Abstract
Cholest-4-en-3-one Δ1-dehydrogenase (AcmB) from Sterolibacterium denitrificans was successfully immobilized on 3-aminopropyltrimethoysilane functionalized mesoporous cellular foam (MCF) and Santa Barbara Amorphous (SBA-15) silica supports using adsorption or covalently with glutaraldehyde or divinyl sulfone linkers. The best catalyst, AcmB on MCF linked covalently with glutaraldehyde, retained the specific activity of the homogenous enzyme while exhibiting a substantial increase of the operational stability. The immobilized enzyme was used continuously in the fed-batch reactor for 27 days, catalyzing 1,2-dehydrogenation of androst-4-en-3-one to androst-1,4-dien-3-one with a final yield of 29.9 mM (8.56 g/L) and 99% conversion. The possibility of reuse of the immobilized catalyst was also demonstrated and resulted in a doubling of the product amount compared to that in the reference homogenous reactor. Finally, it was shown that molecular oxygen from the air can efficiently be used as an electron acceptor either reoxidizing directly the enzyme or the reduced 2,4-dichlorophenolindophenol (DCPIPH2).
1. Introduction
Steroids are an important group of naturally occurring or synthetic compounds belonging to nonsaponifiable lipids. Their structure is based on a cyclopenta[a]phenanthrene carbon skeleton which can be partially unsaturated and is usually substituted with a methyl group at C10 and C13 as well as with an alkyl group at C17 [1]. Steroid compounds are important cell membrane components, precursors for the synthesis of vitamins and hormones. As a result, steroid derivatives form one of the largest group of drugs currently on the market and their synthesis and modification are of utmost importance for the pharmaceutical industry.
Due to their structural complexity and often multiple substituents, synthesis and modification of steroids were never entirely based on solely chemical methods. For example, the first efficient synthetic methods of progesterone and cortisone started with natural sapogenin, diosgenin, derived from a plant source (Cabeza de negro, Annona purpurea). Progesterone was obtained by chemical means (via Marker degradation) and finally cortisone by fermentation with Rhizopus mold (Upjohn’s process) [2,3]. In addition, 1,2-dehydrogenation, for example for androst-4-en-3-one (AD) to androst-1,4-dien-3-one (ADD) conducted by means of microbial biotransformation, has a very long history [4]. Currently, sex hormones and corticosteroid drugs are synthesized by a combination of chemical and biotechnological methods [5]. For several decades, biotransformation is gaining more importance in the synthesis of steroid APIs and these methods have been recently reviewed by Fernández-Cabezón et al. [6].
Most of the already developed biotechnological approaches are based on biotransformation or application of the whole-cells [7,8,9]. However, in this paper, we decided to focus on the application of the immobilized cholest-4-en-3-one Δ1-dehydrogenase of the 3-ketosteroid dehydrogenase family to regioselective dehydrogenation of 3-ketosteroids. The application of natural bacterial cells for biotransformation or a recombinant system for biocatalysis is often cheaper and simpler. However, the immobilized enzymes have several important advantages over bacterial catalysts such as lack of side reaction and toxins, simplified product purification, enhanced stability of the enzyme, the possibility of reuse of the catalyst and easy conversion from the batch to the continuous process mode.
Cholest-4-en-3-one Δ1-dehydrogenase (AcmB) from Sterolibacterium denitrificans is a surprisingly versatile FAD-containing oxidoreductase capable of 1,2-dehydrogenation of an unusually wide group of 3-ketosteroids i.e., not only the standard C20–C22 3-ketosteroids but also significantly bulkier compounds with the undegraded C17 aliphatic side chain such as cholest-4-en-3-one derivatives or even 3-ketosaponins such as (25R)-spirosta-4-en-3-one (diosgenone) [10,11,12]. In this paper, we demonstrate how AcmB covalently immobilized on silica support can be applied to dehydrogenation of 4-androstene-3,17-dione to 1,4-androstadiene-3,17-dione (Figure 1), an industrially important process. We demonstrate how immobilization influences the overall productivity of the system compared to a homogenous enzyme. To the best of our knowledge, this is the first report on the immobilization of a KSTD family member on a solid carrier. Furthermore, we focus on the application of atmospheric O2 as an efficient reoxidant of the enzyme that can support or potentially even replace 2,4-dichlorophenolindophenol (also called 2,6-dichloroindophenol or DCPIP), which is customarily used to reoxidize FADH2 in KSTD enzymes (Figure 1).
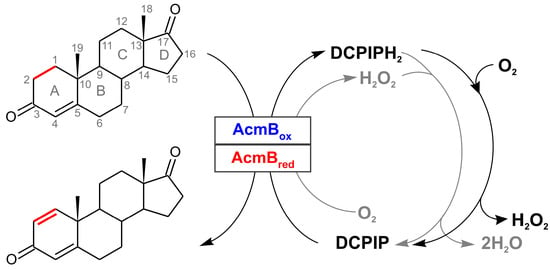
Figure 1.
The schematic representation of reaction catalyzed by AcmB: Dehydrogenation of 4-androstene-3,17-dione to 1,4-androstadiene-3,17-dione and reoxidation of the enzyme by DCPIP or O2 as well as the reoxidation of DCPIPH2 by H2O2 formed in situ or O2.
2. Results
2.1. Selection of Carrier with the Highest Yield and Immobilization Efficiency
Purified AcmB with the specific activity of 12 mM min−1 mg−1 and protein concentration of 0.91 mg mL−1 was immobilized on mesoporous cellular foam (MCF) and Santa Barbara Amorphous (SBA-15) silica carriers functionalized with 3-aminopropyltrimethoysilane (APTS), which provides –NH2 functional groups on the carrier surface (Figure 2). The immobilization was achieved either by simple adsorption or via covalent binding to amine groups using glutaraldehyde (GA) or to hydroxyl groups using divinyl sulfone (DVS). In each case, the same ratio of the enzyme (i.e., 0.455 mg of AcmB) to the carrier (10.5 mg) was used. After immobilization, the amount of unbounded protein was determined with the Bradford method, giving the yield of protein binding. However, AcmB activity was not detected in the wash fractions collected after the immobilization process. After immobilization, the activity of immobilized AcmB was determined in 2 mL vials using the HPLC method (Table 1). Based on the determined activity of the AcmB-carrier catalyst and the initial volume activity of homogenous AcmB, we were able to determine the activity recovery (AR).
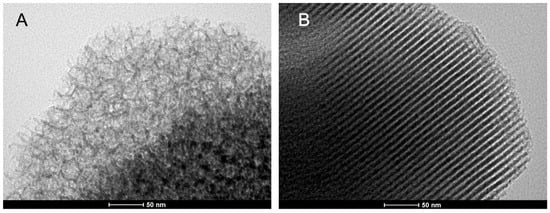
Figure 2.
The TEM image of synthesized carriers (A). MCF (B). SBA-15 before functionalization.

Table 1.
Immobilization of AcmB on silica carriers: Mesoporous cellular foam (MCF) and Santa Barbara Amorphous (SBA). The AcmB activity was measured in the presence of 1.5 mM of androst-4-en-3,17-dione. The activity is calculated for the total amount of the immobilized catalyst (110 µL of the support) and compared to the total amount of the homogenous enzyme used for immobilization (0.455 mg).
In general, the application of GA linker for AcmB immobilization gave a better AR for both carrier types than DVS and adsorption (Table 1). The highest AR was obtained for AcmB immobilized on MCF activated with GA (99.8%), followed by adsorption on MCF (53%), SBA activated with GA (51%), and MCF activated with DVS (48%). In the case of adsorption on SBA and SBA activated with DVS, values of AR were below 40%. Interestingly, we observed retention of the very high specific activity of the immobilized enzyme on supports activated with GA (29.4 ± 4.2 U/mg and 24.3 ± 3.5 U/mg), although the increase of SA above the level of activity measured for homogenous enzyme most probably stems from the errors in the estimation of activity or concentration of the protein. Based on the obtained results, we chose as the best carrier for further AcmB immobilization the MCF activated with GA.
MCF carriers bound 20–30% more protein than SBA-15, even despite a very similar specific surface area of both carriers (353 vs. 316 m2/g, Table 2). However, MCF and SBA-15 significantly differ in size and shape of pores. SBA-15 is of the characteristic hexagonal structure whereas MCF could be described as a foam-like structure (Figure 2). Additionally, the pore diameter of MCF is almost 3–4 times higher than for SBA-15 (22 nm vs. 5.5 nm). The shape and size of the pores seem to also affect the activity of the enzyme after immobilization although, in this case, the enzyme immobilization method (adsorption, GA or DVS) turns out to be much more important. Generally, we can observe that carriers (MCF and SBA-15) activated with DVS bound a little higher amount of enzyme (0.37 vs. 0.39 and 0.23 vs. 0.27 mg) than the same supports activated with GA, whereas the measured activity showed an opposite correlation.

Table 2.
Characterization of the silica carriers used in immobilization.
2.2. Operational Stability of Immobilized AcmB
Enzyme immobilization usually increases enzyme operation stability. To test if the immobilization of AcmB indeed increased its operational stability, we prepared two 5 mL batch reaction systems each containing 5.1 mM DCPIP, one with the best-immobilized catalyst (AcmB:MCF/GA, activity 10.53 U) and the other with homogeneous AcmB with the same amount of enzyme as used for immobilization (activity 10.9 U). Both reaction mixtures were warmed up to 30 °C and supplemented with 0.5 mM of androst-4-en-3-one (AD). The initial dose of the substrate was consumed within 0.5 h. Afterward, the reactors were kept at 30 °C for another 47 h in order to subject the enzyme to prolonged operational conditions. After 47.5 h of incubation, the reactors were supplemented with 5 mM of AD (Figure 3). The reaction with homogeneous AcmB proceeded for another 21 h and resulted in the formation of 1.7 mM of androst-1,4-dien-3-one (ADD), i.e., 32% conversion. Meanwhile, the reaction with immobilized AcmB proceeded for another 23 h and resulted in 99% conversion of the substrate yielding 5.2 mM of ADD. In the homogeneous reactor, the reaction stopped due to the enzyme inactivation, while in the reactor with an immobilized enzyme the catalyst converted the whole substrate. To test if the immobilized enzyme was still active after 70 h, yet another portion of AD (approx. 1.6 mM) was introduced into the reactor. After 110 h, we observed almost 6 mM of the product (total conversion of 88%), which indicated that the enzyme was still active. Based on the increase of the product concentration after the addition of the 5 mM dose (i.e., after 47 h of incubation at 30 °C), we estimated the average activity during the first hour. The apparent average activity of the homogeneous enzyme turned out to be 8.5-times lower than in the case of the immobilized enzyme (0.16 mM/h vs. 1.37 mM/h, respectively), which demonstrates higher operational stability of the immobilized catalyst. Moreover, the estimated activity of the immobilized catalyst after 47 h incubation under reaction conditions was roughly the same as at the beginning of the reaction.
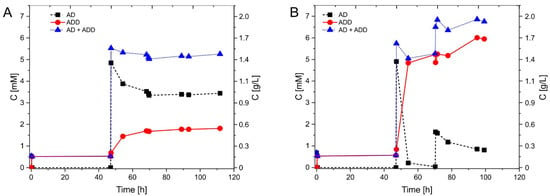
Figure 3.
Progress curves of 1-dehydrogenation of androst-4-en-3-one (AD) to androst-1,4-dien-3-one (ADD) by (A) homogeneous AcmB and (B) immobilized MCF/GA/AcmB. The reaction was started with the addition of 0.5 mM AD and reinitiated at 47.5 h by the addition of 5 mM of AD. For AcmB: MCF/GA, additional 1.6 mM of AD was added at 70 h.
2.3. Long-Term ADD Production
To demonstrate the industrial capacity of our catalyst for the synthesis of ADD, we conducted a long-term 27-days fed-batch experiment with AcmB:MCF/GA immobilized catalyst. AD was fed to the reactor only when its concentration in the reactor was close to zero. The reaction was prepared as previously and monitored by HPLC (Figure 4). The reaction was carried out under aerobic conditions with 5 mM of DCPIP.
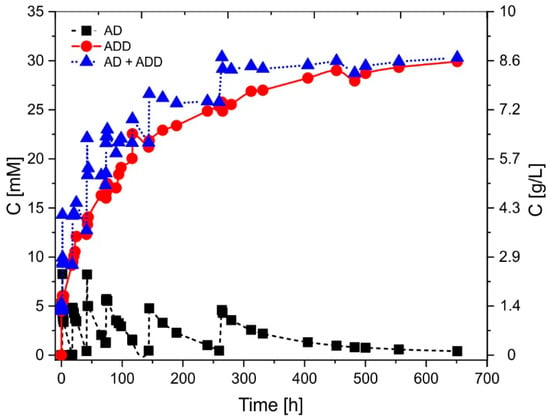
Figure 4.
Long-term fed-batch synthesis of ADD from AD by immobilized AcmB at MCF/GA support. The reaction was fed six times with 5 mM AD.
At 18.1, 41.5, 73.3, 143.7, and 259 h, the approx. 5 mM portions of AD were introduced, enabling continuous conversion of AD to ADD. As a result, the final concentration of the fed substrate was 30.3 mM. After 651 h (27 days) of the continuous reaction, the product concentration reached 29.9 mM (99% conversion).
It should be underlined that the overall DCPIP to product ratio was 1:6, which demonstrates the action of an additional reoxidant in the reactor system. We confirmed in separate experiments that AcmB as a FAD-dependent enzyme can use molecular oxygen as an electron acceptor (data not shown) at the characteristic of FAD-dehydrogenases sluggish rate [13,14]. Additionally, we confirmed that DCPIP reduced either by dithionite, electrochemically, or by AcmB can be reoxidized by H2O2 under anaerobic conditions or directly by O2. We took special care to reduce DCPIP by either chemical or electrochemical means to avoid using enzyme which in contact with O2 produces H2O2. That way we proved that DCPIPH2 can be oxidized by molecular oxygen or H2O2 without the help of the enzyme. The direct oxidation of DCPIPH2 by atmospheric oxygen was previously reported as well [15]. As a result, it seems that the presence of O2 in the reaction mixture results in the effective reoxidation of the reduced enzyme and DCPIPH2 enabling high conversion of the substrate, despite the potentially detrimental influence of the reactive oxygen species to enzyme activity.
We compared the productivity of the aerobic reactor with a reference 27-day fed-batch reactor with AcmB:MCF/GA carried under an anaerobic atmosphere. The reaction was started with 10 mM AD and a limiting amount of DCPIP (4.76 mM). It was then supplemented seven times with additional portions of DCPIP (initially as a stock solution, then in form of a solid powder) and the AD. Each time, the reaction stopped after consumption of the reoxidant, indicating a 1:1 ratio of the converted substrate to reoxidant. The final yield of the reactor turned out to be only 16 mM of the ADD, despite a higher apparent activity of the enzyme recorded after each feed of the substrate in anaerobic conditions with respect to the reactor run under aerobic conditions.
2.4. Reusability of AcmB:MCF/GA in ADD Synthesis
Besides enhanced operational stability of the immobilized enzymes, their potential reusability is the main advantage over homogenous or whole-cell catalysts. The potential for reuse of the catalyst is also very important in the case of steroid conversion, which is very often limited by the poor solubility of the reagent in water and reversibility of the dehydrogenation process (i.e., limitation of the reaction progress by reaching the equilibrium of reaction).
To test the reusability of immobilized AcmB on MCF/GA support, we conducted a sequence of three reactions in the fed-batch system. As previously, AcmB:MCF/GA was compared with the same amount of the homogeneous AcmB (0.455 mg). In the case of homogeneous AcmB, the reaction was started by the addition of 4 mM AD and monitored for 557 h (23 days) (Figure 5). During the reaction at 21 and 45 h, the 6 mM and 4.2 mM portions of AD were added, while at 53 h 0.65 mL of 19 mM DCPIP was added to maintain a concentration of the reoxidant. After 48 h, 70% conversion was obtained (10 mM ADD from the 14.2 mM of AD). It should be noted that the addition of the DCPIP stock solution results in a decrease of steroid concentration not only due to normal dilution but also because of decreases in the concentration of steroid solubilizers (HBC and EGME). As a result, the concentration of both steroid components (AD and ADD) dropped to 9.5 mM at 53 h. To restore the initial conditions of the reaction system (e.g. HBC concentration from before the DCPIP addition), a mixture of buffer (1.437 mL) and 40% HBC solution (0.326 mL) was added to the reaction mixture at 74 h. As a result, the concentration of ADD rapidly dropped due to precipitation and it took several hours to stabilize.
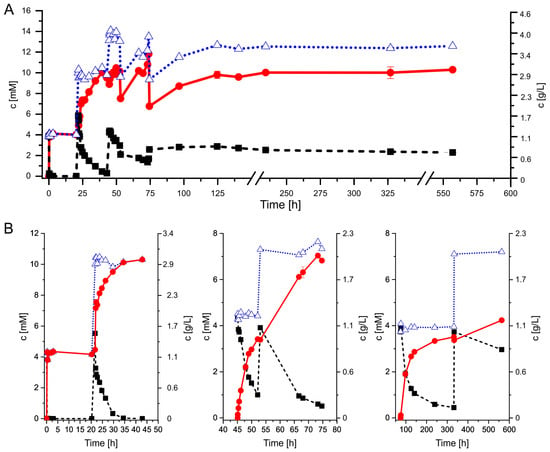
Figure 5.
The conversion of AD (black squares) to ADD (red circles), AD + ADD (open blue triangles) by (A) homogenous AcmB and (B) immobilized AcmB: MCF/GA. The reaction with homogenous AcmB was conducted in one pot fed with the substrate with three portions of AD (4.0, 6.0, 4.2 mM) and one portion of DCPIP at 53 h. The reaction with AcmB: MCF/GA was conducted in three separate reactors in a sequence with one additional AD feed.
In the case of AcmB:MCF/GA, we prepared three fed-batch reactors transferring the catalyst from one reactor to another by centrifugation and washing with 0.1 M KH2PO4/Na2HPO4 pH 7.0 containing 3 mM K3[Fe(CN)6]. Each reactor was initiated with approximately 4 mM of AD (first and second with 4.3 mM, and third with 4.0 mM) and subsequently supplemented with AD (first with 6 mM after 22 h, second with 3.0 mM after 7 h and third with 3.2 mM after 257 h), thereby yielding 10.3 mM of AD in the first reactor, 7.3 mM in the second and 7.2 mM in the third reactor (Table 3). The first reaction was stopped after 34 h (100% conversion), the second after 30 h (93% conversion), and the third after 489 h (58% conversion). The initiation of the reaction in each of the reactors allowed a comparison of the activity of immobilized enzyme under the same reaction conditions (i.e., high concentration of substrate and DCPIP, low concentration of product). The enzyme used in the second run exhibited 8% and in the third run only 1% of the activity recorded for the first reactor.

Table 3.
Comparison of fed-batch reaction with homogeneous AcmB and immobilized AcmB:MCF/GA that was reused in three fed-batch reactors. The efficiency of the process was calculated based on the product concentration and the introduced substrate to the reaction.
The experiment demonstrated the advantage of using an immobilized enzyme over a homogenous one. It was possible to double the yield of the process (10 vs. 21 mM) and simplify the separation of the catalyst from the reaction mixture. Furthermore, restarting the reaction batch avoided problems with reagents solubility.
3. Discussion
We have demonstrated that the immobilization of 3-ketosteroid Δ1-dehydrogenase on solid silica carriers significantly enhances catalyst operational stability while retaining the activity of the homogenous catalyst. However, not all tested supports and immobilization techniques yielded a catalyst of comparable quality to the best AcmB: MCF/GA.
The size and shape of the silica pores seem to have a significant impact on the amount of bound protein. Generally, MCF carriers bound 20–30% more protein than SBA-15 even though their specific surface areas are almost the same (353 vs. 316 m2/g, respectively). This effect may be explained by the relatively lower effective surface of SBA supports, which is available to a relatively big AcmB. The enzyme is of roughly globular shape with axes of 7.3 × 6.0 × 5.1 nm [16] while pores of SBA-15-NH2 material are approx. 5.5 nm. Furthermore, it was shown that AcmB has a high propensity to form aggregates at higher concentrations such as those used during the immobilization process [11,16]. Therefore, we can assume that only the outer surface of the SBA-15 support is effectively available to the enzyme. Meantime, the MCF material with pores in the range of 13–22 nm provides an effectively larger surface. The shape and size of the pores also seem to affect the activity of the enzyme after immobilization although, in this case, the enzyme immobilization method (adsorption, GA or DVS) turns out to be much more important. As was mentioned above, we observed a negative correlation between the amount of bound enzyme and its activity for GA and DVS activators. We have to keep in mind that the covalent immobilization with GA or DVS activators differs significantly. At pH 7, the GA binds to the amine groups on propyl chains while at pH 11 the DVS binds to hydroxyl groups of the support [17]. As a result, for the DVS-activated supports, the enzyme is bound on a relatively short tether to the surface and is surrounded by the aminopropyl brush. Apparently, despite the higher efficiency of such a method in terms of protein binding, the local microenvironment is not favorable for the expression of enzyme activity. Meantime, the GA is attached to aminopropyl chains coming from APTS [18]. GA additionally tends to create dimeric or trimeric structures even further extending the length of the spacer between the matrix and protein surface. Such flexibility as well as a different microenvironment of the surface seems to be responsible for the higher specific activity of the immobilized enzyme [11].
The comparison of homogenous vs. immobilized enzymes demonstrated the higher operational stability of the heterogeneous catalyst. The initial tests show that homogenous enzyme can exhibit higher activity, most probably due to a lack of diffusion limitation. However, after 48 h under reaction conditions, the activity of the homogenous enzyme was 8.5 lower than the activity of the immobilized catalyst. The most probable reason, besides a standard thermal denaturation of the protein that proceeds faster for non-immobilized enzymes, is a gradual aggregation of the enzyme at the optimal pH of the reaction (pH 6.5) [16]. This aggregation is completely inhibited by the immobilization.
As a result, it was possible to run an efficient long-term fed-batch reactor with immobilized AcmB at MCF/GA. The reactor, which contained 455 µg of AcmB per 0.25 mL of support allowed the synthesis of 8.56 g/L of ADD in the medium composed of the water: Organic solvent: HBC (98–88% H2O, 2–12 % of EGME, 5% HBC solubilizer). To our best knowledge, this is the highest steroid concentration reported for such a system used for the in vitro catalytic reaction. It also has to be underlined that such a high conversion simplifies the purification of the product and enhances the overall yield of the process. Similar yields in that range were obtained with the Corynebacterium crenatum whole-cell system containing a recombinant 3-ketosteroid Δ1-dehydrogenase from Mycobacterium neoaurum, which allowed the production of 8.39 g/L ADD with maximum conversion rates of about 83.87% after 10 h of reaction [19]. Finally, it was demonstrated that molecular oxygen from the air can be efficiently used for recycling of the enzyme reoxidant DCPIP.
Multiple applications of the same batch of the immobilized enabled the determination of the enzyme robustness at the beginning of the reaction (i.e., without the influence of the product which gets accumulated during prolonged fed-batch procedure). As previously, we run the experiment in comparison with the homogenous enzyme. Three reactions with AcmB: MCF/GA allowed conversion of 24.8 mM of AD to 21.3 mM of ADD, comparing to homogeneous AcmB where 14.2 mM of AD was converted to 10 mM ADD. During the roughly similar time (53 h vs. 64 h for homogenous vs. heterogeneous catalyst), it was possible to obtained 7.1 mM more of the product using the immobilized catalyst. Although, the total time of reaction with reuse of AcmB: MCF/GA was approx. 10 times longer than in the case of the process with the homogenous enzyme, it resulted in a doubling of the product amount. The overall efficiency of the process was also better in the case of an immobilized enzyme (86 vs. 70%). Moreover, we should take into account that the time of the reaction with the immobilized enzyme was hugely overestimated. The first 4.3 mM portion of AD in the first reaction with AcmB: MCF/GA was consumed within 0.5 h, the second 6.0 mM portion of AD within 17 h. Therefore, the transfer of the catalyst to the second reaction was possible after approx. 18 h instead of 43 h. Additionally, the third reaction was conducted for 20 days to observe the long-term stability of the system. Summing up, it is possible to optimize the total time of the synthesis with reused catalyst and obtain even higher yields. This relatively good performance was achieved despite the observed significant loss of enzyme activity between batches. As such a rapid decrease in activity was not observed in the long-term reactor, we suspect that during catalyst washing, the enzyme was losing the non-covalently bound FAD. Although this hypothesis needs further studies, we have already shown that the enzyme is prone to lose its cofactor during column purification and the FAD content can be reconstituted with help of the detergent [10].
In our recent paper, we demonstrated that recombinantly produced AcmB subjected to FAD reconstitution can exhibit higher activity than the whole-cell system [10]. However, the whole-cell system has a definitive advantage over homogenous enzyme in post-processing of the reaction mixture due to the relatively easy separation of bacteria by centrifugation or filtration. The immobilized enzyme restores easy separation (and reuse) while providing high enzyme activity with additional enhanced operational stability.
The experiment also demonstrated the advantage of the reuse of immobilized AcmB in the production of poorly soluble steroids. This is especially important for other steroids than AD, such as cholest-4-en-3-on derivates or even 3-ketosaponins [10]. Although it was proved previously [10,20,21,22] that HBC and EGME in the reaction medium can solubilize such steroids in the water medium, from the point of view of industrial application, it is better to control the reaction in such a way as to obtain 100% conversion.
4. Materials and Methods
4.1. AcmB Production and Purification
The protein was obtained in heterologous overexpression in E. coli BL21(DE3)Magic with cloned pMCSG7 vector containing acmb gene from S. denitrificans (gi: 157673245) [16]. Overexpression was prepared by the induction with 250 μM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16 °C. Bacterial cell mass was centrifuged, homogenized, and the enzyme was purified according to the methodology obtained in [10]. Protein concertation was determined with the Bradford method.
4.2. Carrier Preparation
The SBA-15 carrier was synthesized according to a previously established protocol [23]. Briefly, 4 g of Pluronic P123 (Aldrich, St. Louis, MO, US) was dissolved in a mixture of 30 mL of water and 120 mL of 2 M HCl solution under stirring at 35 °C. Afterward, 9.1 mL of tetraethylorthosilicate (TEOS) (Aldrich, St. Louis, MO, US) was added and the mixture was incubated for 20 h followed by incubation at 80 °C overnight without stirring. The solid product formed was recovered, washed, and air-dried at room temperature. Calcination was carried out by slowly increasing the temperature from room temperature to 500 °C over 8 h and subsequent heating at 500 °C for 8 h.
The MCF was synthesized according to [24]. Briefly, 4 g of Pluronic P123 was dissolved in a mixture of 30 mL of water and 120 mL of 2 M HCl solution at room temperature. 1,3,5-Trimethylbenzene (Aldrich, St. Louis, MO, US) (11.6 mL) and NH4F (0.046 g) were added under vigorous stirring and the mixture was heated to 40 °C. Following 1 h stirring, TEOS was added (9.4 mL), whereupon the mixture was stirred for 1 h and then stored at 40 °C for 20 h and then at 100 °C for 24 h. After cooling to room temperature, the precipitate was filtered, dried at room temperature calcined at 500 °C for 8 h.
The functionalization was conducted according to [25]. Before grafting, SBA-15 and MCF were contacted with water vapor for 5 h and subsequently dried at 200 °C for 2 h. Amino groups were grafted onto the silica surface by the addition of the organosilane 3-aminopropyltrimethoysilane (Aldrich, St. Louis, MO, US) (APTS) dissolved in toluene. 1.5 mmol organosilane was incubated under reflux (24 h, 85 °C) with 1 g of silica. The products achieved were filtered and dried at 60 °C.
4.3. Immobilization of AcmB
Immobilization by adsorption: 110 µL of the carrier (10.5 mg) was rinsed with distillate water and added to 500 µL of the enzyme solution (0.91 mg mL−1; 10.9 U) in 0.1 M KH2PO4/Na2HPO4 pH 7.0. The solution was gently spun on a rotator (22 rpm) for 2.5 h at room temperature. Then the carrier with immobilized AcmB was separated from the solution by centrifugation (10,000× g, 3 min, RT) and washed four times with 1 mL of cold (7 °C) 0.1 M KH2PO4/Na2HPO4 pH 7.0 containing 3 mM K3[Fe(CN)6]. K3[Fe(CN)6] was used to keep the enzyme in the oxidized form, which prevents FADH2 mediated reduction of O2 and formation of the radical oxygen species. All eluates were collected and analyzed for the presence of protein and activity.
Covalent immobilization: 110 µL of MCF or SBA-15 (10.5 mg) was rinsed subsequently with 1 mL of distilled water and 1 mL of the buffer: 0.1 M KH2PO4/Na2HPO4 pH 7.0 for glutaraldehyde (Aldrich, St. Louis, MO, US) (GA) and 1 M Na2CO3 pH 11.0 for divinyl sulfone (Aldrich, St. Louis, MO, US) (DVS) respectively. 2 mL of 2.5% GA in 0.1 M KH2PO4/Na2HPO4 pH 7.0 and 2 mL of 3% DVS in 1 M Na2CO3 pH 11.0 was used for carrier activation. The activation of functional groups at the surface of carriers proceeded by stirring for 1.5 h at room temperature. After activation, the supports were centrifuged and rinsed with 1 mL distilled water (the procedure repeated 7-times) and subsequently rinsed with appropriate buffers in which the enzyme stock solution was then diluted: (i) 0.1 M KH2PO4/Na2HPO4 pH 7.0 for the supports activated with GA, (ii) 0.1 M Na2HPO4·12H2O pH 8.2 for the supports activated with DVS. As above, carriers were suspended in 500 μL of the solutions containing AcmB enzyme (0.91 mg mL−1) with 3 mM K3[Fe(CN)6] in the appropriate buffer. Tubes with enzyme-carrier suspensions were placed on a rotator (22 rpm) and incubated for 0.5 h at room temperature and another 2 h at 7 °C. The protein that was not bound to the carrier was removed by washing (1 mL of precooled (7 °C) 0.1 M KH2PO4/Na2HPO4 pH 7.0 with 3 mM K3[Fe(CN)6]; 15 min, 22 rpm). The procedure was repeated four times and eluates were collected and analyzed for the presents of protein and activity. After that, the carriers were washed with the same buffer enriched with 0.5 M NaCl and with buffer 0.5 M Tris/HCl buffer pH 7.8 with 3 mM K3[Fe(CN)6 precooled to 7 °C (1 h, 22 rpm, 1 mL of buffer) to block the remaining active groups on the carriers. Finally, immobilized AcmB on a carrier was suspended in 200 µL 0.1 M KH2PO4/Na2HPO4 pH 7.0 with 20 mM DCPIP, stored at 7 °C for further studies.
The AcmB:MCF/GA for experiments in paragraphs of Section 2.2, Section 2.3, Section 2.4 was prepared according to the above procedure using 250 µL of MCF/GA carrier (24 mg) and 500 µL AcmB (0.91 mg/mL; 10.9 U).
4.4. Activity Assays
The enzyme initial activity (U) was defined as the amount of enzyme required to oxidize 1 μmol of the AD to ADD per min at 30 °C using the 1 min of the reaction progress curve. Reagents concentration was determined by HPLC. AcmB activity was determined from the linear fit to the initial linear reaction region of the progress curve.
In Section 2.1, the reactions were carried out in a volume of 2 mL at 30 °C in a water bath with magnetic stirring. Reaction mixture consisted of 89 mM K2HPO4/KH2PO4 pH 6.5, 1.6 mM DCPIP, 1.5 mM AD in 2% EGME, 5% HBC (w/v) and 0.025 mL of the carrier suspension or homogenous enzyme.
4.5. HPLC Measurements
The progress of the reaction was determined with HPLC Agilent 1100 system equipped with a DAD detector (Agilent, CA, US) on Ascentis® RP-Amide Express (Supelco, PA, US) column (75 mm × 4.6 mm, 2.7 μm) at 40 °C using an isocratic mobile phase composed of H2O/CH3CN (Fluka, HPLC grade) (6:4, v/v), 1 mL/min flow rate and injection volumes of 5 μL. Samples for injection were prepared by dilution of 50 μL of the sample in 100 μL ice-cold acetonitrile followed by centrifugation (4 min, 14,500 rpm). The quantitation of the substrate (4-androstene-3,17-dione, AD) and the product (1,4-androstadiene-3,17-dione, ADD) was conducted at 240 nm using external standards for calibration. Retention times of ADD and AD were 2.87 and 4.11 min, respectively. All measurements were conducted in duplicate.
4.6. Reactor Tests
In each reactor experiment, two catalyst types were tested: (i) homogenous AcmB (activity 10.9 U), and (ii) freshly prepared immobilized enzyme on MCF carrier activated with GA, AcmB: MCF/GA (activity 10.53 U). Reactions were run in a 10 mL stirred glass reactor (200 rpm) at room temperature or 30 °C.
In reactions described in Section 2.2, the 5 mL reaction mixtures comprised of 5.1 mM DCPIP, 5% HBC (w/v), 2% of EGME with dissolved 0.5 mM initial dose of AD, respectively 53 or 48 mM K2HPO4/KH2PO4 pH 6.5 buffers for reactions with 0.25 mL of AcmB:MCF/GA or 0.5 mL homogenous AcmB (i.e., the volume of homogenous AcmB was identical to the volume of the enzyme used to perform 0.25 mL of AcmB:MCF/GA). The reaction mixtures were preheated and kept at 30 °C and constantly mixed (200 rpm). To confirm that the immobilized and homogeneous AcmB are catalytically active before exposure to long-term reaction conditions, 0.5 mM of AD was introduced and the reaction progress was tested after 30 min. Afterward, the reactors were kept at 30 °C and constantly mixed (200 rpm) for another 47 h. After 47.5 h of incubation, the reactors were supplemented with 5 mM of AD (0.1 mL 250 mM AD stock solution in EGME). The reaction mixture with immobilized AcmB, after a total conversion of 5 mM of the substrate, was supplemented with another 1.7 mM portion of AD (35 µL 250 mM AD stock solution in EGME).
In reactions described in Section 2.3, the 5 mL reaction mixtures comprised of 4.9 mM DCPIP, 5% HBC (w/v), 2% of EGME with dissolved 5 mM AD in 56 mM K2HPO4/KH2PO4 pH 6.5 buffer. The reaction was initiated by the addition of 0.1 mL of AcmB:MCF/GA catalyst and was conducted in an aerobic atmosphere. When the substrate concentration was close to zero, another portion of AD in EGME was fed. In the same manner, a referential experiment was performed under anaerobic conditions (N2/H2, 97/3%). The initial concentration of AD was 10 mM while DCPIP was 4.76 mM. The reactor was supplemented 7 times with AD in EGME (72, 74.6, 95.4, 118, 145, 190.2 and 280 h) and DCPIP (once at 16 h with 5 mM DCPIP portion—0.675 mL of 18 mM DCPIP in H2O and five times with approx. 8 mg of DCPIP at 73, 96, 121, 145, 190.5 and 404 h).
In reactions described in Section 2.4, the 5 mL reaction mixtures comprised of 5.1 mM DCPIP, 5% HBC (w/v), 2% of EGME with dissolved 4 mM androstene-3,17-dione (AD) and, respectively, 53 or 48 mM K2HPO4/KH2PO4 pH 6.5 buffer for immobilized or homogenized AcmB. The reaction was initiated by the addition of 0.25 mL of AcmB: MCF/GA or 0.5 mL homogenous AcmB (i.e., the volume of homogenous AcmB was identical to the volume of the enzyme used to perform 0.25 mL of AcmB:MCF/GA). Each time the substrate concentration was close to zero, another portion of AD in EGME was fed. In the reactor with AcmB: MCF/GA, when the second fed of AD was consumed, AcmB: MCF/GA was separated from the reaction mixture by centrifugation (5 min, 1400 rpm), washed three times with 0.1 M K2HPO4/KH2PO4 pH 6.5 with 3 mM K3[Fe(CN)6], and used to initiate a new reaction. The reaction with immobilized AcmB was repeated three times using the same reaction mixture composition. In the case of the reaction with homogenous AcmB at 53 h 2.1 mM of DCPIP was added (0.65 mL of 19 mM DCPIP). The increase of the reaction total volume was included in calculations.
4.7. DCPIPH2 Reoxidation Tests
278 μM DCPIP solution in 0.1 M K2HPO4/KH2PO4 pH 6.5 buffer was anaerobized and electrochemically reduced in a galvanostatic mode at the constant current of −200 μV in the system comprised of platinum working and counter electrode and Ag/AgCl reference electrode. There, under an argon atmosphere, the reduction proceeded efficiently until the characteristic blue color of DCPIP disappeared. Then it was exposed to aerobic conditions and the change to blue color (oxidized state) was monitored with UV-vis (Shimadzu UV-1280, Kyoto, Japan) (400–800 nm).
95 μM DCPIP solution in 0.1 M K2HPO4/KH2PO4 pH 6.5 buffer was anaerobized and transferred to an anaerobic glovebox (Coy Laboratory Products, MI, US) (H2:N2 5:95 v/v). Then sodium dithionite was added (end concentration 85 μM), resulting in the reduction of 90% of DCPIP to DCPIPH2. Then H2O2 (end concentration 98 mM) was added as an oxidant. Both the reduction and oxidation were controlled by Shimadzu UV-1280spectrometer (under an anaerobic atmosphere in the range of 400–800 nm).
5. Conclusions
It was demonstrated that covalently immobilized 3-ketosteroid dehydrogenase can compete with the homogenous enzyme or even, by extension, with the whole-cells system, which was up to date routinely used in 1,2-dehydrogenation of 3-ketosteroids. In the fed-batch reactor, we were able also to achieve a very high concentration of the steroid product (8.56 g/L). It was also shown that running the process under the aerobic atmosphere is overall more advantageous to productivity as molecular oxygen reoxidizes the enzyme and electron acceptor. This not only increases the overall yield but also reduces the cost of the process.
Author Contributions
Conceptualization, M.S.; methodology, M.T., M.S. and K.S.; validation, M.S. and A.M.W.; formal analysis, M.T., A.M.W. and M.S.; investigation, K.Z., M.T. and P.W., resources, K.S.; writing—original draft preparation, M.T., A.M.W., K.S., M.S.; writing—review and editing, M.S., K.S.; visualization, A.M.W.; supervision, M.T., M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre Poland under the OPUS grant number UMO-2016/21/B/ST4/03798. P.W. acknowledges the fellowship with project no. POWR.03.02.00-00-I013/16. M.T. acknowledges ICSC PAS Statutory Fund. Authors acknowledge the help of Grzegorz Mordarski and Dimitry Kharitonov in the electrochemical reduction of DCPIP.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nič, M. IUPAC Compendium of Chemical Terminology. In Gold Book, 2nd ed.; IUPAC: Research Triagle Park, NC, USA, 1997. [Google Scholar] [CrossRef]
- Hogg, J.A. Steroids, the steroid community, and Upjohn in perspective: A profile of innovation. Steroids 1992, 57, 593–616. [Google Scholar] [CrossRef]
- Marker, R.E.; Rohrmann, E. Sterols. LXXXVIII. Pregnanediols from Sarsasapogenin. J. Am. Chem. Soc. 1940, 62, 518–520. [Google Scholar] [CrossRef]
- Vischer, E.; Wettstein, A. Mikrobiologische Reaktionen. Experientia 1953, 9, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I. Chemical Pathways of Corticosteroids, Industrial Synthesis from Sapogenins. Methods Mol. Biol. 2017, 1645, 15–27. [Google Scholar]
- Fernández-Cabezón, L.; Galán, B.; García, J.L. New Insights on Steroid Biotechnology. Front. Microbiol. 2018, 9, 958. [Google Scholar] [CrossRef]
- Koshcheyenko, K.A.; Turkina, M.V.; Skryabin, G.K. Immobilization of living microbial cells and their application for steroid transformations. Enzym. Microb. Tech. 1983, 5, 14–21. [Google Scholar] [CrossRef]
- Donova, M.V. Transformation of steroids by actinobacteria: A review. Appl. Biochem. Microbiol. 2007, 43, 1–14. [Google Scholar] [CrossRef]
- Freeman, A.; Lilly, M.D. The effect of water-miscible solvents on the Δ1-dehydrogenase activity of free and PAAH-entrapped Arthrobacter simplex. Appl. Microbiol. Biotechnol. 1987, 25, 495–501. [Google Scholar] [CrossRef]
- Wojtkiewicz, A.M.; Wójcik, P.; Procner, M.; Flejszar, M.; Oszajca, M.; Hochołowski, M.; Tataruch, M.; Mrugała, B.; Janeczko, T.; Szaleniec, M. The efficient Δ1-dehydrogenation of a wide spectrum of 3-ketosteroids in a broad pH range by 3-ketosteroid dehydrogenase from Sterolibacterium denitrificans. J. Steroid Biochem. Mol. Biol. 2020, 105731. [Google Scholar] [CrossRef]
- Chiang, Y.R.; Ismail, W.; Heintz, D.; Schaeffer, C.; Van Dorsselaer, A.; Fuchs, G. Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J. Bacteriol. 2008, 190, 905–914. [Google Scholar] [CrossRef]
- Lin, C.W.; Wang, P.H.; Ismail, W.; Tsai, Y.W.; El Nayal, A.; Yang, C.Y.; Yang, F.C.; Wang, C.H.; Chiang, Y.R. Substrate Uptake and Subcellular Compartmentation of Anoxic Cholesterol Catabolism in Sterolibacterium denitrificans. J. Biol. Chem. 2015, 290, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Bruice, T.C. Oxygen-Flavin Chemistry. Isr. J. Chem. 1984, 24, 54–61. [Google Scholar] [CrossRef]
- Fagan, R.L.; Palfey, B.A. Flavin-Dependent Enzymes. In Comprehensive Natural Products II, 1st ed.; Mander, L., Liu, H.-W., Eds.; Elsevier Science: Amsterdam, NL, USA, 2010; pp. 37–113. [Google Scholar]
- García-Castiñeiras, S.; Velázquez, S.; Martínez, P.; Torres, N. Aqueous humor hydrogen peroxide analysis with dichlorophenol-indophenol. Exp. Eye Res. 1992, 55, 9–19. [Google Scholar] [CrossRef]
- Sofińska, K.; Wojtkiewicz, A.M.; Wójcik, P.; Zastawny, O.; Guzik, M.; Winiarska, A.; Waligórski, P.; Cieśla, M.; Barbasz, J.; Szaleniec, M. Investigation of quaternary structure of aggregating 3-ketosteroid dehydrogenase from Sterolibacterium denitrificans: In the pursuit of consensus of various biophysical techniques. Biochim. Biophys. Acta Gen. Sub. 2019, 1863, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Pepper, D.S. Some alternative coupling chemistries for affinity chromatography. Mol. Biotechnol. 1994, 2, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, K.; Bryjak, J.; Mrowiec-Białoń, J.; Jarzębski, A.B. Application and properties of siliceous mesostructured cellular foams as enzymes carriers to obtain efficient biocatalysts. Microporous Mesoporous Mater. 2007, 99, 167–175. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Yang, T.; Xu, M.; Rao, Z. Over-expression of Mycobacterium neoaurum 3-ketosteroid-Δ1-dehydrogenase in Corynebacterium crenatum for efficient bioconversion of 4-androstene-3,17-dione to androst-1,4-diene-3,17-dione. Electron. J. Biotechnol. 2016, 24, 84–90. [Google Scholar] [CrossRef]
- Rugor, A.; Tataruch, M.; Staron, J.; Dudzik, A.; Niedzialkowska, E.; Nowak, P.; Hogendorf, A.; Michalik-Zym, A.; Napruszewska, D.B.; Jarzebski, A.; et al. Regioselective hydroxylation of cholecalciferol, cholesterol and other sterol derivatives by steroid C25 dehydrogenase. Appl. Microbiol. Biotechnol. 2017, 101, 1163–1174. [Google Scholar] [CrossRef]
- Hesselink, P.G.M.; van Vliet, S.; de Vries, H.; Witholt, B. Optimization of steroid side chain cleavage by Mycobacterium sp. in the presence of cyclodextrins. Enzym. Microb. Tech. 1989, 11, 398–404. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Shen, Y.; Ma, Y.; Zheng, Y.; Luo, J. Effects of hydroxypropyl-β-cyclodextrin on steroids 1-en-dehydrogenation biotransformation by Arthrobacter simplex TCCC 11037. J. Mol. Catal. B Enzym. 2009, 59, 58–63. [Google Scholar] [CrossRef]
- Oelschlägel, M.; Riedel, A.; Zniszczoł, A.; Szymańska, K.; Jarzębski, A.B.; Schlömann, M.; Tischler, D. Immobilization of an integral membrane protein for biotechnological phenylacetaldehyde production. J. Biotechnol. 2014, 174, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rekuc, A.; Bryjak, J.; Szymanska, K.; Jarzebski, A.B. Laccase immobilization on mesostructured cellular foams affords preparations with ultra high activity. Process. Biochem. 2009, 44, 191–198. [Google Scholar] [CrossRef]
- Szymanska, K.; Bryjak, J.; Jarzebski, A.B. Immobilization of Invertase on Mesoporous Silicas to Obtain Hyper Active Biocatalysts. Top. Catal. 2009, 52, 1030–1036. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).