Pt Nanoclusters Anchored on Hollow Ag-Au Nanostructures for Electrochemical Oxidation of Methanol
Abstract
1. Introduction
2. Results and Discussion
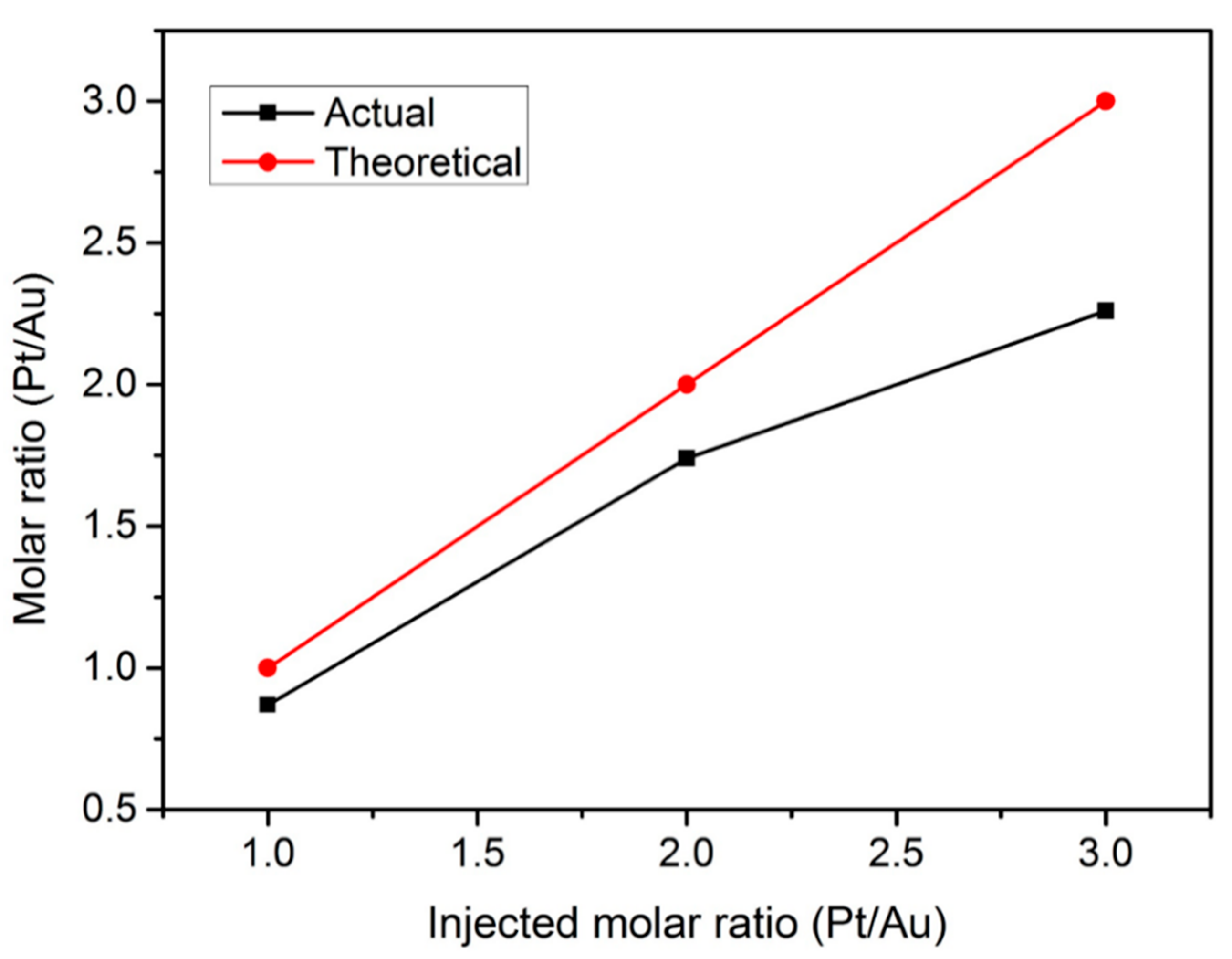
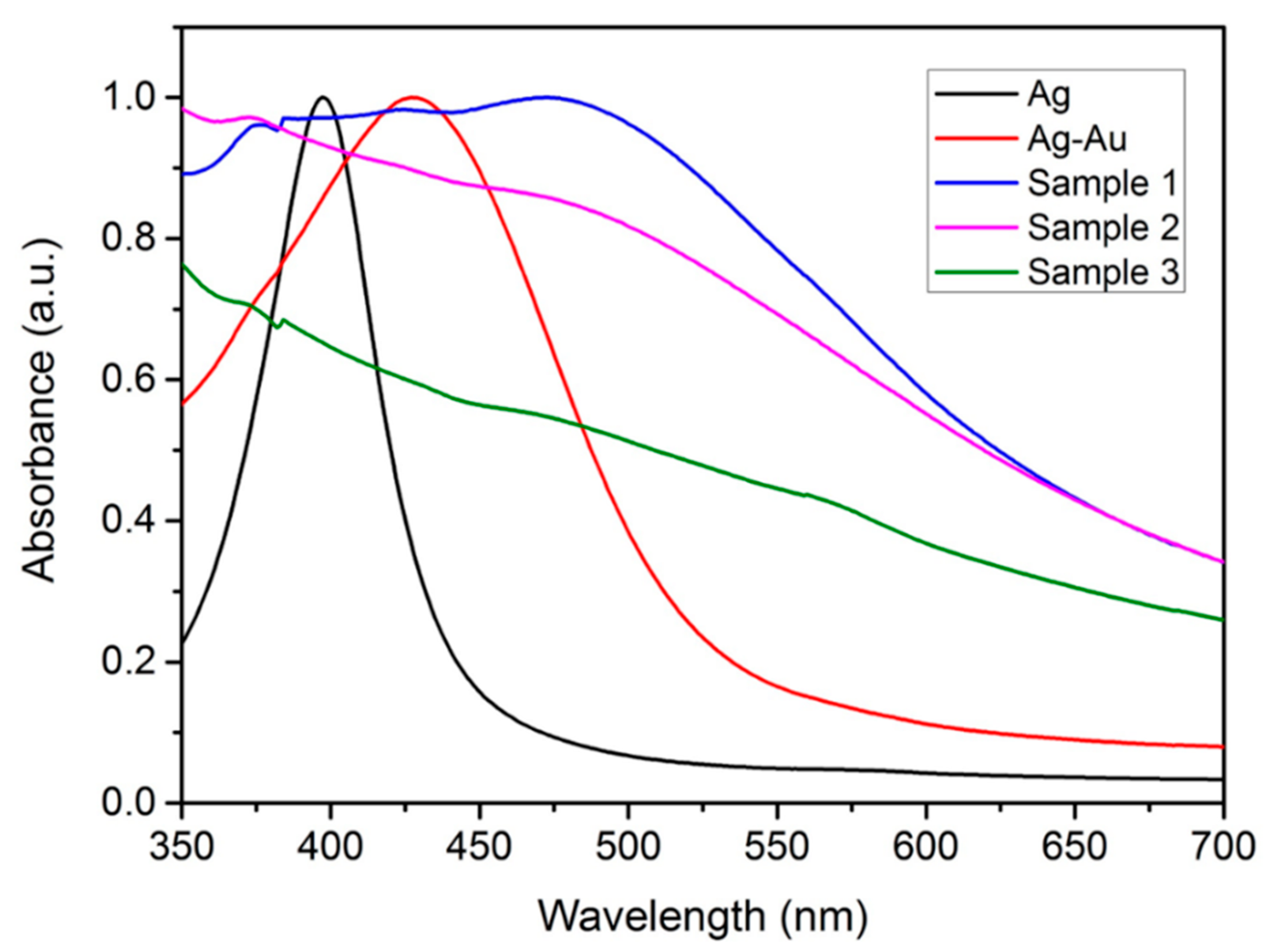
2.1. Physical and Chemical Characterizations
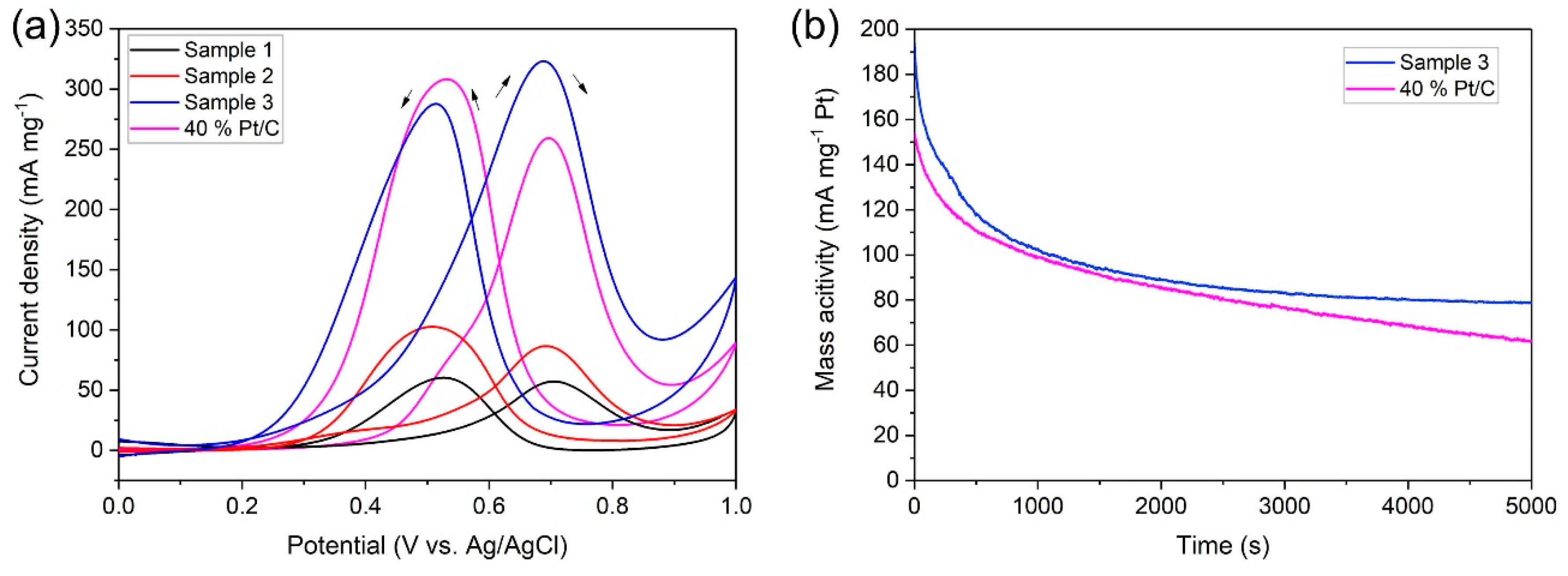
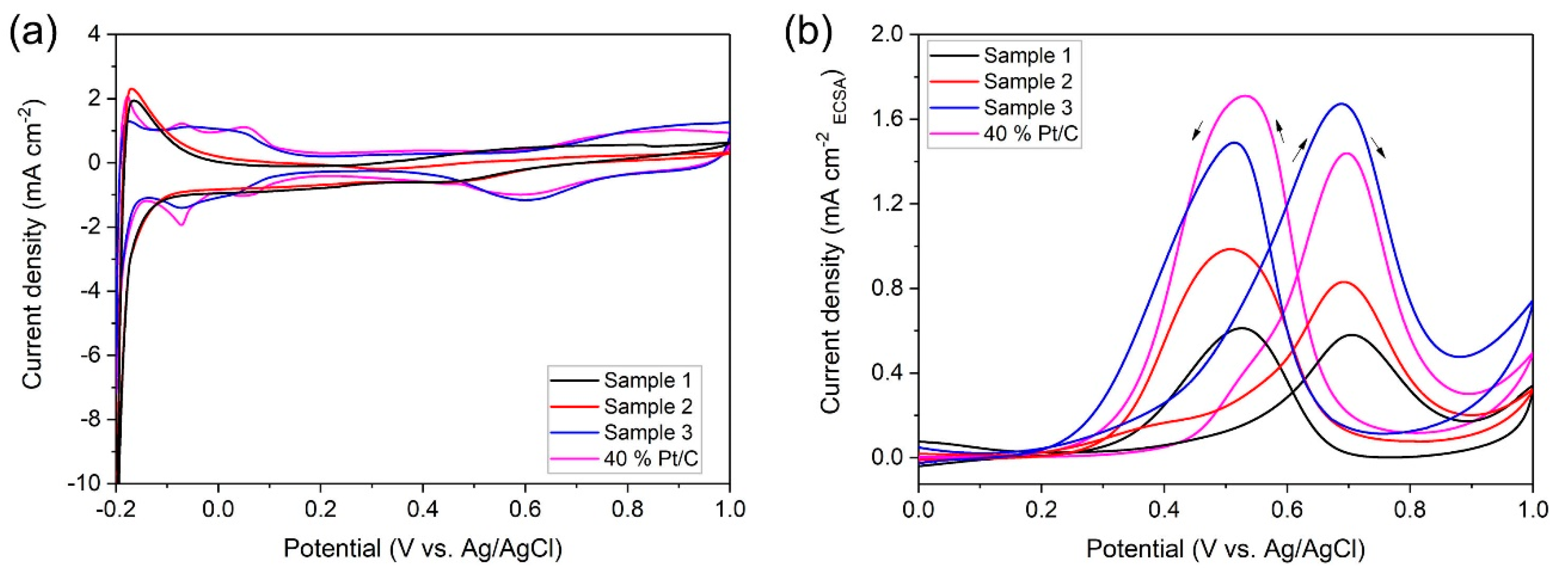
2.2. Electrocatalytic Characterization
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Uniform Ag Nanospheres
3.3. Synthesis of Hollow Ag-Au Nanostructures
3.4. Synthesis of Pt Nanocluster-Anchored Hollow Ag-Au Nanostructures
3.5. Physical and Chemical Characterizations
3.6. Electrocatalytic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wasmus, S.; Küver, A. Methanol oxidation and direct methanol fuel cells: A selective review. J. Electroanal. Chem. 1999, 461, 14–31. [Google Scholar] [CrossRef]
- Aricò, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 226, 223–240. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Wang, X.; Hu, J.; Liu, Y.; Fu, G.; Tang, Y. Concave PtCo nanocrosses for methanol oxidation reaction. Appl. Catal. B 2020, 277, 119135. [Google Scholar] [CrossRef]
- Bonzel, H.P.; Ku, R. Mechanisms of the catalytic carbon monoxide oxidation on Pt (110). Surf. Sci. 1972, 33, 91–106. [Google Scholar] [CrossRef]
- Ertl, G.; Neumann, M.; Streit, K.M. Chemisorption of CO on the Pt(111) surface. Surf. Sci. 1977, 64, 393–410. [Google Scholar] [CrossRef]
- Batista, E.A.; Malpass, G.R.P.; Motheo, A.J.; Iwasita, T. New mechanistic aspects of methanol oxidation. J. Electroanal. Chem. 2004, 571, 273–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, X. Nanoporous bimetallic Pt-Au alloy nanocomposites with superior catalytic activity towards electro-oxidation of methanol and formic acid. Nanoscale 2011, 3, 1663–1674. [Google Scholar] [CrossRef]
- Liao, S.; Holmes, K.A.; Tsaprailis, H.; Birss, V.I. High performance PtRuIr catalysts supported on carbon nanotubes for the anodic oxidation of methanol. J. Am. Chem. Soc. 2006, 128, 3504–3505. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.; Liu, J.; Zou, Z.; Li, Z.; Yang, H. One-step synthesis of carbon-supported Pd–Pt alloy electrocatalysts for methanol tolerant oxygen reduction. Electrochem. Commun. 2008, 10, 1396–1399. [Google Scholar] [CrossRef]
- Wang, S.; Kristian, N.; Jiang, S.; Wang, X. Controlled synthesis of dendritic Au@Pt core-shell nanomaterials for use as an effective fuel cell electrocatalyst. Nanotechnology 2009, 20, 25605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, S.; Zhou, W.; Wang, G.; Jiang, L.; Li, W.; Song, S.; Liu, J.; Sun, G.; Xin, Q. Novel synthesis of highly active Pt/C cathode electrocatalyst for direct methanol fuel cell. Chem. Commun. 2003, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Maye, M.M.; Petkov, V.; Kariuki, N.N.; Wang, L.; Njoki, P.; Mott, D.; Lin, Y.; Zhong, C.-J. Phase Properties of Carbon-Supported Gold−Platinum Nanoparticles with Different Bimetallic Compositions. Chem. Mater. 2005, 17, 3086–3091. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.-N.; Kim, H.; Song, S.; Lee, W.-H. The preparation of Pt/C catalysts using various carbon materials for the cathode of PEMFC. J. Power Sources 2006, 163, 93–97. [Google Scholar] [CrossRef]
- Park, I.-S.; Lee, K.-S.; Jung, D.-S.; Park, H.-Y.; Sung, Y.-E. Electrocatalytic activity of carbon-supported Pt–Au nanoparticles for methanol electro-oxidation. Electrochim. Acta 2007, 52, 5599–5605. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, B.Q. Enhancement of Pt utilization in electrocatalysts by using gold nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 4955–4959. [Google Scholar] [CrossRef]
- Luo, J.; Wang, L.; Mott, D.; Njoki, P.N.; Lin, Y.; He, T.; Xu, Z.; Wanjana, B.N.; Lim, I.I.S.; Zhong, C.-J. Core/Shell Nanoparticles as Electrocatalysts for Fuel Cell Reactions. Adv. Mater. 2008, 20, 4342–4347. [Google Scholar] [CrossRef]
- Kristian, N.; Wang, X. Ptshell–Aucore/C electrocatalyst with a controlled shell thickness and improved Pt utilization for fuel cell reactions. Electrochem. Commun. 2008, 10, 12–15. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Wang, L.; Nemoto, Y.; Yamauchi, Y. Synthesis of Bimetallic Au@Pt Nanoparticles with Au Core and Nanostructured Pt Shell toward Highly Active Electrocatalysts. Chem. Mater. 2010, 22, 6310–6318. [Google Scholar] [CrossRef]
- Kim, Y.; Noh, Y.; Lim, E.J.; Lee, S.; Choi, S.M.; Kim, W.B. Star-shaped Pd@Pt core–shell catalysts supported on reduced graphene oxide with superior electrocatalytic performance. J. Mater. Chem. A 2014, 2, 6976–6986. [Google Scholar] [CrossRef]
- Xie, R.; Chen, M.; Wang, J.; Mei, S.; Pan, Y.; Gu, H. Facile synthesis of Au–Pt bimetallic nanocomplexes for direct oxidation of methanol and formic acid. RSC Adv. 2015, 5, 650–653. [Google Scholar] [CrossRef]
- Ye, H.; Crooks, R.M. Effect of elemental composition of PtPd bimetallic nanoparticles containing an average of 180 atoms on the kinetics of the electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2007, 129, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V.; Hien, T.D.; Asaka, T.; Ohtaki, M.; Nogami, M. Synthesis and characterization of Pt–Pd alloy and core-shell bimetallic nanoparticles for direct methanol fuel cells (DMFCs): Enhanced electrocatalytic properties of well-shaped core-shell morphologies and nanostructures. Int. J. Hydrog. Energy 2011, 36, 8478–8491. [Google Scholar] [CrossRef]
- Hills, C.W.; Mack, N.H.; Nuzzo, R.G. The Size-Dependent Structural Phase Behaviors of Supported Bimetallic (Pt−Ru) Nanoparticles. J. Phys. Chem. B 2003, 107, 2626–2636. [Google Scholar] [CrossRef]
- Alayoglu, S.; Zavalij, P.; Eichhorn, B.; Wang, Q.; Frenkel, A.I.; Chupas, P. Structural and architectural evaluation of bimetallic nanoparticles: A case study of Pt-Ru core-shell and alloy nanoparticles. ACS Nano 2009, 3, 3127–3137. [Google Scholar] [CrossRef]
- Park, J.Y.; Zhang, Y.; Grass, M.; Zhang, T.; Somorjai, G.A. Tuning of catalytic CO oxidation by changing composition of Rh-Pt bimetallic nanoparticles. Nano Lett. 2008, 8, 673–677. [Google Scholar] [CrossRef]
- Huan, T.N.; Shinde, D.V.; Kim, S.; Han, S.-H.; Artero, V.; Chung, H. Forest of Pt–Au–Ag tri-metallic nanodendrites as an efficient electrocatalyst for methanol oxidation reaction. RSC Adv. 2015, 5, 6940–6944. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Zhao, G.; Wang, Z. Facile synthesis of gold–platinum dendritic nanostructures with enhanced electrocatalytic performance for the methanol oxidation reaction. RSC Adv. 2016, 6, 51569–51574. [Google Scholar] [CrossRef]
- Lou, Y.; Maye, M.M.; Han, L.; Luo, J.; Zhong, C.-J. Gold–platinum alloy nanoparticle assembly as catalyst for methanol electrooxidation. Chem. Commun. 2001, 473–474. [Google Scholar] [CrossRef]
- Chen, G.; Xia, D.; Nie, Z.; Wang, Z.; Wang, L.; Zhang, L.; Zhang, J. Facile Synthesis of Co−Pt Hollow Sphere Electrocatalyst. Chem. Mater. 2007, 19, 1840–1844. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Z.; Yang, H.; Liu, Q.; Zhang, J.; Fang, J.; Zou, S.; Sun, K. Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum-copper nanocubes. Angew. Chem. Int. Ed. 2009, 48, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Stamenkovic, V.R.; Soled, S.; Henao, J.D.; Sun, S. Synthesis of Pt3Sn Alloy Nanoparticles and Their Catalysis for Electro-Oxidation of CO and Methanol. ACS Catal. 2011, 1, 1719–1723. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energ. Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Asset, T.; Job, N.; Busby, Y.; Crisci, A.; Martin, V.; Stergiopoulos, V.; Bonnaud, C.; Serov, A.; Atanassov, P.; Chattot, R.; et al. Porous Hollow PtNi/C Electrocatalysts: Carbon Support Considerations To Meet Performance and Stability Requirements. ACS Catal. 2018, 8, 893–903. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, P.; Zhang, H.; Cai, C. Synthesis of graphene-supported hollow Pt–Ni nanocatalysts for highly active electrocatalysis toward the methanol oxidation reaction. Electrochim. Acta 2012, 85, 314–321. [Google Scholar] [CrossRef]
- Fang, C.; Zhao, J.; Jiang, R.; Wang, J.; Zhao, G.; Geng, B. Engineering of Hollow PdPt Nanocrystals via Reduction Kinetic Control for Their Superior Electrocatalytic Performances. ACS Appl. Mater. Interfaces 2018, 10, 29543–29551. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.T.; Xia, Y. Template-Engaged Replacement Reaction: A One-Step Approach to the Large-Scale Synthesis of Metal Nanostructures with Hollow Interiors. Nano Lett. 2002, 2, 481–485. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. A general method for the rapid synthesis of hollow metallic or bimetallic nanoelectrocatalysts with urchinlike morphology. Chem. Eur. J. 2008, 14, 4689–4695. [Google Scholar] [CrossRef]
- Song, H.M.; Anjum, D.H.; Sougrat, R.; Hedhili, M.N.; Khashab, N.M. Hollow Au@Pd and Au@Pt core–shell nanoparticles as electrocatalysts for ethanol oxidation reactions. J. Mater. Chem. 2012, 22, 25003–25010. [Google Scholar] [CrossRef]
- Choi, Y.; Hong, S.; Liu, L.; Kim, S.K.; Park, S. Galvanically replaced hollow Au-Ag nanospheres: Study of their surface plasmon resonance. Langmuir 2012, 28, 6670–6676. [Google Scholar] [CrossRef]
- Li, W.; Kuai, L.; Chen, L.; Geng, B. “Re-growth etching” to large-sized porous gold nanostructures. Sci. Rep. 2013, 3, 2377. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, B.; Imura, M.; Malgras, V.; Yamauchi, Y. Mesoporous Pt hollow cubes with controlled shell thicknesses and investigation of their electrocatalytic performance. Chem. Commun. 2014, 50, 15337–15340. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Fang, Y.; Dong, S.; Wang, E. High-Efficiency and Low-Cost Hybrid Nanomaterial as Enhancing Electrocatalyst: Spongelike Au/Pt Core/Shell Nanomaterial with Hollow Cavity. J. Phys. Chem. C 2007, 111, 17104–17109. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Y.; Yu, L.; Dong, L.; Shi, C.; Hu, M.J.; Xu, Y.J.; Wen, L.P.; Yu, S.H. Hydrophilic Co@Au yolk/shell nanospheres: Synthesis, assembly, and application to gene delivery. Adv. Mater. 2010, 22, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.; Armyanov, S.; Valova, E.; Hubin, A.; Steenhaut, O.; Pavlidou, E.; Kokkinidis, G.; Sotiropoulos, S. Methanol Oxidation at Pt−Cu, Pt−Ni, and Pt−Co Electrode Coatings Prepared by a Galvanic Replacement Process. J. Phys. Chem. C 2010, 114, 5217–5223. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th anniversary article: Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Mallin, M.P.; Murphy, C.J. Solution-Phase Synthesis of Sub-10 nm Au−Ag Alloy Nanoparticles. Nano Lett. 2002, 2, 1235–1237. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef]
- Kim, M.H.; Lu, X.; Wiley, B.; Lee, E.P.; Xia, Y. Morphological Evolution of Single-Crystal Ag Nanospheres during the Galvanic Replacement Reaction with HAuCl(4). J. Phys. Chem. C 2008, 112, 7872–7876. [Google Scholar] [CrossRef]
- Jung, N.; Chung, D.Y.; Ryu, J.; Yoo, S.J.; Sung, Y.-E. Pt-based nanoarchitecture and catalyst design for fuel cell applications. Nano Today 2014, 9, 433–456. [Google Scholar] [CrossRef]
- Kuai, L.; Wang, S.; Geng, B. Gold-platinum yolk-shell structure: A facile galvanic displacement synthesis and highly active electrocatalytic properties for methanol oxidation with super CO-tolerance. Chem. Commun. 2011, 47, 6093–6095. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Zhang, F.; Liu, Z.; Fang, J. Free-Standing Pt–Au Hollow Nanourchins with Enhanced Activity and Stability for Catalytic Methanol Oxidation. ACS Catal. 2014, 4, 2829–2835. [Google Scholar] [CrossRef]
- Tan, C.; Sun, Y.; Zheng, J.; Wang, D.; Li, Z.; Zeng, H.; Guo, J.; Jing, L.; Jiang, L. A self-supporting bimetallic Au@Pt core-shell nanoparticle electrocatalyst for the synergistic enhancement of methanol oxidation. Sci. Rep. 2017, 7, 6347. [Google Scholar] [CrossRef]
- Shervani, Z.; Ikushima, Y.; Sato, M.; Kawanami, H.; Hakuta, Y.; Yokoyama, T.; Nagase, T.; Kuneida, H.; Aramaki, K. Morphology and size-controlled synthesis of silver nanoparticles in aqueous surfactant polymer solutions. Colloid Polym. Sci. 2007, 286, 403–410. [Google Scholar] [CrossRef]
- Lee, W.-K.; Cha, S.-H.; Kim, K.-H.; Kim, B.-W.; Lee, J.-C. Shape-controlled synthesis of gold icosahedra and nanoplates using Pluronic P123 block copolymer and sodium chloride. J. Solid State Chem. 2009, 182, 3243–3248. [Google Scholar] [CrossRef]
- Holah, D.G.; Hughes, A.N.; Hui, B.C. Ligand effects upon the reactions of Ni(II) with sodium tetrahydroborate: Ni(I) complexes of bipyridyl and 1,10-phenanthroline. Can. J. Chem. 1977, 55, 4048–4055. [Google Scholar] [CrossRef]
- Au, L.; Lu, X.; Xia, Y. A Comparative Study of Galvanic Replacement Reactions Involving Ag Nanocubes and AuCl(2) or AuCl(4). Adv. Mater. 2008, 20, 2517–2522. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Lee, J.Y.; Zhang, J.; Boothroyd, C. Synthesis of Ag@AgAu metal core/alloy shell bimetallic nanoparticles with tunable shell compositions by a galvanic replacement reaction. Small 2008, 4, 1067–1071. [Google Scholar] [CrossRef]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts Prepared by Galvanic Replacement. Catalysts 2017, 7, 80. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Jiao, Y.; He, X.; Wang, J.; Zhang, Y. Characterization of Ag/Pt core-shell nanoparticles by UV-vis absorption, resonance light-scattering techniques. Spectrochim. Acta A 2007, 68, 484–490. [Google Scholar] [CrossRef]
- Li, K.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Platinum nanoparticles partially-embedded into carbon sphere surfaces: A low metal-loading anode catalyst with superior performance for direct methanol fuel cells. J. Mater. Chem. A 2017, 5, 19857–19865. [Google Scholar] [CrossRef]
- Brankovic, S.R.; Wang, J.X.; Adžić, R.R. Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf. Sci. 2001, 474, L173–L179. [Google Scholar] [CrossRef]
- Zhu, J.; Lyu, Z.; Chen, Z.; Xie, M.; Chi, M.; Jin, W.; Xia, Y. Facile Synthesis and Characterization of Pd@IrnL (n = 1–4) Core–Shell Nanocubes for Highly Efficient Oxygen Evolution in Acidic Media. Chem. Mater. 2019, 31, 5867–5875. [Google Scholar] [CrossRef]
- Yang, L.; Li, G.; Chang, J.; Ge, J.; Liu, C.; Vladimir, F.; Wang, G.; Jin, Z.; Xing, W. Sea urchin-like Aucore@Pdshell electrocatalysts with high FAOR performance: Coefficient of lattice strain and electrochemical surface area. Appl. Catal. B 2020, 260, 118200. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, X. Facile fabrication and electrocatalytic activity of Pt0.9Pd0.1 alloy film catalysts. J. Power Sources 2007, 170, 13–19. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Wu, P.; Zhang, H.; Zhou, B.; Cai, C. Bimetallic Pt-Au nanocatalysts electrochemically deposited on graphene and their electrocatalytic characteristics towards oxygen reduction and methanol oxidation. Phys. Chem. Chem. Phys. 2011, 13, 4083–4094. [Google Scholar] [CrossRef]
- Sanders, C.I.; Martin, D.S., Jr. Acid Hydrolysis of [PtCl4]= and [PtCl3(H2O)]−1. J. Am. Chem. Soc. 1961, 83, 807–810. [Google Scholar] [CrossRef]
- Yu, Y.-T.; Xu, B.-Q. Effect of Precursor Hydrolysis on Shape-controlled Synthesis of Pt Nanocrystals. Acta Chim. Sin. 2003, 6, 1758–1764. [Google Scholar]





| Sample 1 | Sample 2 | Sample 3 | |
|---|---|---|---|
| Ag/ppb (%) | 1376.3 (13.68) | 1598.9 (11.42) | 1769.0 (10.26) |
| Au/ppb (%) | 4666.9 (46.40) | 4569.9 (32.62) | 4782.5 (27.73) |
| Pt/ppb (%) | 4015.0 (39.92) | 7839.9 (55.96) | 10,696.4 (62.01) |
| Molar ratio (Pt/Au) | 0.87 | 1.74 | 2.26 |
| Theoretical Pt mass/μg | 66.33 | 132.66 | 198.98 |
| Actual Pt mass/μg | 40.15 | 78.40 | 106.96 |
| Pt loss (%) | 39.47 | 40.90 | 46.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Qin, X.; Yan, B.; Huang, H.; Zhang, W.; Piao, Y. Pt Nanoclusters Anchored on Hollow Ag-Au Nanostructures for Electrochemical Oxidation of Methanol. Catalysts 2020, 10, 1440. https://doi.org/10.3390/catal10121440
Li X, Qin X, Yan B, Huang H, Zhang W, Piao Y. Pt Nanoclusters Anchored on Hollow Ag-Au Nanostructures for Electrochemical Oxidation of Methanol. Catalysts. 2020; 10(12):1440. https://doi.org/10.3390/catal10121440
Chicago/Turabian StyleLi, Xinghe, Xinyu Qin, Bingyi Yan, Huiling Huang, Wang Zhang, and Yuanzhe Piao. 2020. "Pt Nanoclusters Anchored on Hollow Ag-Au Nanostructures for Electrochemical Oxidation of Methanol" Catalysts 10, no. 12: 1440. https://doi.org/10.3390/catal10121440
APA StyleLi, X., Qin, X., Yan, B., Huang, H., Zhang, W., & Piao, Y. (2020). Pt Nanoclusters Anchored on Hollow Ag-Au Nanostructures for Electrochemical Oxidation of Methanol. Catalysts, 10(12), 1440. https://doi.org/10.3390/catal10121440






