Abstract
Chiral amines are key building blocks for pharmaceuticals. Economic assessment of commercial potential of bioprocesses is needed for guiding research. Biosynthesis of (S)-α-methylbenzylamine (MBA) was selected as case study. For transamination route, transaminase coupled with glucose dehydrogenase and lactate dehydrogenase catalyzed the reaction with NADH (Nicotinamide adenine dinucleotide) regeneration. Amine dehydrogenase coupled with NADH oxidase, which catalyzed the reductive amination process. Comparison of biosynthesis cost by reductive amination and transamination routes was carried out. Economic assessment based on the framework of cost analysis and preliminary process information revealed that cost is greatly dependent on enzyme price. The results indicated that enhancing the activity of amine dehydrogenase by 4–5 folds can drop the unit price of reductive amination to $0.5–0.6/g, which make it competitive with transamination route.
1. Introduction
Chiral amines are key building blocks for many new pharmaceutical products. Transaminases have emerged as an attractive alternative for chiral amine synthesis, due to their high enantioselectivity. However, there are still many challenges that need to be overcome to make the technology more widely applicable such as unfavorable equilibrium, low substrate solubility, low activity, low stability of the biocatalyst as well as product, and substrate inhibition [1].
The success of a biocatalytic process ultimately depends on the economic profile of the process. Economic assessment in early-stage can guide research and development activities to achieve commercial potential by identifying process bottlenecks. Once these bottlenecks are identified, prioritizing research and development (R&D) efforts to overcome these barriers has the greatest impact on the process. Therefore, such an evaluation can guarantee efficient utilization of limited resources to attain competitiveness in an industrial environment [2]. However, compared with chemical process, the data and modeling for unit operation of biomanufacturing are insufficient.
Increasing awareness for economic analysis of biotechnology has a profound and lasting significance for the biomanufacturing development [3]. Application of economic analysis in decision-making in research and design is a key factor to fulfill the economic requirements for commercial operation [4]. This paper demonstrates an approach of economic analysis of biocatalytic processes and illustrates it using a case study. Using the framework of cost analysis and preliminary process information, economic assessments of two multi-enzyme system for chiral amine production were studied, namely, transamination process and reductive amination by transaminase and amine dehydrogenase system, respectively [5]. Amine dehydrogenases dependent on NADH can catalyze the direct reductive amination of ketones with high enantioselectivity. Asymmetric synthesis of chiral amines by amine dehydrogenases is an ideal route for chiral amines production coupled with a cofactor recycling system with the consumption of an inexpensive agent, such as free ammonia [6]. Multi-enzyme biosynthesis of chiral amine based on amine dehydrogenases is with the advantages of simple reaction system and easy co-enzyme regeneration [7,8].
2. Results and Discussion
General assumptions were made as a plant to produce 600 kg (S)-α-methylbenzylamine (MBA) (99% ee) per year at concentrations by commercially available transaminases. The plant uses a 2 m3 reactor and operates with a 50-h process cycle time.
Assume that the equipment cost and waste disposal cost are the same, the reaction time of the reductive amination is 48 h, and the reaction time of the transamination is 50 h, which can be roughly regarded as the same time. Therefore, the difference in total cost lies in the price of raw materials and the enzyme activity of the reaction.
2.1. Multi-Enzyme System of Transamination
Biosynthesis of chiral amines from their corresponding ketones by a multi-enzyme system, which consists of transaminases, glucose dehydrogenase, and lactate dehydrogenase with cofactor regeneration, is selected as a case study (Figure 1). The amine donor is alanine. The generated pyruvate is reduced into L-lactate by lactate dehydrogenase (LDH), which eliminates pyruvate inhibition to the transaminase and lifts the reaction equilibrium. NADH regeneration is achieved by glucose dehydrogenase (GDH). Ion-exchange resin is applied for in situ product removal. The desired product is separated with a more than 90% yield [5].
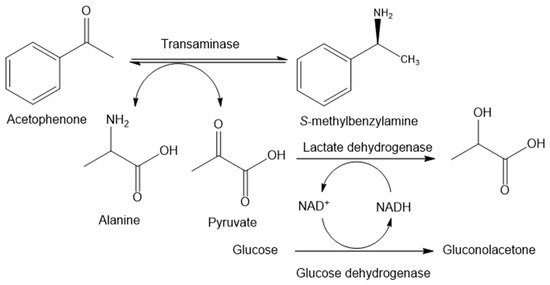
Figure 1.
Multi-enzyme catalyzed transamination [5].
The bioprocess shown in Figure 2 is as follows: (1) bioconversion of the ketone to amine and cofactor regeneration, (2) separation of the ion-exchange resin from the reaction mixture by filtration, and (3) isolation and purification of product amine.
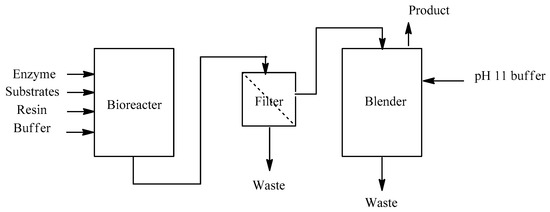
Figure 2.
Overview of the biocatalytic transamination process.
Reaction condition: 100 mM potassium phosphate buffer (pH 7.5), 1 g/L NAD+ (Nicotinamide adenine dinucleotide), 0.5 g/L pyridoxal-5-phosphate, 90 g/L glucose, 90 g/L alanine, 50 g/L acetophenone, 1 g/L glucose dehydrogenase (GDH), 1 g/L lactate dehydrogenase (LDH), 5 g/L transaminase (ATA), and 200 g/L ion-exchange resin (Amberlite XAD 1180). Reactions were run at 30 °C in a Multimax reactor system with overhead mechanical stirring at 400 rpm.
Route cost analysis:

Table 1.
Raw material costs per batch of transamination process.

Table 2.
Major equipment purchase and installed costs of transamination process.
Energy consumption, including stirring, heating, and also waste treatment during the process of transamination is calculated (Table 3). The labor costs are shown in Table 4.

Table 3.
Cost of energy consumption and waste disposal of transamination process.

Table 4.
Labor cost estimation of transamination process.
The price of electricity is 12 ct/kw.
The cost price of raw materials consumed in each batch is $302,820. The purchase cost of the equipment is $25,000. The installation cost (TIC) of the entire process line is $125,000. The cost of equipment maintenance is calculated at 10% of the TIC, which is approximately $12,500 per year. The price of the annuity (10-year lifetime) is $27,500 per year. Calculated according to 100 batches per year, the average annuity per batch is $275. The total cost of energy consumption and waste disposal is $22.8/batch. The total labor cost per batch is $1000/batch. Summarizing the above data, we can get an average total cost of $304,117.8/batch.
2.2. Multi-Enzyme System of Reductive Amination
Amine dehydrogenases which dependent on NADH can catalyze reductive amination of ketones with high enantioselectivity. When coupled a co-enzyme regeneration system with alcohol dehydrogenase (ADH), amine dehydrogenase implemented a redox self-sufficient reaction which generated water as the sole by-product (Figure 3) [9].
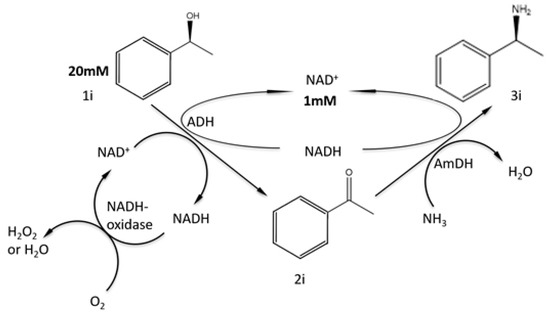
Figure 3.
Multi-enzyme catalyzed reductive amination with amine dehydrogenase [9].
Reaction condition: L-Phenylethanol, 20 mM; NH4Cl buffer, 2 M (pH 8.7); NAD+ 1 mM, 30 °C, 200 rpm, 48 h.

Table 5.
Raw material costs per batch of reductive amination.

Table 6.
Major equipment purchase and installed costs of reductive amination.
Energy consumption, including stirring, heating, and also waste treatment during the process of transamination is calculated (Table 7).

Table 7.
Cost of energy consumption and waste disposal of reductive amination.
The labor costs are shown in Table 8. The price of electricity is 12 ct/kw:

Table 8.
Labor cost estimation of reductive amination.
The cost price of raw materials consumed in each batch is $203,802. The cost of the equipment is $25,000. The installation cost (TIC) of the entire process line is $125,000. The cost of equipment maintenance is calculated at 10% of the TIC, which is approximately $12,500 per year. The price of the annuity (10-year lifetime) is $27,500 per year. Calculated according to 100 batches per year, the average annuity per batch is $275. The total cost of energy consumption and waste disposal is $22.8/batch. The total labor cost per batch is $960/batch. Therefore, an average total cost of $205,059.8/batch.
2.3. Economic Constraints Analysis
Economic analysis of the reaction determined that the performance criteria needed to make the biocatalysis cost-effective. Cost distribution for chiral amine production by multi-enzyme system was analyzed to address the bottleneck [10]. Comprehensive analysis of these two synthetic routes indicated that the amine dehydrogenase route is cost effective than the transamination route. In the case of other costs, they are basically the same; the cost difference is dependent on raw material costs, especially the enzyme cost.
As showed in Table 9, for transamination, 99.57% of the total cost was raw materials, of which 92.3% was the biocatalyst cost. For reductive amination process, 99.39% of the total cost was raw materials, of which 96.39% was the enzyme cost. The cost of transaminase route was $306,967.8 for 600 kg product with the unit price of $0.51/g. Amine dehydrogenase route cost $205,091.8 for 99.5 kg product, with unit price of $2.06/g. Due to high enzyme activity, the conversion rate was 90% for transamination, while the conversion rate was 31% for reductive amination. The ees of amines were measured by GC (Gas Chromatography) using an Agilent 7890 A GC system (Beijing Keyi Hengda Technology Co. LTD, Beijing, China), equipped with an FID detector and using a Varian Chrompack Chiracel Dex-CB column (Beijing Keyi Hengda Technology Co. LTD, Beijing, China) (25 m, 320 μm, 0.25 μm). The purity was determined by GC-FID (Gas Chromatography-Flame Ionization Detector) and NMR (Nuclear Magnetic Resonance) (1H-NMR, 13C-NMR) [8,9].

Table 9.
Cost distribution for chiral amine production by each conversion method.
Obviously, the cost of transaminase is much lower than that of amine dehydrogenase, and it is lower than the current commercial price ($0.99/g, data from https://www.trc-canada.com/product-detail/?M288801), which has high economic benefits. It was due to the activity of amine dehydrogenase being much lower than that of transaminase. Improving amine dehydrogenase 4–5-fold, the conversion rate can be increased from 31% to 80–90%. Therefore, the unit price will drop to $0.5–0.6/g. Therefore, reductive amination has a greater advantage than transamination in the case of the same bioprocess cost [11].
In general, two general strategies can reduce the enzyme cost, namely, improving enzyme efficiency and reusing enzyme. Immobilization has been widely applied for enzyme reuse due to the advantage of low cost and easy scale-up. Considering the rising cost caused by immobilization, the last cost will be 12–20% of the initial cost. Economic constraints analysis improved efficiency by focusing efforts to biocatalyst improvement on R&D aspects and has the greatest impact on the process.
3. Materials and Methods
The total cost of a bioprocess consists of two main components, fixed costs which are mainly equipment (instrumentation) cost, and variable costs, including raw materials cost, labor cost, energy consumption, and waste disposal cost [8].
3.1. Raw Material Costs
Raw materials consist of fixed raw materials and variable raw materials. Fixed raw materials are the ones that remain at a constant concentration in the reaction, such as buffer. Variable raw materials are the enzyme and the substrate, whose use could increase or decrease depend on process improvements. Therefore, substrates’ and enzymes’ concentration are the key factor of the raw materials cost. Possible price sources include supplier, internal data, literature, and sales catalogues [12].
3.2. Equipment Cost
Equipment cost is the cost of all the required equipment for the whole process from biocatalysis to final product isolation and purification. Bioreactors, centrifuges, extractors, distillation columns, and dryers are typical bioprocessing equipment for enzyme-catalyzed processes and product purification. The cost of instrumentation depends on the required equipment and the cycle time. Possible price sources of equipment include supplier, previous projects, literature, default values simulation software, etc. Process costs can be estimated from previous projects and analogous sources.
3.3. Labor Cost
The total labor amount was calculated by defining the number of people per shift and the number of shifts. The cost of off-line analysis, quality control (QC) and quality assurance (QA) were taken into account. The demand for each process step was estimated. Hourly cost can be obtained from the internal company average value or literature, etc. International labor comparisons provided labor cost data.
3.4. Energy Consumption and Waste Disposal
Typical energy consumptions include heating, cooling, and bioreactor agitation in typical enzyme-catalyzed reaction. In the downstream processing, evaporation, distillation, and centrifugation are energy-consuming.
Waste disposal cost is involved with waste treatment and emissions. Treatment is mandated by environmental laws. Waste types include solid waste and liquid waste. The waste emissions cost contribution is dependent on the composition, namely hazardous and non-hazardous waste, and greatly affected by local laws.
3.5. Materials
Multi-enzyme catalyzed transamination: Biosynthesis was carried out at 30 °C in a stirred-tank bioreactor (2 m3) with overhead mechanical stirring at 400 rpm. The reaction volume was 1 m3. Stirred bioreactors are widely applied for biosynthesis due to the advantages such as easy scale-up, good fluid mixing, and alternative impellers. The substrates and enzymes concentrations were listed in Table 1. The substrates and enzymes were dissolved in the 100 mM potassium phosphate buffer, while the pH was controlled at 7.5 with automated addition of 2 M NaOH. Upon complete conversion, the ion-exchange resin was recovered from the reaction mixture by filtration and washed with phosphate buffer solution (pH 11, Na3PO4/Na2HPO4, 0.05 M) to recover the methylbenzylamine product. The concentration of (S)-α-methylbenzylamine (MBA) (99% ee) was up to 6 g/L [8,13].
Multi-enzyme system of reductive amination: For ease of comparison, the synthetic route of transaminase uses uniform sites and instruments. The reaction was performed in ammonium chloride buffer (pH 8.7, final concentration 2 M) containing catalytic NAD+ (final concentration 1 mM). The AA-ADH enzyme (ADH originated from Aromatoleum aromaticum, 44.5 mg/mL) solution and the Ch1-AmDH (chimeric amine dehydrogenase generating through domain shuffling, 2.95 kU) solution were added. 1-Phenylethanol was finally added to start the reaction. Reaction was shaken at 30 °C, in an orbital shaker at 200 rpm, for 48 h. Reactions were stopped by the addition of KOH and extracted with CH2Cl2 to obtain methylbenzylamine.
4. Conclusions
Economic assessment based on the framework of cost analysis and preliminary process information revealed that the total cost greatly depends on enzyme cost. For this case study, enhancing the activity of amine dehydrogenase by 4–5 folds can drop the unit price of reductive amination to $0.5–0.6/g, which can compete with transamination. Economic assessment should follow each of the bioprocess development stages in order to guide research towards optimal process technology [14]. In addition, the case study keeps up to date on industrial and academic activity in bioprocess simulation to develop a methodology by rapidly estimating the production cost of biocatalysis [15].
Author Contributions
S.W. designed an experiment to confirm the writing idea of the article. H.H. calculated the cost, first and revised the subsequent manuscript, G.Y. collected the data needed for the article; S.W. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21776233, No. 22078273), the Natural Science Foundation of Fujian Province of China (No. 2018J01013), and the Fundamental Research Funds for the Central Universities (No. 20720170033).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tufvesson, P.; Lima-Ramos, J.; Jensen, J.S.; Al-Haque, N.; Neto, W.; Woodley, J.M. Process considerations for the asymmetric synthesis of chiral amines using transaminases. Biotechnol. Bioeng. 2011, 108, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Dahms, A.S. Biotechnology: What it is, what it is not, and the challenges in reaching a national or global consensus. Biochem. Mol. Biol. Educ. 2010, 32, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S. Successful engineers and indifferent economists. J. Eng. Educ. 2001, 90, 261–266. [Google Scholar] [CrossRef]
- Gruber, P.; Carvalho, F.; Marques, M.P.C.; O’Sullivan, B.; Subrizi, F.; Dobrijevic, D.; Ward, J.; Hailes, H.C.; Fernandes, P.; Wohlgemuth, R.; et al. Enzymatic synthesis of chiral amino-alcohols by coupling transketolase and transaminase-catalyzed reactions in a cascading continuous-flow microreactor system. Biotechnol. Bioeng. 2018, 115, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S. Amine dehydrogenases occur in nature. Nat. Catal. 2019, 2, 288–289. [Google Scholar] [CrossRef]
- Caparco, A.A.; Pelletier, E.; Petit, J.L.; Jouenne, A.; Bommarius, B.R.; Berardinis, V.; Zaparucha, A.; Champion, J.A.; Bommarius, A.S.; Vergne-Vaxelaire, C. Metagenomic mining for amine dehydrogenase discovery. Adv. Synth. Catal. 2020, 362, 2427–2436. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z. Enhancing cofactor recycling in the bioconversion of racemic alcohols to chiral amines with alcohol dehydrogenase and amine dehydrogenase by coupling cells and cell-free system. Biotechnol. Bioeng. 2019, 116, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Mutti, F.G.; Knaus, T.; Scrutton, N.S.; Breuer, M.; Turner, N.J. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades. Science 2015, 349, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Petrides, D.; Carmichael, D.; Siletti, C.; Koulouris, A. Bioprocess Simulation and Economics, Essentials in Fermentation Technology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Tseliou, V.; Knaus, T.; Masman, M.F.; Corrado, M.L.; Mutti, F.G. Generation of amine dehydrogenases with increased catalytic performance and substrate scope from ε-deaminating L-Lysine dehydrogenase. Nat. Commun. 2019, 10, 3717. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.S. Plant Design and Economics for Chemical Engineers; Max, S.P., Klaus, D., Timmerhaus, R.E.W., Eds.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Truppo, M.D.; Rozzell, J.D.; Turner, N.J. Efficient production of enantiomerically pure chiral amines at concentrations of 50 g/L using transaminases. Org. Process Res. Dev. 2010, 14, 234–237. [Google Scholar] [CrossRef]
- Wenda, S.; Illner, S.; Mell, A.; Kragl, U.J.G.C. Industrial biotechnology—The future of green chemistry? Green Chem. 2011, 13, 3007–3047. [Google Scholar] [CrossRef]
- Bucak, S.; Tuiebakhova, Z.; Kadirgan, N. Bridging the gap between chemical engineering education and industrial practice. Int. J. Eng. Educ. 2007, 23, 1219–1231. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).