The Improvement of Coralline-Like ZnGa2O4 by Cocatalysts for the Photocatalytic Degradation of Rhodamine B
Abstract
1. Introduction
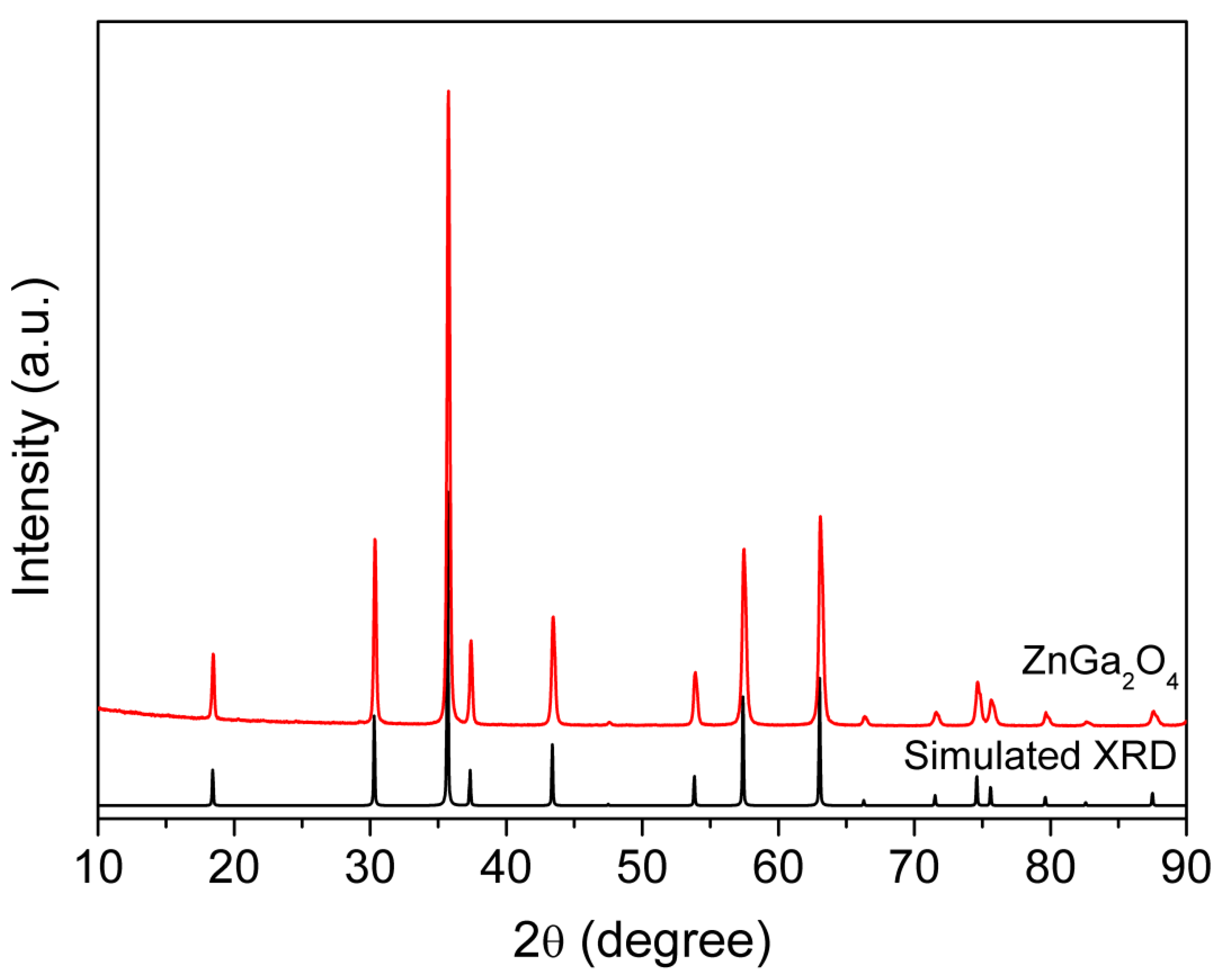
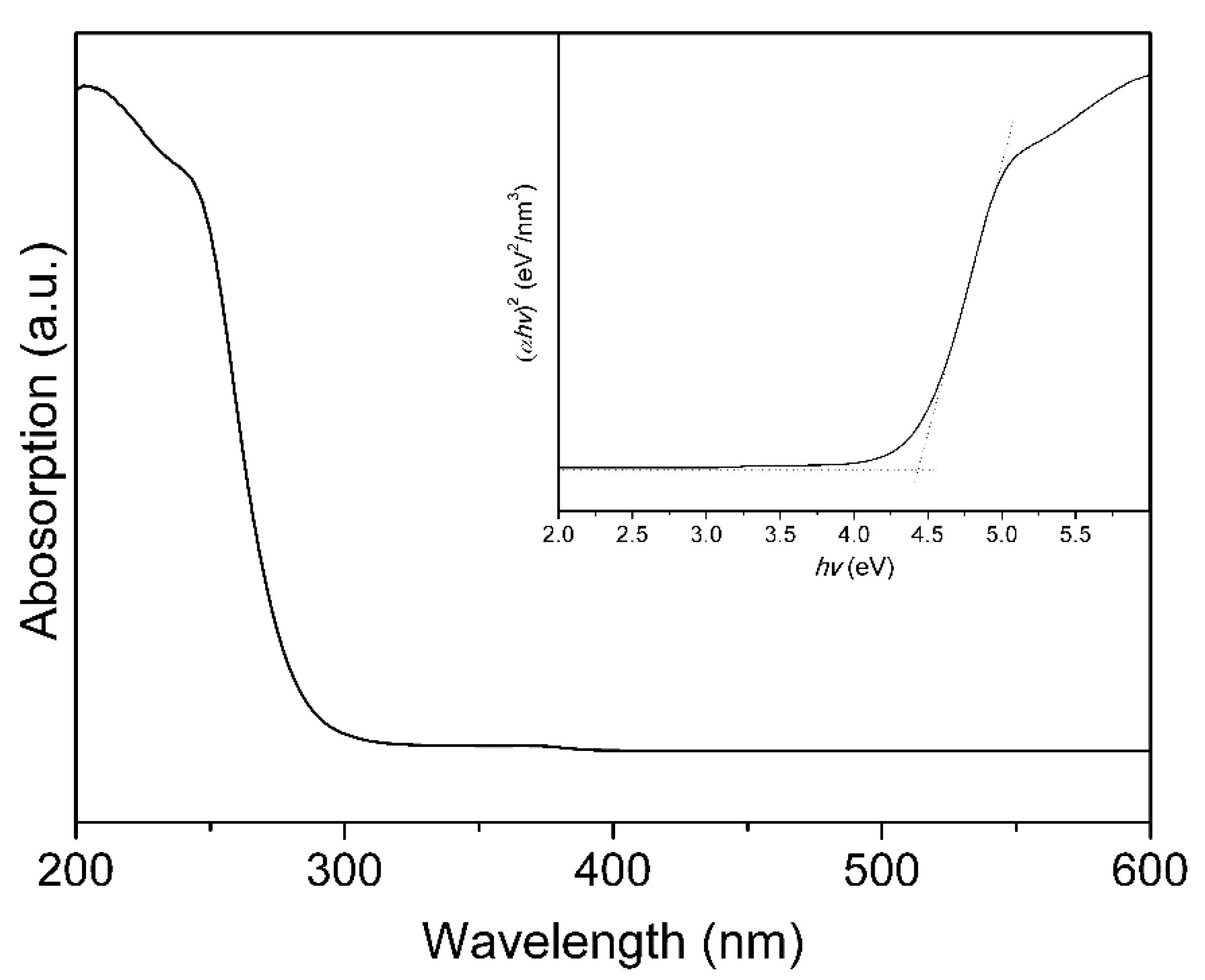
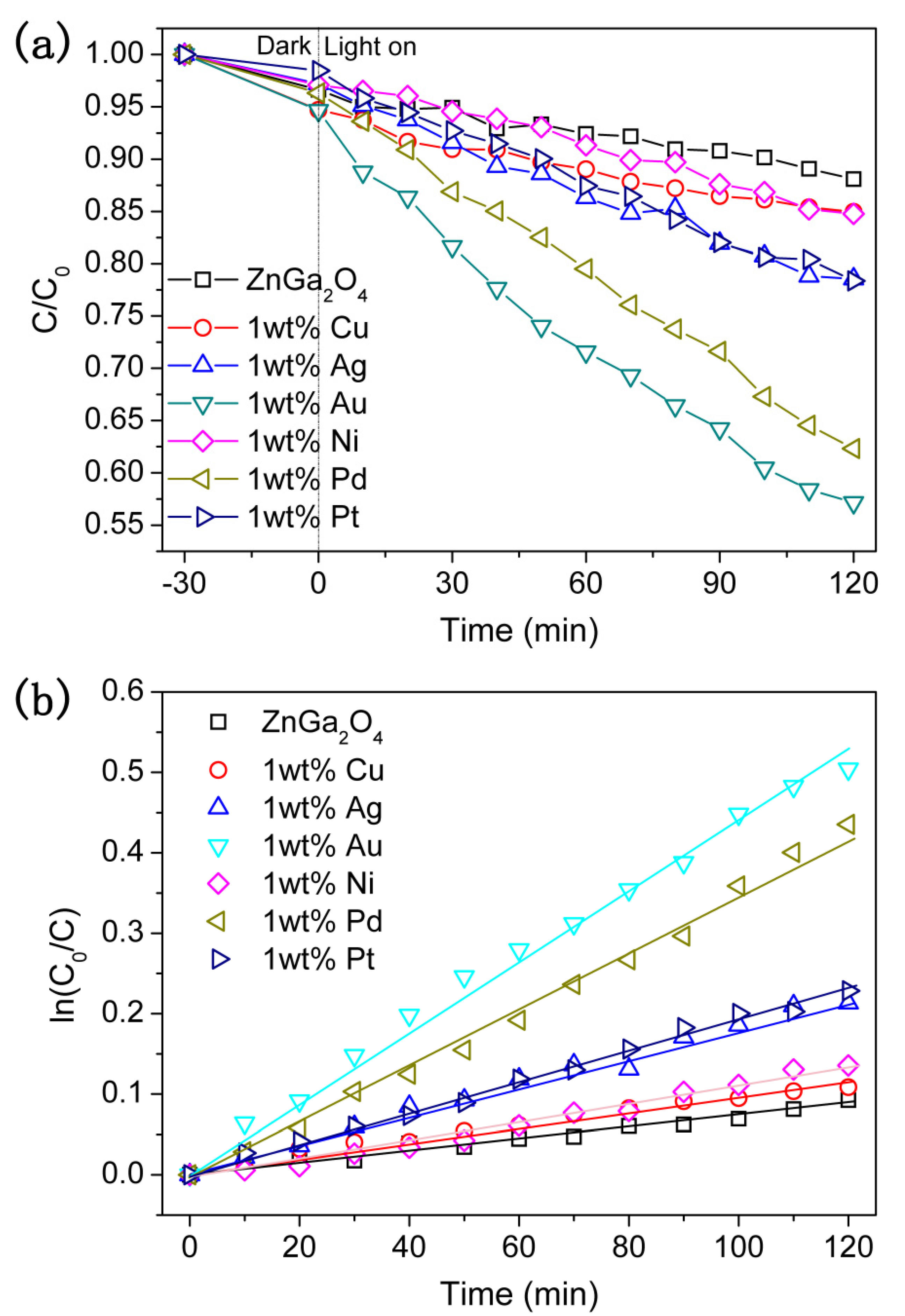
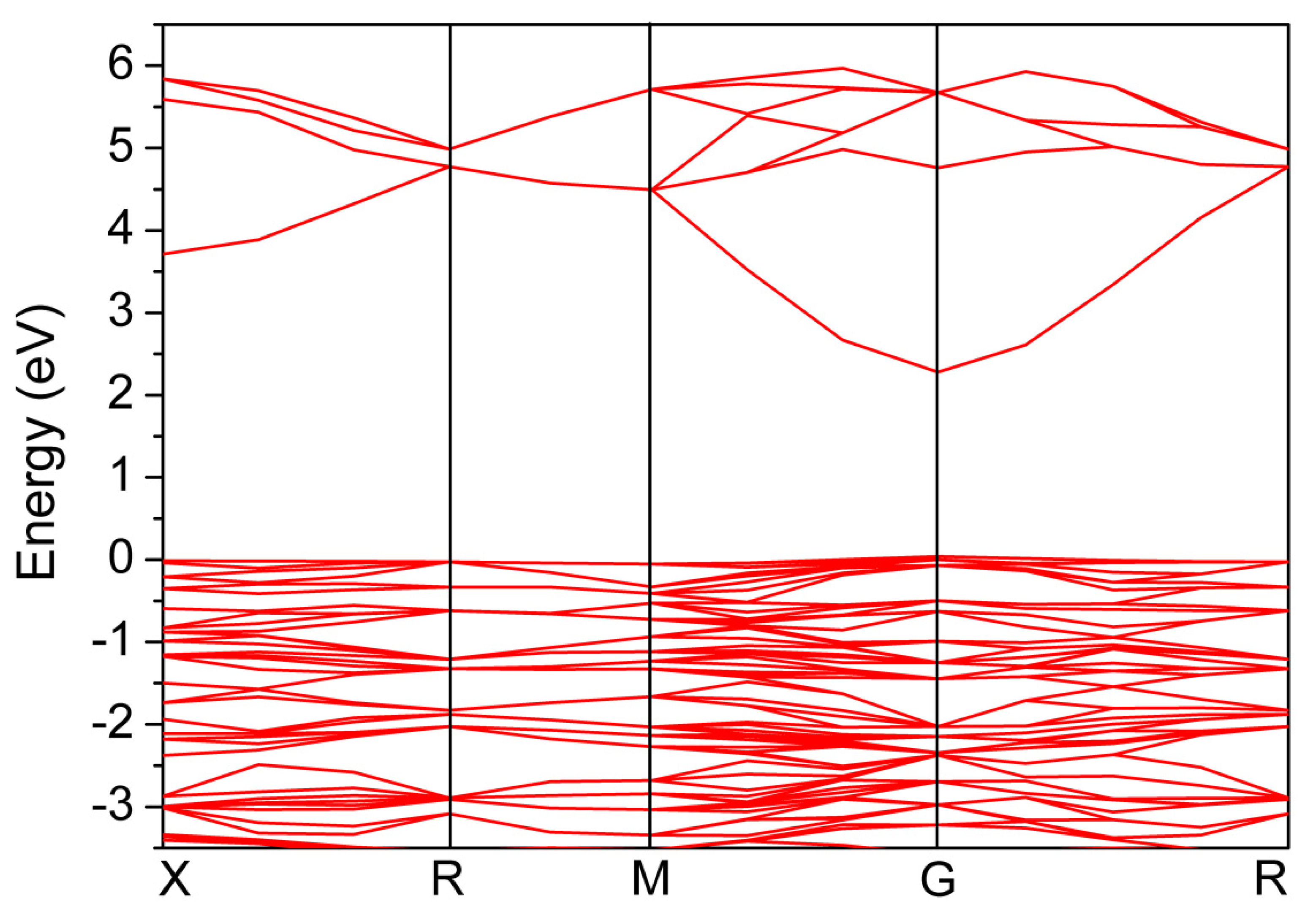
2. Results and Discussion
3. Experimental Section
3.1. Preparation of the Photocatalyst
3.2. Characterizations of the Photocatalyst
3.3. Calculations of Band Structure
3.4. Photocatalytic Degradation Performance Measurements
3.5. Cocatalysts
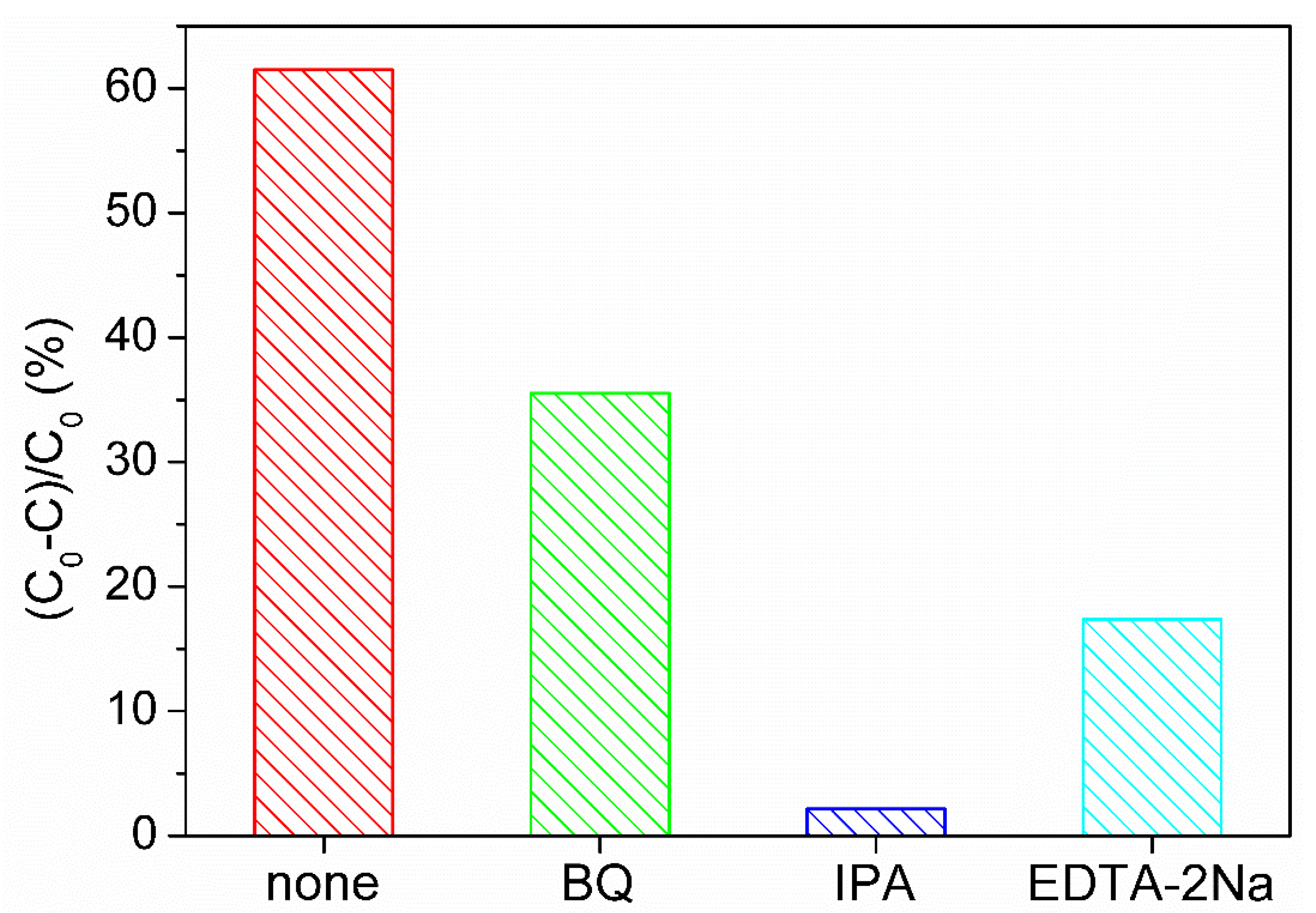
3.6. Reactive Species Scavenging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.I.; Kottaisamy, M.; Rama, N.; Ramachandra, R.; Bhattacharya, S.S. Thin film luminescence of ZnGa2O4: Mn deposited by PLD. Scr. Mater. 2006, 54, 237–240. [Google Scholar] [CrossRef]
- Cao, M.; Djerdj, I.; Antonietti, M.; Niederberger, M. Nonaqueous synthesis of colloidal ZnGa2O4 nanocrystallites and their photoluminescence properties. Chem. Mater. 2007, 19, 1239, 5830–5832. [Google Scholar]
- Teng, Y.; Song, L.X.; Liu, W.; Xu, Z.Y.; Wang, Q.S.; Ruan, M.M. Monodispersed hierarchical ZnGa2O4 microflowers for self-powered solar-blind detection. J. Mater. Chem. C 2016, 4, 3113–3118. [Google Scholar] [CrossRef]
- Bessiere, A.; Sharma, S.K.; Basavaraju, N.; Priolkar, K.R.; Binet, L.; Viana, B.; Bos, A.J.J.; Maldiney, T.; Richard, C.; Scherman, D.; et al. Storage of visible light for long-lasting phosphorescence in chromium-doped Zinc Gallate. Chem. Mater. 2014, 26, 1365–1373. [Google Scholar] [CrossRef]
- Yan, S.C.; Ouyang, S.X.; Gao, J.; Yang, M.; Feng, J.Y.; Fan, X.X.; Wan, L.J.; Li, Z.S.; Ye, J.H.; Zhou, Y.; et al. A room-temperature reactive-template route to mesoporous ZnGa2O4 with improved photocatalytic activity in reduction of CO2. Angew. Chem. Int. Ed. 2010, 49, 6400. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.M.; Hu, T.; Hou, N.J.; Liu, S.Y.; Gao, W.L.; Cong, R.H.; Yang, T. Photocatalytic pure water splitting activities for ZnGa2O4 synthesized by various methods. Mater. Res. Bull. 2015, 61, 481–485. [Google Scholar] [CrossRef]
- Zheng, T.T.; Xia, Y.G.; Jiao, X.L.; Wang, T.; Chen, D.R. Enhanced photocatalytic activities of single-crystalline ZnGa2O4 nanoprisms by the coexposed {111} and {110} facets. Nanoscale 2017, 9, 3206–3212. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Tang, L.Q.; Li, Y.Q.; Zhou, Y.; Liu, J.M.; Zou, Z.G. Robust, double-shelled ZnGa2O4 hollow spheres for photocatalytic reduction of CO2 to methane. Dalton Trans. 2017, 46, 10564–10568. [Google Scholar] [CrossRef]
- Liu, J.; Wei, L.; Wu, H.Z.; Jin, L.; Hu, B.; Li, L.L.; Wang, Z.L. In situ synthesis of rice-like ZnGa2O4 for the photocatalytic removal of organic and inorganic pollutants. Mater. Sci. Semicond. Process. 2016, 56, 251–259. [Google Scholar] [CrossRef]
- Chen, X.; Xue, H.; Li, Z.H.; Wu, L.; Wang, X.X.; Fu, X.Z. Ternary wide band gap p-block metal semiconductor ZnGa2O4 for photocatalytic benzene degradation. J. Phys. Chem. C 2017, 112, 20393–20397. [Google Scholar] [CrossRef]
- Tien, L.C.; Tseng, C.C.; Chen, Y.L.; Ho, C.H. Direct vapor transport synthesis of ZnGa2O4 nanowires with superior photocatalytic activity. J. Alloy. Compd. 2013, 555, 325–329. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, H.; Meng, S.G.; Zhang, L.; Fu, X.L.; Chen, S.F. One-pot hydrothermal synthesis of highly efficient SnOx/Zn2SnO4 composite photocatalyst for the degradation of methyl orange and gaseous benzene. Appl. Catal. B Environ. 2017, 200, 19–30. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.H.; Li, H.; Huang, H.J.; Zhao, H. Suppression of photocatalysis and long-lasting luminescence in ZnGa2O4 by Cr3+ doping. RSC Adv. 2015, 5, 57193–57200. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.Z.; Zhang, W.J.; Chen, Z.X.; Huang, H.J.; Li, W.J.; He, Y.H.; Fu, X.Z. Rapid microwave hydrothermal synthesis of ZnGa2O4 with high photocatalytic activity toward aromatic compounds in air and dyes in liquid water. J. Solid State Chem. 2012, 190, 135–142. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhang, J.Y.; Chen, Z.Y.; Wang, T.M. Photocatalytic degradation of methylene blue by ZnGa2O4 thin films. Catal. Commun. 2009, 10, 1781–1785. [Google Scholar] [CrossRef]
- Liu, L.L.; Huang, J.F.; Cao, L.Y.; Wu, J.P.; Fei, J.; Ouyang, H.B. Synthesis of ZnGa2O4 octahedral crystallite by hydrothermal method with the aid of CTAB and its photocatalytic activity. Mater. Lett. 2013, 95, 160–163. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Huang, J.J.; Tu, W.X.; Huang, S.M. Synthesis of uniform ZnGa2O4 nanoparticles with high photocatalytic activity. J. Alloy. Compd. 2014, 616, 461–467. [Google Scholar] [CrossRef]
- Liu, L.L.; Cao, L.Y.; Huang, J.F.; Zhang, X.W.; Wu, J.P.; Wang, K.T. Preparation and Photocatalytic Properties of the Octahedral ZnGa2O4 Crystallites. Chin. J. Inorg. Chem. 2012, 28, 2091–2096. [Google Scholar]
- Li, X.F.; Zhang, X.Y.; Zheng, X.Z.; Shao, Y.; He, M.; Wang, P.; Fu, X.Z.; Li, D.Z. A facile preparation of ZnGa2O4 photonic crystals with enhanced light absorption and photocatalytic activity. J. Mater. Chem. A 2014, 2, 15796–15802. [Google Scholar] [CrossRef]
- Ma, B.; Cong, R.H.; Gao, W.L.; Yang, T. Photocatalytic overall water splitting over an open-framework gallium borate loaded with various cocatalysts. Catal. Commun. 2015, 71, 17–20. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, P.F.; Yue, M.F.; Yang, D.F.; Cong, R.H.; Gao, W.L.; Yang, T. Bi2Ga4O9: An undoped single-phase photocatalyst for overall water splitting under visible light. J. Catal. 2017, 345, 236–244. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, Q.M.; Dai, C.H.; Zhou, M.J.; Liu, Y.N. Preparation and characterization of noble metal (Pt, Ag, Ru) loaded ZnGa2O4 and its photocatalytic and photoelectric performance. J. Mater. Sci. Mater. Electron. 2017, 28, 17917–17924. [Google Scholar] [CrossRef]
- Huang, X.; Jing, Y.; Yang, J.; Ju, J.; Cong, R.H.; Gao, W.L.; Yang, T. Flower-like nanostructure MNb2O6 (M = Mn, Zn) with high surface area: Hydrothermal synthesis and enhanced photocatalytic performance. Mater. Res. Bull. 2014, 51, 271–276. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Song, K.; Yang, J.; Sun, Y.; Wang, Z.Y.; Wang, L.; Cong, R.H.; Yang, T. Improving photocatalytic water reduction activity for In2TiO5 by loading metal cocatalysts. J. Alloy. Compd. 2015, 646, 277–282. [Google Scholar] [CrossRef]
- Ikarashi, K.; Sato, J.; Kobayashi, H.; Saito, N.; Inoue, Y. Photocatalysis for water decomposition by RuO2-dispersed ZnGa2O4 with d(10) configuration. J. Phys. Chem. B 2002, 106, 9048–9053. [Google Scholar] [CrossRef]
- Yang, H.X.; Shan, B.Q.; Zhang, L. A new composite membrane based on Keggin polyoxotungstate/poly (vinylidene fluoride) and its application in photocatalysis. RSC Adv. 2014, 4, 61226–61231. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.R.; Zeng, C.M.; Deng, Q.H.; Hu, Y.L.; Zeng, T.; Shi, J.W. Effect of La-doped scheelite-type SrWO4 for photocatalytic H2 production. Ionics 2019, 25, 5083–5089. [Google Scholar] [CrossRef]
- Qian, K.; Sweeny, B.C.; Johnston-Peck, A.C.; Niu, W.X.; Graham, J.O.; DuChene, J.S.; Qiu, J.J.; Wang, Y.C.; Engelhard, M.H.; Su, D.; et al. Surface plasmon-driven water reduction: Gold nanoparticle size matters. J. Am. Chem. Soc. 2014, 136, 9842–9845. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Ouyang, S.X.; Ye, J.H. Simple room-temperature mineralization method to SrWO4 micro/nanostructures and their photocatalytic properties. J. Phys. Chem. C. 2011, 115, 15778–15784. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.R.; Zeng, C.M.; Wang, X.T.; Hu, Y.L.; Zeng, T.; Shi, J.W. Highly improved photocatalytic degradation of rhodamine B over Bi2Ga4-xFexO9 solid solutions under visible light irradiation. RSC Adv. 2019, 9, 2945–2949. [Google Scholar] [CrossRef]
- Regulska, E.; Breczko, J.; Basa, A. Pristine and graphene-quantum-dots-decorated spinel nickel aluminate for water remediation from dyes and toxic pollutants. Water 2019, 11, 953. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.L.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-Principles Simulation: Ideas, Illustrations and the CASTEP Code. J. Phys. Condens. Matter 2002, 14, 2717–2743. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wang, G.J.; Jing, Y.; Ju, J.; Yang, D.F.; Yang, J.; Gao, W.L.; Cong, R.H.; Yang, T. Ga4B2O9: An efficient borate photocatalyst for overall water splitting without cocatalyst. Inorg. Chem. 2015, 54, 2945–2949. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sun, X.; Yang, W.; Zhu, M.; Shi, J. The Improvement of Coralline-Like ZnGa2O4 by Cocatalysts for the Photocatalytic Degradation of Rhodamine B. Catalysts 2020, 10, 221. https://doi.org/10.3390/catal10020221
Yang J, Sun X, Yang W, Zhu M, Shi J. The Improvement of Coralline-Like ZnGa2O4 by Cocatalysts for the Photocatalytic Degradation of Rhodamine B. Catalysts. 2020; 10(2):221. https://doi.org/10.3390/catal10020221
Chicago/Turabian StyleYang, Jia, Xiaorui Sun, Wanxi Yang, Meixia Zhu, and Jianwei Shi. 2020. "The Improvement of Coralline-Like ZnGa2O4 by Cocatalysts for the Photocatalytic Degradation of Rhodamine B" Catalysts 10, no. 2: 221. https://doi.org/10.3390/catal10020221
APA StyleYang, J., Sun, X., Yang, W., Zhu, M., & Shi, J. (2020). The Improvement of Coralline-Like ZnGa2O4 by Cocatalysts for the Photocatalytic Degradation of Rhodamine B. Catalysts, 10(2), 221. https://doi.org/10.3390/catal10020221




