Impregnation of CaO from Eggshell Waste with Magnetite as a Solid Catalyst (Fe3O4/CaO) for Transesterification of Palm Oil Off-Grade
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of Palm Oil Off-Grade
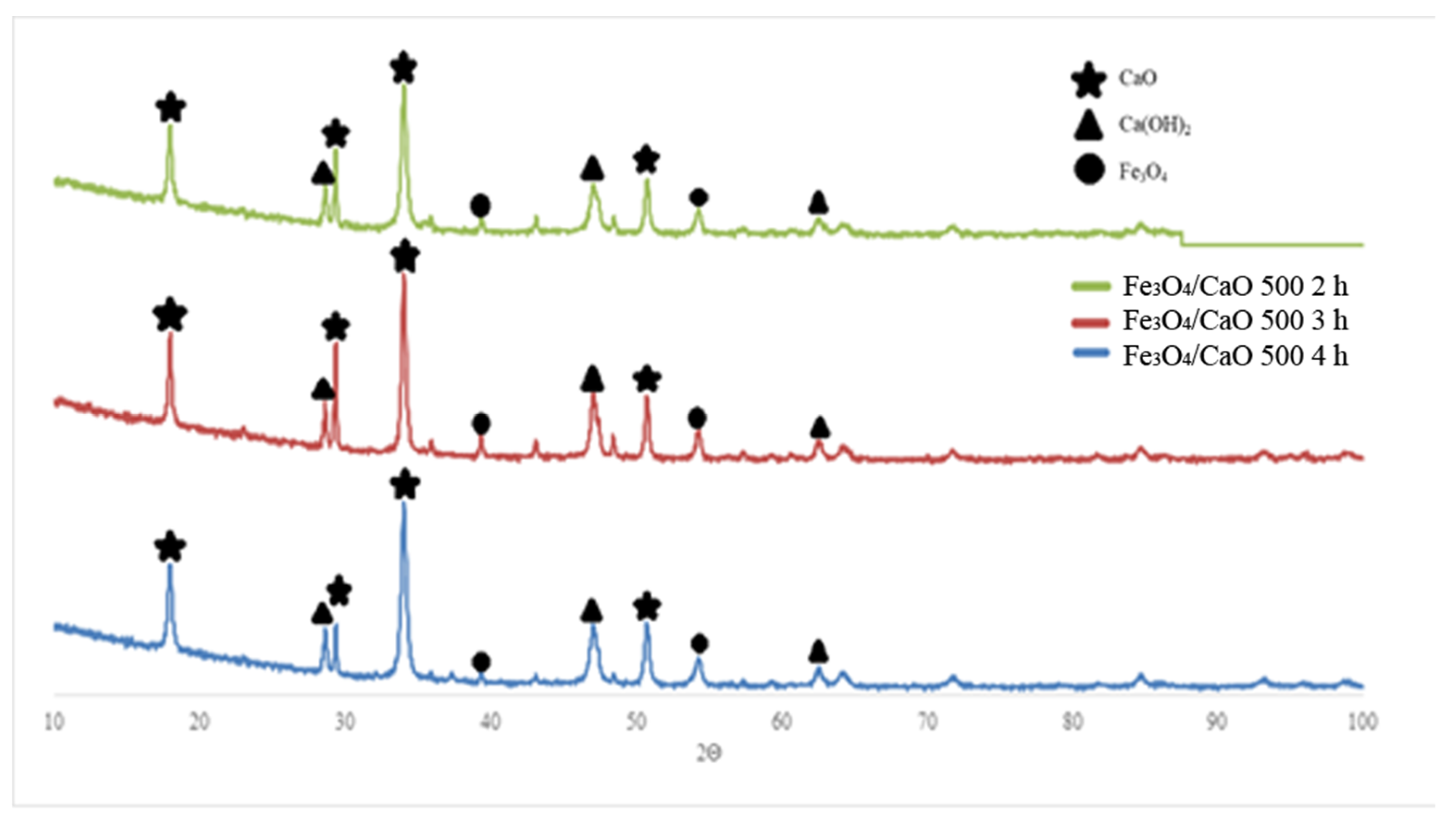
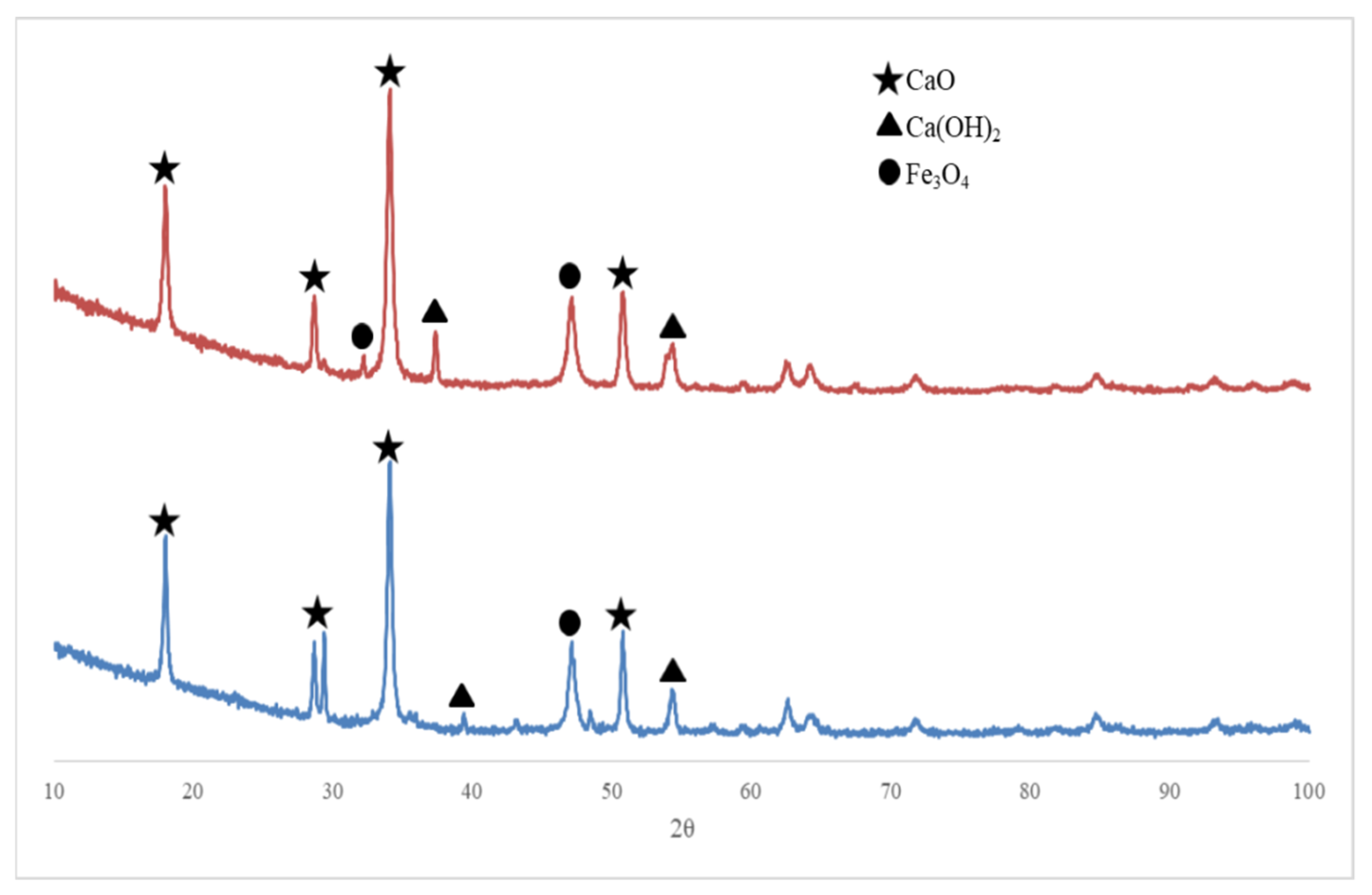
2.1.1. Catalyst Characterization
2.1.2. Hammett Indicator Titration
2.1.3. Braunaeur–Emmet–Teller (BET)
2.2. Catalytic Study of Fe3O4/CaO
2.2.1. Biodiesel Yield
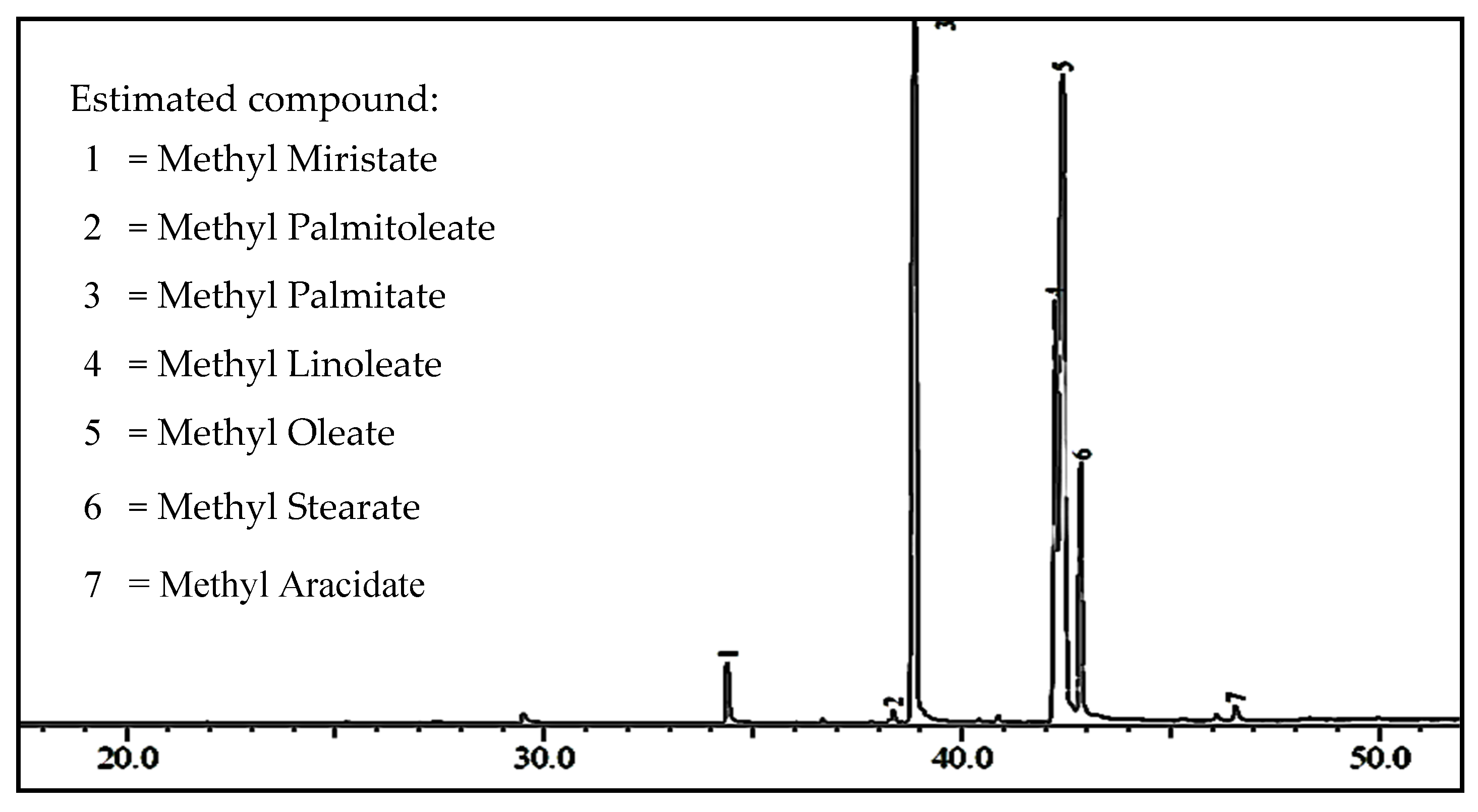
2.2.2. Biodiesel Characterization
2.3. Separation of Fe3O4/CaO Catalyst
3. Experimental Section
3.1. Materials
3.2. Equipment
3.3. Raw Material Preparation
3.4. Preparation of Fe3O4/CaO Base Catalysts
3.5. Catalytic Study
3.6. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Kusumo, F.; Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Siswantoro, J.; Mahlia, T.M.I. Optimization of transesterification process for Ceiba pentandra oil: A comparative study between kernel-based extreme learning machine and artificial neural networks. Energy 2017, 134, 24–34. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Chong, W.T.; Mahlia, T.M.I.; Sebayang, A.H.; Berawi, M.A.; Nur, H. Second generation bioethanol potential from selected Malaysia’s biodiversity biomasses: A review. Waste Manag. 2016, 47, 46–61. [Google Scholar] [CrossRef]
- Silitonga, A.; Mahlia, T.M.; Ong, H.C.; Riayatsyah, T.M.; Kusumo, F.; Ibrahim, H.; Dharma, S.; Gumilang, D. A comparative study of biodiesel production methods for Reutealis trisperma biodiesel. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 2006–2014. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Saiful, S.; Alatas, M.; Subhan, S.; Lala, A.; Muslem, M.; Suhendra, R.; Idroes, G.M.; Marwan, M.; et al. Geochemistry Exploration and Geothermometry Application in the North Zone of Seulawah Agam, Aceh Besar District, Indonesia. Energies 2019, 12, 4442. [Google Scholar] [CrossRef] [Green Version]
- Schloer, S.; Bredmose, H.; Bingham, H.B. The influence of fully nonlinear wave forces on aero-hydro-elastic calculations of monopile wind turbines. Mar. Struct. 2016, 50, 162–188. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Techno-economic analysis of an optimized photovoltaic and diesel generator hybrid power system for remote houses in a tropical climate. Energy Convers. Manag. 2013, 69, 163–173. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.M. Phase change materials (PCM) for solar energy usages and storage: an overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Preparation and properties of highly conductive palmitic acid/graphene oxide composites as thermal energy storage materials. Energy 2013, 58, 628–634. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Idroes, R.; Yusuf, M.; Alatas, M.; Lala, A.; Suhendra, R.; Idroes, G.M. Marwan Geochemistry of Sulphate spring in the Ie Jue geothermal areas at Aceh Besar district, Indonesia. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 12 September 2019; Volume 523, p. 012012. [Google Scholar]
- Idroes, R.; Yusuf, M.; Alatas, M.; Lala, A.; Suhendra, R.; Idroes, G.M. Geochemistry of hot springs in the Ie Seu’um hydrothermal areas at Aceh Besar district, Indonesia. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 12 September 2018; Volume 334, p. 12002. [Google Scholar]
- Idroes, R.; Yusuf, M.; Alatas, M.; Lala, A.; Suhendra, R.; Idroes, G.M.; Riza, M. Geochemistry of warm springs in the Ie Brôuk hydrothermal areas at Aceh Besar district. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 12 September 2019; Volume 523, p. 12010. [Google Scholar]
- Nuraskin, C.; Idroes, R.; Soraya, C. Djufri Identification of secondary metabolite of laban leaf extract (Vitex pinnata l) from geothermal areas and non-geothermal of agam mountains in Aceh Besar, Aceh province, Indonesia. Rasayan J. Chem. 2020, 13, 18–23. [Google Scholar]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Santos, A.F.; Arim, A.L.; Lopes, D.V.; Gando-Ferreira, L.M.; Quina, M.J. Recovery of phosphate from aqueous solutions using calcined eggshell as an eco-friendly adsorbent. J. Environ. Manag. 2019, 238, 451–459. [Google Scholar] [CrossRef]
- Yaakob, Z.; Sukarman, I.S.B.; Narayanan, B.; Abdullah, S.R.S.; Ismail, M. Utilization of palm empty fruit bunch for the production of biodiesel from Jatropha curcas oil. Bioresour. Technol. 2012, 104, 695–700. [Google Scholar] [CrossRef]
- Liu, C.; Lv, P.; Yuan, Z.; Yan, F.; Luo, W. The nanometer magnetic solid base catalyst for production of biodiesel. Renew. Energy 2010, 35, 1531–1536. [Google Scholar] [CrossRef]
- Kesica, Z.; Lukic, I.; Zdujic, M.; Liu, H.; Skala, D. Mechanochemically Synthesized CaO ZnO Catalyst For Biodiesel Production. Procedia Eng. 2012, 42, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Zhang, Y.; Li, S.; Tian, S.; Wang, B. Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process. Technol. 2012, 95, 84–89. [Google Scholar] [CrossRef]
- Zhang, P.; Han, Q.; Fan, M.; Jiang, P. Magnetic solid base catalyst CaO/CoFe2O4 for biodiesel production: Influence of basicity and wettability of the catalyst in catalytic performance. Appl. Surf. Sci. 2014, 317, 1125–1130. [Google Scholar] [CrossRef]
- Istadi, I.; Prasetyo, S.A.; Nugroho, T.S. Characterization of K2O/CaO-ZnO Catalyst for Transesterification of Soybean Oil to Biodiesel. Procedia Environ. Sci. 2015, 23, 394–399. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@CaO. Fuel 2016, 164, 314–321. [Google Scholar] [CrossRef]
- Hafiz, F.; Helwani, Z.; Saputra, E. Sintesis Katalis Basa Padat Nanomagnetik CaO/Serbuk Besi untuk Reaksi Transesterifikasi Minyak Sawit Off Grade menjadi Biodiesel. Ph.D. Thesis, Riau University, Riau, Indonesia, 2017. [Google Scholar]
- Niju, S.; Meera, K.M.; Begum, S.; Anantharaman, N. Modification of egg shell and its application in biodiesel production. J. Saudi Chem. Soc. 2014, 18, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.C.; Tan, Y.P.; Taufiq-Yap, Y.H.; Ramli, I.; Tee, H.S. Biodiesel production via transesterification of palm oil by using CaO–CeO2 mixed oxide catalysts. Fuel 2015, 162, 288–293. [Google Scholar] [CrossRef]
- Umdu, E. Methyl Ester Production From Vegetable Oils on Heterogeneous Basic Catalysts; İzmir Institute of Technology: Urla, Turkey, 2008. [Google Scholar]
- Mutreja, V.; Singh, S.; Ali, A. Potassium impregnated nanocrystalline mixed oxides of La and Mg as heterogeneous catalysts for transesterification. Renew. Energy 2014, 62, 226–233. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Watkins, R.S.; Lee, A.F.; Wilson, K. Li–CaO catalysed tri-glyceride transesterification for biodiesel applications. Green Chem. 2004, 6, 335–340. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S.; Tan, S.H. Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energy Convers. Manag. 2014, 88, 1167–1178. [Google Scholar] [CrossRef]
- Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Md Sani, N.D.; Safitri, E.; Yook Heng, L.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Ali, A. Nanocrystalline K–CaO for the transesterification of a variety of feedstocks: Structure, kinetics and catalytic properties. Biomass Bioenergy 2012, 46, 459–468. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Improving transesterification acitvity of CaO with hydration technique. Bioresour. Technol. 2010, 101, 3784–3786. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Development and characterisation of novel heterogeneous palm oil mill boiler ash-based catalysts for biodiesel production. Bioresour. Technol. 2012, 125, 158–164. [Google Scholar] [CrossRef]
- Earlia, N.; Rahmad, R.; Amin, M.; Prakoeswa, C.; Khairan, K.; Idroes, R. The potential effect of fatty acids from Pliek U on epidermal fatty acid binding protein: Chromatography and bioinformatic studies. Sains Malaysiana 2019, 48, 1019–1024. [Google Scholar] [CrossRef]
- Rahmad, R.; Earlia, N.; Nabila, C.; Inayati, I.; Amin, M.; Prakoeswa, C.R.S.; Khairan, K.; Idroes, R. Antibacterial cream formulation of ethanolic Pliek U extracts and ethanolic residue hexane Pliek U extracts against Staphylococcus aureus. IOP Conf. Ser. Mater. Sci. Eng. 2019, 523, 012011. [Google Scholar] [CrossRef]
- Earlia, N.; Suhendra, R.; Amin, M.; Prakoeswa, C.R.S.; Idroes, R. GC/MS Analysis of Fatty Acids on Pliek U Oil and Its Pharmacological Study by Molecular Docking to Filaggrin as Drug Candidate in Atopic Dermatitis Treatment. Sci. World J. 2019, 2019, 8605743. [Google Scholar] [CrossRef]
- Hasanah, U.; Md Sani, N.D.; Yook Heng, L.; Idroes, R.; Safitri, E. Construction of a Hydrogel Pectin-Based Triglyceride Optical Biosensor with Immobilized Lipase Enzymes. Biosensors 2019, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Iraj, K.; Mosivand, S. Phase Transition of Electrooxidized Fe3O4 to gamma and alpha-Fe2O3 Nanoparticles Using Sintering Treatment. Acta Phys. Pol. Ser. A 2014, 125, 1210–1214. [Google Scholar]
- Hindryawati, N.; Maniam, G.P.; Karim, M.R.; Chong, K.F. Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst. Eng. Sci. Technol. Int. J. 2014, 17, 95–103. [Google Scholar] [CrossRef]


| No. | Parameter | Unit | Value | Standard CPO SNI 01-2901-2006 |
|---|---|---|---|---|
| 1 2 3 4 5 | Density (40°) Viscosity (40°) Moisture FFA Color | kg/m3 mm2/s % % | 894 29.50 3.5 6.9 Orange | - - Max 0.5 Max 0.5 Orange |
| Dehydration Temperature (°C) | Dehydration (h) | Strength (H_) |
|---|---|---|
| 500 °C | 2 | 7.2 < H_ < 9.3 |
| 3 | 7.2 < H_ < 9.3 | |
| 4 | 7.2 < H_ < 9.3 | |
| 600 °C | 2 | 9.3 < H_ < 12.2 |
| 3 | 12.2 < H_ < 15.0 | |
| 4 | 7.2 < H_ < 9.3 | |
| 700 °C | 2 | 7.2 < H_ < 9.3 |
| 3 | 7.2 < H_ < 9.3 | |
| 4 | 7.2 < H_ < 9.3 |
| Dehydration Temperature (°C) | Dehydration Time (h) | Surface Area (m2/g) |
|---|---|---|
| 500 °C | 2 | 27.70 |
| 3 | 31.55 | |
| 4 | 46.75 | |
| 600 °C | 2 | 148.65 |
| 3 | 265.37 | |
| 4 | 49.43 | |
| 700 °C | 2 | 15.67 |
| 3 | 35.67 | |
| 4 | 44.24 |
| No. | Characteristics | Unit | Result | SNI 7182:2015 |
|---|---|---|---|---|
| 1 | Density | kg/m3 | 871.59 | 850–890 |
| 2 | Kinematic viscosity | mm2/s | 3.81 | 2.3–6.0 |
| 3 | Flash point | °C | 135 | Min. 100 |
| 4 5 | Acid number Cetane number | mg-KOH/g | 0.423 53 | Max. 0.5 >48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helwani, Z.; Ramli, M.; Saputra, E.; Bahruddin, B.; Yolanda, D.; Fatra, W.; Idroes, G.M.; Muslem, M.; Mahlia, T.M.I.; Idroes, R. Impregnation of CaO from Eggshell Waste with Magnetite as a Solid Catalyst (Fe3O4/CaO) for Transesterification of Palm Oil Off-Grade. Catalysts 2020, 10, 164. https://doi.org/10.3390/catal10020164
Helwani Z, Ramli M, Saputra E, Bahruddin B, Yolanda D, Fatra W, Idroes GM, Muslem M, Mahlia TMI, Idroes R. Impregnation of CaO from Eggshell Waste with Magnetite as a Solid Catalyst (Fe3O4/CaO) for Transesterification of Palm Oil Off-Grade. Catalysts. 2020; 10(2):164. https://doi.org/10.3390/catal10020164
Chicago/Turabian StyleHelwani, Zuchra, Muliadi Ramli, Edy Saputra, Bahruddin Bahruddin, Delvi Yolanda, Warman Fatra, Ghazi Mauer Idroes, Muslem Muslem, Teuku Meurah Indra Mahlia, and Rinaldi Idroes. 2020. "Impregnation of CaO from Eggshell Waste with Magnetite as a Solid Catalyst (Fe3O4/CaO) for Transesterification of Palm Oil Off-Grade" Catalysts 10, no. 2: 164. https://doi.org/10.3390/catal10020164
APA StyleHelwani, Z., Ramli, M., Saputra, E., Bahruddin, B., Yolanda, D., Fatra, W., Idroes, G. M., Muslem, M., Mahlia, T. M. I., & Idroes, R. (2020). Impregnation of CaO from Eggshell Waste with Magnetite as a Solid Catalyst (Fe3O4/CaO) for Transesterification of Palm Oil Off-Grade. Catalysts, 10(2), 164. https://doi.org/10.3390/catal10020164








