Mercury(II)-Catalyzed Cleavage, Isomerization and Depurination of RNA and DNA Model Compounds and Desulfurization of Their Phosphoromonothioate Analogs
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Reaction Kinetics
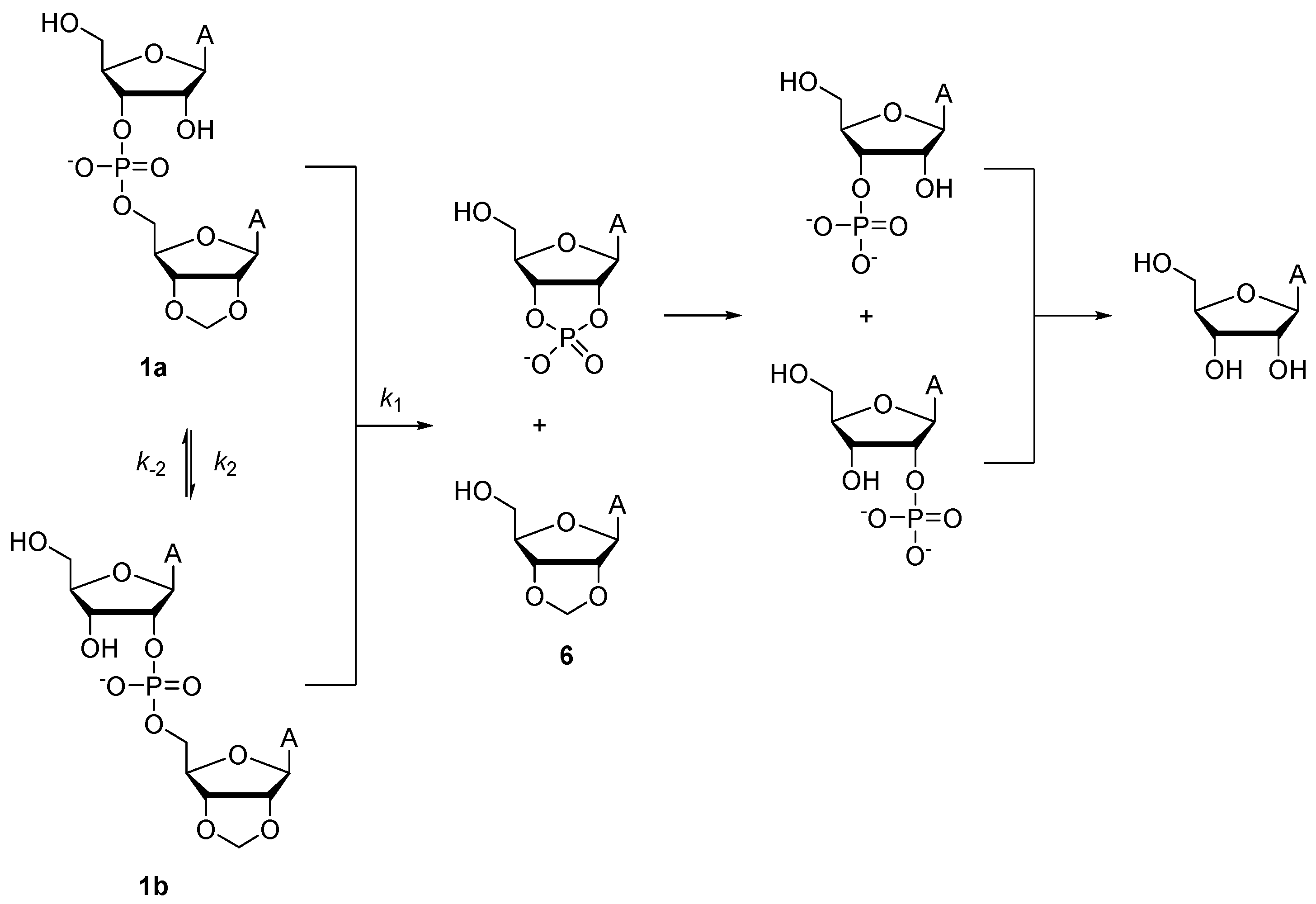
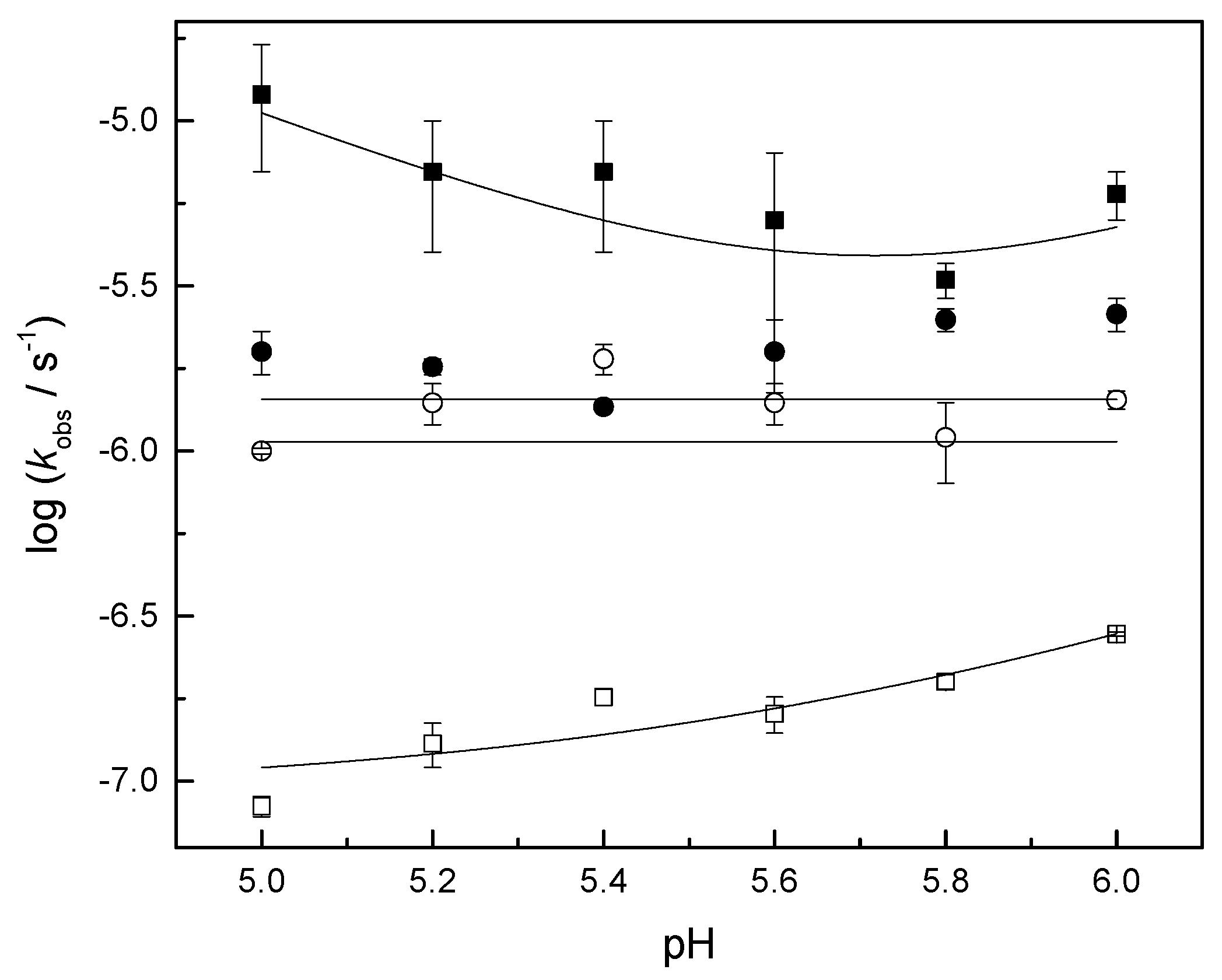
2.2.1. Cleavage and Isomerization of Adenylyl-3′,5′-(2′,3′-O-Methyleneadenosine) (1a)
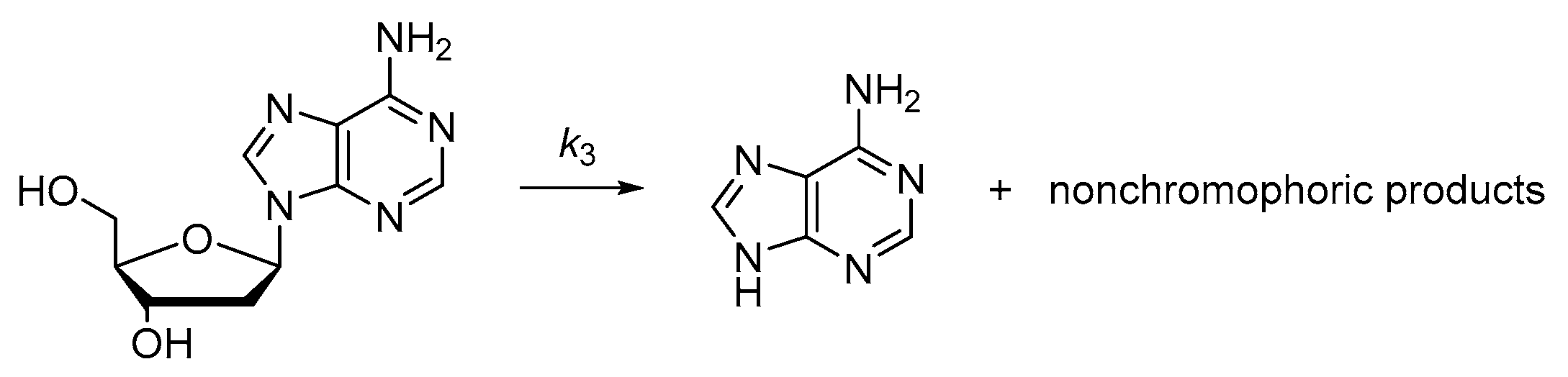
2.2.2. Depurination of 2′-Deoxyadenosine
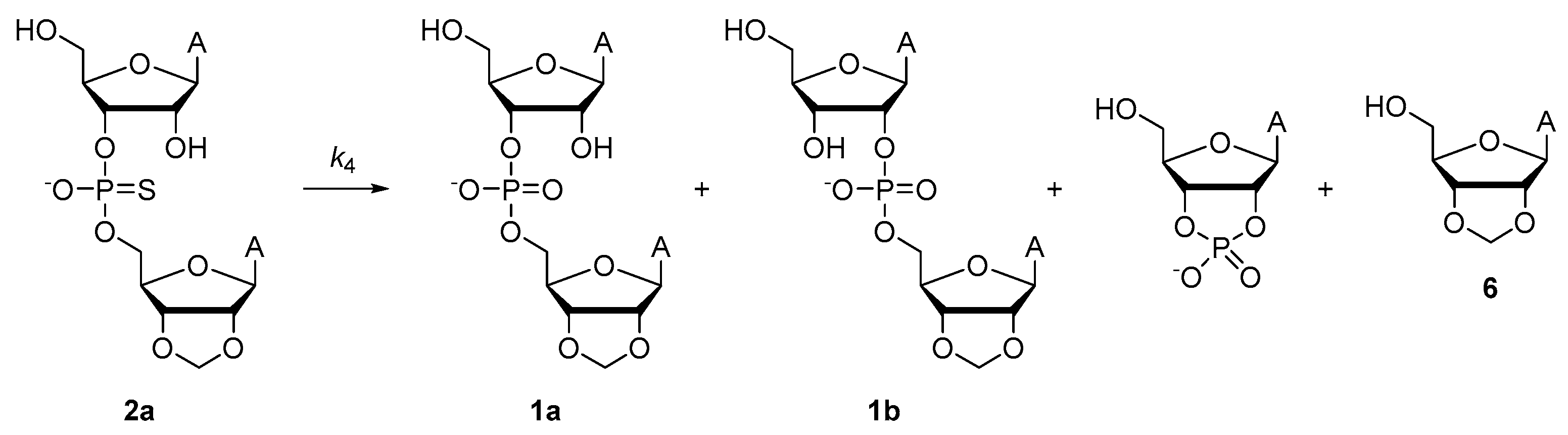
2.2.3. Desulfurization of Adenylyl-3′,5′-(2′,3′-O-Methyleneadenosine) Phosphoromonothioate (2a)
3. Materials and Methods
3.1. General
3.2. Materials
3.3. Kinetic Measurements
3.4. 2′-O-(tert-Butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)-adenylyl-3′,5′-(2′,3′-O-methyleneadenosine) (4)
3.5. 2′-O-(tert-Butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)-adenylyl-3′,5′-(2′,3′-O-methyleneadenosine) phosphoromonothioate (5)
3.6. Adenylyl-3′,5′-(2′,3′-O-methyleneadenosine) (1a)
3.7. Adenylyl-3′,5′-(2′,3′-O-methyleneadenosine) phosphoromonothioate (2a)
3.8. N6,N6-dibenzoyl-2′,3′-O-methyleneadenosine (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dallas, A.; Vlassov, A.V.; Kazakov, S.A. Principles of Nucleic Acid Cleavage by Metal Ions. In Artificial Nucleases; Zenkova, M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 61–88. [Google Scholar]
- Lönnberg, T. Understanding Catalysis of Phosphate-Transfer Reactions by the Large Ribozymes. Chem. Eur. J. 2011, 17, 7140–7153. [Google Scholar] [CrossRef] [PubMed]
- Forconi, M.; Herschlag, D. Metal ion-based RNA cleavage as a structural probe. Methods Enzymol. 2009, 468, 91–106. [Google Scholar] [PubMed]
- Pyle, A.M. Ribozymes: A distinct class of metalloenzymes. Science 1993, 261, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Sigel, R.K.O.; Sigel, H. 3.21-Metal-Ion Interactions with Nucleic Acids and Their Constituents. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 623–660. [Google Scholar]
- Ghidini, A.; Murtola, M.; Strömberg, R. Oligonucleotide Based Artificial Ribonucleases (OBANs). DNA Supramol. Chem. Nanotechnol. 2014. [Google Scholar] [CrossRef]
- Komiyama, M. Cut-and-paste of DNA using an artificial restriction DNA cutter. Int. J. Mol. Sci. 2013, 14, 3343–3357. [Google Scholar] [CrossRef]
- Aiba, Y.; Sumaoka, J.; Komiyama, M. Artificial DNA cutters for DNA manipulation and genome engineering. Chem. Soc. Rev. 2011, 40, 5657–5668. [Google Scholar] [CrossRef]
- Kuzuya, A.; Komiyama, M. Site-selective artificial ribonucleases and their applications. Curr. Org. Chem. 2007, 11, 1450–1459. [Google Scholar] [CrossRef]
- Morrow, J.R.; Iranzo, O. Synthetic metallonucleases for RNA cleavage. Curr. Opin. Chem. Biol. 2004, 8, 192–200. [Google Scholar] [CrossRef]
- Mikkola, S.; Lönnberg, T.; Lönnberg, H. Phosphodiester models for cleavage of nucleic acids. Beilstein J. Org. Chem. 2018, 14, 803–837. [Google Scholar] [CrossRef]
- Ora, M.; Lönnberg, T.; Lönnberg, H. Thio Effects as a Tool for Mechanistic Studies of the Cleavage of RNA Phosphodiester Bonds: The Chemical Basis. In From Nucleic Acids Sequences to Molecular Medicine; Erdmann, V.A., Barciszewski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–65. [Google Scholar]
- Harris, M.E. Identification and Characterization of Metal Ion Coordination Interactions with RNA by Quantitative Analysis of Thiophilic Metal Ion Rescue of Site-Specific Phosphorothioate Modifications. In Handbook of RNA Biochemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2014; Volumes 1 and 2, pp. 285–299. [Google Scholar]
- Forconi, M.; Herschlag, D. Use of phosphorothioates to identify sites of metal-ion binding in RNA. Methods Enzymol. 2009, 468, 311–333. [Google Scholar]
- Frederiksen, J.K.; Piccirilli, J.A. Identification of catalytic metal ion ligands in ribozymes. Methods 2009, 49, 148–166. [Google Scholar] [CrossRef]
- Christian, E.L. Identification and Characterization of Metal Ion Binding by Thiophilic Metal Ion Rescue. In Handbook of RNA Biochemistry; Wiley-VCH: Weinheim, Germany, 2008; pp. 319–344. [Google Scholar]
- Huang, P.-J.J.; Wang, F.; Liu, J. Cleavable Molecular Beacon for Hg2+ Detection Based on Phosphorothioate RNA Modifications. Anal. Chem. 2015, 87, 6890–6895. [Google Scholar] [CrossRef]
- Sjöberg, S. Metal-Complexes with Mixed-Ligands 11 formation of ternary mononuclear and polynuclear mercury (II) complexes in system Hg2+-Cl-OH-potentiometric study in 3.0 M (Na)ClO4, Cl media. Acta Chem. Scand. 1977, 31, 705–717. [Google Scholar] [CrossRef][Green Version]
- Lönnberg, H. Mechanisms for the solvolytic decompositions of nucleoside analogs.4. The effect of metal-ions on the acidic hydrolysis of 9-(1-ethoxyethyl) purine. Acta Chem. Scand. 1980, 34, 703–708. [Google Scholar] [CrossRef]
- Almer, H.; Stawinski, J.; Strömberg, R.; Thelin, M. Synthesis of diribonucleoside phosphorothioates via stereospecific sulfuration of H-phosphonate diesters. J. Org. Chem. 1992, 57, 6163–6169. [Google Scholar] [CrossRef]
- Järvinen, P.; Oivanen, M.; Lönnberg, H. Interconversion and phosphoester hydrolysis of 2′,5′-and 3′,5′-dinucleoside monophosphates: Kinetics and mechanisms. J. Org. Chem. 1991, 56, 5396–5401. [Google Scholar] [CrossRef]
- Simpson, R.B. Association Constants of Methylmercuric and Mercuric Ions with Nucleosides. J. Am. Chem. Soc. 1964, 86, 2059–2065. [Google Scholar] [CrossRef]
- Leroy, J.L.; Guéron, M. Demonstration of different complexation modes between cobalt and 5′ AMP, by direct NMR observation of the low-temperature complex. Biochimie 1982, 64, 297–299. [Google Scholar] [CrossRef]
- Leroy, J.L.; Guéron, M. Demonstration and characterization of two complexes of cobalt(II) to mononucleotides by phosphorus-31 and proton NMR. J. Am. Chem. Soc. 1986, 108, 5753–5759. [Google Scholar] [CrossRef]
- Collins, A.D.; Demeester, P.; Goodgame, D.M.L.; Skapski, A.C. Site of metal-ion binding in a nickel derivative of adenosine 5′-monophosphate-X-ray study. Biochim. Biophys. Acta 1975, 402, 1–6. [Google Scholar] [CrossRef]
- Chapman, W.H.; Breslow, R. Selective Hydrolysis of Phosphate Esters, Nitrophenyl Phosphates and UpU, by Dimeric Zinc Complexes Depends on the Spacer Length. J. Am. Chem. Soc. 1995, 117, 5462–5469. [Google Scholar] [CrossRef]
- Wall, M.; Hynes, R.C.; Chin, J. Double Lewis Acid Activation in Phosphate Diester Cleavage. Angew. Chem. Int. Ed. 1993, 32, 1633–1635. [Google Scholar] [CrossRef]
- Liu, S.D.; Hamilton, A. Rapid and highly base selective RNA cleavage by a dinuclear Cu(II) complex. Chem. Commun. 1999, 587–588. [Google Scholar] [CrossRef]
- Iranzo, O.; Elmer, T.; Richard, J.P.; Morrow, J.R. Cooperativity between Metal Ions in the Cleavage of Phosphate Diesters and RNA by Dinuclear Zn(II) Catalysts. Inorg. Chem. 2003, 42, 7737–7746. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Mareque-Rivas, J.C.; Williams, N.H. Comparing a mononuclear Zn(ii) complex with hydrogen bond donors with a dinuclear Zn(ii) complex for catalysing phosphate ester cleavage. Chem. Commun. 2006, 1845–1847. [Google Scholar] [CrossRef]
- Feng, G.; Natale, D.; Prabaharan, R.; Mareque-Rivas, J.C.; Williams, N.H. Efficient Phosphodiester Binding and Cleavage by a ZnII Complex Combining Hydrogen-Bonding Interactions and Double Lewis Acid Activation. Angew. Chem. Int. Ed. 2006, 45, 7056–7059. [Google Scholar] [CrossRef]
- Linjalahti, H.; Feng, G.; Mareque-Rivas, J.C.; Mikkola, S.; Williams, N.H. Cleavage and Isomerization of UpU Promoted by Dinuclear Metal Ion Complexes. J. Am. Chem. Soc. 2008, 130, 4232–4233. [Google Scholar] [CrossRef]
- Korhonen, H.; Mikkola, S.; Williams, N.H. The Mechanism of Cleavage and Isomerisation of RNA Promoted by an Efficient Dinuclear Zn2+ Complex. Chem. Eur. J. 2012, 18, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Huang, D.L. Effects of metal ions, including Mg2+ and lanthanides, on the cleavage of ribonucleotides and RNA model compounds. Proc. Natl. Acad. Sci. USA 1991, 88, 4080. [Google Scholar] [CrossRef] [PubMed]
- Oivanen, M.; Ora, M.; Almer, H.; Strömberg, R.; Lönnberg, H. Hydrolytic Reactions of the Diastereomeric Phosphoromonothioate Analogs of Uridylyl(3′,5′)uridine: Kinetics and Mechanisms for Desulfurization, Phosphoester Hydrolysis, and Transesterification to the 2′,5′-Isomers. J. Org. Chem. 1995, 60, 5620–5627. [Google Scholar] [CrossRef]
- Norman, D.G.; Reese, C.B.; Serafinowska, H.T. 2′,3′-O-Methylene derivatives of ribonucleosides. Synthesis 1985, 1985, 751–754. [Google Scholar] [CrossRef]
- Busca, P.; Etheve-Quelquejeu, M.; Valéry, J.-M. Synthesis of 2′-O,3′-O bicyclic adenosine analogues using ring closing metathesis. Tetrahedron Lett. 2003, 44, 9131–9134. [Google Scholar] [CrossRef]

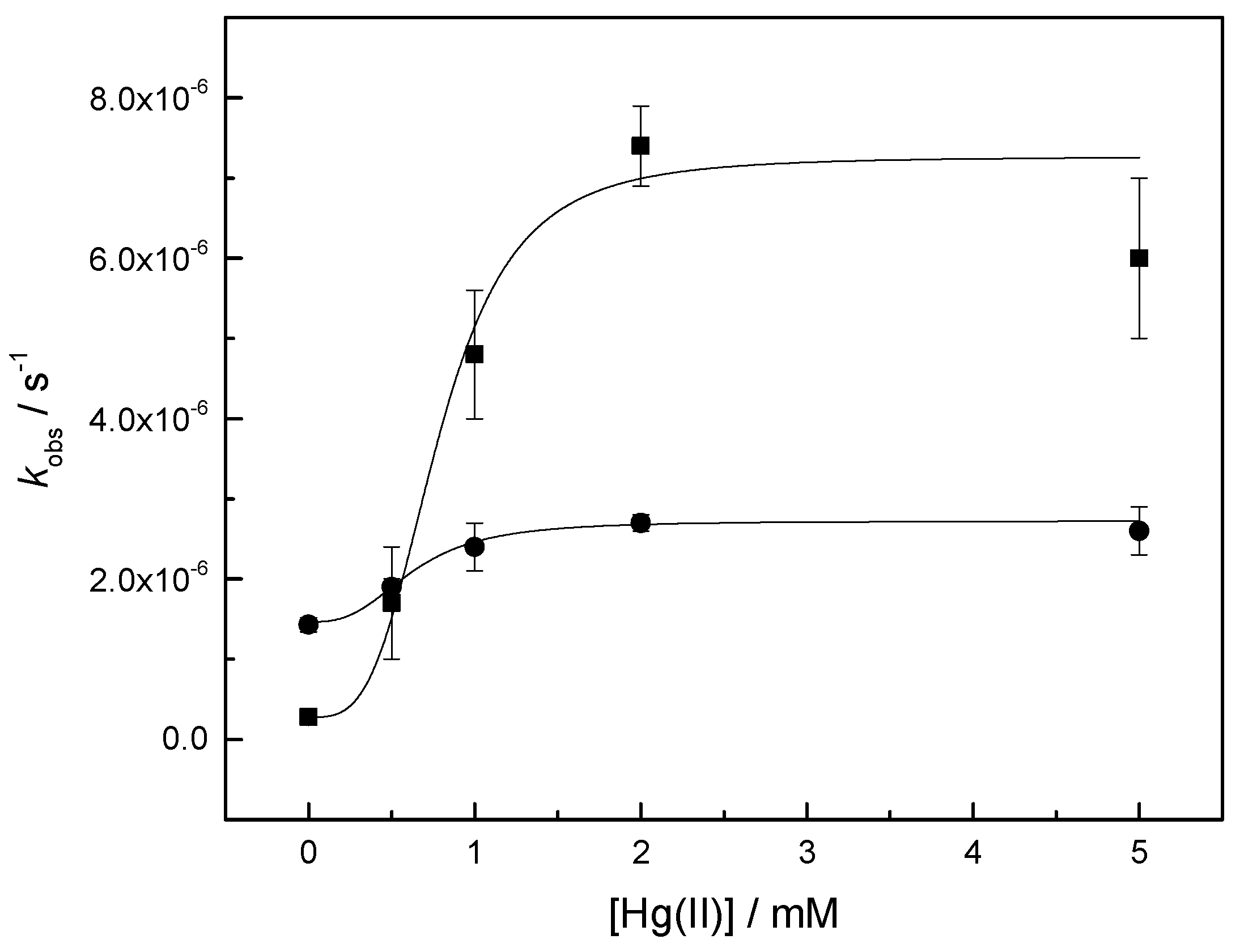

| k0/10−7 s−1 | kHg/10−6 M−n s−1 | n | |
|---|---|---|---|
| k1 | 2.8 ± 0.1 | 7 ± 2 | 3 ± 10 |
| k2 + k−2 | 14 ± 1 | 1.3 ± 0.2 | 3 ± 6 |
| Hg(II) | kH/M−1 s−1 | kW/10−8 s−1 | kOH/M−1 s−1 | |||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| k1 | n.a. | 1.0 ± 0.3 | 9 ± 2 | n.a. | 0.30 ± 0.04 | 6 ± 1 |
| k2 + k−2 | n.a. | n.a. | 107 ± 8 | 140 ± 10 | n.a. | n.a. |
| k2 | n.a. | n.a. | 59 ± 4 | n.a. | n.a. | n.a. |
| k−2 | n.a. | n.a. | 48 ± 4 | n.a. | n.a. | n.a. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, L.Y.; Ora, M.; Lönnberg, T. Mercury(II)-Catalyzed Cleavage, Isomerization and Depurination of RNA and DNA Model Compounds and Desulfurization of Their Phosphoromonothioate Analogs. Catalysts 2020, 10, 219. https://doi.org/10.3390/catal10020219
Saleh LY, Ora M, Lönnberg T. Mercury(II)-Catalyzed Cleavage, Isomerization and Depurination of RNA and DNA Model Compounds and Desulfurization of Their Phosphoromonothioate Analogs. Catalysts. 2020; 10(2):219. https://doi.org/10.3390/catal10020219
Chicago/Turabian StyleSaleh, Lange Yakubu, Mikko Ora, and Tuomas Lönnberg. 2020. "Mercury(II)-Catalyzed Cleavage, Isomerization and Depurination of RNA and DNA Model Compounds and Desulfurization of Their Phosphoromonothioate Analogs" Catalysts 10, no. 2: 219. https://doi.org/10.3390/catal10020219
APA StyleSaleh, L. Y., Ora, M., & Lönnberg, T. (2020). Mercury(II)-Catalyzed Cleavage, Isomerization and Depurination of RNA and DNA Model Compounds and Desulfurization of Their Phosphoromonothioate Analogs. Catalysts, 10(2), 219. https://doi.org/10.3390/catal10020219







