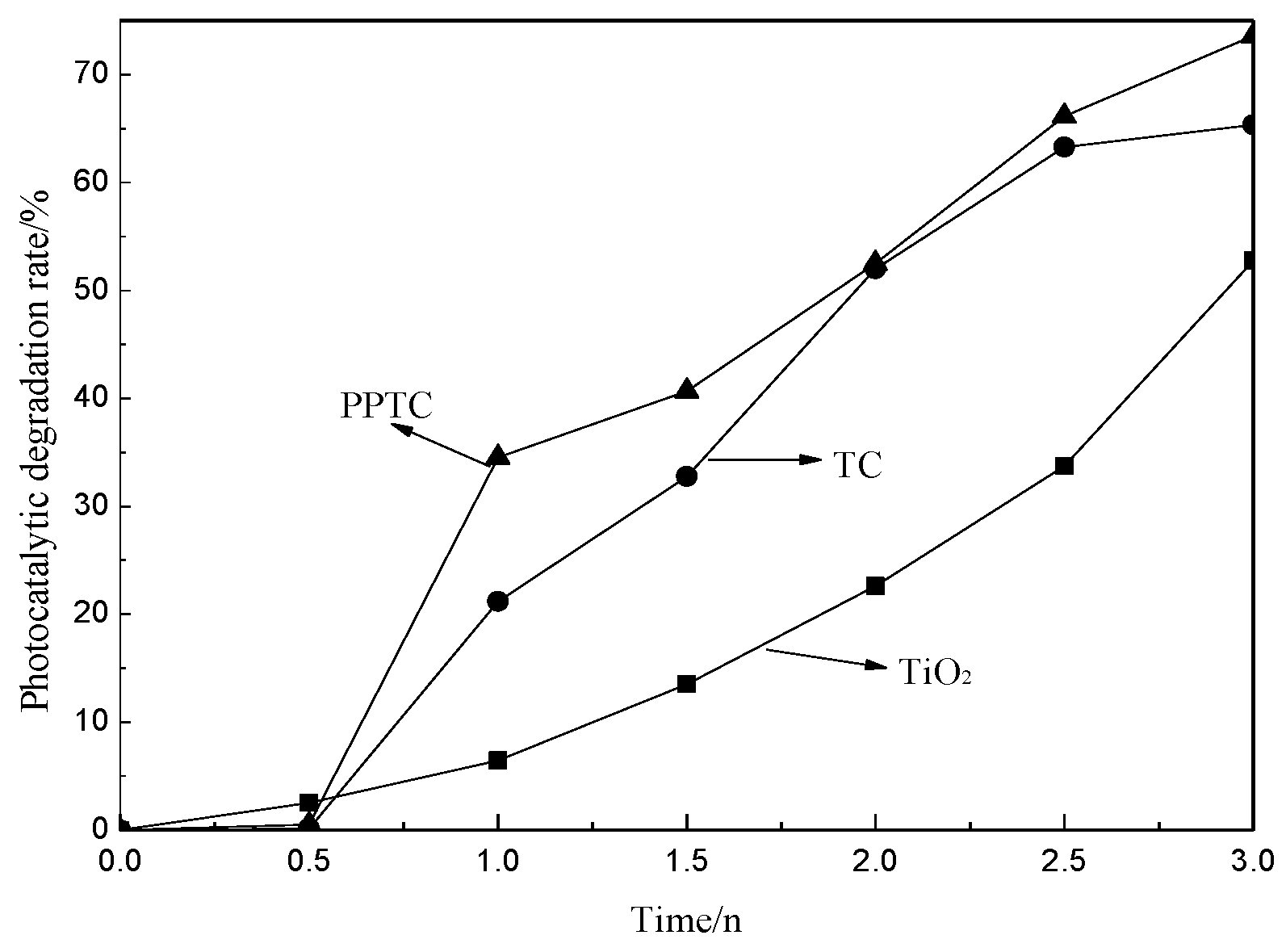
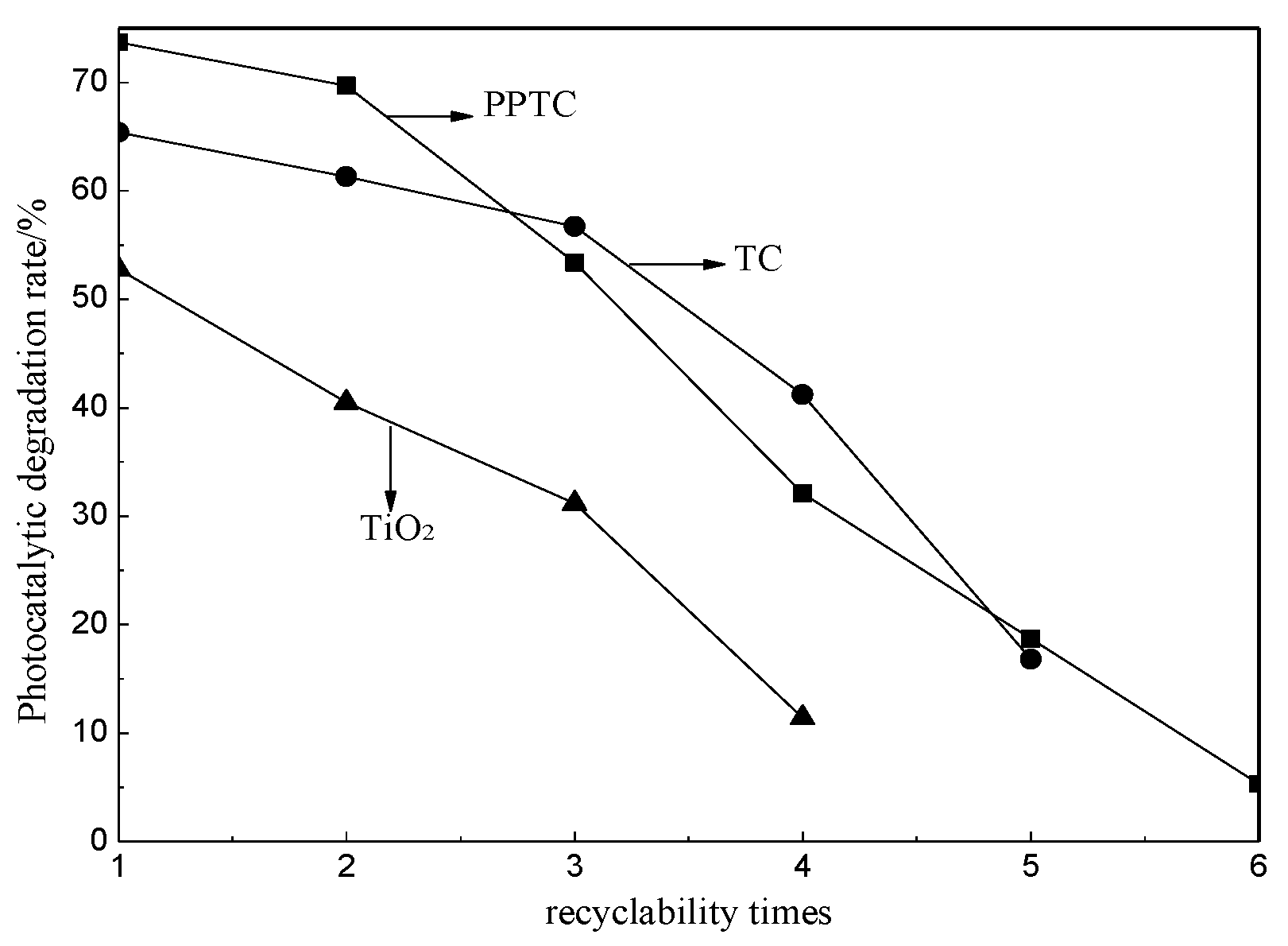
2.3. Photocatalytic Performance of TiO2, TC and PPTC
3. Experimental
3.1. Chemicals
3.2. Preparation of TiO2, TC, and PPTC
3.3. Characterization and Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. Weinheim 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Saravanan, R.; Sacari, E.; Gracia, F.; Khan, M.M.; Mosquera, E.; Gupta, V.K. Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 2016, 221, 1029–1033. [Google Scholar] [CrossRef]
- Liu, L.; Ding, L.; Liu, Y.; An, W.; Lin, S.; Liang, Y.-H.; Cui, W. A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with π-π conjugation. Appl. Catal. B 2017, 201, 92–104. [Google Scholar] [CrossRef]
- Krbal, M.; Ng, S.; Motola, M.; Hromadko, L.; Dvorak, F.; Prokop, V.; Sopha, H.; Macak, J.M. Sulfur treated 1D anodic TiO2 nanotube layers for significant photo- and electroactivity enhancement. Appl. Mater. Today 2019, 17, 104–111. [Google Scholar] [CrossRef]
- Sopha, H.; Hromadko, L.; Motola, M.; Macak, J.M. Fabrication of TiO2 nanotubes on Ti spheres using bipolar electrochemistry. Electrochem. Commun. 2020, 111, 106669. [Google Scholar] [CrossRef]
- Su, R.; Dimitratos, N.; Liu, J.; Carter, E.; Althahban, S.; Wang, X.; Shen, Y.; Wendt, S.; Wen, X.-D.; Hans Niemantsverdriet, J.W.; et al. Mechanistic insight into the interaction between a titanium dioxide photocatalyst and Pd cocatalyst for improved photocatalytic performance. ACS Catal. 2016, 6, 4239–4247. [Google Scholar] [CrossRef]
- Hernandez, J.V.; Coste, S.; Murillo, A.G.; Romo, F.D.J.C.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Wan, D.; Xue, C.; Zhao, J.; Shao, G. Direct evidence of 2D/1D heterojunction enhancement on photocatalytic activity through assembling MoS2 nanosheets onto super-long TiO2 nanofibers. Appl. Surf. Sci. 2020, 504, 144361. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Diak, M.; Grabowska, E.; Zaleska, A. Synthesis, characterization and photocatalytic activity of noble metal-modified TiO2 nanosheets with exposed {0 0 1} facets. Appl. Surf. Sci. 2015, 347, 275–285. [Google Scholar] [CrossRef]
- Zhou, X.; Häublein, V.; Liu, N.; Nguyen, N.T.; Zolnhofer, E.; Tsuchiya, H.; Killian, M.S.; Meyer, K.; Frey, L.; Schmuki, P. TiO2 nanotubes: Nitrogen-ion implantation at low dose provides noble-metal-free photocatalytic H2-evolution activity. Angew. Chem. Int. Ed. Engl. 2016, 55, 3763–3767. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Noble metal modified reduced graphene oxide/TiO2 ternary nanostructures for efficient visible-light-driven photoreduction of carbon dioxide into methane. Appl. Catal. B 2015, 166–167, 251–259. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@C-TiO2 direct Z-scheme heterojunction. Chin. J. Catal. 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Üzüm, Ç.; Shahwan, T.; Eroglu, A.E.; Hallam, K.; Scott, T.; Lieberwirth, I. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl. Clay Sci. 2009, 43, 172–181. [Google Scholar] [CrossRef]
- Doong, R.A.; Liao, C.Y. Enhanced visible-light-responsive photodegradation of bisphenol A by Cu, N-codoped titanate nanotubes prepared by microwave-assisted hydrothermal method. J. Hazard. Mater. 2017, 322, 254–262. [Google Scholar] [CrossRef]
- Xu, Q.J.; Li, X.; Zhang, Z. Preparation of copper nanoparticle-improved polyamide 6 composites by an in situ solution route with cupric oxide as the metallic copper source and investigation of their properties. New J. Chem. 2015, 39, 3015–3020. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K.; Lee, C.H. The advanced removal of benzene from aerosols by photocatalytic oxidation and adsorption of Cu–TiO2/PU under visible light irradiation. Appl. Catal. B Environ. 2016, 182, 172–183. [Google Scholar] [CrossRef]
- Ishibashi, K.-I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, P.; Fan, J.; Hu, J.; Shao, G. Constructing 2D layered MoS2 nanosheets-modified Z-scheme TiO2/WO3 nanofibers ternary nanojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 466–474. [Google Scholar] [CrossRef]
- An, C.-S.; Zhang, B.; Tang, L.-B.; Xiao, B.; He, Z.-J.; Zheng, J. Binder-free carbon-coated TiO2@graphene electrode by using copper foam as current collector as a high-performance anode for lithium ion batteries. Ceram. Int. 2019, 45, 13144–13149. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Saran, S.; Devipriya, S.P. Recovery and reuse of TiO2 photocatalyst from aqueous suspension using plant based coagulant-A green approach. Korean J. Chem. Eng. 2016, 33, 2107–2113. [Google Scholar] [CrossRef]
- Liu, H.; Yu, D.; Sun, T.; Du, H.; Jiang, W.; Muhammad, Y.; Huang, L. Fabrication of surface alkalinized g-C3N4 and TiO2 composite for the synergistic adsorption-photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2019, 473, 855–863. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]



| Photocatalyst | Brunner Emmet Teller (BET) Multi-Point Contact (m2/g) | Langmuir Single-Point Contact (m2/g) | Total Pore Volume of Single-Point Adsorption (cm3/g) | Average Adsorption Pore Size (nm) |
|---|---|---|---|---|
| TiO2 | 61.9 | 93.7 | 0.3 | 19.5 |
| TC | 385.4 | 594.7 | 0.4 | 4.7 |
| PPTC | 486.7 | 693.6 | 0.5 | 4.1 |
| Photocatalyst | Degradation Rate of DMF | ||
|---|---|---|---|
| No Light | Xenon Light | Sunlight | |
| PPTC | 26.0% | 73.7% | 64.0% |
| TC | 21.2% | 65.4% | 57.6% |
| TiO2 | 6.5% | 52.8% | 48.3% |
| No. | Photocatalyst | Organic Pollutant | Adsorption Rate/% a | Degradation Rate/% b |
|---|---|---|---|---|
| 1 | TiO2 | DMF | 6.5 | 52.8 |
| 2 | TC | DMF | 21.2 | 65.4 |
| 3 | PPTC | DMF | 26.0 | 73.7 |
| 4 | TiO2 | Methyl orange | 3.6 | 55.5 |
| 5 | TC | Methyl orange | 29.7 | 91.6 |
| 6 | PPTC | Methyl orange | 53.9 | 97.8 |
| 7 | TiO2 | Methylene blue | 40.0 | 90.9 |
| 8 | TC | Methylene blue | 66.3 | 94.3 |
| 9 | PPTC | Methylene blue | 94.9 | 100 |
| 10 | TiO2 | Phenol | 32.1 | 83.6 |
| 11 | TC | Phenol | 46.3 | 90.7 |
| 12 | PPTC | Phenol | 58.7 | 98.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Wang, Y.; Chi, M.; Hu, W.; Zhang, N.; He, W. Porous Polymer-Titanium Dioxide/Copper Composite with Improved Photocatalytic Activity toward Degradation of Organic Pollutants in Wastewater: Fabrication and Characterization as Well as Photocatalytic Activity Evaluation. Catalysts 2020, 10, 310. https://doi.org/10.3390/catal10030310
Xu Q, Wang Y, Chi M, Hu W, Zhang N, He W. Porous Polymer-Titanium Dioxide/Copper Composite with Improved Photocatalytic Activity toward Degradation of Organic Pollutants in Wastewater: Fabrication and Characterization as Well as Photocatalytic Activity Evaluation. Catalysts. 2020; 10(3):310. https://doi.org/10.3390/catal10030310
Chicago/Turabian StyleXu, Qijie, Yan Wang, Mei Chi, Wenbin Hu, Ning Zhang, and Weiwei He. 2020. "Porous Polymer-Titanium Dioxide/Copper Composite with Improved Photocatalytic Activity toward Degradation of Organic Pollutants in Wastewater: Fabrication and Characterization as Well as Photocatalytic Activity Evaluation" Catalysts 10, no. 3: 310. https://doi.org/10.3390/catal10030310
APA StyleXu, Q., Wang, Y., Chi, M., Hu, W., Zhang, N., & He, W. (2020). Porous Polymer-Titanium Dioxide/Copper Composite with Improved Photocatalytic Activity toward Degradation of Organic Pollutants in Wastewater: Fabrication and Characterization as Well as Photocatalytic Activity Evaluation. Catalysts, 10(3), 310. https://doi.org/10.3390/catal10030310




