Hydrogenation of Carbon Dioxide on Supported Rh Catalysts
Abstract
1. Introduction
2. Hydrogenation of CO2 on Supported Metal Catalysts
3. Adsorption and Dissociation of CO2 on Rh and on Supported Rh Catalysts
4. Reaction of CO2 with H2 on Unsupported Rh Catalysts
5. Reaction of CO2 with H2 on Supported Rh Catalysts
5.1. Reaction of CO2 with H2 on Titania-Supported Rh Catalysts
5.2. Reaction of CO2 with H2 on Silica Supported Rh Catalysts
5.3. Reaction of CO2 with H2 on Alumina Supported Rh Catalysts
5.4. Reaction of CO2 with H2 on Other Supported Rh Catalysts
| D% | Particle Size (nm) | Ea kJ/mol | x | y | Reference | |
|---|---|---|---|---|---|---|
| 5% Rh/SiO2 | 22.8 | 72.4 | 0.64 | 0.27 | [66] | |
| 3.4% Rh/SiO2 | 45 | 2.2 | 69.5 | 0.53 | −0.46 | [39] |
| 2.3% Rh/SiO2 | 27 | 66.6 | - | 0.4 | [68] | |
| 1.047% Rh/SiO2 | 99.2 | - | - | [84] | ||
| 2.3% Rh/ZrO2 | 51 | 62.4 | 0.4 | [67,68] | ||
| 5% Rh/Al2O3 | 30.2 | 67.8 | 0.61 | 0.26 | [12,65] | |
| 1% Rh/Al2O3 | 30 | 3.6 | 95 | [79] | ||
| 2% Rh/Al2O3 | 18 | 6.1 | 66.1 | [79] | ||
| 3% Rh/Al2O3 | 7.1 | 15.4 | 61.1 | [79] | ||
| 2.3% Rh/Al2O3 | 6 | 71.2 | [67,68] | |||
| 1% Rh/TiO2 | 22.3 | 81.1 | [65] | |||
| 1% Rh/TiO2 | 7.8 | 103 | [77] | |||
| 1% Rh/TiO2 + 2% WO3 | 16.9 | 72.8 | [77] | |||
| 0.5% Rh/TiO2 | 102.8 for CH4 54.8 for CO | 0 1 | 0.49 1.1 | [78] | ||
| 0.5% Rh/TiO2 + 0.45% W6+ | 69.4 for CH4 26.3 for CO | 0 1 | 0.53 0.95 | [78] | ||
| 0.5% Rh/TiO2 | 61 | 2 | 120.2 | 0.83 | −0.36 | [69] |
| 1% Rh/TiO2 | 24 | 5 | 81.2 | 0.75 | −0.15 | [69] |
| 3% Rh/TiO2 | 6 | 17 | 71.2 | 0.58 | 0.01 | [69] |
| 5% Rh/TiO2 | 6 | 19 | 68.2 | [69] | ||
| 2.3% Rh/MgO | 27 | 96.7 | [67,68] | |||
| 0.67% Rh/MgO | 29 | 92 | [106] | |||
| 2.3% Rh/Nb2O5 | 6 | 69.9 | [67] | |||
| Rh foil | 71.2 | 0.5 | 0.2 | [54] | ||
| Rh foil | 67 | - | - | [25] | ||
| Rh foil | 75 | [57] |
6. Surface Species Formed in the Interaction and in the Reaction of CO2 with H2 on Supported Rh

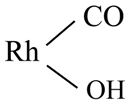
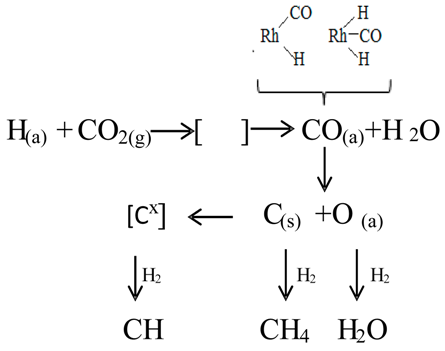
7. The Proposed Mechanism for the CO2 Hydrogenation on Supported Rh Catalysts
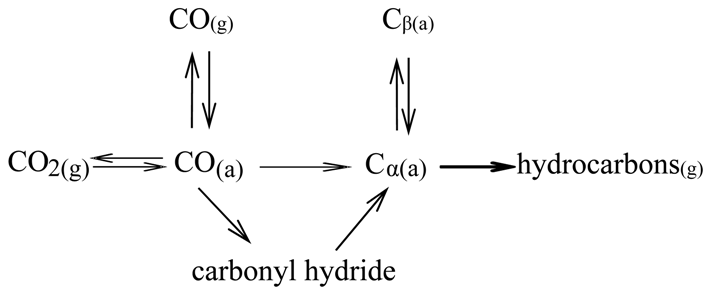
8. Conclusions
Conflicts of Interest
References
- Earth System Research Laboratory Global Monitoring Division. Available online: https://www.esrl.noaa.gov/gmd/ccgg/aggi.html (accessed on 7 January 2020).
- CO2 Earth. Available online: https://www.co2.earth/daily-co2 (accessed on 7 January 2020).
- Sabatier, P.; Senderens, J.-B. Hydrogénation directe des oxydes du carbone en présence de divers méltaux divisés. Comptes Rendus 1902, 134, 689–691. [Google Scholar]
- Mills, G.A.; Steffgen, F.W. Catalytic Methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schluter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation–From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Fechete, I.; Védrine, J.C. Nanoporous Materials as New Engineered Catalysts for the Synthesis of Green Fuels. Molecules 2015, 20, 5638–5666. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Fierro-Gonzalez, J.C. Mechanistic Insights into the CO2 Methanation Catalyzed by Supported Metals: A Review. J. Nanosci. Nanotechnol. 2019, 19, 3110–3123. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Younas, M.; Kong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Sidik, S.M. Methanation of carbon dioxide on metal-promoted mesostructured silica nanoparticles. Appl. Catal. A Gen. 2014, 486, 115–122. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdőhelyi, A. Hydrogenation of CO2 to CH4 over alumina-supported noble metals. J. Mol. Catal. 1980, 8, 471–474. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A Gen. 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported noble metal catalysts: Effects of the nature of the metallic phase on catalytic performance. Appl. Catal. A Gen. 2008, 344, 45–54. [Google Scholar] [CrossRef]
- Martin, N.M.; Velin, P.; Skoglundh, M.; Bauer, M.; Carlsson, P.-A. Catalytic hydrogenation of CO2 to methane over supported Pd, Rh and Ni catalysts. Catal. Sci. Technol. 2017, 7, 1086–1094. [Google Scholar]
- Muhammad, Y.; Sethupathi, S.; Kong, L.L.; Mohamed, A.R. CO2 methanation over Ni and Rh based catalysts: Process optimization at moderate temperature. Int. J. Energy Res. 2018, 42, 3289–3302. [Google Scholar]
- Wambach, J.; Baiker, A.; Wokaun, A. CO2 hydrogenation over metal/zirconia catalysts. Phys. Chem. Chem. Phys. 1999, 1, 5071–5080. [Google Scholar] [CrossRef]
- De Leitenburg, C.; Trovarelli, A.; Kašpar, J. A Temperature-Programmed and Transient Kinetic Study of CO2 Activation and Methanation over CeO2 Supported Noble Metals. J. Catal. 1997, 166, 98–107. [Google Scholar] [CrossRef]
- Yaccato, K.; Carhart, R.; Hagemeyer, A.; Lesik, A.; Strasser, P.; Volpe, A.F., Jr.; Turner, A.F.; Weinberg, H.; Grasselli, R.K.; Brooks, C. Competitive CO and CO2 methanation over supported noble metal catalysts in high throughput scanning mass spectrometer. Appl. Catal. A Gen. 2005, 296, 30–48. [Google Scholar] [CrossRef]
- Solymosi, F. The bonding, structure and reactions of CO2 adsorbed on clean and promoted metal surfaces. J. Mol. Catal. 1991, 65, 337–358. [Google Scholar] [CrossRef]
- Freund, H.-J.; Roberts, M.W. Surface chemistry of carbon dioxide. Surf. Sci. Rep. 1996, 25, 225–273. [Google Scholar] [CrossRef]
- Collins, A.C.; Trapnell, B.M.W. CO2 chemisorption on evaporated metal films. Trans. Faraday Soc. 1957, 53, 1476. [Google Scholar] [CrossRef]
- Campbell, C.T.; White, J.M. The adsorption, desorption, and reactions of CO and O2 on Rh. J. Catal. 1978, 54, 289–302. [Google Scholar] [CrossRef]
- Yang, C.; Garland, C.W. Infrared Studies of Carbon Monoxide Chemisorbed on Rhodium. J. Phys. Chem. 1957, 61, 1504–1512. [Google Scholar] [CrossRef]
- Sexton, B.; Somorjai, G.A. The hydrogenation of CO and CO2 over polycrystalline rhodium: Correlation of surface composition, kinetics and product distributions. J. Catal. 1977, 46, 167–189. [Google Scholar] [CrossRef]
- Dubois, L.; Somorjai, G.A. The dissociative chemisorption of carbon dioxide on rhodium surfaces. Surf. Sci. 1979, 88, L13–L17. [Google Scholar] [CrossRef][Green Version]
- Dubois, L.H.; Somorjai, G.A. The chemisorption of CO and CO2 on Rh(111) studied by high resolution electron energy loss spectroscopy. Surf. Sci. 1980, 91, 514–532. [Google Scholar] [CrossRef]
- Weinberg, W.H. Why CO2 does not disWhy CO2 does not dissociate on Rh at low temperature. Surf. Sci. 1983, 128, L224–L230. [Google Scholar] [CrossRef]
- Goodman, D.W.; Peebles, D.E.; White, J.M. CO2 dissociation on rhodium: Measurement of the specific rates on Rh(111). Surf. Sci. 1984, 140, L239–L243. [Google Scholar] [CrossRef]
- Solymosi, F.; Kiss, J. Impurity effects in the adsorption and dissociation of CO2 on Rh. Surf. Sci. 1985, 149, 17–32. [Google Scholar] [CrossRef][Green Version]
- Solymosi, F.; Kiss, J. The effect of boron impurity on the adsorption and dissociation of CO2 on Rh surfaces. Chem. Phys. Lett. 1984, 110, 639–642. [Google Scholar] [CrossRef][Green Version]
- Kiss, J.; Révész, K.; Solymosi, F. Photoelectron spectroscopic studies of the adsorption of CO2 on potassium-promoted Rh(111) surface. Surf. Sci. 1988, 207, 36–54. [Google Scholar] [CrossRef][Green Version]
- Solymosi, F.; Bugyi, L. Adsorption and dissociation of CO2 on a potassium-promoted Rh(111) surface. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 2015–2033. [Google Scholar] [CrossRef]
- Hendrickx, H.A.C.M.; Jongenelis, A.P.J.M.; Nieuwenhuys, B.E. Adsorption and dissociation of carbon dioxide on rhodium surfaces. Surf. Sci. 1985, 154, 503–523. [Google Scholar]
- Van Tol, M.F.H.; Gielbert, A.; Nieuwenhuys, B.E. The adsorption and dissociation of CO2 on Rh. Appl. Surf. Sci. 1993, 67, 166–178. [Google Scholar] [CrossRef]
- Lambeets, S.V.; Barroo, C.; Owczarek, S.; Jacobs, L.; Genty, E.; Gilis, N.; Kruse, N.; De Bocarmé, T.V. Adsorption and Hydrogenation of CO2 on Rh Nanosized Crystals: Demonstration of the Role of Interfacet Oxygen Spillover and Comparative Studies with O2, N2O, and CO. J. Phys. Chem. C 2017, 121, 16238–16249. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdőhelyi, A.; Bánsági, T. Infrared study of the surface interaction between H2 and CO2 over rhodium on various supports. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 2645. [Google Scholar] [CrossRef]
- Henderson, M.A.; Worley, S.D. An infrared study of the hydrogenation of carbon dioxide on supported rhodium catalysts. J. Phys. Chem. 1985, 89, 1417–1423. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. A Comparative Study of CO and CO2 Hydrogenation over Rh/SiO2. J. Catal. 1996, 162, 54–65. [Google Scholar] [CrossRef]
- Tanaka, Y.; Iizuka, T.; Tanabe, K. Infrared spectroscopic study of CO and CO2 adsorption on Rh-ZrO2, Rh-Al2O3 and Rh-MgO. J. Chem. Soc. Faraday Trans. 1982, 78, 2215–2225. [Google Scholar] [CrossRef]
- Heyl, D.; Rodemerck, U.; Bentrup, U. Mechanistic study of low-temperature CO2 hydrogenation over modified Rh/Al2O3 catalysts. ACS Catal. 2016, 6, 6275–6284. [Google Scholar] [CrossRef]
- Novák, É.; Fodor, K.; Szailer, T.; Oszkó, A.; Erdőhelyi, A. CO2 hydrogenation on Rh/TiO2 previously reduced at different temperatures. Top. Catal. 2002, 20, 107–117. [Google Scholar]
- Tóth, M.; Kiss, J.; Oszkó, A.; Pótári, G.; László, B.; Erdőhelyi, A. Hydrogenation of carbon dioxide on Rh, Au and Au-Rh bimetallic clusters supported on titanate nanotubes, nanowires and TiO2. Top. Catal. 2012, 55, 747–756. [Google Scholar]
- Ruiz-García, J.R.; Fierro-Gonzalez, J.C.; Handy, B.E.; Hinojosa-Reyes, L.; Río, D.A.D.H.D.; Lucio-Ortiz, C.J.; Valle-Cervantes, S.; Flores-Escamilla, G.A. An In Situ Infrared Study of CO2 Hydrogenation to Formic Acid by Using Rhodium Supported on Titanate Nanotubes as Catalysts. ChemistrySelect 2019, 4, 4206–4216. [Google Scholar]
- Zhang, Z.L.; Kladi, A.; Verykios, X.E. Surface species formed during CO and CO2 hydrogenation over Rh/TiO2 (W6+) catalysts investigated by FTIR and mass-spectroscopy. J. Catal. 1995, 156, 37–50. [Google Scholar] [CrossRef]
- Jacquemin, M.; Beuls, A.; Ruiz, P. Catalytic production of methane from CO2 and H2 at low temperature: Insight on the reaction mechanism. Catal. Today 2010, 157, 462–466. [Google Scholar] [CrossRef]
- Basini, L.; Marchionna, M.; Aragno, A. Drift and mass spectroscopic studies on the reactivity of rhodium clusters at the surface of polycrystalline oxides. J. Phys. Chem. 1992, 96, 9431–9441. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdőhelyi, A.; Kocsis, M. Surface interaction between H2 and CO2 on Rh/Al2O3, studied by adsorption and infrared spectroscopic measurements. J. Catal. 1980, 65, 428–436. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Hexen, G.; Karelovič, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Solymosi, F.; Pásztor, M.; Rákhely, G. Infrared studies of the effects of promoters on CO-induced structural changes in Rh. J. Catal. 1988, 110, 413–415. [Google Scholar] [CrossRef]
- Trautmann, S.; Baerns, M. Infrared Spectroscopic Studies of CO Adsorption on Rhodium Supported by SiO2, Al2O3, and TiO2. J. Catal. 1994, 150, 335–344. [Google Scholar] [CrossRef]
- Van’t Blik, H.F.J.; Van Zon, J.B.A.D.; Huizinga, T.; Vis, J.C.; Koningsberger, D.C.; Prins, R. Structure of rhodium in an ultradispersed Rh/Al2O3 catalyst as studied by EXAFS and other techniques. J. Am. Chem. Soc. 1985, 107, 3139–3147. [Google Scholar] [CrossRef]
- Amariglio, A.; Elbiache, A.; Amariglio, H. Effect of oxidizing pretreatments on the behavior of a rhodium powder in CO2 chemisorption and methanation. J. Catal. 1986, 98, 355–366. [Google Scholar] [CrossRef]
- Williams, K.J.; Boffa, A.B.; Salmeron, M.; Bell, A.T.; Somorjai, G.A. The kinetics of CO2 hydrogenation on a Rh foil promoted by titania overlayers. Catal. Lett. 1991, 9, 415–426. [Google Scholar] [CrossRef]
- Arandiyan, H.; Kani, K.; Wang, Y.; Jiang, B.; Kim, J.; Yoshino, M.; Rezaei, M.; Rowan, A.E.; Dai, H.; Yamauchi, Y. Highly Selective Reduction of Carbon Dioxide to Methane on Novel Mesoporous Rh Catalysts. ACS Appl. Mater. Interfaces 2018, 10, 24963–24968. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, Y.; Pan, H.; Wu, L.; Zhang, W.; Cao, L.; Zhang, J.; Zheng, L.; Yao, T. Grain boundaries modulating active sites in RhCo porous nanospheres for efficient CO2 hydrogenation. Nano Res. 2018, 11, 2357–2365. [Google Scholar] [CrossRef]
- Kohl, A.; Linsmeier, C.; Taglauer, E.; Knözinger, H. Influence of support and promotor on the catalytic activity of Rh/VOx/SiO2 model catalysts. Phys. Chem. Chem. Phys. 2001, 3, 4639–4643. [Google Scholar] [CrossRef]
- Boffa, A.; Lin, C.; Bell, A.T.; Somorjai, G.A. Promotion of CO and CO2 Hydrogenation over Rh by Metal Oxides: The Influence of Oxide Lewis Acidity and Reducibility. J. Catal. 1994, 149, 149–158. [Google Scholar] [CrossRef]
- Boffa, A.; Bell, A.T.; Somorjai, G.A. Vanadium Oxide Deposited on an Rh Foil: CO and CO2 Hydrogenation Reactivity. J. Catal. 1993, 139, 602–610. [Google Scholar] [CrossRef]
- Kusama, H.; Okabe, K.; Sayama, K.; Arakawa, H. CO2 hydrogenation to ethanol over promoted Rh/SiO2 catalysts. Catal. Today 1996, 28, 261–266. [Google Scholar] [CrossRef]
- Inoue, T.; Iizuka, T.; Tanabe, K. Hydrogenation of carbon dioxide and carbon monoxide over supported rhodium catalysts under 10 bar pressure. Appl. Catal. 1989, 46, 1–9. [Google Scholar] [CrossRef]
- Izumi, Y. Selective ethanol synthesis from carbon dioxide; roles of rhodium catalytic sites. Platinum Met. Rev. 1997, 41, 166–170. [Google Scholar]
- Nozaki, F.; Sodesawa, T.; Satoh, S.; Kimura, K. Hydrogenation of carbon dioxide into light hydrocarbons at atmospheric pressure over Rh/Nb2O5 or Cu/SiO2-Rh/Nb2O5 catalyst. J. Catal. 1987, 104, 339–346. [Google Scholar] [CrossRef]
- Trovarelli, A.; Mustazza, C.; Dolcetti, G.; Kašpar, J.; Graziani, M. Carbon dioxide hydrogenation on rhodium supported on transition metal oxides. Appl. Catal. 1990, 65, 129–142. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdőhelyi, A.; Bánsági, T. Methanation of CO2 on supported rhodium catalyst. J. Catal. 1981, 68, 371–382. [Google Scholar] [CrossRef]
- Solymosi, F. Importance of the Electric Properties of Supports in the Carrier Effect. Catal. Rev. 1968, 1, 233–255. [Google Scholar] [CrossRef]
- Iizuka, T.; Tanaka, Y.; Tanabe, K. Hydrogenation of carbon monoxide and carbon dioxide over supported rhodium catalysts. J. Mol. Catal. 1982, 17, 381–389. [Google Scholar] [CrossRef]
- Iizuka, T.; Tanaka, Y.; Tanabe, K. Hydrogenation of CO and CO2 over rhodium catalysts supported on various metal oxides. J. Catal. 1982, 76, 1–8. [Google Scholar] [CrossRef]
- Karelovič, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Yang, V.N.; Christopher, P. Isolated Metal Active Site Concentration and Stability Control Catalytic CO2 Reduction Selectivity. J. Am. Chem. Soc. 2015, 137, 3076–3084. [Google Scholar] [CrossRef]
- Ma, S.; Song, W.; Liu, B.; Zheng, H.; Deng, J.; Zhong, W.; Liu, J.; Gong, X.-Q.; Zhao, Z. Elucidation of the high CO2 reduction selectivity of isolated Rh supported on TiO2: A DFT study. Catal. Sci. Technol. 2016, 6, 6128–6136. [Google Scholar] [CrossRef]
- Bando, K.K.; Ichikuni, N.; Soga, K.; Kunimori, K.; Arakawa, H.; Asakura, K. Characterization of Rh Particles and Li-Promoted Rh Particles in Y Zeolite during CO2 Hydrogenation—A New Mechanism for Catalysis Controlled by the Dynamic Structure of Rh Particles and the Li Additive Effect. J. Catal. 2000, 194, 91–104. [Google Scholar] [CrossRef]
- Deleitenburg, C.; Trovarelli, A. Metal-Support Interactions in Rh/CeO2, Rh/TiO2, and Rh/Nb2O5 Catalysts as Inferred from CO2 Methanation Activity. J. Catal. 1995, 156, 171–174. [Google Scholar] [CrossRef]
- Tauster, S.L.; Fung, S.C.; Garten, R.L. Strong metal-support interaction. Group 8 noble metals supported on TiO2. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Zhang, S.; DeRita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 2017, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chandler, B. D Strong metal-support interactions; An external layer of complexity. Nat. Chem. 2017, 9, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, F.; Tombácz, I.; Koszta, J. Effects of variation of electric properties of TiO2 support on hydrogenation of CO and CO2 over Rh catalysts. J. Catal. 1985, 95, 578–586. [Google Scholar] [CrossRef]
- Zhang, Z.; Kladi, A.; Verykios, X. Effects of Carrier Doping on Kinetic Parameters of CO2 Hydrogenation on Supported Rhodium Catalysts. J. Catal. 1994, 148, 737–747. [Google Scholar] [CrossRef]
- Karelovič, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B Environ. 2012, 113–114, 237–249. [Google Scholar]
- Kurakata, H.; Izumi, Y.; Aika, K.I. Ethanol synthesis from carbon dioxide on TiO2-supported [Rh10Se] catalyst. Chem. Commun. 1996, 389, 389–390. [Google Scholar] [CrossRef]
- Szailer, T.; Novák, É.; Oszkó, A.; Erdőhelyi, A. Effect of H2S on the hydrogenation of carbon dioxide over supported Rh catalysts. Top. Catal. 2007, 46, 79–86. [Google Scholar] [CrossRef]
- Kusama, H.; Bando, K.K.; Okabe, K.; Arakawa, H. Effect of metal loading on CO2 hydrogenation reactivity over Rh/SiO2 catalysts. Appl. Catal. A Gen. 2000, 197, 255–268. [Google Scholar] [CrossRef]
- Kusama, H.; Bando, K.K.; Okabe, K.; Arakawa, H. CO2 hydrogenation reactivity and structure of Rh/SiO2 catalysts prepared from acetate, chloride and nitrate precursors. Appl. Catal. A Gen. 2001, 205, 285–294. [Google Scholar] [CrossRef]
- Ichikawa, S. Reactive chemisorption and methanation of carbon dioxide on rhodium particles approaching atomic dispersion. J. Mol. Catal. 1989, 53, 53–65. [Google Scholar] [CrossRef]
- Trovarelli, A.; Deleitenburg, C.; Dolcetti, G.; Lorca, J. CO2 Methanation Under Transient and Steady-State Conditions over Rh/CeO2 and CeO2-Promoted Rh/SiO2: The Role of Surface and Bulk Ceria. J. Catal. 1995, 151, 111–124. [Google Scholar] [CrossRef]
- Arakawa, H.; Takeuchi, K.; Matsuzaki, T.; Sugi, Y. Effect of the metal dispersion on the activity and selectivity of Rh/SiO2 catalyst for high pressure CO hydrogenation. Chem. Lett. 1984, 13, 1607–1610. [Google Scholar] [CrossRef]
- Bhasin, M.M.; Bartley, W.J.; Ellgen, P.C.; Wilson, T.P. Synthesis gas conversion over supported rhodium and rhodium-iron catalysts. J. Catal. 1978, 54, 120–128. [Google Scholar] [CrossRef]
- Kusama, H.; Okabe, K.; Sayama, K.; Arakawa, H. Ethanol synthesis by catalytic hydrogenation of CO2 over Rh-Fe/SiO2 catalysts. Energy 1997, 22, 343–348. [Google Scholar] [CrossRef]
- Arakawa, H. Research and development on new synthetic routes for basic chemicals by catalytic hydrogenation of CO2. Stud. Surf. Sci. Catal. 1998, 114, 19–30. [Google Scholar]
- Gogate, M.R.; Davis, R.J. Comparative study of CO and CO2 hydrogenation over supported Rh–Fe catalysts. Catal. Commun. 2010, 11, 901–906. [Google Scholar] [CrossRef]
- Kusama, H.; Okabe, K.; Sayama, K.; Arakawa, H. Alcohol synthesis by catalytic hydrogenation of CO2 over Rh-Co/SiO2. Appl. Organometal. Chem. 2000, 14, 836–840. [Google Scholar] [CrossRef]
- Kusama, H.; Okabe, K.; Arakawa, H. Characterization of Rh-Co/SiO2 catalysts for CO2 hydrogenation with TEM, XPS and FT-IR. Appl. Catal. A Gen. 2001, 207, 85–94. [Google Scholar] [CrossRef]
- Gronchi, P.; Marengo, S.; Mazzocchia, C.; Tempesti, E.; Del Rosso, R. On the formation of oxygenated products by CO hydrogenation with lanthana promoted rhodium catalysts. React. Kinet. Catal. Lett. 1997, 60, 79–88. [Google Scholar] [CrossRef]
- Ikehara, N.; Hara, K.; Satsuma, A.; Hattori, T.; Murakami, Y. Unique Temperature Dependence of Acetic Acid Formation in CO2 Hydrogenation on Ag-promoted Rh/SiO2 Catalyst. Chem. Lett. 1994, 23, 263–264. [Google Scholar] [CrossRef]
- Bowker, M. On the mechanism of ethanol synthesis on rhodium. Catal. Today 1992, 15, 77–100. [Google Scholar] [CrossRef]
- Ding, Y.-H.; Huang, W.; Wang, Y.-G. Direct synthesis of acetic acid from CH4 and CO2 by a step-wise route over Pd/SiO2 and Rh/SiO2 catalysts. Fuel Process. Technol. 2007, 88, 319–324. [Google Scholar] [CrossRef]
- Erdőhelyi, A.; Kocsis, M.; Bánsági, T.; Solymosi, F. Hydrogenation of CO2 on Rh/Al2O3. Acta Chim. Acad. Sci. Hung. 1982, 111, 591–605. [Google Scholar]
- Bowker, M.; Cassidy, T.; Ashcroft, A.; Cheetham, A. The Methanation of CO and CO2 over a Rh/Al2O3 Catalyst Using a Pulsed-Flow Microreactor. J. Catal. 1993, 143, 308–313. [Google Scholar] [CrossRef]
- Martin, N.M.; Hemmingsson, F.; Wang, X.; Merte, L.R.; Hejral, U.; Gustafson, J.; Skoglundh, M.; Meira, D.M.; Dippel, A.-C.; Gutowski, O.; et al. Structure–function relationship during CO2 methanation over Rh/Al2O3 and Rh/SiO2 catalysts under atmospheric pressure conditions. Catal. Sci. Technol. 2018, 8, 2686–2696. [Google Scholar] [CrossRef]
- Tripol’skii, A.I. Effect of modifying additive to rhodium–containing catalyst on the kinetics of CO2 hydrogenation. Theor. Exp. Chem. 2011, 47, 331–336. [Google Scholar]
- Büchel, R.; Baiker, A.; Pratsinis, S.E. Effect of Ba and K addition and controlled spatial deposition of Rh in Rh/Al2O3 catalysts for CO2 hydrogenation. Appl. Catal. A Gen. 2014, 477, 93–101. [Google Scholar]
- Karelovič, A.; Ruiz, P. Improving the Hydrogenation Function of Pd/γ-Al2O3 Catalyst by Rh/γ-Al2O3 Addition in CO2 Methanation at Low Temperature. ACS Catal. 2013, 3, 2799–2812. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Martin, N.M.; Hemmingsson, F.; Schaefer, A.; Ek, M.; Merte, L.R.; Hejral, U.; Gustafson, J.; Skoglundh, M.; Dippel, A.-C.; Gutowski, O.; et al. Structure–function relationship for CO2 methanation over ceria supported Rh and Ni catalysts under atmospheric pressure conditions. Catal. Sci. Technol. 2019, 9, 1644–1653. [Google Scholar] [CrossRef]
- Kai, T.; Matsumura, T.; Takahashi, T. The effect of support structure on CO2 hydrogenation over a rhodium catalyst supported on niobium oxide. Catal. Lett. 1992, 16, 129–135. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kanno, T.; Konishi, Y.; Ohashi, H. Dynamic kinetics of the common intermediates formed in CO and CO2 hydrogenation on Rh/MgO. Chem. Eng. Commun. 1988, 71, 189–193. [Google Scholar] [CrossRef]
- Reyes, P.; Concha, I.; Pecchi, G.; Fierro, J. Changes induced by metal oxide promoters in the performance of Rh–Mo/ZrO2 catalysts during CO and CO2 hydrogenation. J. Mol. Catal. A Chem. 1998, 129, 269–278. [Google Scholar] [CrossRef]
- Boaro, M.; Colussi, S.; Trovarelli, A. Ceria-based materials in hydrogenation and reforming reactions for CO2 valorization. Front. Chem. 2019, 7, 28. [Google Scholar] [CrossRef]
- Varga, E.; Pusztai, P.; Oszkó, A.; Baán, K.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Stability and Temperature-Induced Agglomeration of Rh Nanoparticles Supported by CeO2. Langmuir 2016, 32, 2761–2770. [Google Scholar] [CrossRef]
- Kai, T.; Yamasaki, Y.; Takahashi, T.; Masumoto, T.; Kimura, H. Increase in the thermal stability during the methanation of CO2 over a Rh catalyst prepared from an amorphous alloy. Can. J. Chem. Eng. 1998, 76, 331–335. [Google Scholar] [CrossRef]
- Kishida, M.; Fujita, T.; Umakoshi, K.; Ishiyama, J.; Nagata, H.; Wakabayashi, K. Novel preparation of metal-supported catalysts by colloidal microparticles in a water-in-oil microemulsion; catalytic hydrogenation of carbon dioxide. J. Chem. Soc. Chem. Commun. 1995, 763–764. [Google Scholar] [CrossRef]
- Yan, B.; Wu, Q.; Cen, J.; Timoshenko, J.; Frenkel, A.I.; Su, D.; Chen, X.; Parise, J.B.; Stach, E.; Orlov, A.; et al. Highly active sub-nanometer Rh clusters derived from Rh-doped SrTiO3 for CO2 reduction. Appl. Catal. B Environ. 2018, 237, 1003–1011. [Google Scholar] [CrossRef]
- Bando, K.K.; Soga, K.; Kunimori, K.; Ichikuni, N.; Okabe, K.; Kusama, H.; Sayama, K.; Arakawa, H. CO2 hydrogenation activity and surface structure of zeolite-supported Rh catalysts. Appl. Catal. A Gen. 1998, 173, 47–60. [Google Scholar] [CrossRef]
- Wang, C.; Guan, E.; Wang, L.; Chu, X.; Wu, Z.; Zhang, J.; Yang, Z.; Jiang, Y.; Zhang, L.; Meng, X.; et al. Product Selectivity Controlled by Nanoporous Environments in Zeolite Crystals Enveloping Rhodium Nanoparticle Catalysts for CO2 Hydrogenation. J. Am. Chem. Soc. 2019, 141, 8482–8488. [Google Scholar] [CrossRef] [PubMed]
- Bando, K.K.; Soga, K.; Kunimori, K.; Arakawa, H. Effect of Li additive on CO2 hydrogenation reactivity of zeolite supported Rh catalysts. Appl. Catal. A Gen. 1998, 175, 67–81. [Google Scholar] [CrossRef]
- Lavalley, J. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Szanyi, J.; Kwak, J.H. Dissecting the steps of CO2 reduction: 1. The interaction of CO and CO2 with γ-Al2O3: An in situ FTIR study. Phys. Chem. Chem. Phys. 2014, 16, 15117–15125. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, F.; Bánsági, T.; Erdőhelyi, A. Infrared study of the reaction of adsorbed formate ion with H2 on supported Rh catalysts. J. Catal. 1981, 72, 166–169. [Google Scholar] [CrossRef]
- Benitez, J.J.; Carrizosa, I.; Odriozola, J.A. HCOOH hydrogenation over lanthanide-oxide-promoted Rh/A12O3 catalysts. Appl. Surf. Sci. 1993, 68, 565–573. [Google Scholar] [CrossRef]
- Worley, S.D.; Rice, C.A.; Curtis, C.V.; Guin, J.A. Effect of support material on Rh catalysts. J. Phys Chem. 1982, 86, 2714–2717. [Google Scholar] [CrossRef]
- Solymosi, F.; Lancz, M. Effects of different surface species on the infrared spectrum of CO adsorbed on Rh/Al2O3. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 883. [Google Scholar] [CrossRef]
- Henderson, M.A.; Worley, S.D. An infrared study of isotopic exchange during methanation over supported rhodium catalysts: An inverse spillover effect. J. Phys. Chem. 1985, 89, 392–394. [Google Scholar] [CrossRef]
- Iizuka, T.; Tanaka, Y. Dissociative adsorption of CO2 on supported rhodium catalyst: Comment on surface interaction between H2 and CO2 on Rh-Al2O3. J. Catal. 1981, 70, 440–441. [Google Scholar] [CrossRef]
- Benitez, J.J.; Alvero, R.; Carrizosa, I.; Odriozola, J.A. “In situ” DRIFTS study of adsorbed species in the hydrogenation of carbon oxides. Catal. Today 1991, 9, 53–60. [Google Scholar] [CrossRef]
- Yoshida, H.; Narisawa, S.; Fujita, S.-I.; Ruixia, L.; Arai, M. In situ FTIR study on the formation and adsorption of CO on alumina-supported noble metal catalysts from H2 and CO2 in the presence of water vapor at high pressures. Phys. Chem. Chem. Phys. 2012, 14, 4724. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.C.J.; Vannice, M.A. Metal-support interactions during the CO2 reforming of CH4 over model TiOx/Pt catalysts. Catal. Lett. 1997, 48, 31–38. [Google Scholar] [CrossRef]
- Stevenson, S.A.; Lisitsyn, A.; Knoezinger, H. Adsorption of carbon monoxide on manganese-promoted rhodium/silica catalysts as studied by infrared spectroscopy. J. Phys. Chem. 1990, 94, 1576–1581. [Google Scholar] [CrossRef]
- Chuang, S.S.C.; Stevens, R.W., Jr.; Khatri, R. Mechanism of C2+ oxygenate synthesis on Rh catalysts. Top. Catal., 2005, 32, 225–232. [Google Scholar] [CrossRef]
- Raskó, J.; Kecskés, T.; Kiss, J. Adsorption and reaction of formaldehyde on TiO2-supported Rh catalysts studied by FTIR and mass spectrometry. J. Catal. 2004, 226, 183–191. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, S.; Wang, S. In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2. Cat. Sci. Technol. 2014, 4, 502–509. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active Site Dependent Reaction Mechanism over Ru/CeO2 Catalyst toward CO2 Methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef]
- Erdőhelyi, A.; Solymosi, F. Effects of the support on the adsorption and dissociation of CO and on the reactivity of surface carbon on Rh catalysts. J. Catal. 1983, 84, 446–460. [Google Scholar] [CrossRef][Green Version]
- Solymosi, F.; Tombácz, I.; Kocsis, M. Hydrogenation of CO on supported Rh catalysts. J. Catal. 1982, 75, 78–93. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdőhelyi, A. Hydrogenation of Carbon Dioxide on Supported Rh Catalysts. Catalysts 2020, 10, 155. https://doi.org/10.3390/catal10020155
Erdőhelyi A. Hydrogenation of Carbon Dioxide on Supported Rh Catalysts. Catalysts. 2020; 10(2):155. https://doi.org/10.3390/catal10020155
Chicago/Turabian StyleErdőhelyi, András. 2020. "Hydrogenation of Carbon Dioxide on Supported Rh Catalysts" Catalysts 10, no. 2: 155. https://doi.org/10.3390/catal10020155
APA StyleErdőhelyi, A. (2020). Hydrogenation of Carbon Dioxide on Supported Rh Catalysts. Catalysts, 10(2), 155. https://doi.org/10.3390/catal10020155




