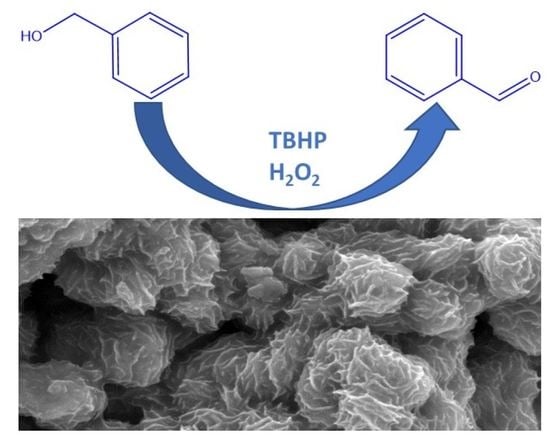
Selective Catalytic Oxidation of Benzyl Alcohol by MoO2 Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
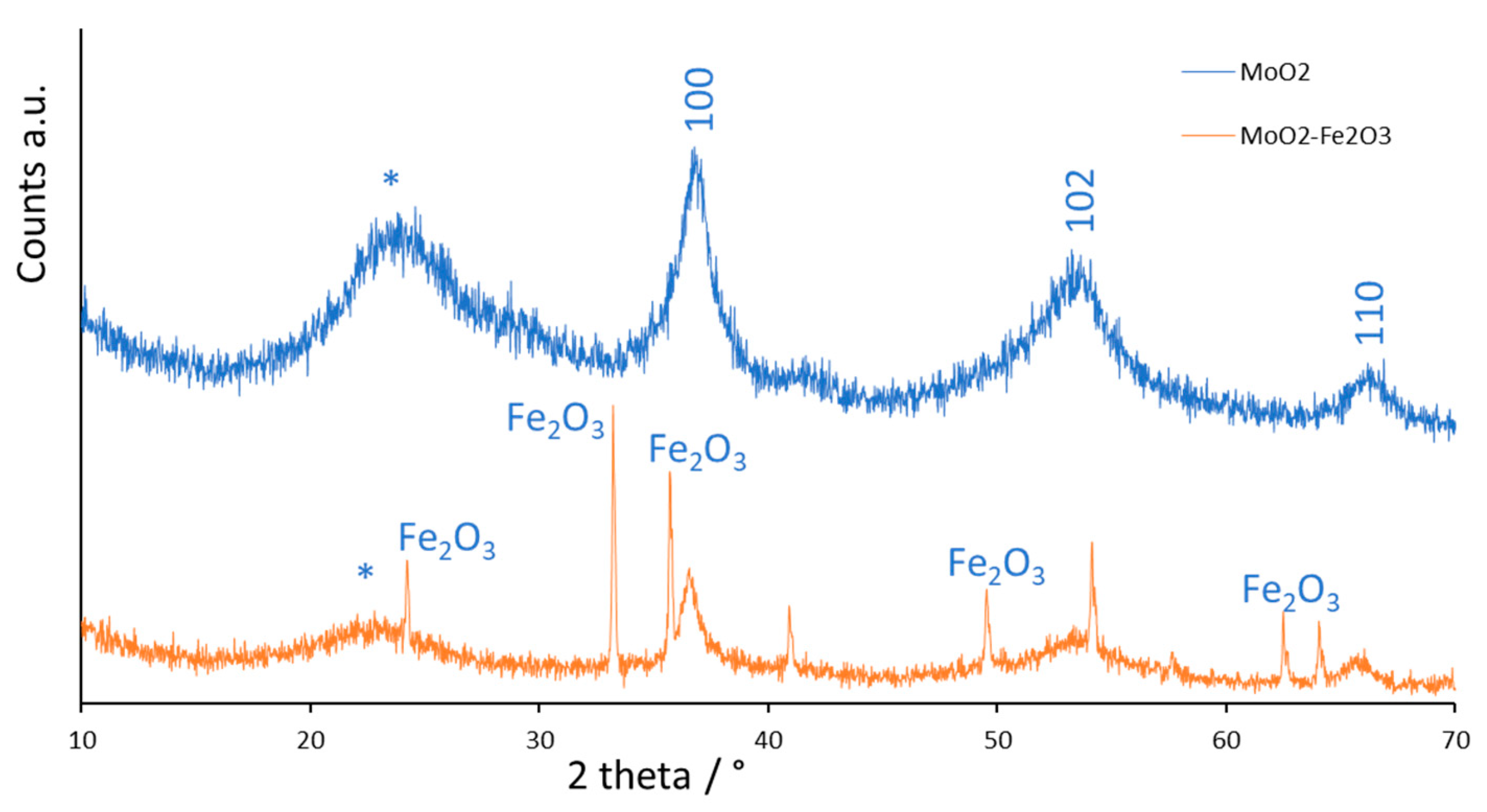
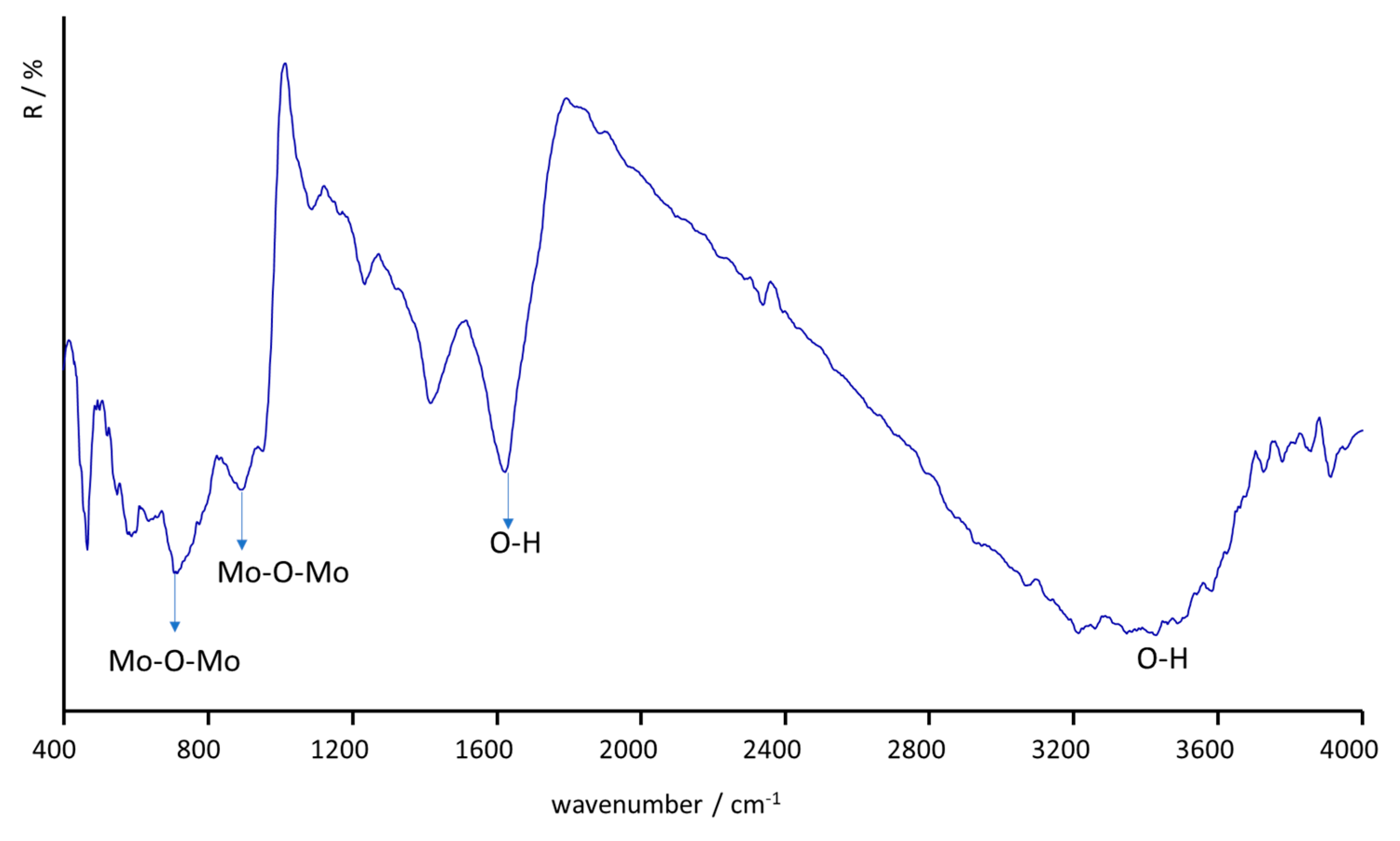
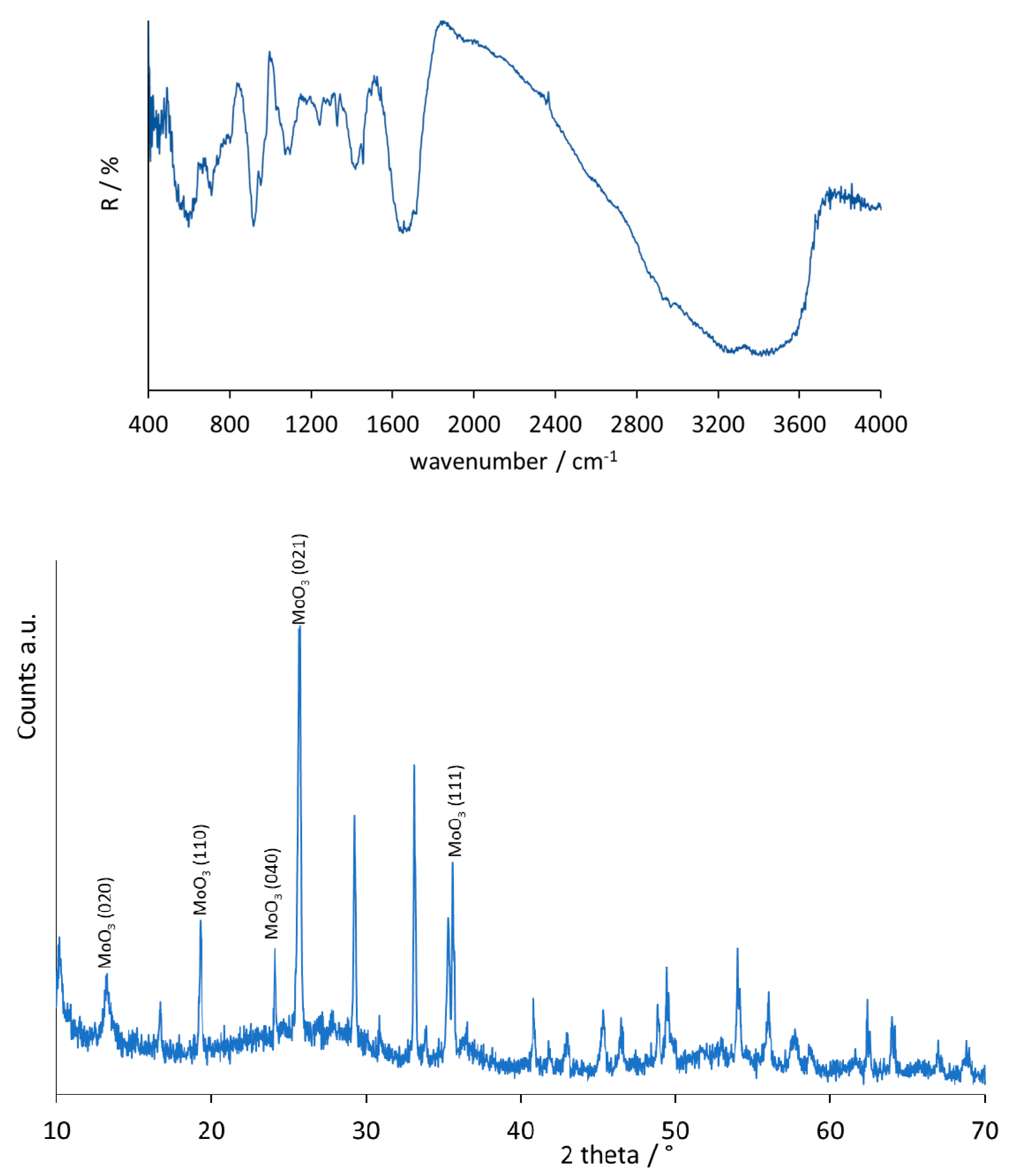
2.1. Characterization of the Catalyst
2.2. Catalytic Tests and Optimization of Alcohol Oxidation Conditions
3. Mechanistic Study
4. Materials and Methods
4.1. General
4.2. Catalytic Tests
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mahmoud, M.A.; Narayanan, R.; El-Sayed, M.A. Enhancing Colloidal Metallic Nanocatalysis: Sharp Edges and Corners for Solid Nanoparticles and Cage Effect for Hollow Ones. Acc. Chem. Res. 2013, 46, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Akbaria, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano Struct. Nano Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- Mai, L.; Yang, F.; Zhao, Y.; Xu, X.; Xu, L.; Hu, B.; Luo, Y.; Liu, H. Molybdenum oxide nanowires: Synthesis & properties. Mater. Today 2011, 14, 346–352. [Google Scholar]
- Kroschwitz, J.I.; Othmer, K. Encyclopedia of Chemical Technology; Wiley-Interscience Publications: New York, NY, USA, 1992; Volume 17, pp. 64–72. [Google Scholar]
- Nowicka, E.; Althahban, S.; Leah, T.D.; Shaw, G.; Morgan, D.; Kiely, C.J.; Roldan, A.; Hutchings, G.J. Benzyl alcohol oxidation with Pd-Zn/TiO2: Computational and experimental studies. Sci. Technol. Adv. Mater. 2019, 20, 367–378. [Google Scholar] [CrossRef]
- Albonetti, S.; Mazzoni, R.; Cavani, F. Homogeneous, Heterogeneous and Nanocatalysis. In Transition Metal Catalysis in Aerobic Alcohol Oxidation; Green Chemistry Series; RSC: London, UK, 2014; pp. 1–39. [Google Scholar]
- Ley, S.V.; Madin, A. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 7. [Google Scholar]
- Beller, M. The Current Status and Future Trends in Oxidation Chemistry. Adv. Synth. Catal. 2004, 346, 107–108. [Google Scholar] [CrossRef]
- Yang, L.C.; Gao, Q.S.; Zhang, Y.H.; Tang, Y.; Wu, Y.P. Tremella-like molybdenum dioxide consisting of nanosheets as an anode material for lithium ion battery. Electrochem. Commun. 2008, 10, 118–122. [Google Scholar] [CrossRef]
- Bento, A.; Sanches, A.; Medina, E.; Nunes, C.D.; Vaz, P.D. MoO2 nanoparticles as highly efficient olefin epoxidation catalysts. App. Catal. A Gen. 2015, 504, 399–407. [Google Scholar] [CrossRef]
- Shylesh, S.; Schweizer, J.; Demeshko, S.; Schunemann, V.; Ernst, S.; Thiel, W.R. Nanoparticle Supported, Magnetically Recoverable Oxodiperoxo Molybdenum Complexes: Efficient Catalysts for Selective Epoxidation Reactions. Adv. Synth. Catal. 2009, 351, 1789–1795. [Google Scholar] [CrossRef]
- Fernandes, C.I.; Carvalho, M.D.; Ferreira, L.P.; Nunes, C.D.; Vaz, P.D. Organometallic Mo complex anchored to magnetic iron oxide nanoparticles as highly recyclable epoxidation catalyst. J. Organomet. Chem. 2014, 760, 2–10. [Google Scholar] [CrossRef]
- Bento, A.; Sanches, A.; Vaz, P.D.; Nunes, C.D. Catalytic application of Fe-doped MoO2 tremella-like nanosheets. Top. Catal. 2016, 59, 1123–1131. [Google Scholar] [CrossRef]
- Nunes, C.D.; Rudic, S.; Vaz, P.D. Probing the relevance of MoO2 nanoparticles’ synthesis on their catalytic activity by inelastic neutron scattering. Phys. Chem. Chem. Phys. 2020, 22, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Uplane, M.D.; Patil, P.S. Structural and optical properties of electrodeposited molybdenum oxide thin films. Appl. Surf. Sci. 2006, 252, 8050–8056. [Google Scholar] [CrossRef]
- Wen, B.H.; Chernova, N.A.; Zhang, R.B.; Wang, Q.; Omenya, F.; Fang, J.; Whittingham, M.S. Layered Molybdenum (Oxy)Pyrophosphate as Cathode for Lithium-Ion Batteries. Chem. Mater. 2013, 25, 3513–3521. [Google Scholar] [CrossRef]
- Xia, T.A.; Li, Q.; Liu, X.D.; Meng, J.A.; Cao, X.Q. Morphology-Controllable Synthesis and Characterization of Single-Crystal Molybdenum Trioxide. J. Phys. Chem. B 2006, 110, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Metal-Catalyzed Epoxidations of Olefins with Hydroperoxides. In Aspects of Homogeneous Catalysis; Ugo, R., Ed.; Springer: Dordrecht, The Netherlands, 1981; Volume IV. [Google Scholar]
- Silva, N.U.; Fernandes, C.I.; Nunes, T.G.; Saraiva, M.S.; Nunes, C.D.; Vaz, P.D. Performance evaluation of mesoporous host materials in olefin epoxidation using Mo(II) and Mo(VI) active species—Inorganic vs. hybrid matrix. Appl. Catal. A Gen. 2011, 408, 105–116. [Google Scholar] [CrossRef]
- Doornkamp, C.; Ponec, V. The universal character of the Mars and Van Krevelen mechanism. J. Mol. Catal. A Chem. 2000, 162, 19–32. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Hatefi-Ardakani, M.; Saeednia, S.; Pakdin-Parizi, Z.; Rafeezadeh, M. Efficient and selective oxidation of alcohols with tert-BuOOH catalyzed by a dioxomolybdenum(VI) Schiff base complex under organic solvent-free conditions. Res. Chem. Intermed. 2016, 42, 7223–7230. [Google Scholar] [CrossRef]
- Biradar, A.V.; Dongare, M.K.; Umbarkar, S.B. Selective oxidation of aromatic primary alcohols to aldehydes using molybdenum acetylide oxo-peroxo complex as catalyst. Tetrahedron Lett. 2009, 50, 2885–2888. [Google Scholar] [CrossRef]
- Shojaei, A.F.; Tabatabaeian, K.; Zanjanchi, M.A.; Moafi, H.F.; Modirpanah, N. Synthesis, characterization and study of catalytic activity of Silver doped ZnO nanocomposite as an efficient catalyst for selective oxidation of benzyl alcohol. J. Chem. Sci. 2015, 127, 481–491. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Santos, A.; Teodoro, O.M.N.D.; Luque, R. Catalytic applications of a versatile magnetically separable Fe–Mo (Nanocat-Fe–Mo) nanocatalyst. Green Chem. 2013, 15, 682–689. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Q.; Zhao, H.; Zou, S. Efficient Room-Temperature Selective Oxidation of Benzyl Alcohol into Benzaldehyde over Pt/BiOCl Nanocomposite. Catal. Lett. 2018, 148, 1093–1099. [Google Scholar] [CrossRef]
- Khawaji, M.; Chadwick, D. Au-Pd Bimetallic Nanoparticles Immobilised on Titanate Nanotubes: A Highly Active Catalyst for Selective Oxidation. ChemCatChem 2017, 9, 4353–4363. [Google Scholar] [CrossRef]
- Kimi, M.; Jaidie, M.M.H.; Pang, S.C. Bimetallic Cu-Ni nanoparticles supported on activated carbon for catalytic oxidation of benzyl alcohol. J. Phys. Chem. Solids 2018, 112, 50–53. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Wang, H.; Li, Z.; Zheng, K.; Li, S.; Li, G. Transition metal-mediated catalytic properties of gold nanoclusters in aerobic alcohol oxidation. Nano Res. 2018, 11, 2139–2148. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, C.; Chena, R.; Chen, F. Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on epichlorohydrin-modified Fe3O4 microspheres. New J. Chem. 2015, 39, 4924–4932. [Google Scholar] [CrossRef]
- Nagy, G.; Beck, A.; Sáfrán, G.; Schay, Z.; Liu, S.; Li, T.; Qiao, B.; Wang, J.; Lázár, K. Nanodisperse gold catalysts in oxidation of benzyl alcohol: Comparison of various supports under different conditions. React. Kinet. Mech. Catal. 2019, 128, 71–95. [Google Scholar] [CrossRef]
- Boruah, J.J.; Das, S.P. Solventless, selective and catalytic oxidation of primary, secondary and benzylic alcohols by a Merrifield resin supported molybdenum(VI) complex with H2O2 as an oxidant. RSC Adv. 2018, 8, 34491–34504. [Google Scholar] [CrossRef]
- de Abreu, W.C.; Garcia, M.A.S.; Nicolodi, S.; de Moura, C.V.R.; de Moura, E.M. Magnesium surface enrichment of CoFe2O4 magnetic nanoparticles immobilized with gold: Reusable catalysts for green oxidation of benzyl alcohol. RSC Adv. 2018, 8, 3903–3909. [Google Scholar] [CrossRef]
- Fernandes, C.I.; Saraiva, M.S.; Nunes, T.G.; Vaz, P.D.; Nunes, C.D. Highly enantioselective olefin epoxidation controlled by helical confined environments. J. Catal. 2014, 309, 21–32. [Google Scholar] [CrossRef]
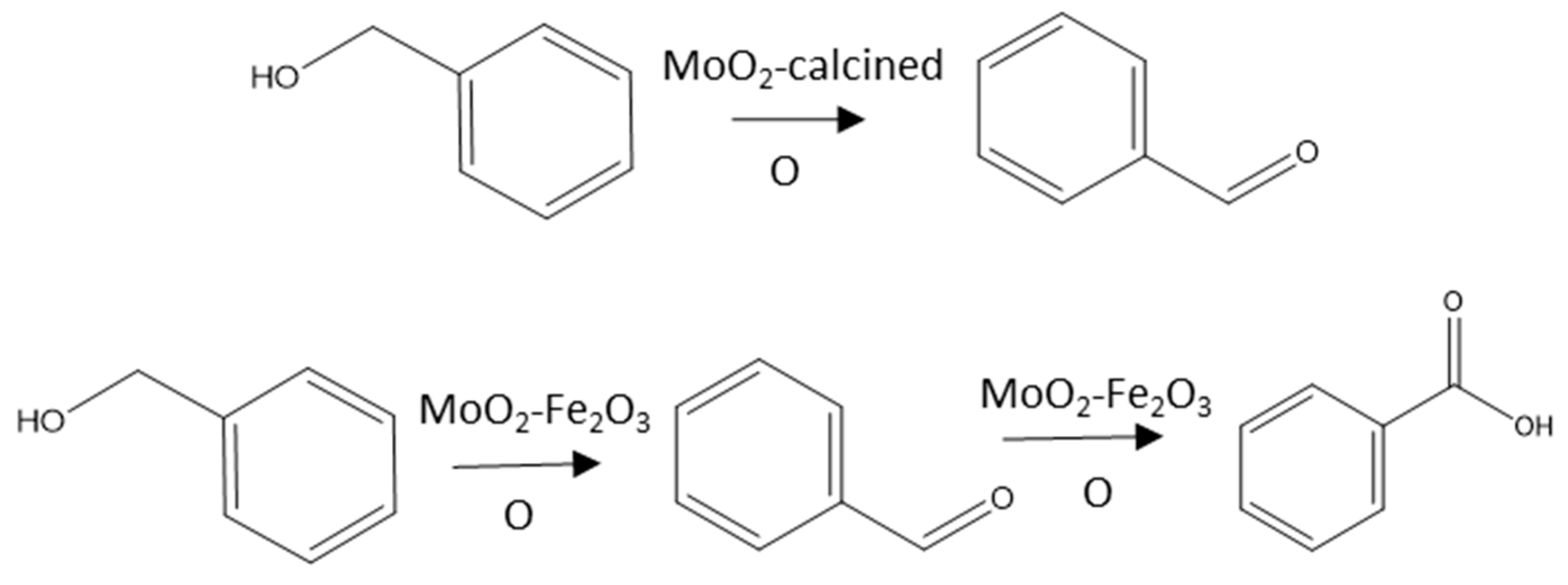

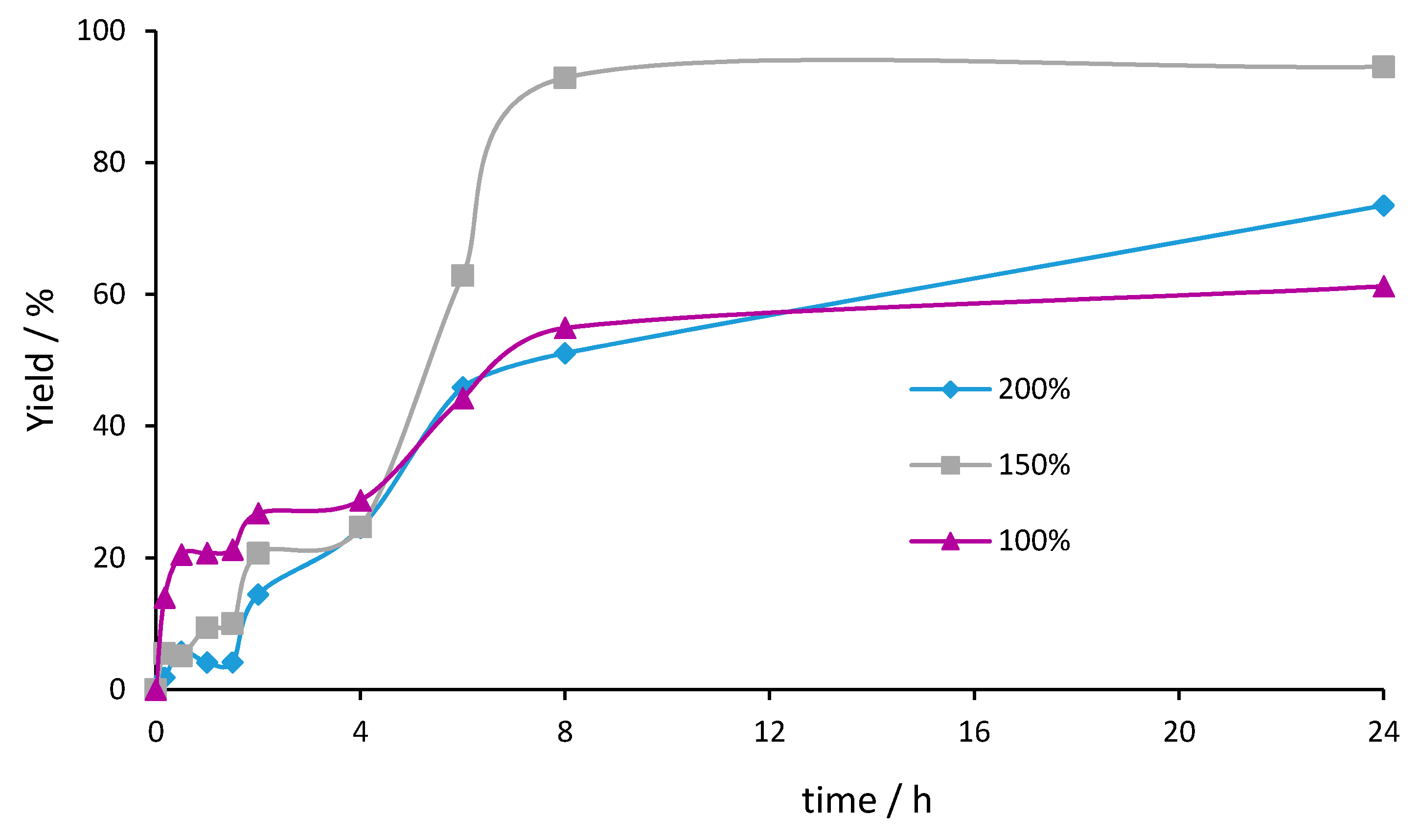
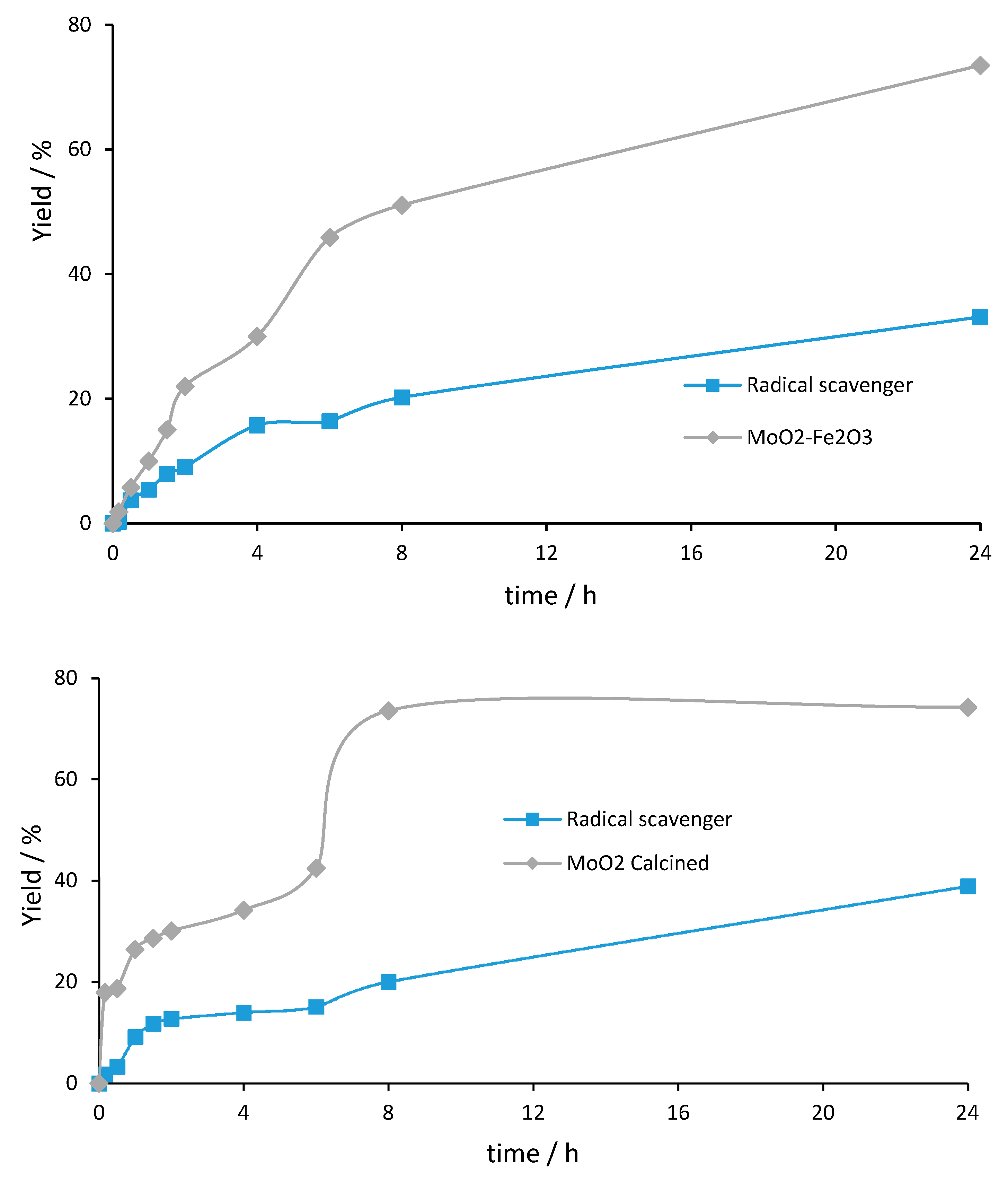
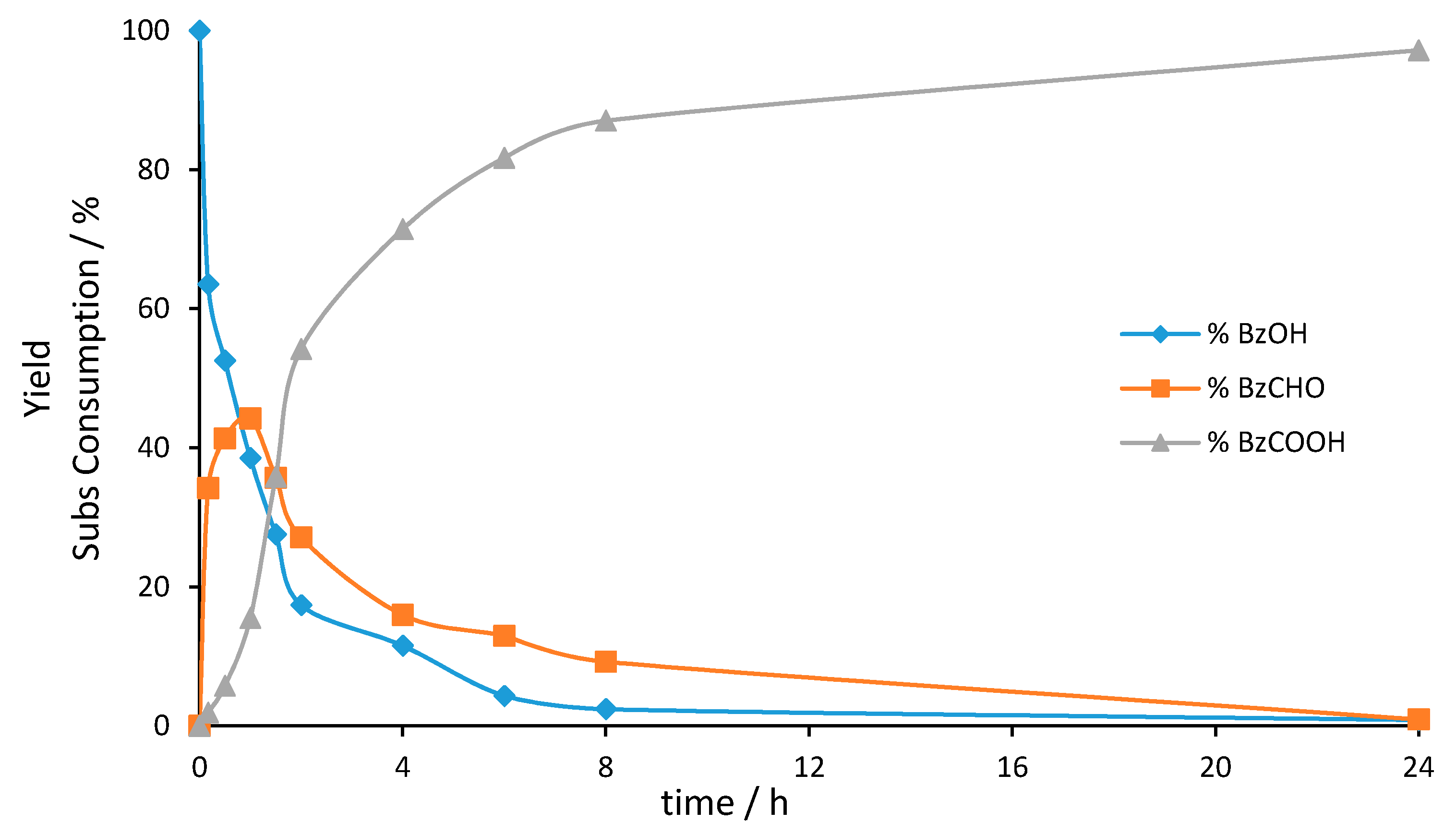
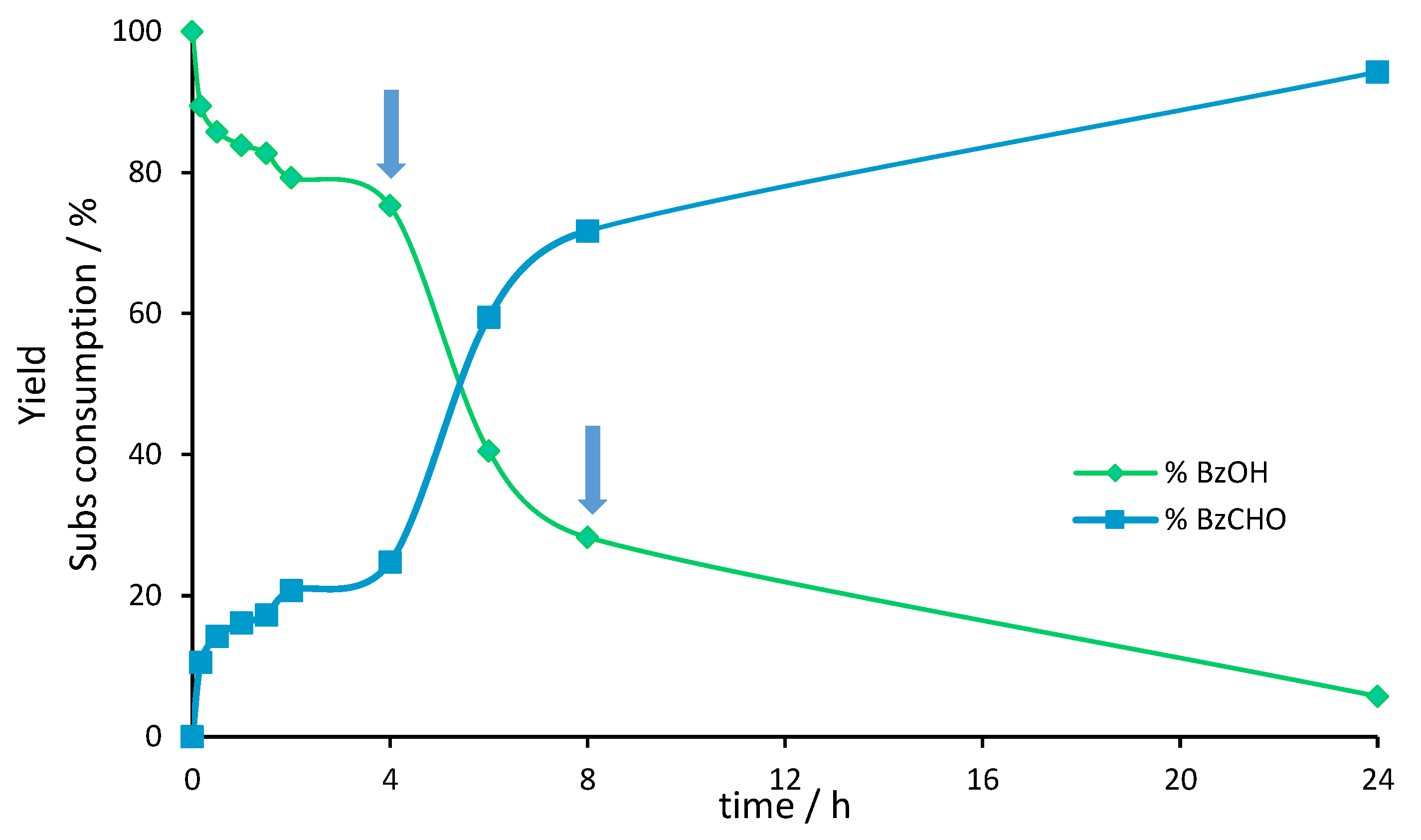
| Entry | Catalyst | Oxidant (mol%) | Yield (%) [a] | Selectivity (%) [b] | |
|---|---|---|---|---|---|
| BzCHO | BzCOOH | ||||
| 1 | MoO2–Fe2O3 | TBHP (200%) | 74 | 91 | 9 |
| 2 | TBHP (150%) | 95 | 92 | 7 | |
| 3 | TBHP (100%) | 61 | 92 | 7 | |
| 4 | Leaching 2 h/24 h | 22/27 | 100/100 | 0 | |
| 5 | MoO2 calcined (MoO2 NPs) | TBHP (200%) | 74 | 100 | 0 |
| 6 | TBHP (150%)(1st/2nd run) | 89/65 | 100/100 | 0/0 | |
| 7 | TBHP (100%) | 82 | 100 | 0 | |
| 8 | TBHP (200%) solventless | 69 | 100 | 0 | |
| 9 | γ-Fe2O3 | TBHP (200%) | 97 | 1 | 99 |
| Entry | Catalyst | Oxidant (mol%) | Conversion (%) [a] | Selectivity (%) [b] | |
|---|---|---|---|---|---|
| BzCHO | BzCOOH | ||||
| 1 | MoO2–Fe2O3 | TBHP (200%) | 74 | 91 | 9 |
| 2 | radical scavenger (200%) | 33 | 88 | 11 | |
| 3 | MoO2 calcined (MoO2 NPs) | TBHP (200%) | 74 | 100 | 0 |
| 4 | radical scavenger (200%) | 39 | 100 | 0 | |
| Entry | Catalyst | Oxidant | Solvent | Yield (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|
| BzCHO | BzCOOH | |||||
| 1 | MoO2–Fe2O3 | H2O2 | Acetonitrile 353 K | 25 | 100 | 0 |
| 2 | Acetonitrile 383 K | 23 | 97 | 1 | ||
| 3 | Decane 383 K | 16 | 96 | 2 | ||
| 4 | Solventless 383 K | 12 | 78 | 11 | ||
| 5 | New H2O2 addition at 4 h Acetonitrile 353 K | 48 | 100 | 0 | ||
| 6 | New H2O2 addition at 4 h and at 8 h Acetonitrile 353 K | 94 | 100 | 0 | ||
| Entry | Catalyst | Oxidant | Temp (°C) | Time (h) | Yield or Conv./% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Fe3O4–MoO3 | TBHP | 80 | 6 | 89 | [26] |
| 2 | Ag–ZnO | TBHP | 80 | 0.5 | 77 | [25] |
| 3 | Mo(VI) peroxo | H2O2 | 80 | 8 | 79 | [24] |
| 4 | Mo(VI)O2-Schif | TBHP | r.t. | 2 | 90 | [23] |
| 5 | Pt/BiOCl | O2 press | r.t. | 5 | 100 | [27] |
| 6 | Au–Pd–TiO2 | O2 4 bar | 120 | 1 | 10–50 | [28] |
| 7 | Cu–Ni–Carbon | H2O2 | 80 | 2 | 14–47 | [29] |
| 8 | Au-M (Cu, Ni, Co, Zn) | K2CO3/O2 | 80 | 16 | 23–81 (Conv) | [30] |
| 9 | Fe3O4–ECH | H2O2 | 100 | 1.5 | 8–34 | [31] |
| 10 | Au-Supported (SiO2, Al2O3, HAP, MgAl2O4, MgO) | O2/K2CO3 O2 5 bar | 60 150 | 3 5 | 60 (Conv) 47–94 (Conv) | [32] |
| 11 | MR–MS–Mo | H2O2 | 65 | 1–5 | 0–99 | [33] |
| 12 | MgO–CoFe2O4–Au | O2 2 bar | 100 | 2.5 | 18–42 | [34] |
| 13 | Pd–Zn–TiO2 | O2 1 bar | 120 | 1 | 3–55 (Conv) | [6] |
| 14 | MoO2–Fe2O3 | TBHP | 110 | 24 | 61–95 | This work |
| 15 | MoO2–Fe2O3 | H2O2 | 110 | 24 | 12–94 | This work |
| 16 | MoO2 calcined | TBHP | 110 | 24 | 74–89 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, F.; Nunes, C.D. Selective Catalytic Oxidation of Benzyl Alcohol by MoO2 Nanoparticles. Catalysts 2020, 10, 265. https://doi.org/10.3390/catal10020265
Gaspar F, Nunes CD. Selective Catalytic Oxidation of Benzyl Alcohol by MoO2 Nanoparticles. Catalysts. 2020; 10(2):265. https://doi.org/10.3390/catal10020265
Chicago/Turabian StyleGaspar, Filipe, and Carla D. Nunes. 2020. "Selective Catalytic Oxidation of Benzyl Alcohol by MoO2 Nanoparticles" Catalysts 10, no. 2: 265. https://doi.org/10.3390/catal10020265
APA StyleGaspar, F., & Nunes, C. D. (2020). Selective Catalytic Oxidation of Benzyl Alcohol by MoO2 Nanoparticles. Catalysts, 10(2), 265. https://doi.org/10.3390/catal10020265