Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light
Abstract
1. Introduction
2. Results and Discussion
2.1. Light Absorption and Fluorescence Properties of the 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives
2.2. The 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives as Photosensitizers of Iodonium Salts: Investigation into the Photo-Induced Electron Transfer Process with the Photo-Oxidation Mechanism
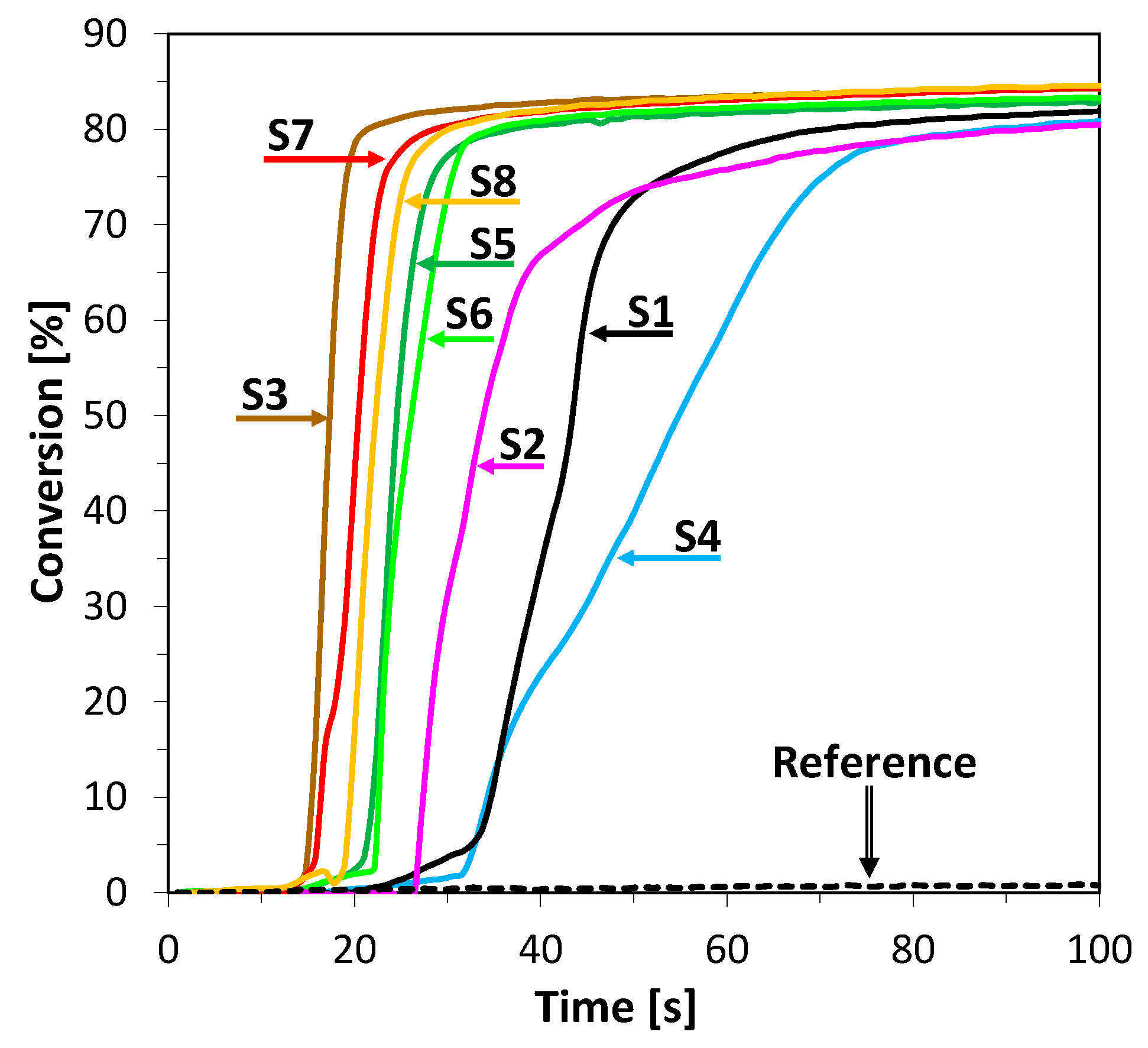
2.3. Performance of 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives as Photosensitizers in Bimolecular Photoinitiating Systems through Photo-Oxidation Mechanisms for Different Types of Photopolymerization
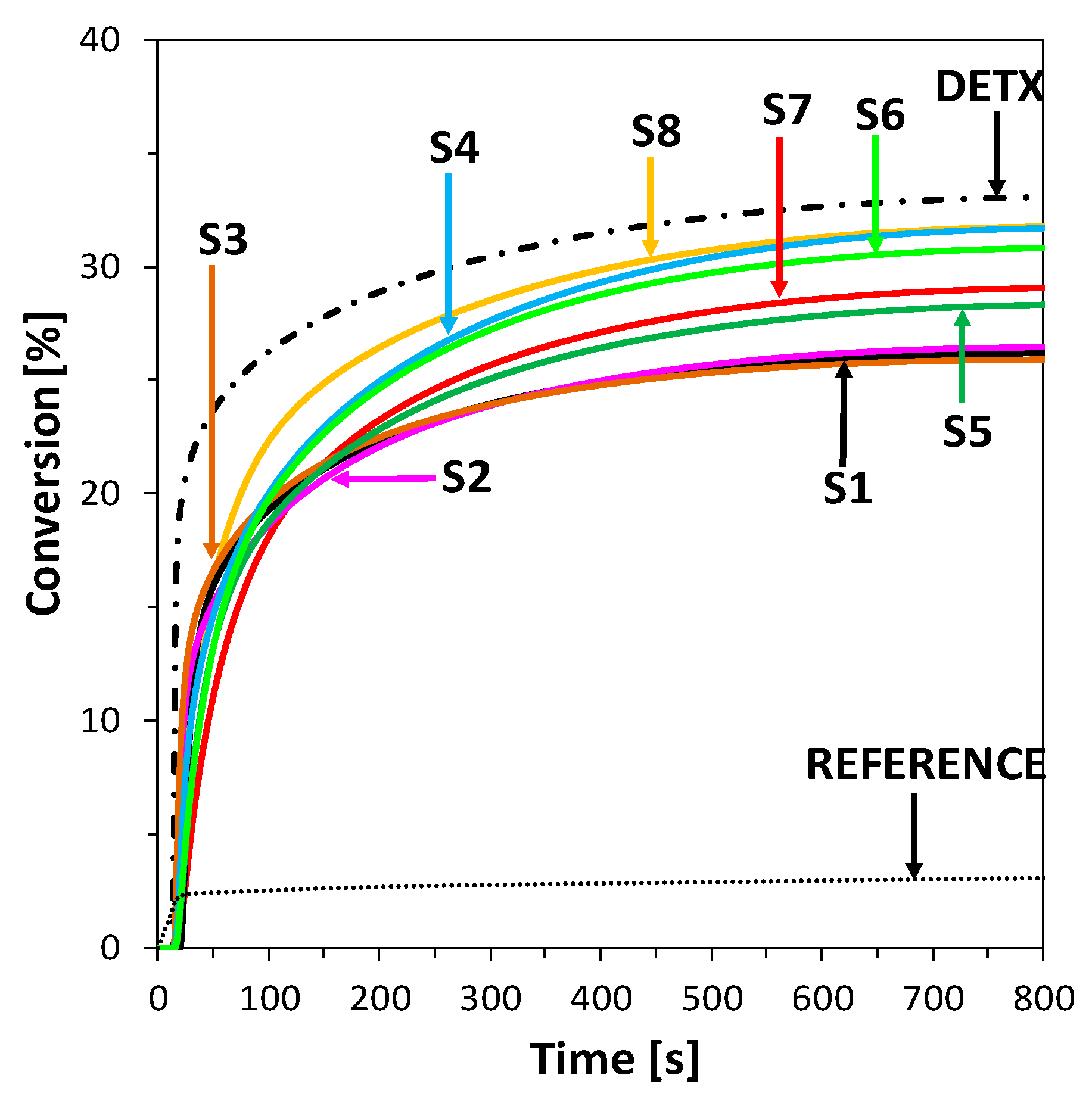
2.3.1. Cationic Photopolymerization (CP) of Vinyl and Cycloaliphatic Epoxide Monomers
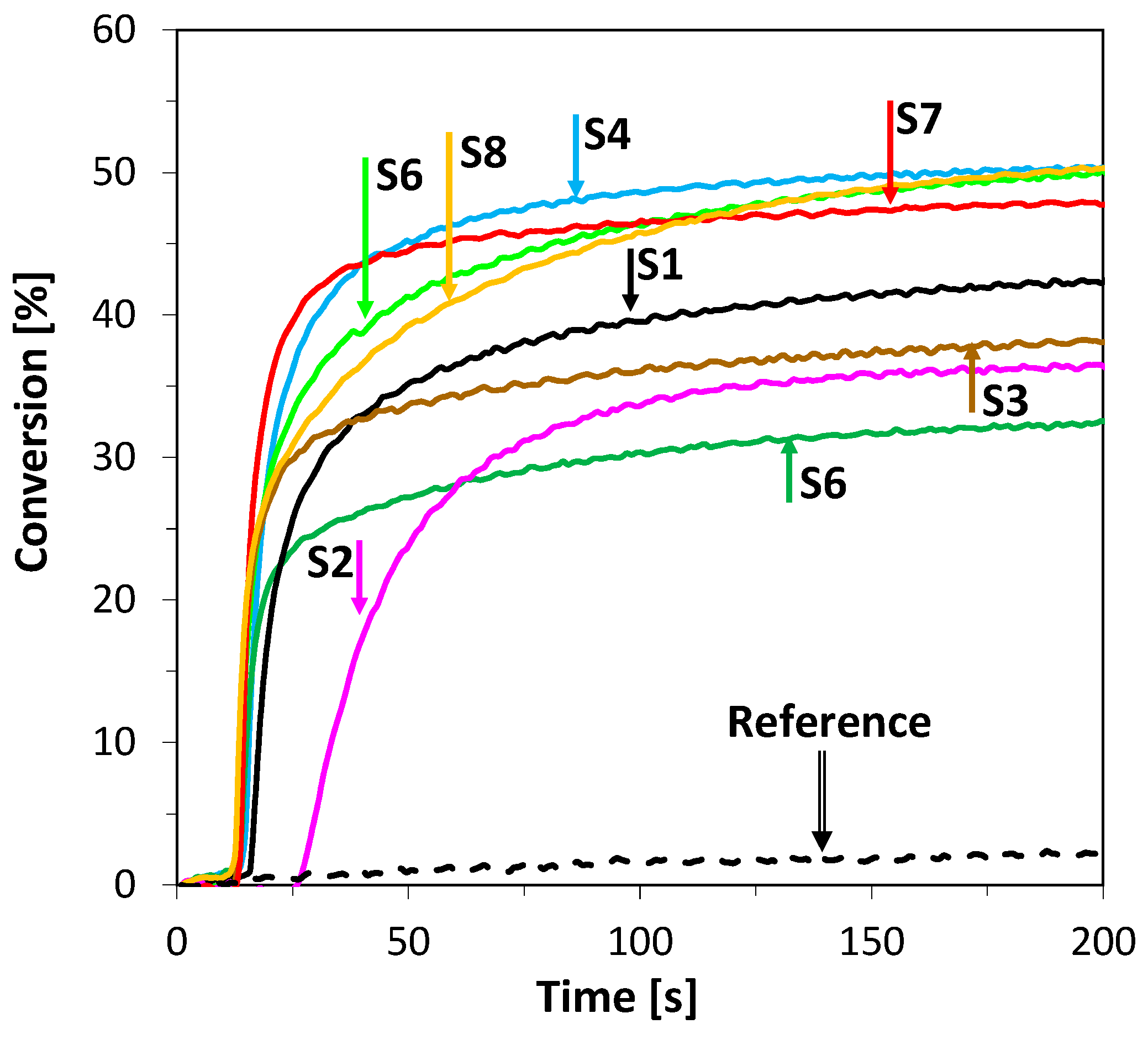
2.3.2. Free-Radical Photopolymerization (FRP) of Acrylate Monomers
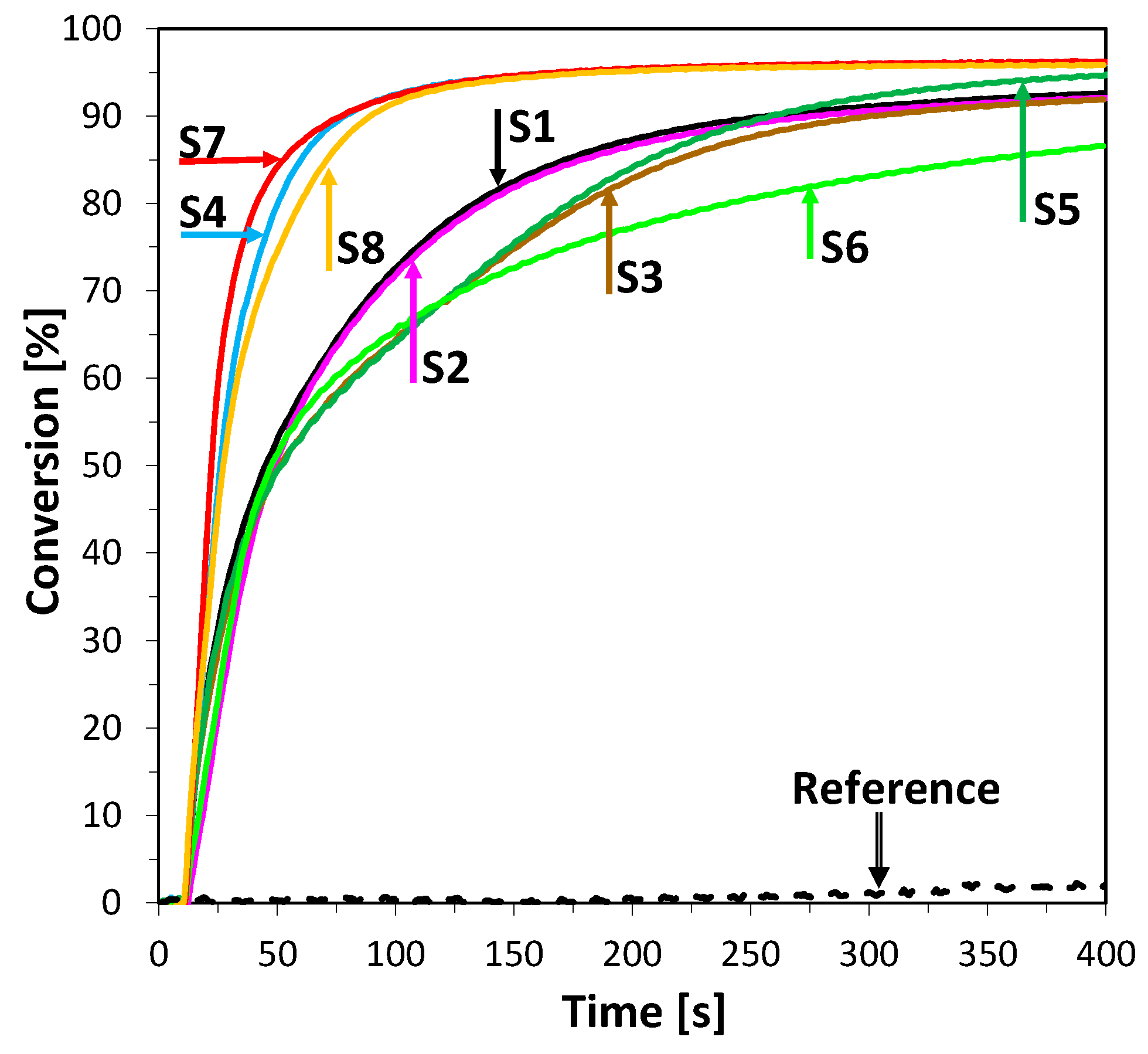
2.3.3. Thiol-Ene Photopolymerization (TEP) of Methacrylate and Thiol Monomers
2.4. Performance of 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives as Photosensitizers in Bimolecular Photoinitiating Systems through Photo-Reduction Mechanisms for Free-Radical Photopolymerization
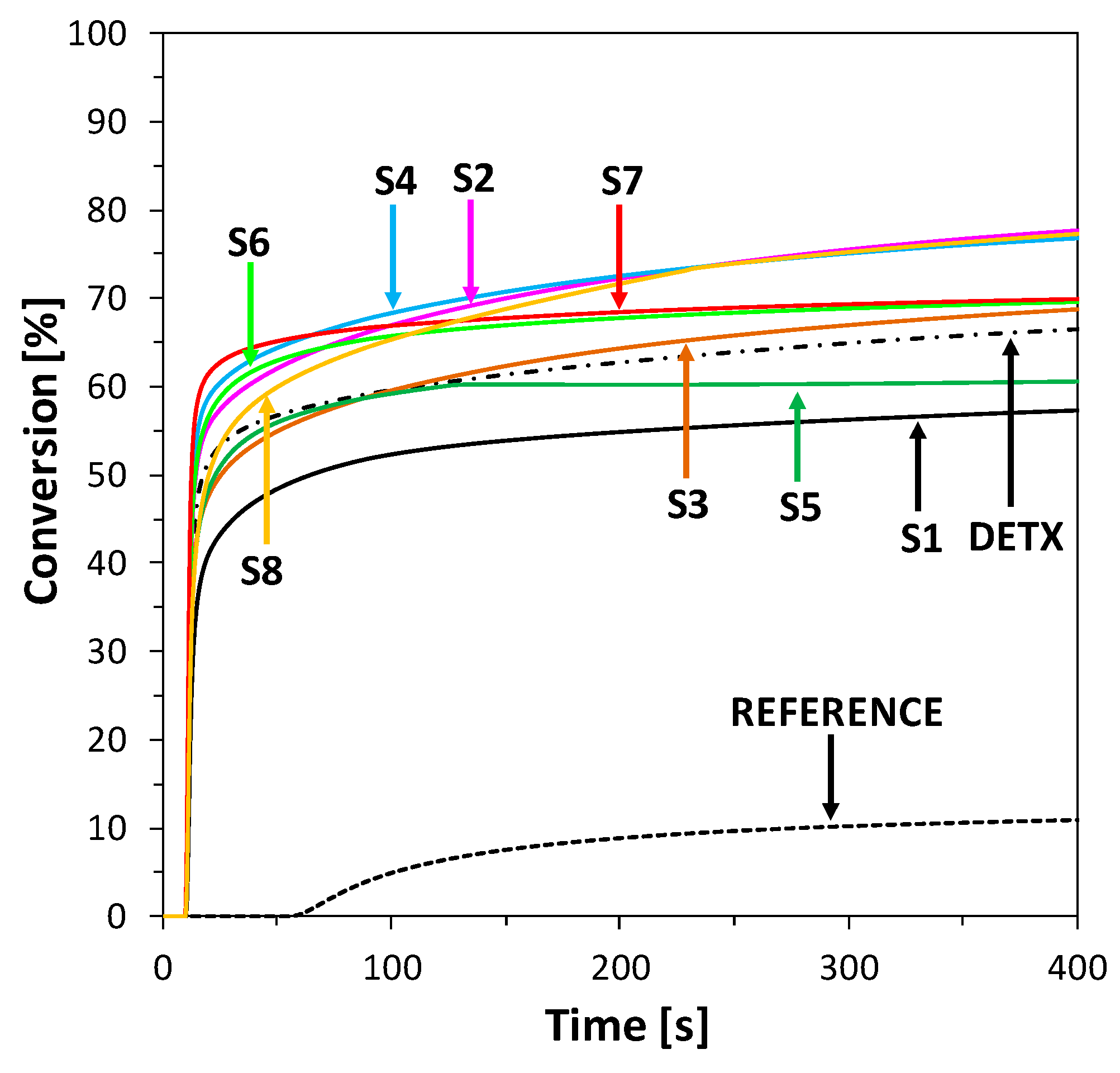
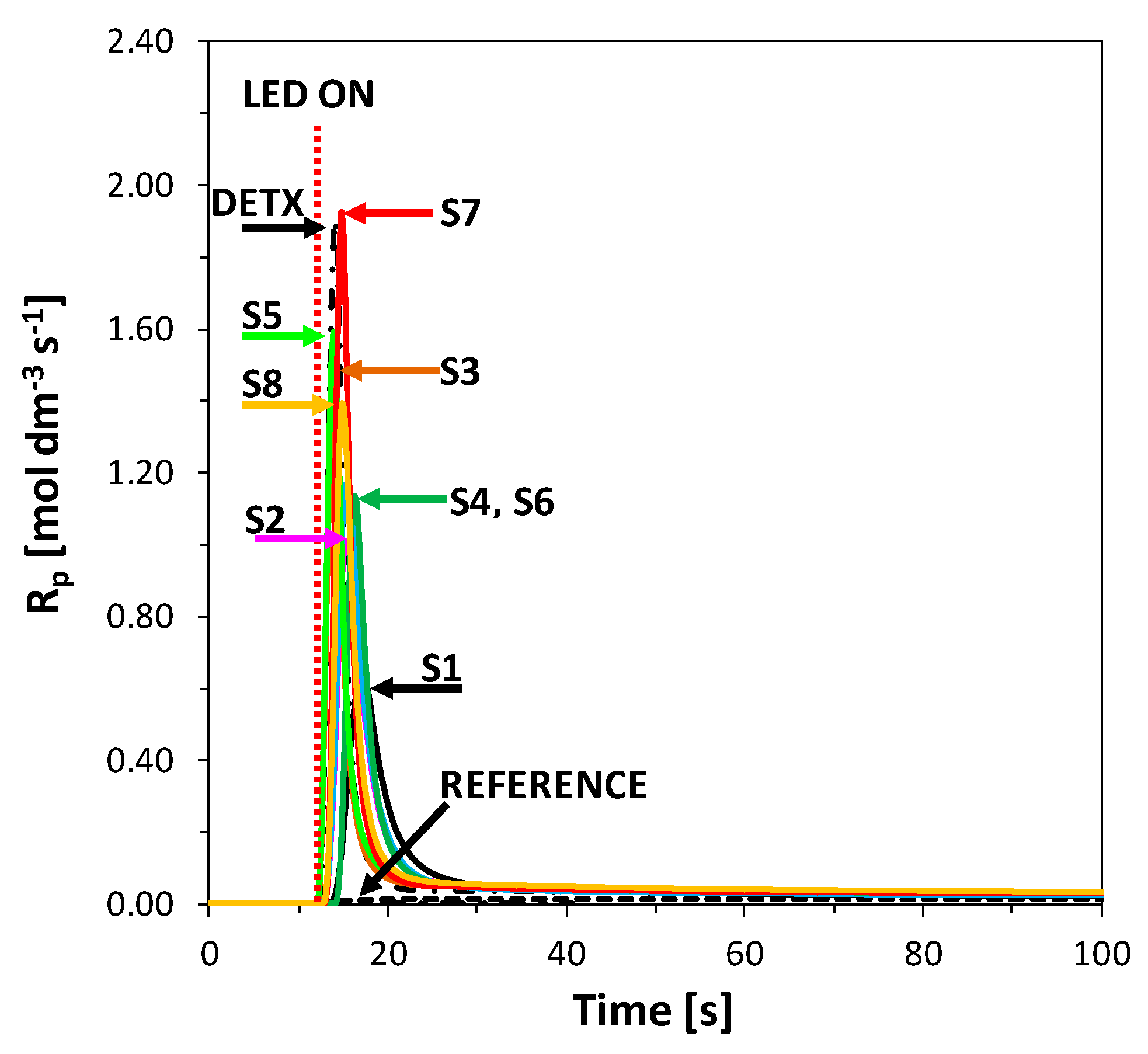
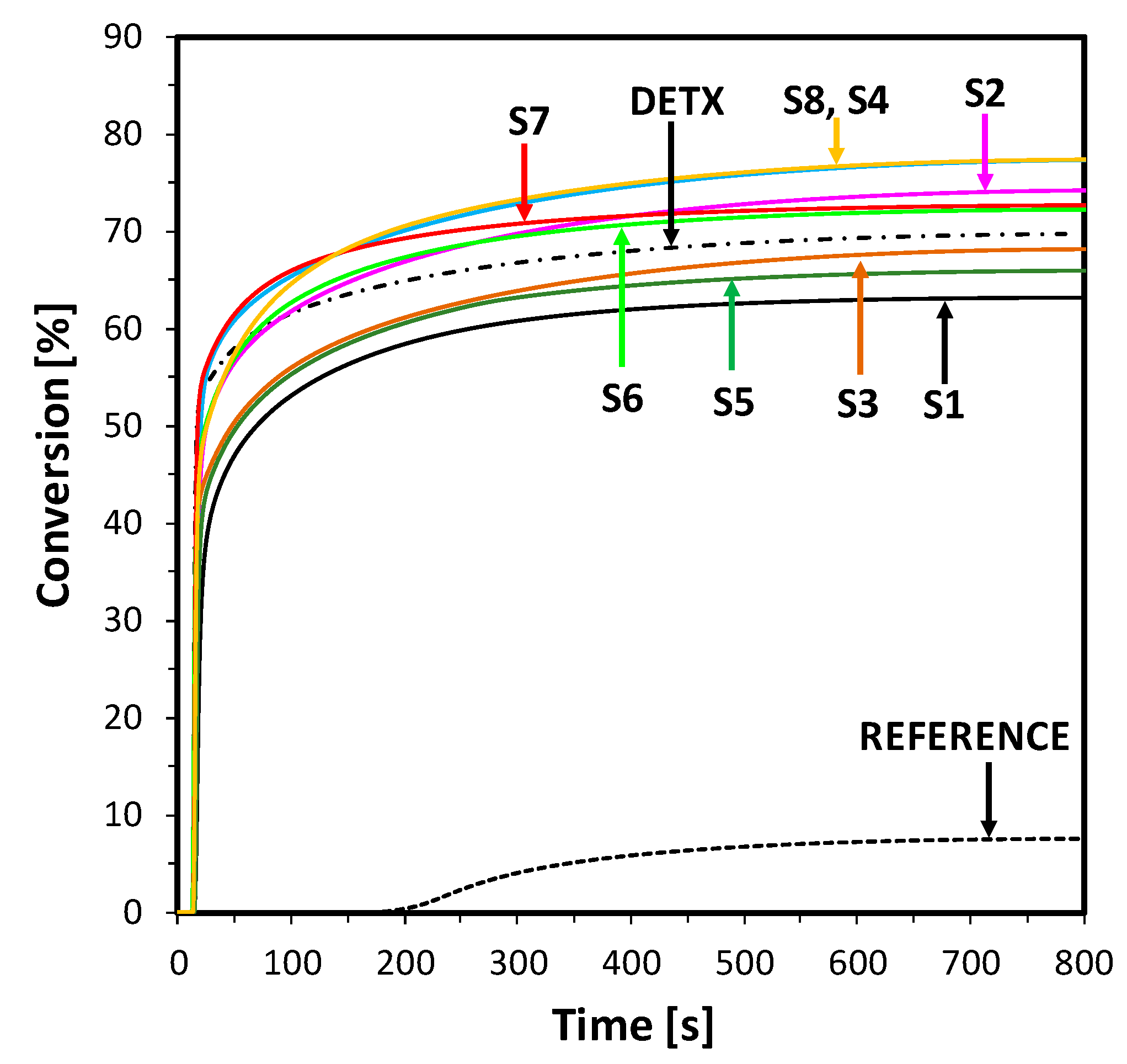
2.5. Performance of 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives as Visible Photosensitizers in Bimolecular Photoinitiating Systems for 3D Printing Processes
2.6. Performance of New Visible Bimolecular Photoinitiating Systems for 3D Printing Experiments
3. Experimental Section
3.1. Materials
| 2-amino-4,6-diphenyl-pyridine-3-carbonitrile | (S1), |
| 2-amino-4-(4-methylthiophenyl)-6-(4-cyanophenyl)-pyridine-3-carbonitrile | (S2), |
| 2-amino-4-(4-cyanophenyl)-6-(4-methylthiophenyl)-pyridine-3-carbonitrile | (S3), |
| 2-amino-4-(4-methylthiophenyl)-6-phenylpyridine-3-carbonitrile | (S4), |
| 2-amino-4-phenyl-6-(4-methylthiophenyl)pyridine-3-carbonitrile | (S5), |
| 2-amino-4,6-bis(4-methylthiophenyl)--pyridine-3-carbonitrile | (S6), |
| 2-amino-4-(4-methylthiophenyl)-6-(4-methoxyphenyl)-pyridine-3-carbonitrile | (S7), |
| 2-amino-4-(4-methoxyphenyl)-6-(4-methylthiophenyl)-pyridine-3-carbonitrile | (S8) |
3.2. Spectroscopic Characteristics of 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives
3.2.1. Molecular Absorption Spectra Measurements
3.2.2. Fluorescence Spectra Measurements
3.2.3. Steady-State Photolysis Measurements
- ⇒
- UV-LED-365 M365L2 (from Thorlabs Inc., Newton, NJ, USA) emitting light with a wavelength at λmax = 365 nm (~190 mW/cm2, current 700 mA) over 30 min;
- ⇒
- 405 nm M405LP1 (from Thorlabs Inc., Newton, NJ, USA) emitting light with a wavelength at λmax = 405 nm (~870 mW/cm2, current 1000 mA) over 30 min.
3.2.4. Fluorescence Quenching Measurements
3.3. Electrochemical Determination of Oxidation Potentials
3.4. Molecular Orbital Calculations
3.5. Preparation of Photocurable Compositions
3.6. Monitoring the Photopolymerization Processes by Real-Time FTIR
3.6.1. Monitoring the Photopolymerization Processes by Real-Time FTIR
3.6.2. Cationic Photopolymerization (CP) Measurements
3.6.3. Free-radical Photopolymerization (FRP) Measurements
3.6.4. Thiol–Ene Photopolymerization (TEP) Measurements
3.6.5. Source of Light for Real-Time FTIR Measurements
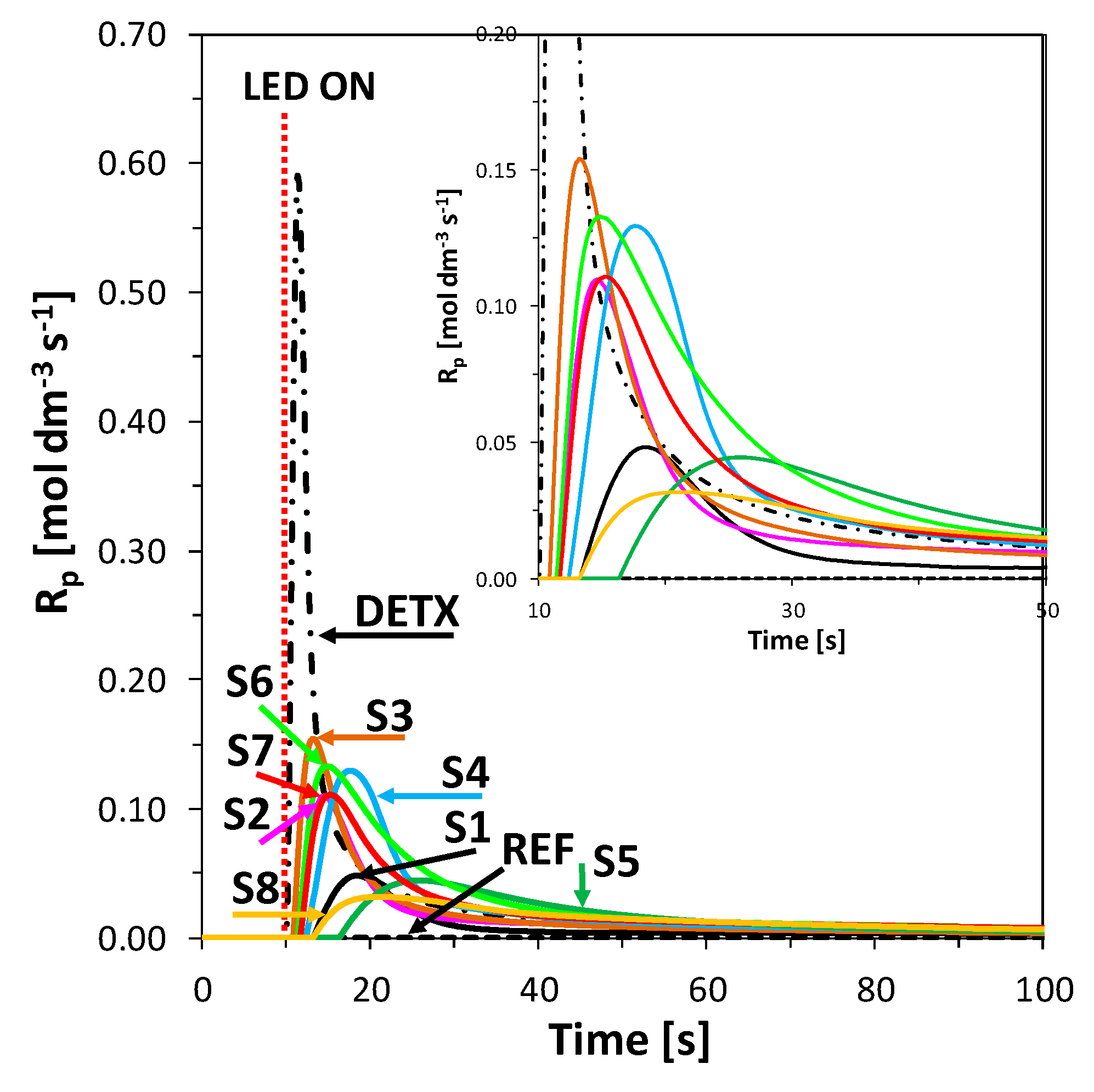
3.7. Monitoring the Photopolymerization Processes by Photo Differential Scanning Calorimetry (Photo-DSC)
Source of Light for Photo-DSC Measurements
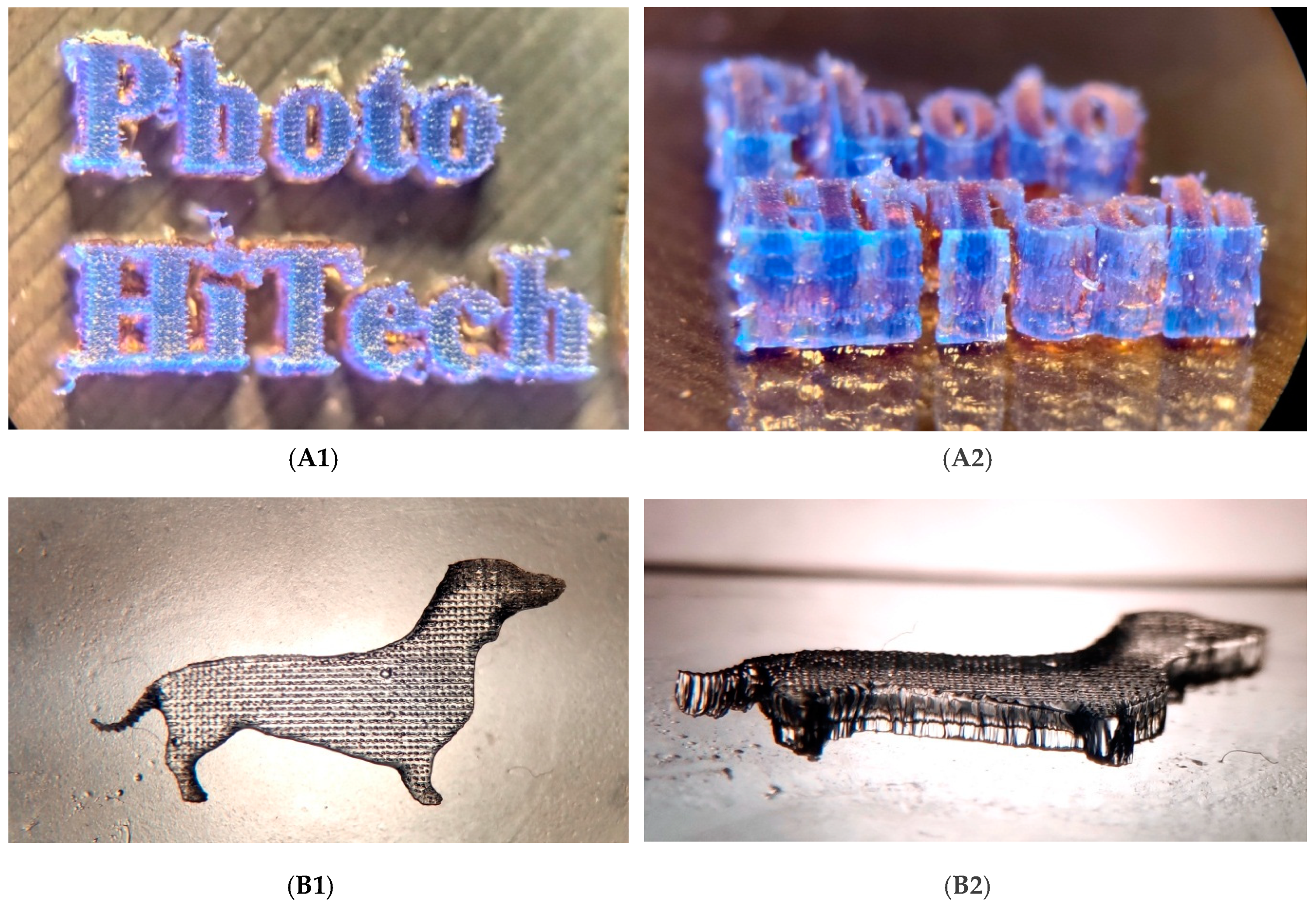
3.8. 3D Printing Equipment with Different Visible Light Sources
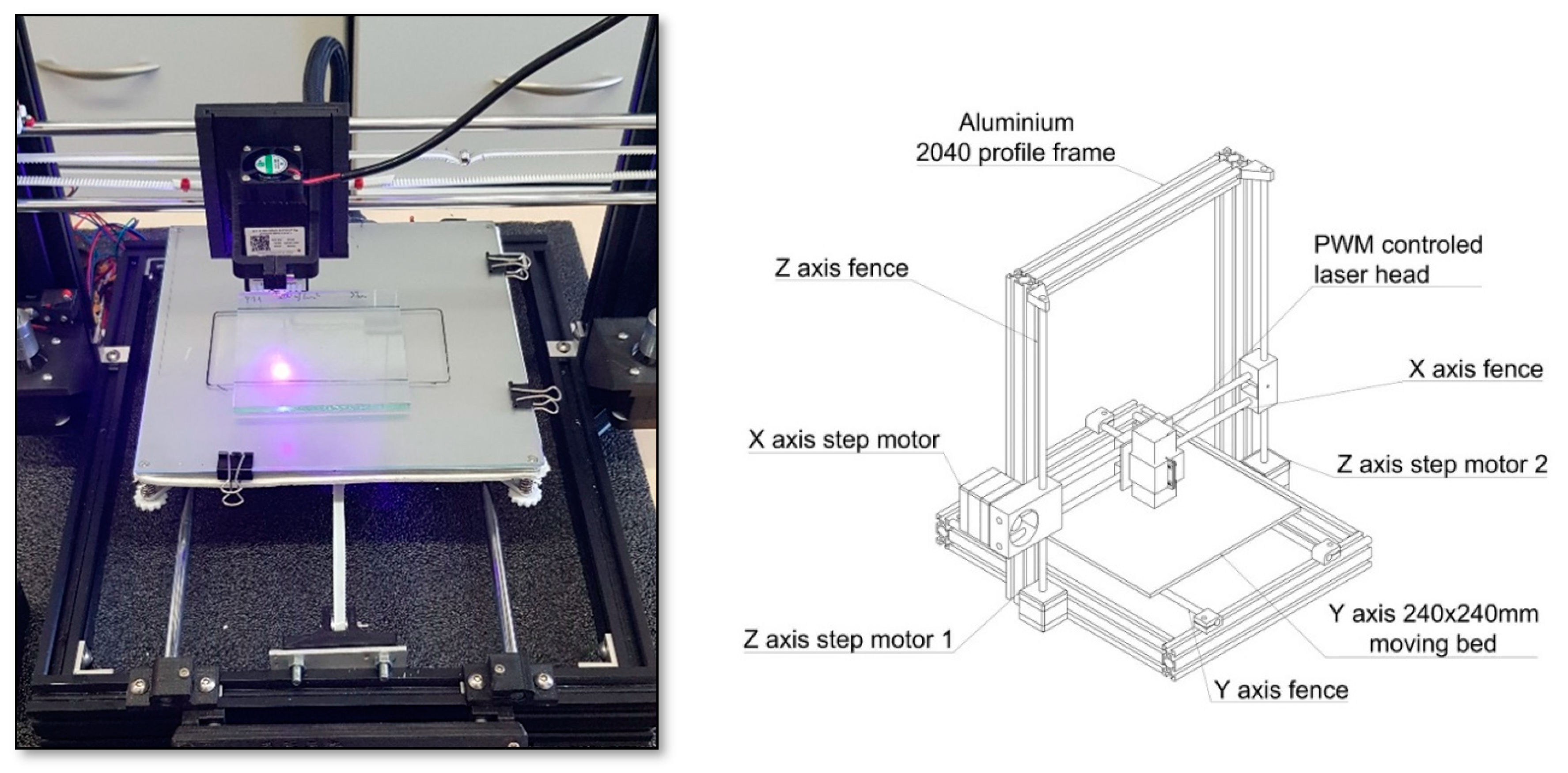
3.8.1. The Laser Engraver Printer Machine
3.8.2. SLA Home-Made 3D Printer
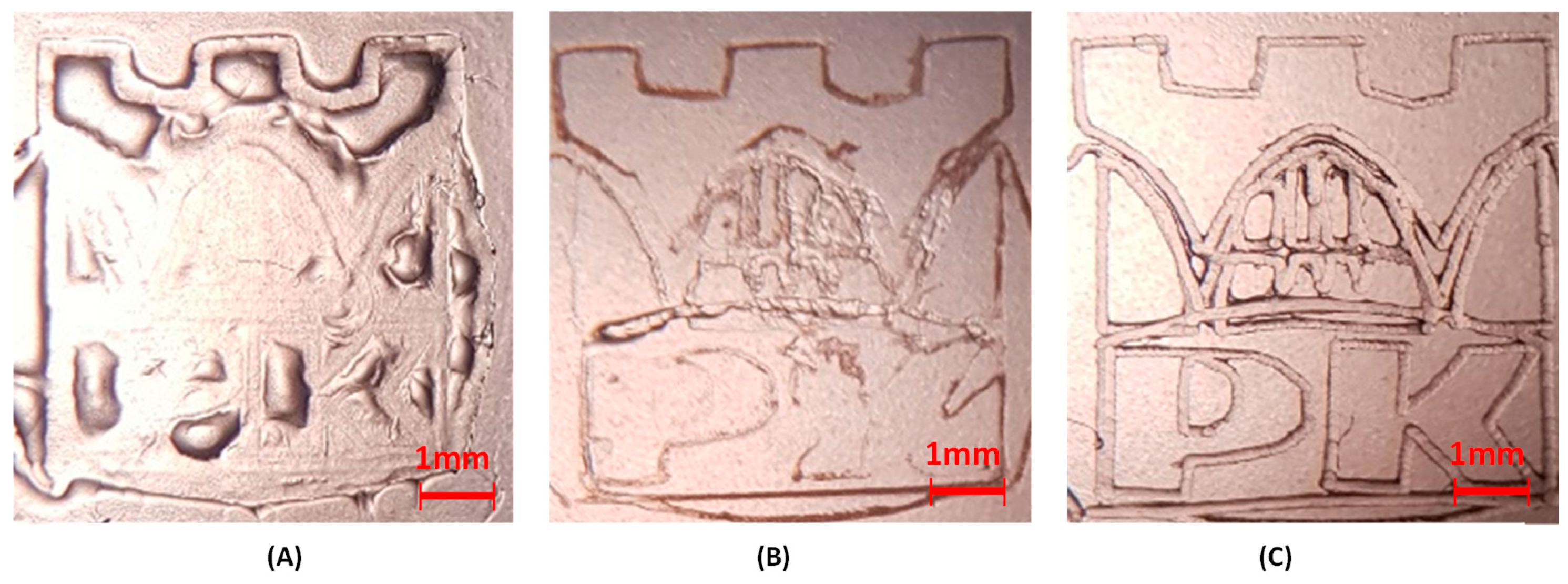
3.9. Microscope Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Seitz, H.; Yang, S.F. A Review on 3D Micro-Additive Manufacturing Technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef] [PubMed]
- Hossam, K.; Soham, W.; Changxue, X.; Fakhrul, A. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous Liquid Interface of 3D Objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Dilag, J.; Chen, T.; Li, S.; Bateman, S.A. Design and direct additive manufacturing of three-dimensional surface micro-structures using material jetting technologies. Addit. Manuf. 2019, 27, 167–174. [Google Scholar] [CrossRef]
- Kerekes, T.W.; Lim, H.; Joe, W.Y.; Yun, H.J. Characterization of process–deformation/damage property relationship of fused deposition modeling (FDM) 3D-printed specimens. Addit. Manuf. 2019, 25, 532–544. [Google Scholar]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bose, S. Additive Manufacturing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D Printing of Photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Ortyl, J. Cationic Photoinitiators. In Photopolymerisation Initiating Systems, 1st ed.; Lalevée, J., Fouassier, J.P., Eds.; Royal Society of Chemistry: Croydon, UK, 2018; pp. 74–130. [Google Scholar]
- Ortyl, J.; Popielarz, R. New photoinitiators for cationic polymerization. Polimery 2012, 57, 7–8. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Hola, M.; Pilch, M.; Galek, M.; Ortyl, J. New versatile bimolecular photoinitiating systems based on amino-m-terphenyl derivatives for cationic, free-radical and thiol-ene photopolymerization under low intensity UV-A and visible light sources. Polym. Chem. 2020, 11, 480–495. [Google Scholar] [CrossRef]
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test. 2013, 32, 708–715. [Google Scholar] [CrossRef]
- Kostrzewska, K.; Ortyl, J.; Dobosz, R.; Kabatc, J. Squarylium dye and onium salts as highly sensitive photoradical generators for blue light. Polym. Chem. 2017, 22, 3464–3474. [Google Scholar] [CrossRef]
- Kabatc, J.; Ortyl, J.; Kostrzewska, K. New kinetic and mechanistic aspects of photosensitization of iodonium salts in photopolymerization of acrylates. RCV Adv. 2017, 66, 41619–41629. [Google Scholar] [CrossRef]
- Tomal, W.; Pilch, M.; Chachaj-Brekiesz, A.; Ortyl, J. Development of new high-performance biphenyl and terphenyl derivatives as versatile photoredox photoinitiating systems and their applications in 3D printing photopolymerization processes. Catalysts 2019, 9, 827. [Google Scholar] [CrossRef]
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative sensitization efficiency of fluorescent probes/sensitizers for monitoring and acceleration of cationic photopolymerization of monomers. Polym. Test. 2015, 48, 151–159. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. The performance of 7-hydroxycoumarin-3-carbonitrile and 7-hydroxycoumarin-3-carboxylic acid as fluorescent probes for monitoring of cationic photopolymerization processes by FPT. J. Appl. Polym. Sci. 2013, 128, 1974–1978. [Google Scholar] [CrossRef]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test. 2015, 42, 99–107. [Google Scholar] [CrossRef]
- Ortyl, J.; Sawicz-Kryniger, K.; Galek, M.; Popielarz, R. Monitoring of cationic photopolymerization with stilbene derivatives as fluorescent probes. Przem. Chem. 2010, 89, 1642–1646. [Google Scholar]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test. 2016, 55, 310–317. [Google Scholar] [CrossRef]
- Ortyl, J.; Galica, M.; Popielarz, R.; Bogdał, D. Application of a carbazole derivative as a spectroscopic fluorescent probe for real time monitoring of cationic photopolymerization. Pol. J. Chem. Technol. 2014, 16, 75–80. [Google Scholar] [CrossRef]
- Hola, E.; Ortyl, J.; Jankowska, M.; Pilch, M.; Galek, M.; Morlet-Savary, F.; Graff, B.; Dietlinc, C.; Lalevée, J. New bimolecular photoinitiating systems based on terphenyl derivatives as highly efficient photosensitizers for 3D printing application. Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Lapim, S.C.; Snyder, J.R.; Sitzmann, E.V.; Barnes, D.K.; Green, G.D. Stereolithography Using Vinyl Ether-Epoxide Polymers. U.S. Patent 5,437,964, 1 August 1995. [Google Scholar]
- Lapim, S.C.; Brautigam, R.J. Stereolithography Using Vinyl Ether Based Polymers. U.S. Patent 5,506,087, 9 April 1996. [Google Scholar]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Goubard, F.; Bui, T.-T.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; et al. Azahelicenes as visible light photoinitiators for cationic and radical polymerization: Preparation of photoluminescent polymers and use in high performance LED projector 3D printing resins. J. Polym. Sci. A Polym. Chem. 2017, 55, 1189–1199. [Google Scholar] [CrossRef]
- Ortyl, J.; Fiedor, P.; Chachaj-Brekiesz, A.; Pilch, M.; Hola, E.; Galek, M. The Applicability of 2-amino-4, 6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors 2019, 19, 1668. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic Polymerization—Iodonium and Sulfonium Salt Photoinitiators. Adv. Polym. Sci. 1984, 62, 1–44. [Google Scholar]
- Rehm, D.; Weller, A.H. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Romańczyk, P.P.; Kurek, S.S. The Reduction Potential of Diphenyliodonium Polymerisation Photoinitiator Is Not −0.2 V vs. SCE. A Computational Study. Electrochim. Acta. 2017, 225, 482–485. [Google Scholar] [CrossRef]
- Strehmel, B.; Ernst, S.; Reiner, K.L.; Keil, D.; Lindauer, H.; Baumann, H. Application of NIR-photopolymers in the graphic industry: From physical chemistry to lithographic applications. Z. Phys. Chem. 2014, 228, 129–153. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevee, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Q.; Wei, G.; Liu, R.; Li, Z. Highly stable thiol-ene systems: From their structure—Property relationship to DLP 3D printing. J. Mater. Chem. C 2018, 6, 11561–11568. [Google Scholar] [CrossRef]
- Sycks, D.G.; Wu, T.; Park, H.S.; Gall, K. Tough, Stable Spiroacetal Thiol-Ene Resin for 3D Printing. J. Appl. Polym. Sci. 2018, 135, 46259. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Wiener, J.; Roth, M.; Moser, A.; Gmeiner, R.; Edler, M.; Pinter, G.; Griesser, T. Tough and Degradable Photopolymers Derived from Alkyne Monomers for 3D Printing of Biomedical Materials. Polym. Chem. 2016, 7, 5169–5180. [Google Scholar] [CrossRef]
- Marx, P.; Romano, A.; Roppolo, I.; Chemelli, A.; Mühlbacher, I.; Kern, W.; Chaudhary, S.; Andritsch, T.; Sangermano, M.; Wiesbrock, F. 3D-Printing of High-κ Thiol-Ene Resins with Spiro-Orthoesters as Anti-Shrinkage Additive. Macromol. Mater. Eng. 2019, 304, 1900515. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Zych-Tomkowiak, D.; Andrzejewski, M.; Hug, G.L.; Marciniak, B. Heteroaromatic Thiols as Co-initiators for Type II Photoinitiating Systems Based on Camphorquinone and Isopropylthioxanthone. Macromolecules 2006, 39, 3777–3785. [Google Scholar] [CrossRef]
- Roberts, D.E. Heats of Polymerization. A Summary of Published Values and Their Relation to Structure, Research Paper RP20073. J. Res. Natl. Bur. Stand. 1950, 44, 221–232. [Google Scholar] [CrossRef]

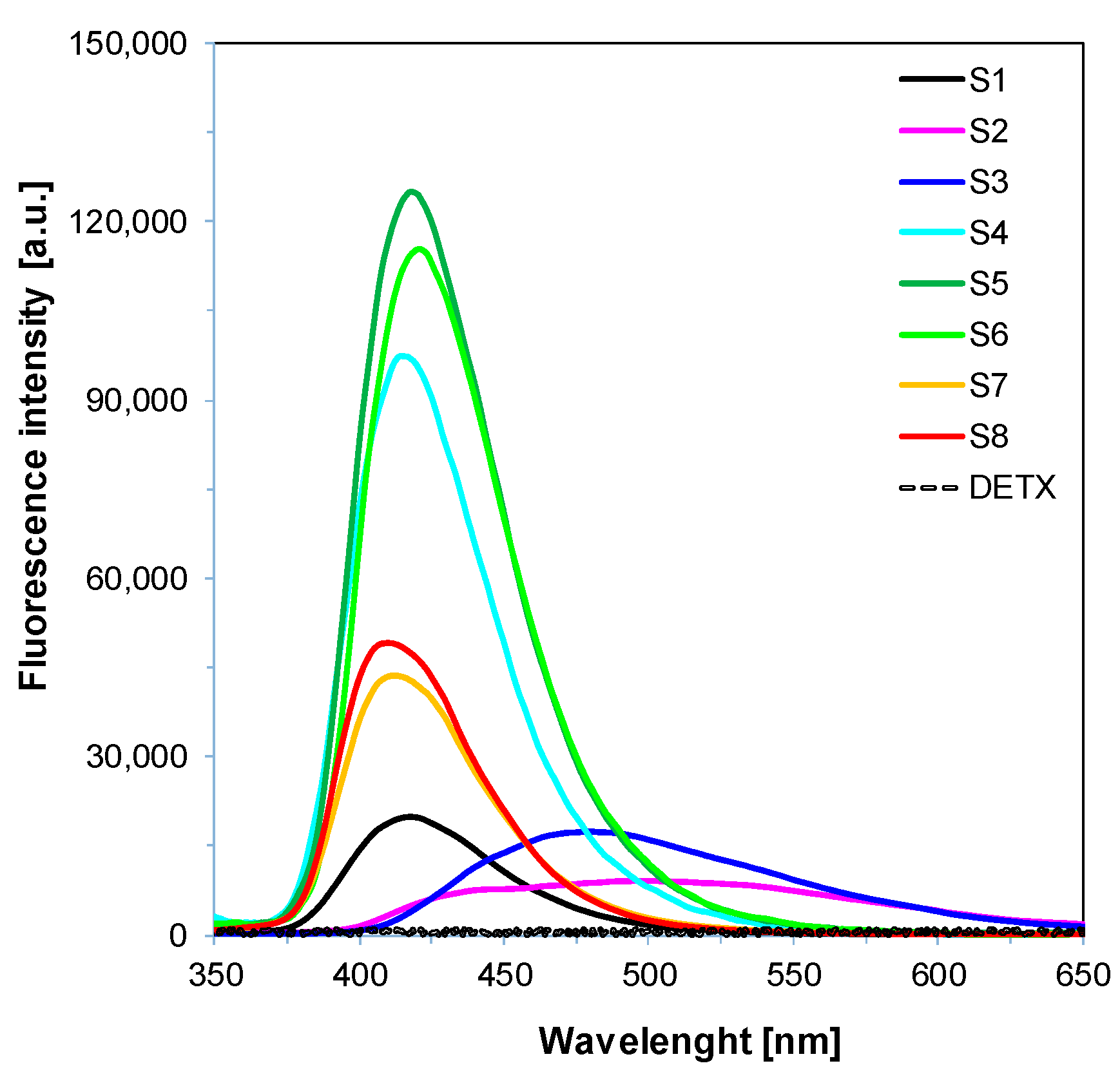


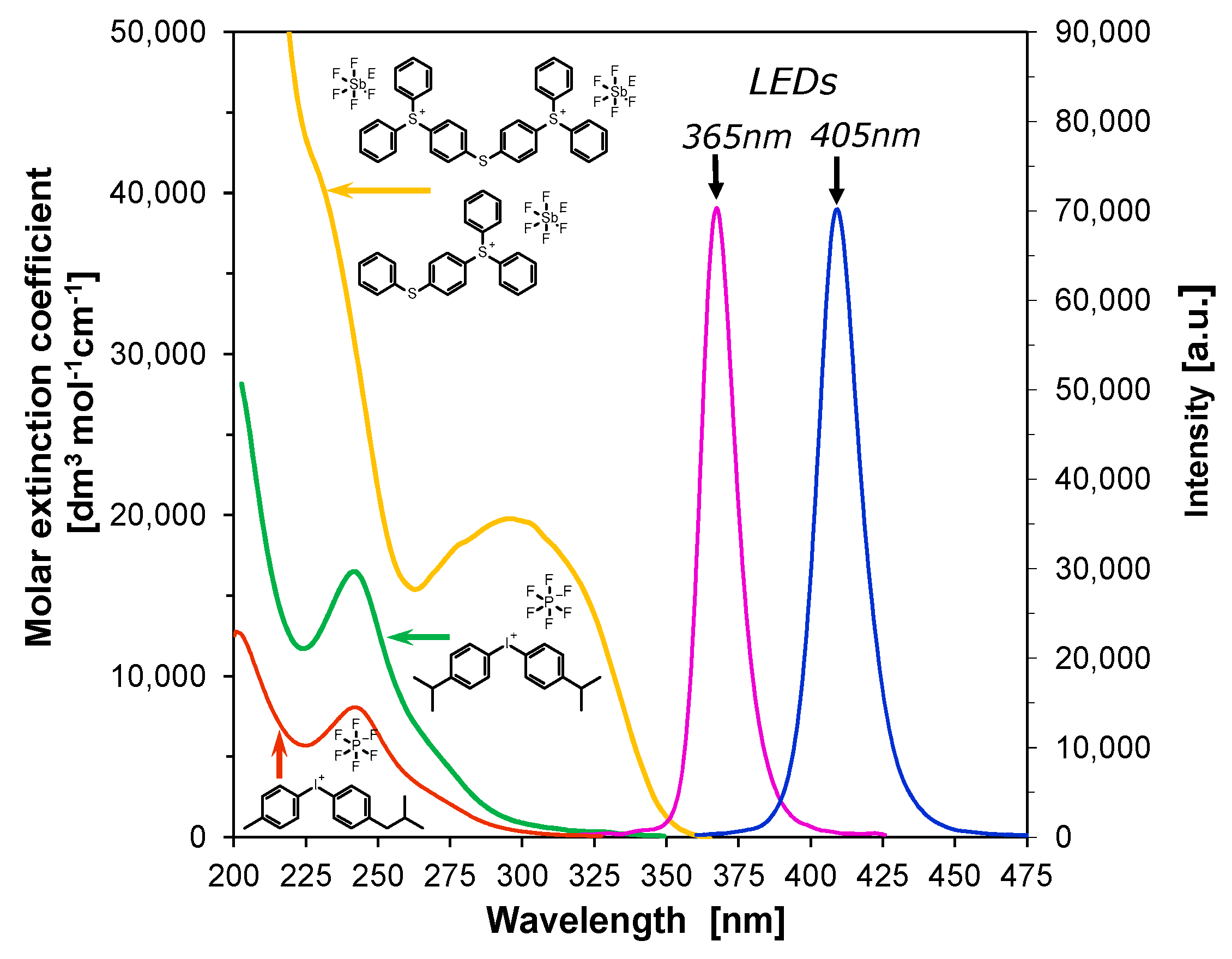







| Abb. | λAb-max [nm] | ε@λmax [dm3·mol−1·cm−1] | ε@365 nm [dm3·mol−1·cm−1] | ε@405 nm [dm3·mol−1·cm−1] | λFl-max (λex= 320 nm) | Stokes Shift [nm] | Intensity @λFl-max [a. u.] |
|---|---|---|---|---|---|---|---|
| S1 | 349 | 12,290 | 8410 | 100 | 418 | 69 | 19,890 |
| S2 | 360 | 15,030 | 14,730 | 950 | 497 | 137 | 9110 |
| S3 | 365 | 25,770 | 25,770 | 2810 | 480 | 115 | 17,410 |
| S4 | 351 | 14,360 | 11,090 | 150 | 414 | 63 | 97,390 |
| S5 | 356 | 20,500 | 19,820 | 310 | 418 | 62 | 125,020 |
| S6 | 361 | 20,170 | 19,980 | 470 | 420 | 59 | 115,380 |
| S7 | 357 | 22,270 | 19,240 | 110 | 411 | 54 | 43,650 |
| S8 | 362 | 24,390 | 24,000 | 120 | 410 | 48 | 49,150 |
| Abb. | Eox1/2 [mV] | E00(S1) [eV] | E00(T1) [eV] | ΔGET(S1) [eV] | ΔGET(T1) [eV] | KSV [M−1] | ΦET(S1) |
|---|---|---|---|---|---|---|---|
| S1 | 1584 | 311 | 2.57 | −0.92 | −0.23 | 35.74 | 0.46 |
| S2 | 1494 | 297 | 2.46 | −0.86 | −0.21 | 27.67 | 0.39 |
| S3 | 1412 | 290 | 2.45 | −0.87 | −0.28 | 40.87 | 0.49 |
| S4 | 1385 | 304 | 2.57 | −1.04 | −0.32 | 61.30 | 0.59 |
| S5 | 1503 | 308 | 2.46 | −0.97 | −0.31 | 33.21 | 0.44 |
| S6 | 1359 | 302 | 2.51 | −0.72 | −0.36 | 42.31 | 0.50 |
| S7 | 1489 | 307 | 2.54 | −0.98 | −0.29 | 38.22 | 0.47 |
| S8 | 1483 | 306 | 2.52 | −0.97 | −0.28 | 27.85 | 0.40 |
| Acronim | Photo-Oxidation Mechanisms of Photopoly Merization | Photo-Reduction Mechanisms | ||||
|---|---|---|---|---|---|---|
| Cationic Polymerization | Free Radical Polymerization | Thiol–ene Polymerization | Free Radical Polymerization | |||
| [%] CADE Monomer after 800 s | [%] TGDVE Monomer after 100 s | [%] TMPTA Monomer after 200 s | [%] TMPTMA Monomer after 200 s | [%] MERCAPTO Monomer after 400 s | [%] TMPTA Monomer after 200 s | |
| @365 nm ~1.59 mW/cm2 | @365 nm ~1.59 mW/cm2 | @365 nm ~1.59 mW/cm2 | @365 nm ~1.59 mW/cm2 | @405 nm ~6 mW/cm2 | ||
| S1 | 67.7 | 81.9 | 42.5 | 92.6 | 44.8 | 2.7 |
| S2 | 80.6 | 80.5 | 36.3 | 92.1 | 44.7 | 43.2 |
| S3 | 72.9 | 84.3 | 38.2 | 91.9 | 44.8 | 46.1 |
| S4 | 86.0 | 80.9 | 50.3 | 96.0 | 47.9 | 38.6 |
| S5 | 56.6 | 82.9 | 32.5 | 94.7 | 44.6 | 44.6 |
| S6 | 81.5 | 83.3 | 49.9 | 86.6 | 40.9 | 48.5 |
| S7 | 81.1 | 84.3 | 47.8 | 96.2 | 48.5 | 39.2 |
| S8 | 78.2 | 84.6 | 50.2 | 95.8 | 48.0 | 34.9 |
| Abb. | E-red1/2 (mV) | E00(S1) (eV) | E00(T1) (eV) | ΔGET(S1) (eV) | ΔGET(T1) (eV) | KSV (M−1) | ΦET(S1) |
|---|---|---|---|---|---|---|---|
| S1 | −1753 | 311 | 2.57 | −0.41 | 0.24 | 62.59 | 0.45 |
| S2 | −1477 | 297 | 2.46 | −0.54 | 0.08 | 13.28 | 0.15 |
| S3 | −1470 | 290 | 2.45 | −0.48 | 0.09 | 33.43 | 0.30 |
| S4 | −1433 | 304 | 2.57 | −0.56 | 0.02 | 24.97 | 0.24 |
| S5 | −1737 | 308 | 2,46 | −0.40 | 0.34 | 24.48 | 0.24 |
| S6 | −1719 | 302 | 2.51 | −0.35 | 0.28 | 23.27 | 0.23 |
| S7 | −1790 | 307 | 2.54 | −0.33 | 0.32 | 21.97 | 0.22 |
| S8 | −1771 | 306 | 2.52 | −0.34 | 0.32 | 22.57 | 0.23 |
| System | Source of Light | Induction Time (s) | Peak Max. Time (s) | Rp-max (mol/dm3s) | ΔH (mJ/mg) | Cphoto-DSC (%) |
|---|---|---|---|---|---|---|
| Cationic photopolymerization | ||||||
| S1 | LED 405 nm | 3.0 | 18.4 | 0.0482 | 120.1 | 16.4 |
| MPM lamp 400–500 nm | 0.7 | 18.6 | 0.0482 | 124.8 | 16.4 | |
| S2 | LED 405 nm | 3.3 | 14.6 | 0.1341 | 185.1 | 25.6 |
| MPM lamp 400–500 nm | 0.3 | 12.0 | 0.1096 | 185.1 | 24.3 | |
| S3 | LED 405 nm | 3.5 | 13.2 | 0.1698 | 179.5 | 25.4 |
| MPM lamp 400–500 nm | 0.3 | 12.2 | 0.1541 | 178.4 | 23.4 | |
| S4 | LED 405 nm | 2.5 | 17.6 | 0.0944 | 235.0 | 31.7 |
| MPM lamp 400–500 nm | 0.8 | 18.1 | 0.1295 | 236.2 | 31.0 | |
| S5 | LED 405 nm | 3.4 | 21.5 | 0.0558 | 208.2 | 28.3 |
| MPM lamp 400–500 nm | 1.4 | 23.8 | 0.0444 | 174.0 | 23.3 | |
| S6 | LED 405 nm | 3.6 | 14.9 | 0.0548 | 307.6 | 30.8 |
| MPM lamp 400–500 nm | 0.2 | 12.3 | 0.1329 | 305.9 | 40.2 | |
| S7 | LED 405 nm | 2.0 | 15.2 | 0.0853 | 257.8 | 31.8 |
| MPM lamp 400–500 nm | 0.2 | 18.0 | 0.1108 | 266.5 | 35.0 | |
| S8 | LED 405 nm | 2.8 | 21.5 | 0.0422 | 180.4 | 29.1 |
| MPM lamp 400–500 nm | 0.2 | 21.5 | 0.0318 | 180.8 | 23.7 | |
| Free-radical photopolymerization | ||||||
| S1 | LED 405 nm | 3.2 | 12.0 | 0.6411 | 366.6 | 63.1 |
| MPM lamp 400–500 nm | 3.0 | 12.1 | 1.1921 | 342.9 | 57.3 | |
| S2 | LED 405 nm | 2.6 | 18.2 | 1.0104 | 430.8 | 74.2 |
| MPM lamp 400–500 nm | 3.3 | 12.0 | 1.9688 | 469.6 | 77.7 | |
| S3 | LED 405 nm | 2.1 | 12.6 | 1.4591 | 395.5 | 68.1 |
| MPM lamp 400–500 nm | 3.6 | 12.5 | 1.7048 | 414.1 | 68.8 | |
| S4 | LED 405 nm | 2.3 | 16.8 | 1.1681 | 449.0 | 77.3 |
| MPM lamp 400–500 nm | 4.1 | 12.2 | 2.1288 | 461.9 | 76.8 | |
| S5 | LED 405 nm | 3.9 | 17.2 | 1.1345 | 382.6 | 65.9 |
| MPM lamp 400–500 nm | 0.1 | 12.8 | 1.5791 | 356.2 | 60.6 | |
| S6 | LED 405 nm | 2.0 | 13.5 | 1.5901 | 419.1 | 72.2 |
| MPM lamp 400–500 nm | 0.3 | 12.9 | 2.0590 | 409.5 | 69.6 | |
| S7 | LED 405 nm | 2.7 | 13.5 | 1.9246 | 421.7 | 72.7 |
| MPM lamp 400–500 nm | 0.2 | 12.9 | 2.4357 | 412.5 | 69.9 | |
| S8 | LED 405 nm | 2.8 | 11.5 | 1.3935 | 449.1 | 77.3 |
| MPM lamp 400–500 nm | 0.3 | 11.6 | 1.5053 | 467.1 | 77.3 | |
| Structures of 2-Amino-4,6-Diphenyl-Pyridine-3-Carbonitrile Derivatives | ||
|---|---|---|
 |  |  |
| S1 | S2 | S3 |
 | 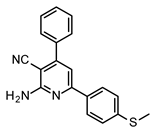 |  |
| S4 | S5 | S6 |
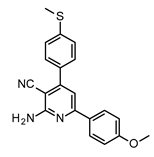 | 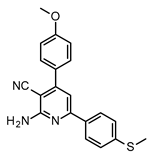 | |
| S7 | S8 | |
| Reference co-initiator | ||
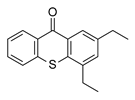 2,4-Diethyl-9H-thioxanthen-9-one (DETX) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Ortyl, J. Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts 2020, 10, 284. https://doi.org/10.3390/catal10030284
Fiedor P, Pilch M, Szymaszek P, Chachaj-Brekiesz A, Galek M, Ortyl J. Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts. 2020; 10(3):284. https://doi.org/10.3390/catal10030284
Chicago/Turabian StyleFiedor, Paweł, Maciej Pilch, Patryk Szymaszek, Anna Chachaj-Brekiesz, Mariusz Galek, and Joanna Ortyl. 2020. "Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light" Catalysts 10, no. 3: 284. https://doi.org/10.3390/catal10030284
APA StyleFiedor, P., Pilch, M., Szymaszek, P., Chachaj-Brekiesz, A., Galek, M., & Ortyl, J. (2020). Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts, 10(3), 284. https://doi.org/10.3390/catal10030284