Photocatalytic Transformation of Triclosan. Reaction Products and Kinetics
Abstract
1. Introduction
2. Results and Discussion
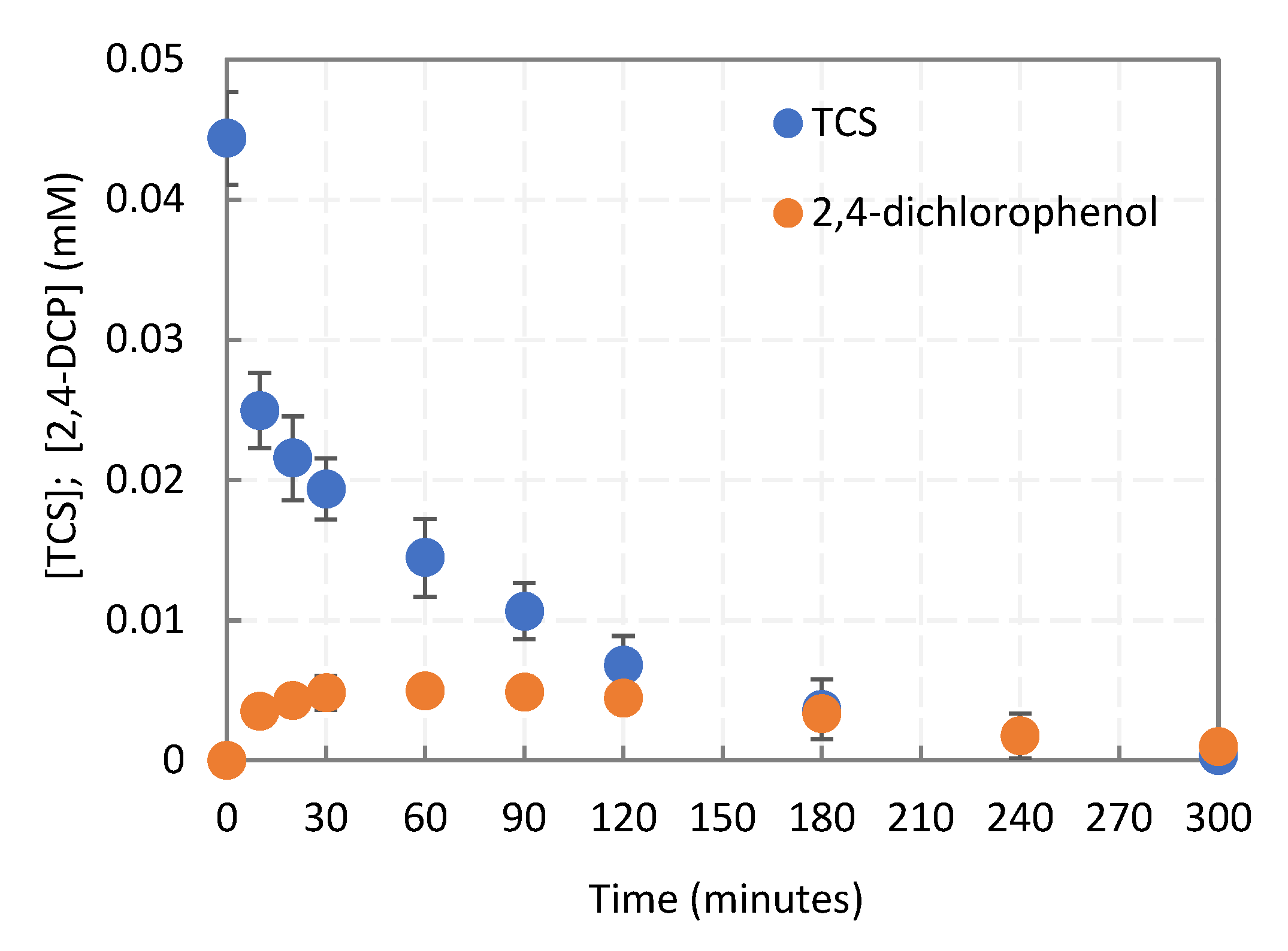
2.1. Degradation Kinetics of the Parent (TCS) Compound
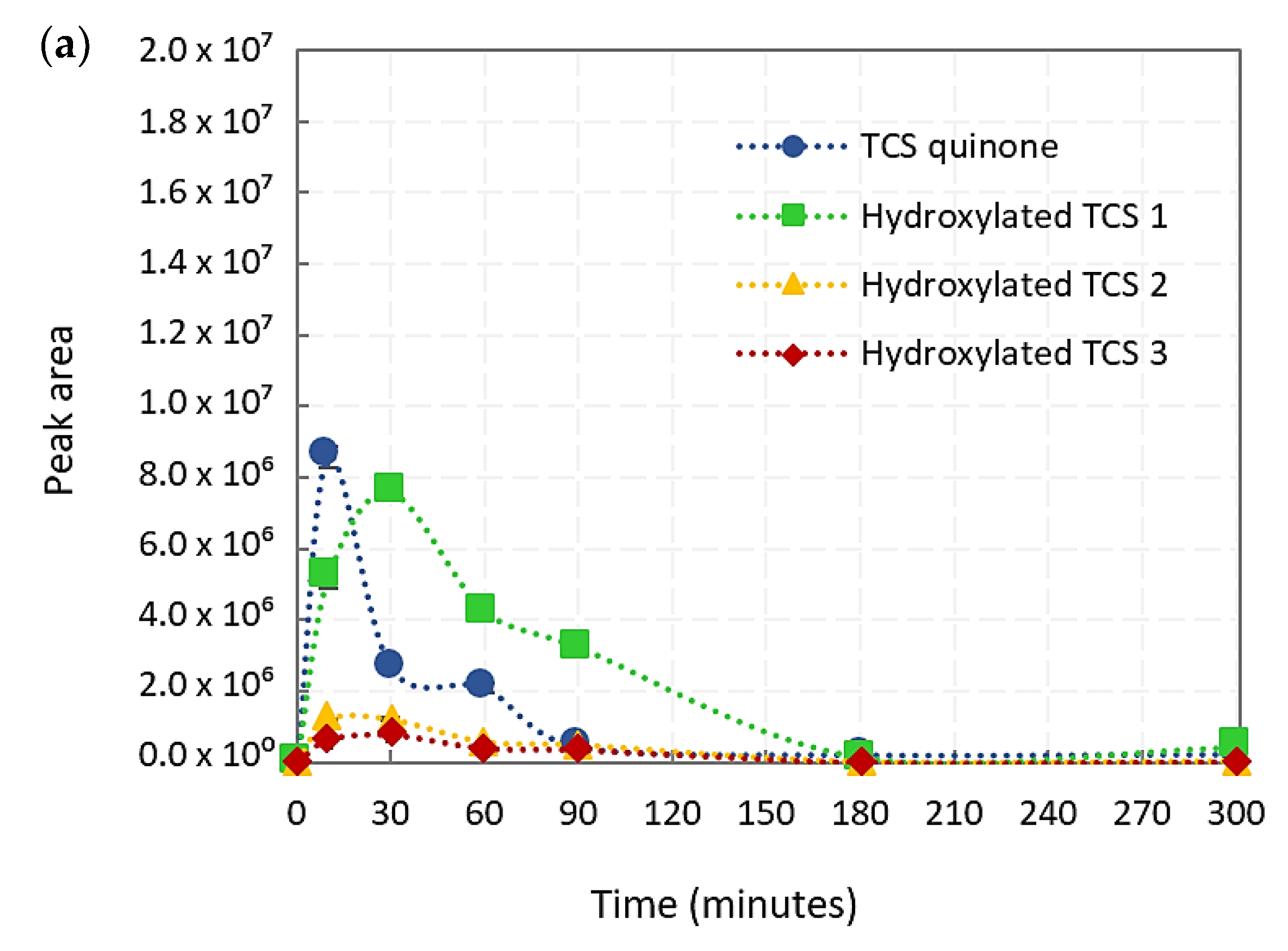
2.1.1. TCS Intermediate Derivatives
2.1.2. TCS Degradation Pathways
2.2. Kinetic Analysis and Modeling
3. Materials and Methods
3.1. Materials and Chemicals
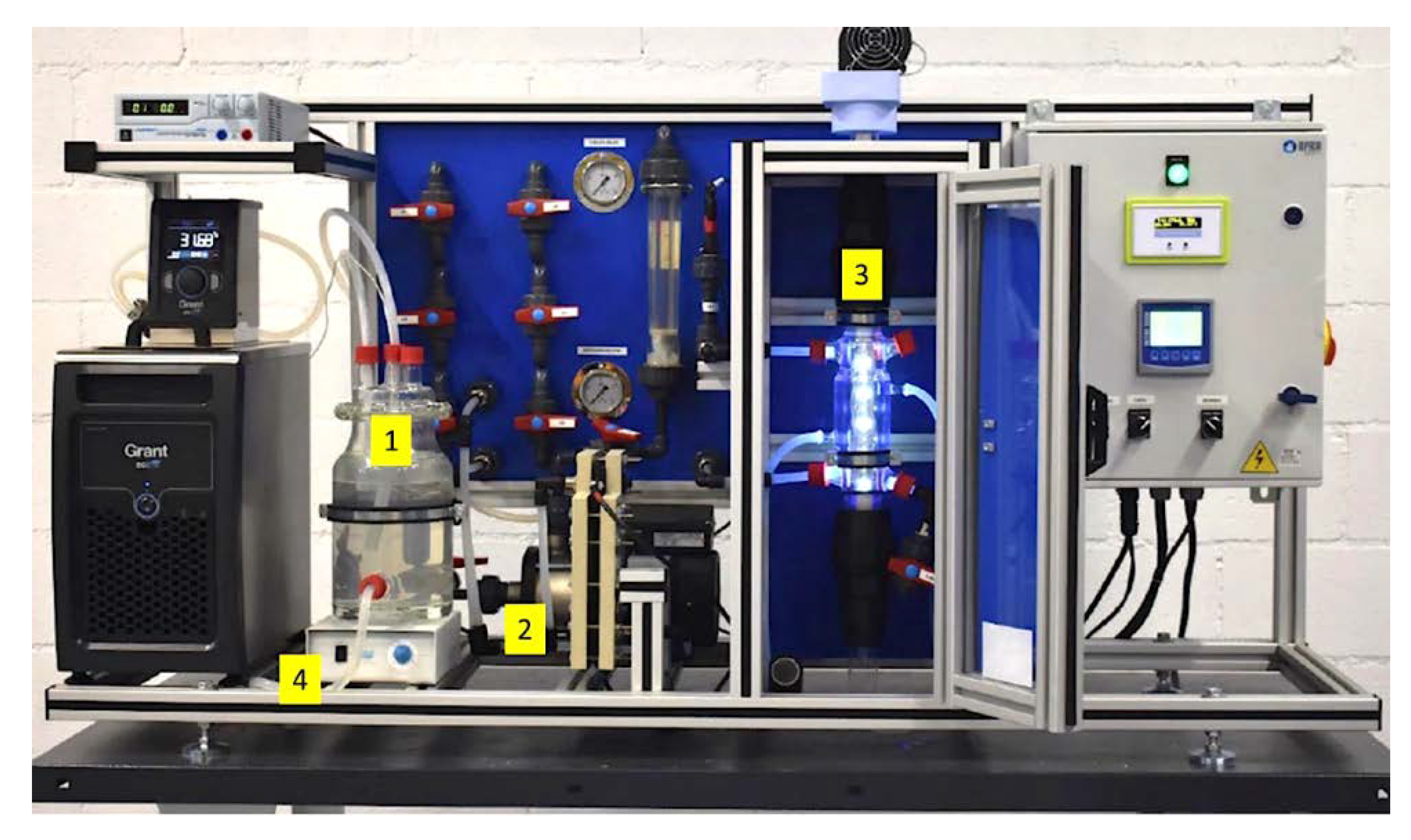
3.2. Photocatalytic Experiments
3.3. Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ortiz Uribe, I.; Mosquera-Corral, A.; Lema Rodicio, J.; Esplugas, S. Advanced technologies for water treatment and reuse. Am. Inst. Chem. Eng. 2015, 61, 3146–3158. [Google Scholar] [CrossRef]
- Solá-Gutiérrez, C.; San Román, M.F.; Ortiz, I. Fate and hazard of the electrochemical oxidation of triclosan. Evaluation of polychlorodibenzo-p-dioxins and polychlorodibenzofurans (PCDD/Fs) formation. Sci. Total Environ. 2018, 626, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.A.; Li, S.; Rodriguez, M.B.; Aschner, M. Is Triclosan a neurotoxic agent? J. Toxicol. Environ. Heal. Part B Crit. Rev. 2017, 20, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Bester, K. Triclosan in a sewage treatment process—Balances and monitoring data. Water Res. 2003, 37, 3891–3896. [Google Scholar] [CrossRef]
- Canosa, P.; Rodríguez, I.; Rubí, E.; Cela, R. Determination of parabens and triclosan in indoor dust using matrix solid-phase dispersion and gas chromatography with tandem mass spectrometry. Anal. Chem. 2007, 79, 1675–1681. [Google Scholar] [CrossRef]
- Chen, J.; Hartmann, E.M.; Kline, J.; Van Den Wymelenberg, K.; Halden, R.U. Assessment of human exposure to triclocarban, triclosan and five parabens in U.S. indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. J. Hazard. Mater. 2018, 360, 623–630. [Google Scholar] [CrossRef]
- Pedrouzo, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Stir-bar-sorptive extraction and ultra-high-performance liquid chromatography-tandem mass spectrometry for simultaneous analysis of UV filters and antimicrobial agents in water samples. Anal. Bioanal. Chem. 2010, 397, 2833–2839. [Google Scholar] [CrossRef]
- Kookana, R.S.; Ying, G.G.; Waller, N.J. Triclosan: Its occurrence, fate and effects in the Australian environment. Water Sci. Technol. 2011, 63, 598–604. [Google Scholar] [CrossRef]
- Villaverde-De-Sáa, E.; González-Mariño, I.; Quintana, J.B.; Rodil, R.; Rodríguez, I.; Cela, R. In-sample acetylation-non-porous membrane-assisted liquid-liquid extraction for the determination of parabens and triclosan in water samples. Anal. Bioanal. Chem. 2010, 397, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Balakrishnan, P. Triclosan in treated wastewater from a city wastewater treatment plant and its environmental risk assessment. Water. Air. Soil Pollut. 2019, 230, 69. [Google Scholar] [CrossRef]
- Ricart, M.; Guasch, H.; Alberch, M.; Barceló, D.; Bonnineau, C.; Geiszingerb, A.; Ferrer, J.; Ricciardi, F.; Romaní, A.M.; Morin, S.; et al. Triclosan persistence through wastewater treatment plants and its potential: Toxic effects on river biofilms. Aquat. Toxicol. 2010, 100, 346–353. [Google Scholar] [CrossRef]
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 19, 1044–1065. [Google Scholar] [CrossRef]
- Guruge, K.S.; Goswami, P.; Tanoue, R.; Nomiyama, K.; Wijesekara, R.G.S.; Dharmaratne, T.S. First nationwide investigation and environmental risk assessment of 72 pharmaceuticals and personal care products from Sri Lankan surface waterways. Sci. Total Environ. 2019, 690, 683–695. [Google Scholar] [CrossRef]
- Hewitt, N.J.; Grégoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Géniès, C.; et al. Measurement of the penetration of 56 cosmetic relevant chemicals into and through human skin using a standardized protocol. J. Appl. Toxicol. 2019, 40, 403–415. [Google Scholar] [CrossRef]
- Wang, L.; Asimakopoulos, A.G.; Kannan, K. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ. Int. 2015, 78, 45–50. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Gaskins, A.J. Female exposure to endocrine disrupting chemicals and fecundity: A review. Curr. Opin. Obstet. Gynecol. 2017, 29, 202–211. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Government of Canada Chemical Products Safety—Triclosan. Available online: https://www.canada.ca/en/health-canada/services/chemicals-product-safety/triclosan.html (accessed on 31 August 2020).
- U.S. FDA 84 FR 14847—Safety and Effectiveness of Consumer Antiseptic Rubs; Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Food and Drug Administration: Silver Spring, MD, USA, 2019; Volume 84, No. 71.
- European Commission. Commission Implementing Decision (EU); European Commission: Luxembourg, 2016; Volume 2001, L 21/86. [Google Scholar]
- Fernández-Castro, P.; San Román, M.F.; Ortiz, I. Theoretical and experimental formation of low chlorinated dibenzo-p-dioxins and dibenzofurans in the Fenton oxidation of chlorophenol solutions. Chemosphere 2016, 161, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Solá-Gutiérrez, C.; Schröder, S.; San Román, M.F.; Ortiz, I. PCDD/Fs traceability during triclosan electrochemical oxidation. J. Hazard. Mater. 2019, 369, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; Fernández-Castro, P.; San Román, M.F.; Ortiz, I. Assessment of PCDD/Fs formation in the Fenton oxidation of 2-chlorophenol: Influence of the iron dose applied. Chemosphere 2015, 137, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; San Román, M.F.; Ortiz, I.; Irabien, A. The critical role of the operating conditions on the fenton oxidation of 2-chlorophenol: Assessment of PCDD/Fs formation. J. Hazard. Mater. 2014, 279, 579–585. [Google Scholar] [CrossRef]
- Vallejo, M.; San Román, M.F.; Ortiz, I. Quantitative assessment of the formation of polychlorinated derivatives, PCDD/Fs, in the electrochemical oxidation of 2-chlorophenol as function of the electrolyte type. Environ. Sci. Technol. 2013, 47, 12400–12408. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Ribao, P.; Corredor, J.; Rivero, M.J.; Ortiz, I. Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. Enhanced photocatalytic activity using GO/TiO2 catalyst for the removal of DCA solutions. Environ. Sci. Pollut. Res. 2018, 25, 34893–34902. [Google Scholar] [CrossRef]
- Solá-Gutiérrez, C.; Schröder, S.; San-Román, M.F.; Ortiz, I. Critical review on the mechanistic photolytic and photocatalytic degradation of triclosan. J. Environ. Manag. 2020, 260, 110101. [Google Scholar] [CrossRef]
- European Commission. Decision No 2455/2001/EC of the European Parliament and of the Council of 20 November 2001; European Commission: Luxembourg, 2004; Volume 2001. [Google Scholar]
- U.S. EPA. 126 Priority Pollutants; U.S. EPA: Washington, DC, USA, 2012; Volume 29.
- Constantin, L.A.; Nitoi, I.; Cristea, N.I.; Constantin, M.A. Possible degradation pathways of triclosan from aqueous systems via TiO2 assisted photocatalyis. J. Ind. Eng. Chem. 2018, 58, 155–162. [Google Scholar] [CrossRef]
- Son, H.-S.; Ko, G.; Zoh, K.D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J. Hazard. Mater. 2009, 166, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, N.; Antonopoulou, M.; Hela, D.; Konstantinou, I. Photocatalytic degradation kinetics and mechanisms of antibacterial triclosan in aqueous TiO2 suspensions under simulated solar irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1145–1154. [Google Scholar] [CrossRef]
- Bayarri, B.; Giménez, J.; Curcó, D.; Esplugas, S. Photocatalytic degradation of 2,4-dichlorophenol by TiO2/UV: Kinetics, actinometries and models. Catal. Today 2005, 101, 227–236. [Google Scholar] [CrossRef]
- Yu, J.C.; Kwong, T.Y.; Luo, Q.; Cai, Z. Photocatalytic oxidation of triclosan. Chemosphere 2006, 65, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, C.H. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ. Sci. Technol. 2003, 37, 2421–2430. [Google Scholar] [CrossRef]
- Sankoda, K.; Matsuo, H.; Ito, M.; Nomiyama, K.; Arizono, K.; Shinohara, R. Identification of triclosan intermediates produced by oxidative degradation using TiO2 in pure water and their endocrine disrupting activities. Bull. Environ. Contam. Toxicol. 2011, 86, 470–475. [Google Scholar] [CrossRef]
- Rafqah, S.; Wong-Wah-Chung, P.; Nelieu, S.; Einhorn, J.; Sarakha, M. Phototransformation of triclosan in the presence of TiO2 in aqueous suspension: Mechanistic approach. Appl. Catal. B Environ. 2006, 66, 119–125. [Google Scholar] [CrossRef]
- Ku, Y.; Hsieh, C.-B. Photocatalytic decomposition of 2,4-dichlorophenol in aqueous TiO2 suspensions. Water Res. 1992, 26, 1451–1456. [Google Scholar] [CrossRef]
- Abeish, A.M.; Ang, H.M.; Znad, H. Solar photocatalytic degradation of chlorophenols mixture (4-CP and 2,4-DCP): Mechanism and kinetic modelling. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2015, 50, 125–134. [Google Scholar] [CrossRef]

 Detected in this work. Adapted from the literature [32,35,36,37,39,41,42].
Detected in this work. Adapted from the literature [32,35,36,37,39,41,42].



| Higher Concentration Compounds (HICs) | ||||
|---|---|---|---|---|
| No. | Compounds | m/z | Retention Time (min) | Structure |
| 1 | TCS quinone | 267/269/53/240 | 23.01 |  |
| 2 | Hydroxylated TCS 1 | 234/304/130/95 | 23.85 | 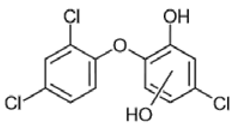 |
| 3 | Hydroxylated TCS 2 | 234/304/162/236 | 23.99 |  |
| 4 | Hydroxylated TCS 3 | 234/304/162/51 | 24.41 |  |
| Lower Concentration Compounds (LOCs) | ||||
| No. | Compounds | m/z | Retention Time (min) | Structure |
| 5 | 2-chloro-p-benzoquinone | 142/54/26 | 8.77 |  |
| 6 | 2,4-dichlorophenol | 162/164/63 | 10.24 | 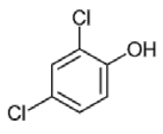 |
| 7 | 2-chlorohydroquinone | 144/52/80 | 12.75 |  |
| 8 | 3,5-dichlorobenzene-1,2-diol | 178/180/114 | 13.3 |  |
| 9 | 4-chlorocatechol | 144/146/63 | 14.2 | 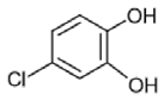 |
| Photocatalysis | |||
|---|---|---|---|
| Conditions | Kinetic Equations and Parameters | Ref. | |
| Kinetic Equations | k (min −1) | ||
| TCS0 = 30 µM (8.7 mg L−1); TiO2 = 0.1 g L−1; V = 0.15 L; pH = neutral; Hg lamp (UV-A) = 450 W, λ = 365 nm; Light intensity = 1.37·10−4 Einstein L−1 min−1 | (k values given at 5 min) | k1 = 1.67·10−1 | [36] |
| TCS0 = 17 µM (4.92 mg L−1); TiO2 = 0.1 g L−1; V = 0.15 L; pH = neutral; Hg lamp (UV-A) = 450 W,λ = 365 nm; Light intensity = 1.37·10−4 Einstein L−1 min−1 | k2 = 1.71·10−1 | ||
| TCS0 = 10 µM (2.89 mg L−1); TiO2 = 0.1 g L−1; V = 0.15 L; pH = neutral; Hg lamp (UV-A) = 450 W, λ = 365 nm; Light intensity = 1.37·10−4 Einstein L−1 min−1 | k3 = 1.87·10−1 | ||
| TCS0 = 7 µM (2.02 mg L−1); TiO2= 0.1 g L−1; V = 0.15 L; pH = neutral; Hg lamp (UV-A) = 450 W, λ = 365 nm; Light intensity = 1.37·10−4 Einstein L−1 min−1 | k4 = 2.02·10−1 | ||
| TCS0 = 3 µM (0.87 mg L−1); TiO2 = 0.1 g L−1; V = 0.15 L; pH = neutral; Hg lamp (UV-A) = 450 W, λ = 365 nm; Light intensity = 1.37·10−4 Einstein L−1 min−1 | k5 = 2.16·10−1 | ||
| TCS0 = 3.45 µM (1 mg L−1); TiO2 = 0.5 g L−1; V = 0.25 L; pH = 6.6; Xe lamp (UV) = 2.2 kW, λ = >290 nm | k1 = 1.50·10−1 t1/2 = 4.61 min | [37] | |
| TCS0 = 109.8 µM (31.8 mg L−1); TiO2 = 0.2 g L−1; pH = 6.5; UV = 300-400 nm; Light intensity = 1.05·10−6 Einstein s−1 | k1 = 2.50·10−2 | [35] | |
| TCS0 = 34.5 µM (10 mg L−1); TiO2 = 1.5 g L−1; V = 1 L; pH = 5.8; LED lamp (UV) = 35 W, λ = 365 nm | k1 = 4.20·10−2 k2 = 1.00·10−1 k3 = 1.95·10−1 k4 = 2.92·100 | This work | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, S.; San-Román, M.-F.; Ortiz, I. Photocatalytic Transformation of Triclosan. Reaction Products and Kinetics. Catalysts 2020, 10, 1468. https://doi.org/10.3390/catal10121468
Schröder S, San-Román M-F, Ortiz I. Photocatalytic Transformation of Triclosan. Reaction Products and Kinetics. Catalysts. 2020; 10(12):1468. https://doi.org/10.3390/catal10121468
Chicago/Turabian StyleSchröder, Sophie, Mª-Fresnedo San-Román, and Inmaculada Ortiz. 2020. "Photocatalytic Transformation of Triclosan. Reaction Products and Kinetics" Catalysts 10, no. 12: 1468. https://doi.org/10.3390/catal10121468
APA StyleSchröder, S., San-Román, M.-F., & Ortiz, I. (2020). Photocatalytic Transformation of Triclosan. Reaction Products and Kinetics. Catalysts, 10(12), 1468. https://doi.org/10.3390/catal10121468






