Pt Nanowire-Anchored Dodecahedral Ag3PO4{110} Constructed for Significant Enhancement of Photocatalytic Activity and Anti-Photocorrosion Properties: Spatial Separation of Charge Carriers and PhotogeneratedElectron Utilization
Abstract
:1. Introduction
2. Results and Discussion
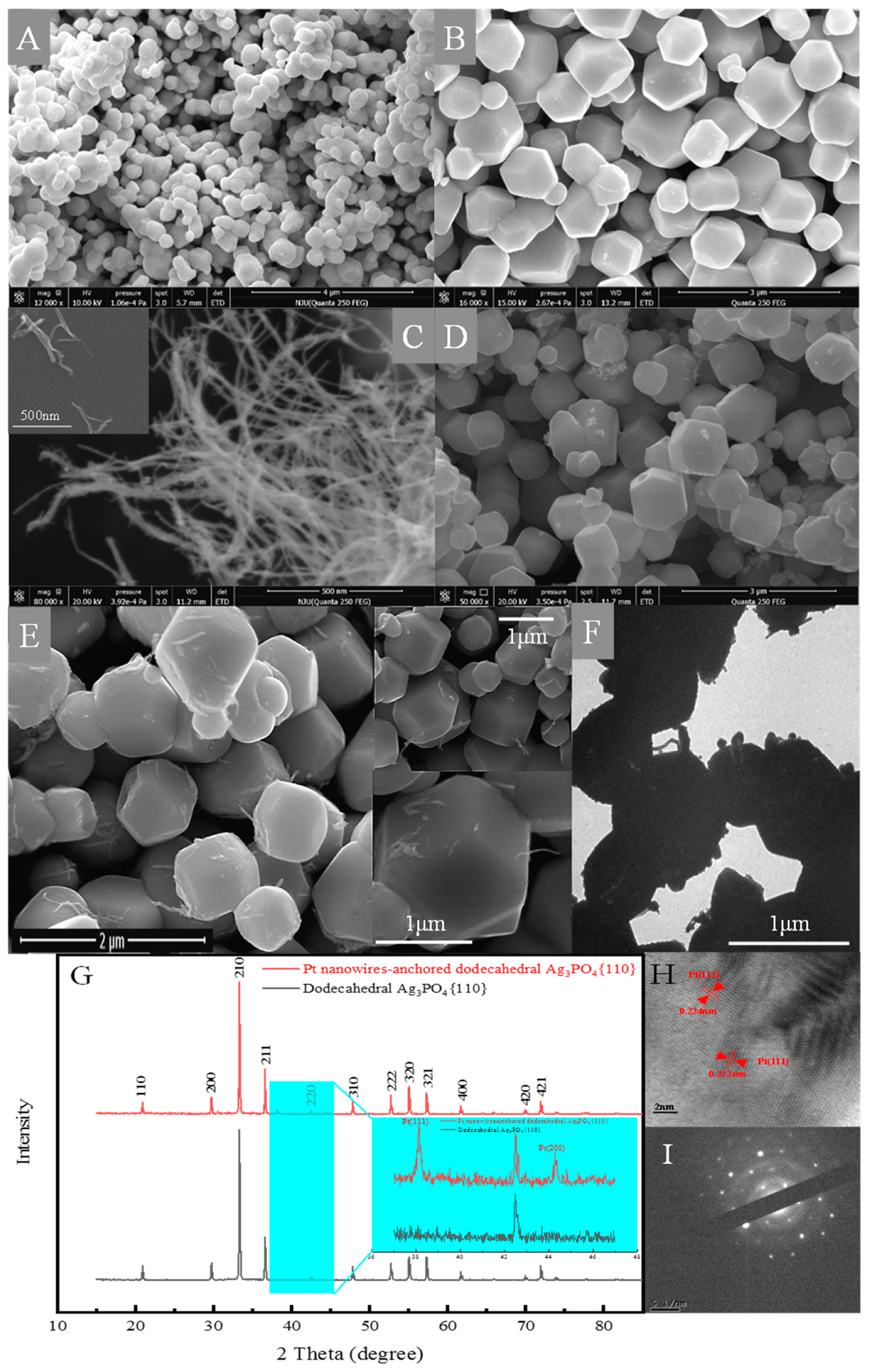
2.1. Structure and Composition
2.2. Photoelectric Properties
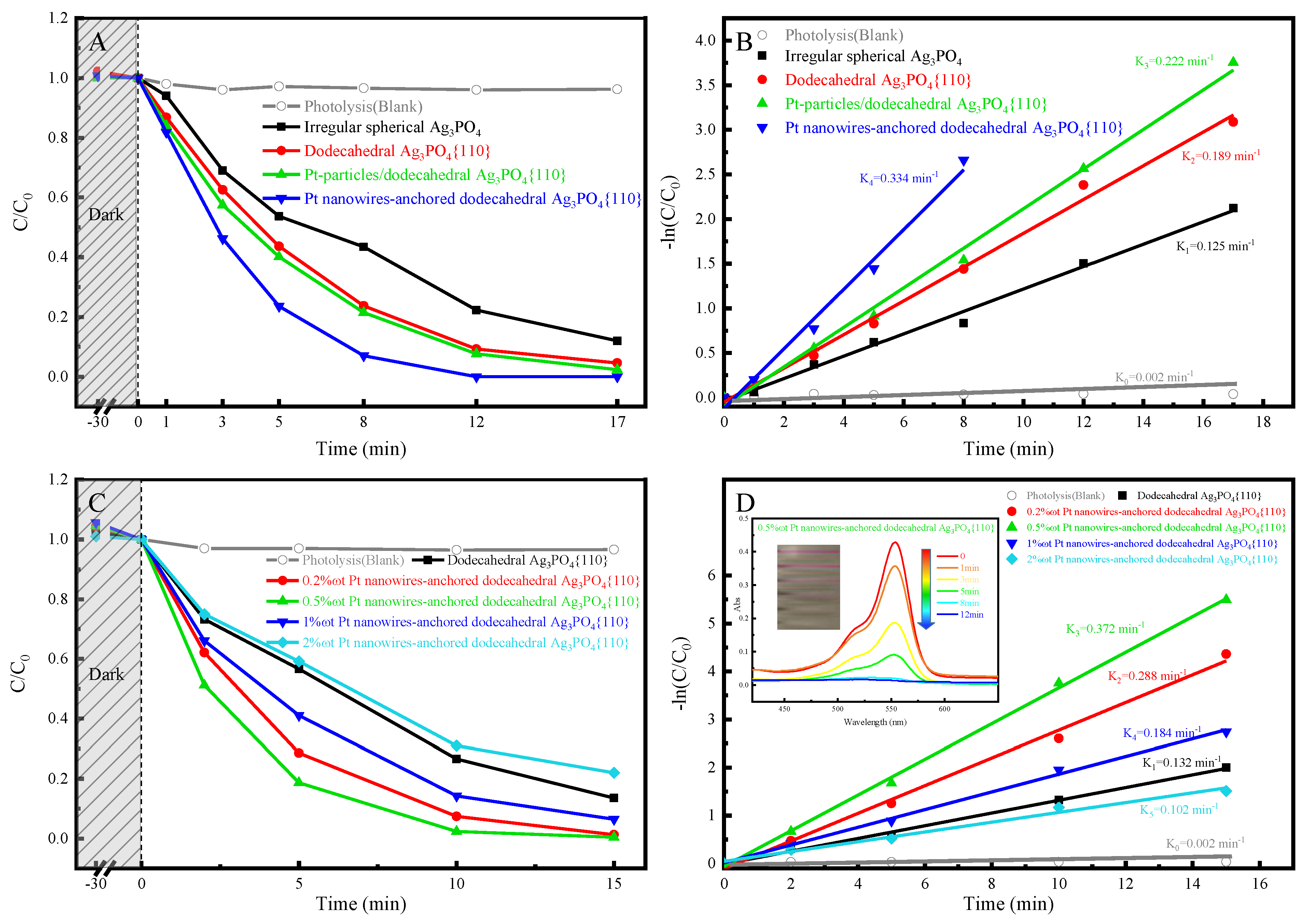
2.3. Photocatalytic Activity and Anti-Photocorrosion
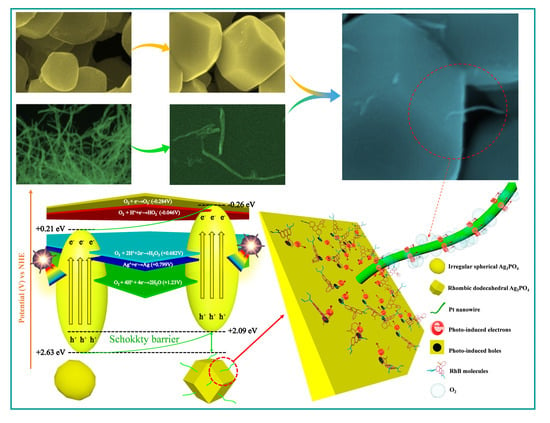
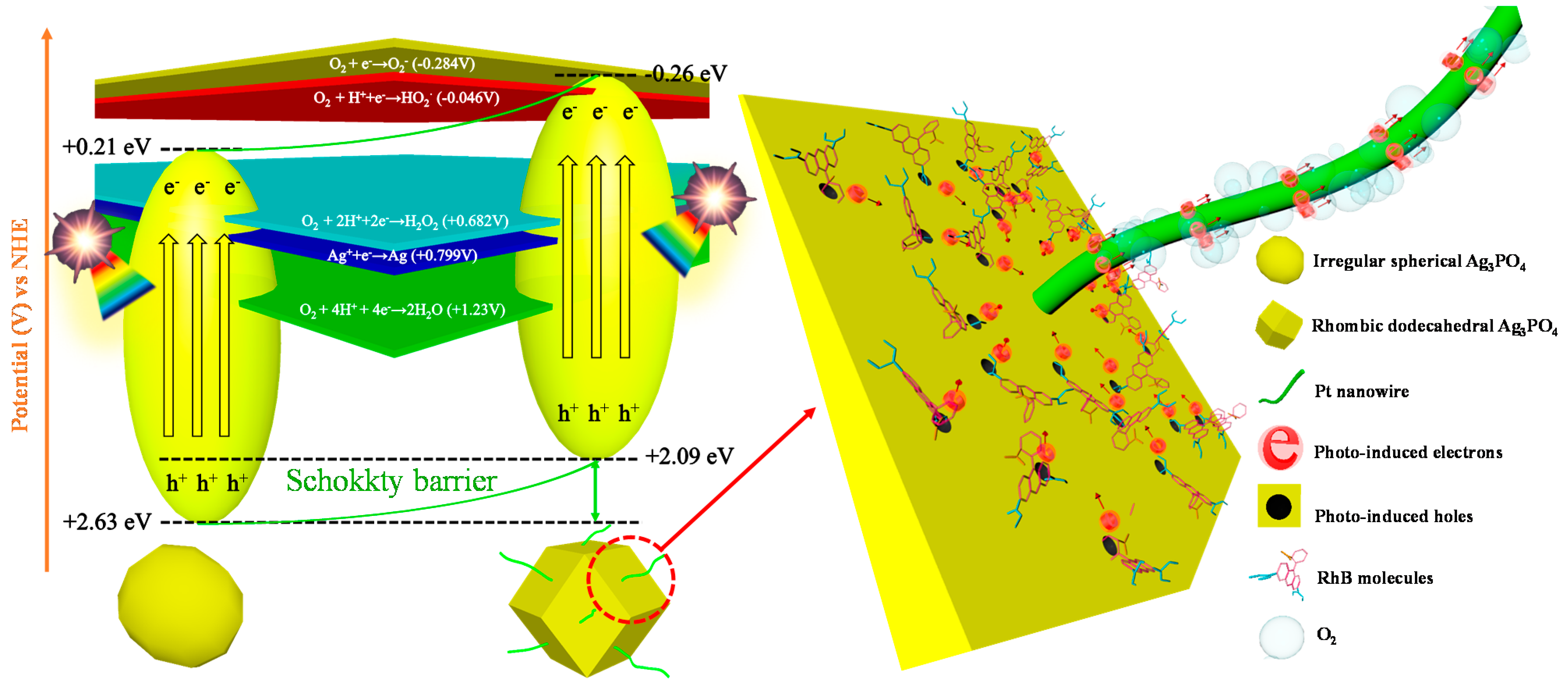
2.4. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Pt Nanowire-Anchored Dodecahedral Ag3PO4{110}
3.3. Characterization of the Catalysts
3.4. Photocatalytic Tests of the Catalysts
3.5. Photocatalytic Mechanism of Pt Nanowire-Anchored Dodecahedral Ag3PO4{110}
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Martin, D.J.; Liu, G.; Moniz, S.J.; Bi, Y.; Beale, A.M.; Ye, J.; Tang, J. Efficient visible driven photocatalyst, silver phosphate: performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Liu, G.; Jimmy, C.Y.; Lu, G.Q.M.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: motivations, advances and unique properties. Chem. Comm. 2011, 47, 6763–6783. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Li, Z.; Xiong, Y. Facet-Engineered Surface and Interface Design of Photocatalytic Materials. Adv. Sci. 2017, 4, 1600216. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef]
- Martin, D.J.; Umezawa, N.; Chen, X.; Ye, J.; Tang, J. Facet engineered Ag3PO4 for efficient water photooxidation. Energy Environ. Sci. 2013, 6, 3380. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Hierarchical Ag3PO4 porous microcubes with enhanced photocatalytic properties synthesized with the assistance of trisodium citrate. CrystEngComm 2012, 14, 2966. [Google Scholar] [CrossRef]
- Li, X.-Z.; Wu, K.-L.; Dong, C.; Xia, S.-H.; Ye, Y.; Wei, X.-W. Size-controlled synthesis of Ag3PO4 nanorods and their high-performance photocatalysis for dye degradation under visible-light irradiation. Mater. Lett. 2014, 130, 97–100. [Google Scholar] [CrossRef]
- Wang, H.; He, L.; Wang, L.; Hu, P.; Guo, L.; Han, X.; Li, J. Facile synthesis of Ag3PO4 tetrapod microcrystals with an increased percentage of exposed {110} facets and highly efficient photocatalytic properties. CrystEngComm 2012, 14, 8342–8344. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Li, H.; Li, H.; Ma, X.; Han, L. Shape-controllable synthesis and morphology-dependent photocatalytic properties of Ag3PO4 crystals. J. Mater. Chem. A 2013, 1, 4651. [Google Scholar] [CrossRef]
- Guo, R.; Fan, Y.; Tang, Y. Interesting Ag3PO4 concave rhombic dodecahedra: the same face with different morphologies and photocatalytic properties. RSC Adv. 2017, 7, 23977–23981. [Google Scholar] [CrossRef] [Green Version]
- Shao, N.; Hou, Z.; Zhu, H.; Wang, J.; François-Xavier, C.P. Novel 3D core-shell structured CQDs/Ag 3 PO 4 @Benzoxazinetetrapods for enhancement of visible-light photocatalytic activity and anti-photocorrosion. Appl. Catal. B: Environ. 2018, 232, 574–586. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C. , et al. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B: Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Wu, L.; Shang, Y.Y.; Liu, W.; Pan, J.; Xiong, X. Hierarchical flower-like SnSe2 supported Ag3PO4 nanoparticles: Towards visible light driven photocatalyst with enhanced performance. Appl. Catal. B: Environ. 2017, 202, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Liu, E.; Wan, J.; Hu, X.; Fan, J. Co3O4 nanoparticles decorated Ag3PO4 tetrapods as an efficient visible-light-driven heterojunction photocatalyst. Appl. Catal. B: Environ. 2016, 181, 707–715. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Yuan, S. In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl. Catal. B: Environ. 2017, 209, 720–728. [Google Scholar] [CrossRef]
- Yang, X.; Tian, L.; Zhao, X.; Tang, H.; Liu, Q.; Li, G. Interfacial optimization of g-C3N4-based Z-scheme heterojunction toward synergistic enhancement of solar-driven photocatalytic oxygen evolution. Appl. Catal. B: Environ. 2019, 244, 240–249. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, K.; Feng, Y.; Chang, Y.; Yang, T.; Xuan, Y.; Lei, D.; Lou, L.-L.; Liu, S. Novel 3DOM-SrTiO 3 /Ag/Ag 3 PO 4 ternary Z-scheme photocatalysts with remarkably improved activity and durability for contaminant degradation. Appl. Catal. B: Environ. 2017, 210, 77–87. [Google Scholar] [CrossRef]
- Shao, N.; Wang, J.; Wang, D.; Corvini, P. Preparation of three-dimensional Ag 3 PO 4 /TiO 2 @MoS 2 for enhanced visible-light photocatalytic activity and anti-photocorrosion. Appl. Catal. B: Environ. 2017, 203, 964–978. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Wang, L.; Zhang, S.; Zeng, Y.; Yuan, J.; Ma, J.; Dong, W.; Liu, C.; Luo, S. Silver phosphate-based Z-Scheme photocatalytic system with superior sunlight photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B: Environ. 2017, 208, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z. Noble metal nanoparticle@metal oxide core/yolk-shell nanostructures as catalysts: Recent progress and perspective. Nanoscale 2014, 6, 3995–4011. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.; Kyaw, H.H.; Sarkar, S.; Pal, S.K.; Dutta, J. Highly efficient ZnO/Au Schottky barrier dye-sensitized solar cells: Role of gold nanoparticles on the charge-transfer process. Beilstein J. Nanotechnol. 2011, 2, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Z.; Chen, F.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Novel Pd/GdCrO 3 composite for photo-catalytic reduction of nitrate to N 2 with high selectivity and activity. Appl. Catal. B: Environ. 2018, 232, 124–134. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.N.K.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef] [Green Version]
- Cademartiri, L.; Ozin, G.A. Ultrathin nanowires - A materials chemistry perspective. Adv. Mater. 2009, 21, 1013–1020. [Google Scholar] [CrossRef]
- Teng, X.; Han, W.Q.; Ku, W.; Hücker, M. Synthesis of ultrathin palladium and platinum nanowires and a study of their magnetic properties. Angew. Chem. 2008, 120, 2085–2088. [Google Scholar] [CrossRef]
- Wang, W.; Lv, F.; Lei, B.; Wan, S.; Luo, M.; Guo, S. Tuning Nanowires and Nanotubes for Efficient Fuel-Cell Electrocatalysis. Adv. Mater. 2016, 28, 10117–10141. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.R.; Sun, Y.; Colmenares, J.C.; Xu, Y.J. One-dimension-based spatially ordered architectures for solar energy conversion. Chem. Soc. Rev. 2015, 44, 5053–5075. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murakami, N.; Ohtani, B. Pristine simple oxides as visible light driven photocatalysts: Highly efficient decomposition of organic compounds over platinum-loaded tungsten oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Li, F.; Chen, Y.; Huang, X. The Advanced Designs of High-Performance Platinum-Based Electrocatalysts: Recent Progresses and Challenges. Adv. Mater. Interfaces 2018, 5, 5. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.Y.; Wu, H.B.; Yan, Y.; Lou, X.W.; Wang, X. Ultrathin and ultralong single-crystal platinum nanowire assemblies with highly stable electrocatalytic activity. J. Am. Chem. Soc. 2013, 135, 9480–9485. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Zhao, K.; Muqsit, A.; Tang, H.; Chang, L.; Zhao, H.; Gao, Y.; Tang, Z. Ultrathin platinum nanowires grown on single-layered nickel hydroxide with high hydrogen evolution activity. Nat. Commun. 2015, 6, 6430. [Google Scholar] [CrossRef]
- Miao, X.; Yue, X.; Ji, Z.; Shen, X.; Zhou, H.; Liu, M.; Xu, K.; Zhu, J.; Zhu, G.; Kong, L. , et al. Nitrogen-doped carbon dots decorated on g-C 3 N 4 /Ag 3 PO 4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl. Catal. B: Environ. 2018, 227, 459–469. [Google Scholar] [CrossRef]
- Ye, C.; Li, J.-X.; Li, Z.-J.; Li, X.-B.; Fan, X.-B.; Zhang, L.-P.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Enhanced Driving Force and Charge Separation Efficiency of Protonated g-C3N4 for Photocatalytic O2 Evolution. ACS Catal. 2015, 5, 6973–6979. [Google Scholar] [CrossRef]
- Lin, R.; Wan, J.; Xiong, Y.; Wu, K.; Cheong, W.C.; Zhou, G.; Wang, D.; Peng, Q.; Chen, C.; Li, Y. Quantitative Study of Charge Carrier Dynamics in Well-Defined WO3 Nanowires and Nanosheets: Insight into the Crystal Facet Effect in Photocatalysis. J. Am. Chem. Soc. 2018, 140, 9078–9082. [Google Scholar] [CrossRef]
- Liu, C.; Jing, L.; He, L.; Luan, Y.; Li, C. Phosphate-modified graphitic C3N4 as efficient photocatalyst for degrading colorless pollutants by promoting O2 adsorption. Chem. Commun. 2014, 50, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Weng, B.; Colmenares, J.C.; Sun, Y.; Xu, Y.J. Multichannel Charge Transfer and Mechanistic Insight in Metal Decorated 2D-2D Bi2 WO6 -TiO2 Cascade with Enhanced Photocatalytic Performance. Small 2017, 13, 1702253. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Ji, Y.; Chen, L.; Bian, W.; Wang, J. Pt Nanowire-Anchored Dodecahedral Ag3PO4{110} Constructed for Significant Enhancement of Photocatalytic Activity and Anti-Photocorrosion Properties: Spatial Separation of Charge Carriers and PhotogeneratedElectron Utilization. Catalysts 2020, 10, 206. https://doi.org/10.3390/catal10020206
Zhu H, Ji Y, Chen L, Bian W, Wang J. Pt Nanowire-Anchored Dodecahedral Ag3PO4{110} Constructed for Significant Enhancement of Photocatalytic Activity and Anti-Photocorrosion Properties: Spatial Separation of Charge Carriers and PhotogeneratedElectron Utilization. Catalysts. 2020; 10(2):206. https://doi.org/10.3390/catal10020206
Chicago/Turabian StyleZhu, Hanxu, Yekun Ji, Lifang Chen, Weilin Bian, and Jinnan Wang. 2020. "Pt Nanowire-Anchored Dodecahedral Ag3PO4{110} Constructed for Significant Enhancement of Photocatalytic Activity and Anti-Photocorrosion Properties: Spatial Separation of Charge Carriers and PhotogeneratedElectron Utilization" Catalysts 10, no. 2: 206. https://doi.org/10.3390/catal10020206
APA StyleZhu, H., Ji, Y., Chen, L., Bian, W., & Wang, J. (2020). Pt Nanowire-Anchored Dodecahedral Ag3PO4{110} Constructed for Significant Enhancement of Photocatalytic Activity and Anti-Photocorrosion Properties: Spatial Separation of Charge Carriers and PhotogeneratedElectron Utilization. Catalysts, 10(2), 206. https://doi.org/10.3390/catal10020206