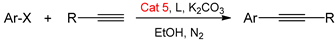
Abstract
In the quest for efficient and recyclable heterogeneous catalysts for Sonogashira coupling reactions, a PEG-OMe (Methoxy Polyene Glycol) - mediated method was developed to immobilize Pd/Cu bimetallic nanoparticles on activated carbon (AC). Catalytic experiments showed that Pd/Cu@AC prepared in a PEG-OMe 500 had the highest activity. The morphology and composition of the catalyst were determined, and the identified crystallized and heterometallic nanoparticles of Pd/Cu were essential for an efficient catalytic cycle. The Pd/Cu@AC catalyst was successfully used in Sonogashira reactions (21 examples) including with aryl bromides as the coupling partner in EtOH at 80–100 °C. The catalyst was recycled at least nine times.
1. Introduction
The Csp–Csp2 cross-coupling reaction, also known as a Sonogashira reaction, has gained much attention because alkynes are important precursors of various pharmaceuticals, natural products, and organic materials [1,2,3]. Despite many attempts to develop a transition-metal-catalyzed Sonogashira coupling reaction using a single metal, such as Cu [4,5], Fe [6,7], Ni [8,9], and Au [10,11,12,13,14], most of today’s transition-metal-catalyzed cross-coupling chemistry still relies on a bimetallic catalyst system of Pd and Cu. In the putative catalytic cycle of a Sonogashira reaction, Pd and Cu are responsible for the oxidative addition of a C–X bond and the transmetalation of an activated triple bond, respectively [15,16]. The Pd/Cu bimetallic catalyst system has excellent redox properties, probably because of the electron-donating and -accepting characteristics of Cu and Pd, respectively [17,18]. The “Cu effect” represents an adjustment or modification of the catalytic ability in Pd-catalyzed Sonogashira coupling reactions [19,20,21]. Recently, several Pd/Cu bimetallic nanoparticles (NPs) were loaded on commercially available insoluble organic or inorganic supports as heterogeneous catalysts, and were successfully used in cross-coupling reactions and other chemical reactions [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Supported bimetallic NP catalysts with minimal or no leaching are necessary for both environmental protection and cost reduction. In other words, it is essential to develop solid-supported Pd/Cu bimetallic NP heterogeneous catalysts using easily available raw materials and simple procedures and with a high catalytic efficiency.
The chemical reductant and solvent of a catalyst system are essential factors to tune the morphology and catalytic activity of heterogeneous Pd catalysts. For a bimetallic Pd/Cu catalyst system, a strong reductant such as sodium borohydride (NaBH4) [34] is used to generate highly uniform Pd/Cu composites. Diverse milder reductants are also used to reduce metal salts, such as DMF [26,28], toluene [36], ethanol [27,28,29,30], and morpholine [35]. The solvent of a catalyst system interacts with the reactants, intermediates, and final products, thus significantly affecting the reaction results. The compatible properties of polyols, water, DMF, oleylamine (OAm), and benzene with common reactants make it possible to use them as solvents and structure modulators to synthesize metal NPs. Interestingly, amines such as DMF, OAm, and polyols can serve as both reductant and solvent [40,41,42].
Polymeric oxygenated solvents such as dioxane and ethylene glycol (EG) with oxygen and hydroxyl groups can reduce Pd/Cu precursors under thermal conditions [9m]. We hypothesized that the polymeric oxygenated solvent PEG-OMe can be used as both reductant and solvent to fabricate uniform Pd/Cu bimetallic catalysts. To the best of our knowledge, PEG-OMe-mediated fabrication of bimetallic Pd/Cu NPs impregnated on activated carbon (AC) and their heterogeneous catalytic activity in cross-coupling reactions have not been reported. Carbonaceous material such as AC is commercially available, inexpensive, has a large surface area, is stable, and separates easily, and therefore could be an excellent support. Here, we report the synthesis and characterization of a new class of heterogeneous Pd/Cu bimetallic NPs supported on AC. This catalyst showed efficient catalytic activity in Sonogashira cross-coupling reactions under mild reaction conditions, and had high recyclability.
2. Results and Discussion
The thermal solvolysis of transition-metal salts in reductive solvents led to the formation of the corresponding NPs. To finely control the composition of Pd/Cu NPs, organic solvents bearing multi-dentated oxygen donors were used to fabricate Pd/Cu@AC catalysts. As shown in Figure 1a, the XRD patterns of Pd and Cu were identified by comparing them with that of amorphous AC. Angles of 2θ at 40.1°, 46.59°, and 68.11° were assigned to Pd (111), (200), and (220) planes, respectively (JCPDS#5-681), and 2θ angles of 43.34°, 50.46°, and 73.99° correspond to Cu (111), (200), and (220) planes, respectively (JCPDS#4-836) (Figure 1b). The reductive solvents significantly affected the crystallization of deposited metallic NPs. In ethanol (Cat 1), only Pd0 deposited on the AC support. With the increase in the length of PEG chains such as PEG 300, PEG-OMe 350, and PEG-OMe 500, the loading of Cu0 increased accordingly. Interestingly, the crystallization of supported metallic NPs was also dependent on the PEGylated solvents. The results indicate that the coordination effect of metal ions with PEGylated solvents induced the aggregation and assembly of Pd0 and Cu0, perhaps because the coordination ability of the O atoms of the solvent helped to capture the metal ions. Cat 5 prepared in PEG-OMe 500 showed the highest crystallization of Pd/Cu NPs, with sharper and more intensive peaks than others.
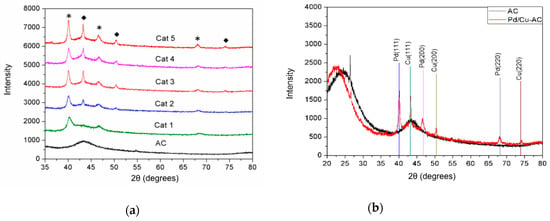
Figure 1.
(a) XRD patterns of activated carbon (AC) and Pd/Cu@AC catalysts prepared using different solvents: (Cat 1) EtOH, (Cat 2) ethylene glycol (EG), (Cat 3) PEG 300, (Cat 4) PEG-OMe 350, and (Cat 5) PEG-OMe 500. (*) Diffraction of Pd in Pd/Cu@AC and (◆) diffraction of Cu in Pd/Cu@AC; (b) XRD patterns of AC and Cat 5 PEG-OMe 500.
The STEM-EDS mapping images (Figure 2) of Pd and Cu show that Pd and Cu homogeneously dispersed on the surface of AC support, respectively. The high-resolution TEM (HRTEM) images of Pd/Cu@AC show that the metallic NPs aggregated on the Cu network after the ultrasonic dispersion of the catalyst (Figure 3A). The particle-size distribution analysis (Figure 3B) indicates that the average diameter of these particles was ~9.3 nm. Three types of lattice with a void image (Figure 3C) correspond to the pure Pd (111) (0.2265 nm), Pd (200) (0.1945 nm), and Cu (111) (0.2088 nm) planes. These results indicate the coexistence of Pd and Cu NPs on the surface of AC, and slight variations of d-spacing might result from the heterometallic interaction between Pd and Cu.
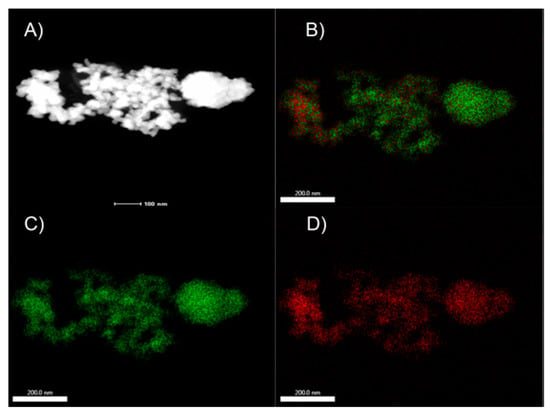
Figure 2.
(A) STEM images and STEM-EDS mapping images of (B) Pd/Cu, (C) Pd, and (D) Cu of the as-prepared Pd/Cu@AC.
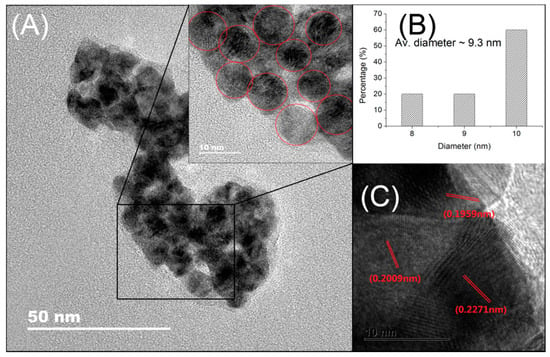
Figure 3.
(A) TEM image of Pd/Cu@AC; (B) particle-size distribution evaluated from (A); (C) high-resolution TEM (HRTEM) image of a part of (B).
XPS measurements for the surface composition of Pd/Cu@AC revealed the valence state of metal NPs. Figure 4 shows the XPS spectra of Pd 3d and Cu 2p in Pd/Cu@AC. The splitting pattern of Pd 3d showed two triple peaks around 332 to 348 eV, corresponding to the Pd 3d5/2 and Pd 3d3/2, respectively. The two lower binding energies at 335.4 and 340.7 eV were related to metallic Pd0, whereas the other two sets of peaks at 336.06 eV, 341.36 eV, and at 337.36 eV, 342.89 eV, were related to two types of Pd oxidant [43]. In Figure 3B, the binding energy of Cu 2p3/2 peak was observed at 932.08 eV, and the Cu 2p1/2 peak centered at 952.01 eV was attributed to Cu0 or Cu+. The shoulder observed on Cu 2p peak can be assigned to CuO. However, probably because of a small amount of CuO, the satellite peaks of CuO at 940–945 eV were not observed [44].
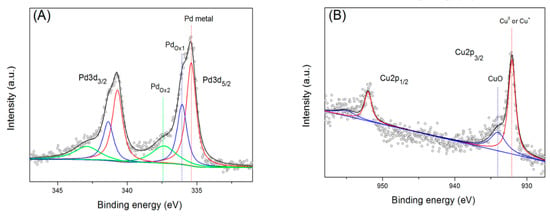
Figure 4.
Core–shell XPS spectra of (A) Pd 3d and (B) Cu 2p.
The activity of Pd/Cu@AC catalysts was evaluated for Sonogashira coupling. Initially, 4-iodotoluene and phenylacetylene were selected for the model reaction to test the activity of Cat 1–Cat 5. As shown in Table 1, Cat 1 prepared using EtOH provided an 87% yield, whereas Cat 2 provided less yield (80%) even though an XRD peak for Cu was clearly observed in Figure 1. This indicates that Pd had a major influence on the bimetallic catalysts of Sonogashira cross-coupling reactions. Furthermore, the activity of catalysts produced by polymeric solvents was better than other catalysts. The activities of solvothermally prepared monometallic catalysts, Pd@AC and Cu@AC (both produced in PEG-OMe 500), were also evaluated. They afforded products in 50% and 61% yields, respectively, much less than Pd/Cu@AC (up to 98% yield). The controlled experiments with mixtures of Pd@Ac and Cu@Ac as catalysts afforded a 67% yield, showing the superior activity of heterometallic catalysts. These results clearly indicate that the cooperative catalysis of Pd and Cu was essential to achieve a significant improvement in catalytic Csp2–Csp coupling reactions. The best result was obtained in the presence of Cat 5, 2 equiv of K2CO3, and 5 mol% of PPh3 in EtOH and N2 atmosphere at 80 °C for 12 h. Therefore, PEG-OMe 500 is an ideal solvent for the preparation of active Pd/Cu@AC catalysts, providing the highest crystallization of both Pd and Cu.

Table 1.
Pd/Cu@AC catalyzed cross-coupling of 4-iodotoluene and phenylacetylene.
To investigate the scope and limitations of Pd/Cu@AC catalyst, the Sonogashira coupling reactions of various aryl iodides/bromides and phenyl/aliphatic acetylenes catalyzed by Cat 5 were studied under the optimized reaction conditions, and the results are shown in Table 2.

Table 2.
Sonogashira cross-coupling of aryl halides and terminal alkynes catalyzed by Pd/Cu@AC.
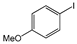
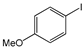
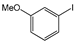
The cross-couplings of aliphatic or aryl alkynes with aryl iodides afforded products in reasonable yields (Table 2, entries 1–14). Both the electron-rich and -deficient aryl iodides afforded products in moderate-to-excellent yields (Table 2, entries 1–6). The aryl iodides bearing electro-donating groups such as –Ome (methoxy), -Me (methyl) and –COMe (O=CCH3) afforded satisfactory yields (Table 2, entries 1–4). The highest yields were provided by 1-iodonaphthalene and 2-iodothiophene, with 92% and 99% yields, respectively. On the other hand, phenylalkynes bearing electron-donating/withdrawing groups provided good results (Table 2, entries 9–14). Aliphatic acetylenes were also successfully coupled to 4-iodoanisole, with yields higher than 60%. The 1-Hexyne provided a 96% yield; the 1-heptyne and 1-octyne provided 84–66% yields (Table 2, entries 15–17).
The above-mentioned procedure was ineffective for less-active aryl bromides. Considering the effect of ligands, six phosphine ligands were selected (Table S1). Notably, Cat 5 successfully catalyzed the cross-coupling of aryl bromides and phenyl acetylene using X-Phos as the ligand at 100 °C for 24 h, affording moderate-to-excellent yields (Table 2, entries 18–21).
Reusability of catalysts is essential for developing heterogeneous catalysts. In this regard, a recycling test was performed for the Pd/Cu@AC catalyst. The catalyst was easily separated by the centrifugation of the reaction mixture. The isolated yields were measured for nine cycles. After eight cycles, the catalyst maintained 99% yields. In the 9th catalytic experiment, the recycled catalyst provided 87% yield. These results clearly indicate the sustainability of the bimetallic catalyst. The Pd and Cu concentrations in the solution were analyzed during each cycle by ICP-MS (Figure 5). The catalyst leached 0.5–1.3 ppm of Pd for each cycle, while the Cu leaching was faster. During the first experiment, the Cu concentration was 30 ppm, and sharply decreased to <8 ppm in the next three cycles. After 9th recycle experiment, the SEM image of recycled Pd/Cu@AC was obtained and compared with fresh catalyst. An obvious aggregation was shown, and it probably led to the yield-decreasing in the 9th recycle and subsequent inactivation.
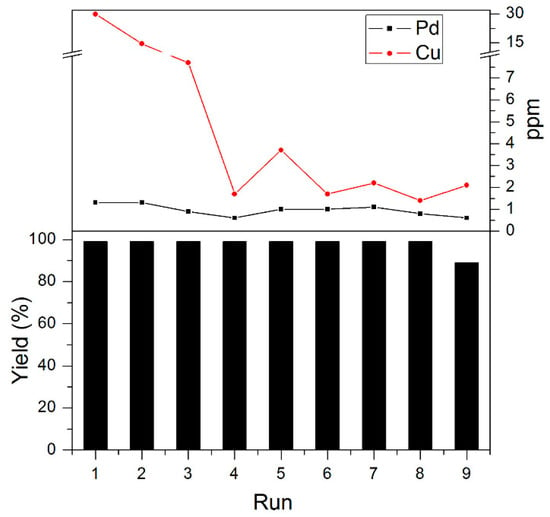
Figure 5.
Recycling experiments using Pd/Cu@AC in the cross-coupling reaction between 4-iodotoluene and phenylacetylene, and the ICP-MS results of Pd/Cu leaching in the solution of 1–9 cycles. The yields were determined by 1H-NMR.
The control experiments using 0.5 ppm Pd and 1 ppm Cu afforded less than 41% yields. However, the Pd and Cu concentrations did not clearly change in the 9th recycle with yield decline, ruling out the possibility that the leached Pd and Cu were the catalytic species. These results showed that the supported bimetallic Pd/Cu NPs were responsible for the catalytic activity.
3. Materials and Methods
3.1. Preparation of Catalysts
Bimetallic Pd/Cu@AC catalysts were prepared as follows: typically, Pd(II) acetate (Pd(OAc)2: 63.3 mg, 0.28 mmol), Cu(II) nitrate trihydrate (Cu(NO3)2•3H2O: 68.1 mg, 0.28 mmol), and AC (1 g) were dispersed in 30 mL of different solvents: EtOH (Cat 1), EG (Cat 2), PEG 300 (Cat 3), PEG-OMe 350 (Cat 4), and PEG-OMe 500 (Cat 5). Then, the mixture was heated in a Teflon-lined stainless-steel autoclave at 170 °C for 24 h. The catalyst was separated by decantation, washed with EtOH (30 mL, 5 times), and dried at 50 °C in an electrothermostatic blast oven.
3.2. General Procedure for Sonogashira Reactions
In a Schlenk tube, K2CO3 (1.0 mmol, 2 equiv), catalyst (3 mol% Pd), and ligand (5 mol% PPh3 or X-Phos) were added under N2 atmosphere. EtOH (5 mL) was transferred using a cannula. After adding the terminal alkyne (0.6 mmol, 1.2 equiv) and aryl halide (0.5 mmol, 1 equiv), the resulting mixture was stirred under N2 atmosphere at 80 °C for 12 h, or at 100 °C for 24 h. The products were isolated via flash chromatography with a selected eluent, such as Hexane: dichloromethane. For instance, after the reaction finished, the solid silica-gel was added into reaction mixture. The solvent was removed under vacuum. The solid residue was loaded on the column filled with a silica gel of 200 mesh. A fraction of the product band was collected. The pure product was contained after the eluent was removed. The pure product was characterized by 1HNMR and 13NMR [26].
3.3. Recycling Experiments
After each catalytic reaction, the catalyst was separated by centrifugation and filtration, washed with H2O (3 mL) and EtOH (9 mL, 3 times), and dried at 50 °C for further recycling experiments. The catalytic experiments using the recycled catalysts were carried out under the same conditions as those of general procedure for the Sonogashira reactions.
4. Conclusions
A facile PEG-OMe-mediated solvothermal method was developed to fabricate highly crystallized Pd/Cu NP catalysts. Pd/Cu@AC (Cat 5) was prepared by supporting Pd/Cu based bimetallic NPs on AC using PEG-OMe 500. Cat 5 showed the best activity and recyclability in Sonogashira cross-coupling reactions. The catalyst was compatible with diverse aryl iodides and bromides, with moderate-excellent yields, and could be recycled at least nine times. The recyclability was consistent with the low-ppm leaching of supported metallic compositions. The AC-supported catalysts have several advantages, such as their recyclability, easy operation, and environmental friendliness, over conventional Pd catalysts. The Pd/Cu@AC catalysts may find applications in various other C-C coupling reactions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/2/192/s1. Figure S1: The XRD patterns of the activated carbon (AC) and Pd/Cu@AC Cat 5; Table S1: The reaction results between 4-Bromoanisole and phenylacetylene; NMR spectra data for the Sonogashira-cross coupling products.
Author Contributions
Conceptualization, Z.X., W.Z., and Z.G.; methodology, Z.W. and Z.X.; validation, Z.W., Y.W., J.Y., and H.S.; formal analysis, G.Z., W.Z., and L.X.; investigation, Z.W.; writing—original draft preparation, Z.W.; writing—review and editing, Z.X., L.G., and Y.J.; supervision, L.X., W.Z., and Z.G.; project administration, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from National Natural Science Foundation of China (21771122, 21602129, 21571121), the 111 Project (B14041), Key Research and Development Project of Shaanxi Science and Technology Department (2017SF-064, 2017GY-124), Fundamental Research Funds for the Central Universities under Grant (GK201903026), and Projects of Xi′an Modern Institute of Chemistry (204-J-2018-315-4/6, 204-J-2019-0387-3/6-6).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, S.H. Sonogashira coupling in natural product synthesis. Org. Chem. Front. 2014, 1, 556–566. [Google Scholar] [CrossRef]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent advances and perspectives in copper catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Carril, M.; Correa, A.; Bolm, C. Iron-Catalyzed Sonogashira Reactions. Angew. Chem. Int. Ed. 2008, 47, 4862–4865. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Okada, Y.; Yoshimoto, Y.; Nakamura, M. Tuning Chemoselectivity in Iron-Catalyzed Sonogashira-Type Reactions Using a Bisphosphine Ligand with Peripheral Steric Bulk: Selective Alkynylation of Nonactivated Alkyl Halides. Angew. Chem. Int. Ed. 2011, 50, 10973–10976. [Google Scholar] [CrossRef]
- Wang, L.; Lia, P.; Zhang, Y. The Sonogashira coupling reaction catalyzed by ultrafine nickel(0) powder. Chem. Commun. 2004, 514–515. [Google Scholar] [CrossRef]
- Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C–O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726. [Google Scholar] [CrossRef]
- Kyriakou, G.; Beaumont, S.K.; Humphrey, S.M.; Antonetti, C.; Lambert, R.M. Sonogashira Coupling Catalyzed by Gold Nanoparticles: Does Homogeneous or Heterogeneous Catalysis Dominate? ChemCatChem 2010, 2, 1444–1449. [Google Scholar] [CrossRef]
- Lauterbach, T.; Livendahl, M.; Rosellón, A.; Espinet, P.; Echavarren, A.M. Unlikeliness of Pd-Free Gold(I)-Catalyzed Sonogashira Coupling Reactions. Org. Lett. 2010, 12, 3006–3009. [Google Scholar] [CrossRef] [PubMed]
- Kanuru, V.K.; Kyriakou, G.; Beaumont, S.K.; Papageorgiou, A.C.; Watson, D.J.; Lambert, R.M. Sonogashira Coupling on an Extended Gold Surface in Vacuo: Reaction of Phenylacetylene with Iodobenzene on Au(111). J. Am. Chem. Soc. 2010, 132, 8081–8086. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, S.K.; Kyriakou, G.; Lambert, R.M. Identity of the Active Site in Gold Nanoparticle-Catalyzed Sonogashira Coupling of Phenylacetylene and Iodobenzene. J. Am. Chem. Soc. 2010, 132, 12246–12248. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Abroshan, H.; Liu, C.; Zhu, M.; Li, G.; Haruta, M. Sonogashira cross-coupling on the Au(1 1 1) and Au(1 0 0) facets of gold nanorod catalysts: Experimental and computational investigation. J. Catal. 2015, 330, 354–361. [Google Scholar] [CrossRef]
- Karak, M.; Barbosa, L.C.A.; Hargaden, G.C. Recent mechanistic developments and next generation catalysts for the sonogashira coupling reaction. RSC Adv. 2014, 4, 53442. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Gazzola, S.; Mazza, A. Recent advances in heterobimetallic palladium(ii)/copper(ii) catalyzed domino difunctionalization of carbon–carbon multiple bonds. Org. Biomol. Chem. 2014, 12, 6767–6789. [Google Scholar] [CrossRef] [PubMed]
- Shih, Z.Y.; Wang, C.W.; Xu, G.; Chang, H.T. Porous palladium copper nanoparticles for the electrocatalytic oxidation of methanol in directmethanol fuel cells. J. Mater. Chem. A 2013, 1, 4773–4778. [Google Scholar] [CrossRef]
- Myers, S.V.; Frenkel, A.I.; Crooks, R.M. X-ray Absorption Study of PdCu Bimetallic Alloy Nanoparticles Containing an Average of ∼64 Atoms. Chem. Mater. 2009, 21, 4824–4829. [Google Scholar] [CrossRef]
- Espinet, P.; Echavarren, A.M. The Mechanisms of the Stille Reaction. Angew. Chem. Int. Ed. 2004, 43, 4704–4734. [Google Scholar]
- Peng, Y.; Li, W.D.Z. cine Substitution and the Cu Effect in Stille Cross-Coupling Reactions: Mechanistic Perspectives and Synthetic Utility. Eur. J. Org. Chem. 2010, 35, 6703–6708. [Google Scholar]
- Pérez-Temprano, M.H.; Casares, J.A.; Espinet, P. Bimetallic Catalysis using Transition and Group 11 Metals: An Emerging Tool for C-C Coupling and Other Reactions. Chem. Eur. J. 2012, 18, 1864–1884. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcίa, H.; Primo, A. Palladium and copper supported on mixed oxides derived from hydrotalcite as reusable solid catalysts for the Sonogashira coupling. J. Catal. 2006, 241, 123–131. [Google Scholar] [CrossRef]
- Cano, R.; Yus, M.; Ramόn, D.J. Impregnated copper or palladium–copper on magnetite as catalysts for the domino and stepwise Sonogashira-cyclization processes: A straightforward synthesis of benzo[b] furans and indoles. Tetrahedron 2012, 68, 1393–1400. [Google Scholar] [CrossRef]
- Cintas, P.; Cravotto, G.; Gaudino, E.C.; Orio, L.; Boffa, L. Reticulated Pd(II)/Cu(I) cyclodextrin complexes as recyclable green catalyst for Sonogashira alkynylation. Catal. Sci. Technol. 2012, 2, 85–87. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Abazari, R.; Balalaie, S. Preparation of monometallic (Pd, Ag) and bimetallic (Pd/Ag, Pd/Ni, Pd/Cu) nanoparticles via reversed micelles and their use in the Heck reaction. Tetrahedron 2012, 68, 3001–3011. [Google Scholar] [CrossRef]
- Xu, W.; Sun, Y.L.; Guo, M.; Zhang, W.Q.; Gao, Z.W. Montmorillonite Supported Pd/Cu Bimetallic Nanoparticle Catalyzed Sonogashira Coupling. Chin. J. Org. Chem. 2013, 33, 820. [Google Scholar] [CrossRef][Green Version]
- Xu, W.; Sun, H.M.; Yu, B.; Zhang, G.F.; Zhang, W.Q.; Gao, Z.W. Sonogashira Couplings on the Surface of Montmorillonite-Supported Pd/Cu Nanoalloys. ACS Appl. Mater. Interfaces 2014, 6, 20261–20268. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Wang, X.; Mawdsley, J.R.; Ferrandon, M.S.; Niyogi, S.G.; Vaughey, J.T.; Myers, D.J. Colloidal synthesis and characterization of carbon-supported Pd-Cu nanoparticle oxygen reduction electrocatalysts. Chem. Mater. 2010, 22, 4144–4152. [Google Scholar] [CrossRef]
- Behmenyar, G.; Akın, A.N. Investigation of carbon supported Pd-Cu nanoparticles as anode catalysts for direct borohydride fuel cell. J. Power Sources 2014, 249, 239–246. [Google Scholar] [CrossRef]
- Gong, Q.; Gong, S.; Zhang, T.; Cheng, X.; Li, H. Achieving High Activity and Stability of Carbon Supported Pd-Cu Alloyed Catalysts for Fuel Cell Applications. J. Electrochem. Soc. 2019, 166, F906–F913. [Google Scholar] [CrossRef]
- Sengupta, D.; Saha, J.; De, G.; Basu, B. Pd/Cu bimetallic nanoparticles embedded in macroporous ion-exchange resins: An excellent heterogeneous catalyst for the Sonogashira reaction. J. Mater. Chem. A. 2014, 2, 3986–3992. [Google Scholar] [CrossRef]
- Korzec, M.; Bartczak, P.; Niemczyk, A.; Szade, J.; Kapkowski, M.; Zenderowska, P.; Balin, K.; Lelątko, J.; Polanski, J. Bimetallic nano-Pd/PdO/Cu system as a highly effective catalyst for the Sonogashira reaction. J. Catal. 2014, 313, 1–8. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Jalehb, B.; Ehsani, A. Preparation of carbon supported CuPd nanoparticles as novel heterogeneous catalysts for the reduction of nitroarenes and the phosphine-free Suzuki–Miyaura coupling reaction. New J. Chem. 2015, 39, 1148–1153. [Google Scholar] [CrossRef]
- Gholinejad, M.; Ahmadi, J. Assemblies of Copper Ferrite and Palladium Nanoparticles on Silica Microparticles as a Magnetically Recoverable Catalyst for Sonogashira Reaction under Mild Conditions. ChemPlusChem 2015, 6, 973–979. [Google Scholar] [CrossRef]
- Diyarbakir, S.; Can, H.; Metin, O. Reduced graphene oxide-supported CuPd alloy nanoparticles as efficient catalysts for the Sonogashira cross-coupling reactions. ACS Appl. Mater. Interfaces. 2015, 7, 3199–3206. [Google Scholar] [CrossRef]
- Gholinejad, M.; Jeddi, N.; Pullithadathil, B. Agarose functionalized phosphorus ligand for stabilization of smallsized palladium and copper nanoparticles: Efficient heterogeneous catalyst for Sonogashira reaction. Tetrahedron 2016, 72, 2491–2500. [Google Scholar] [CrossRef]
- Gholinejad, M.; Ahmadi, J.; Najera, C.; Seyedhamzeh, M.; Zareh, F.; Kompany-Zareh, M. Graphene Quantum Dots Modified Fe3O4 Nanoparticles Supported PdCu with Enhanced Catalytic Activity for Sonogashira Reaction. ChemCatChem 2017, 8, 1442–1449. [Google Scholar] [CrossRef]
- Evangelisti, C.; Balerna, A.; Psaro, R.; Fusini, G.; Carpita, A.; Benfatto, M. Characterization of a Poly-4-vinylpyridine-Supported CuPd Bimetallic Catalyst for Sonogashira Coupling Reactions. ChemPhysChem 2017, 14, 1921–1928. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárováa, K.; Vávra, I.; Sotáka, T.; Mičusík, E.D.M. Carbon supported Pd–Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B Environ. 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, M.; Zhang, Y.; Pang, G.; Feng, S. Influence of Polyols on the Formation of Iron Oxide Nanoparticles in Solvothermal System. J. Nanosci. Nanotechnol. 2010, 10, 8405–8407. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Qin, F.; Wang, R.; Chen, H.S.R. Tunable BiOCl hierarchical nanostructures for high-efficient photocatalysis under visible light irradiation. Chem. Eng. J. 2013, 220, 228–236. [Google Scholar] [CrossRef]
- Lai, J.; Niu, W.; Luque, R.; Xu, G. Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 2015, 10, 240–267. [Google Scholar] [CrossRef]
- Gabasch, H.; Hayek, K.; Klötzer, B.; Unterberger, W.; Kleimenov, E.; Teschner, D.; Zafeiratos, S.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; et al. Methane Oxidation on Pd(111): In Situ XPS Identification of Active Phase. J. Phys. Chem. C 2007, 111, 7957–7962. [Google Scholar] [CrossRef]
- Espinós, J.P.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J.P.; González-Elipe, A.R. Interface Effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS Determination of the Valence State of Copper in Cu/SiO2 and Cu/ZrO2 Catalysts. J. Phys. Chem. B 2002, 106, 6921–6929. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).