Reaction Mechanism of Simultaneous Removal of H2S and PH3 Using Modified Manganese Slag Slurry
Abstract
1. Introduction
2. Results and Discussion
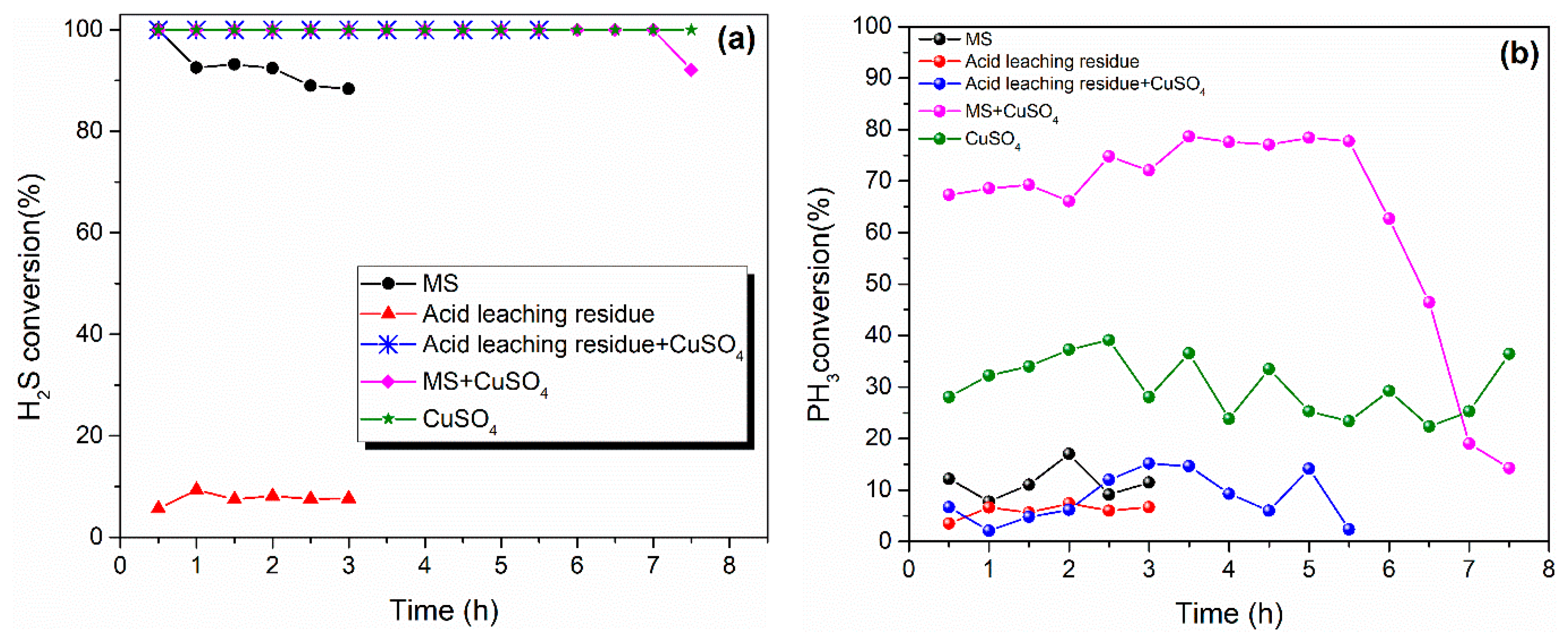
2.1. Effect of Different Component Slurry after Acid Leaching on Simultaneous Removal of H2S and PH3
2.2. Effect of Simulated Modified MS Slurry on Simultaneous Removal of H2S and PH3
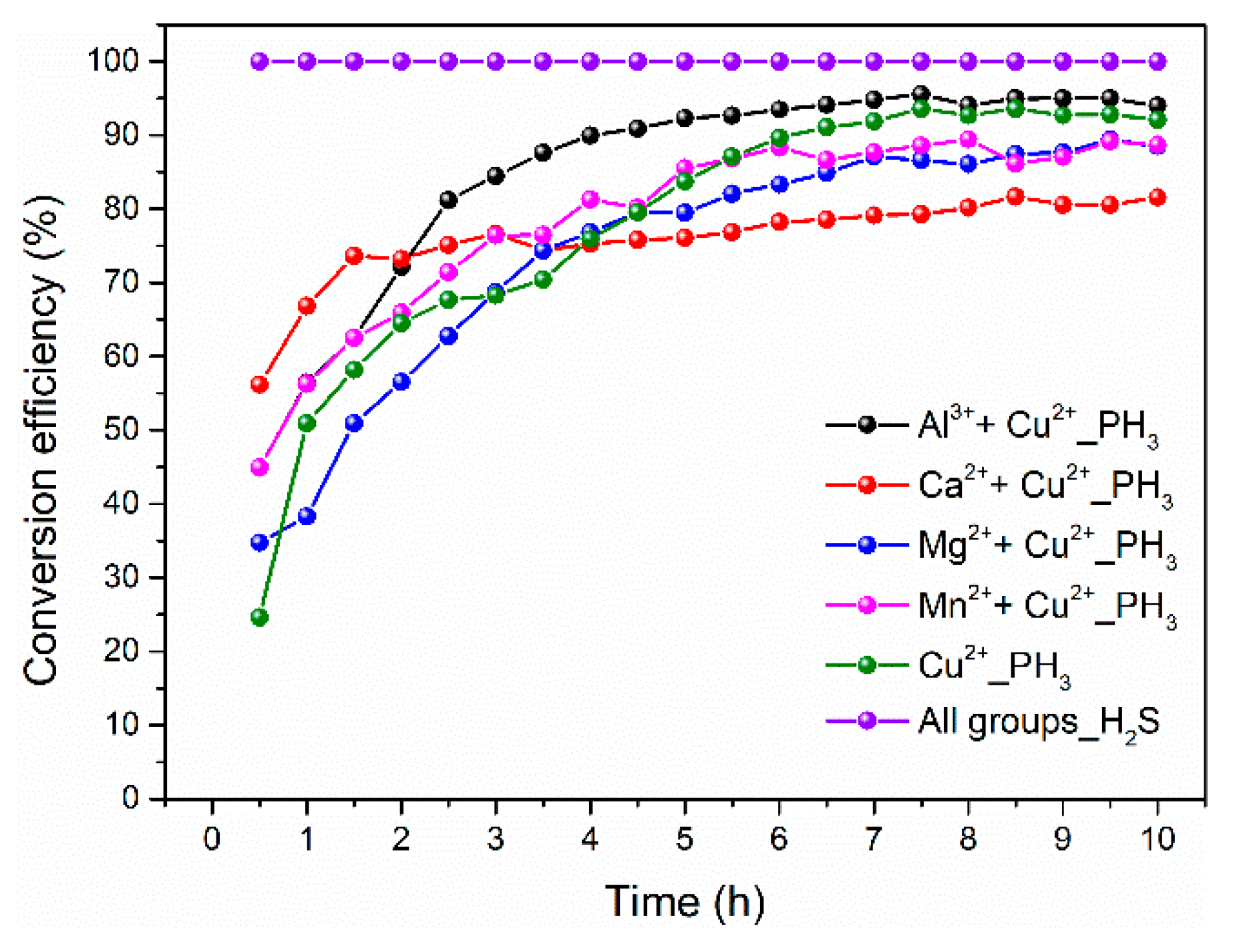
2.3. Effect of Single and Multi-Metal Ions on Simultaneous Removal of H2S and PH3
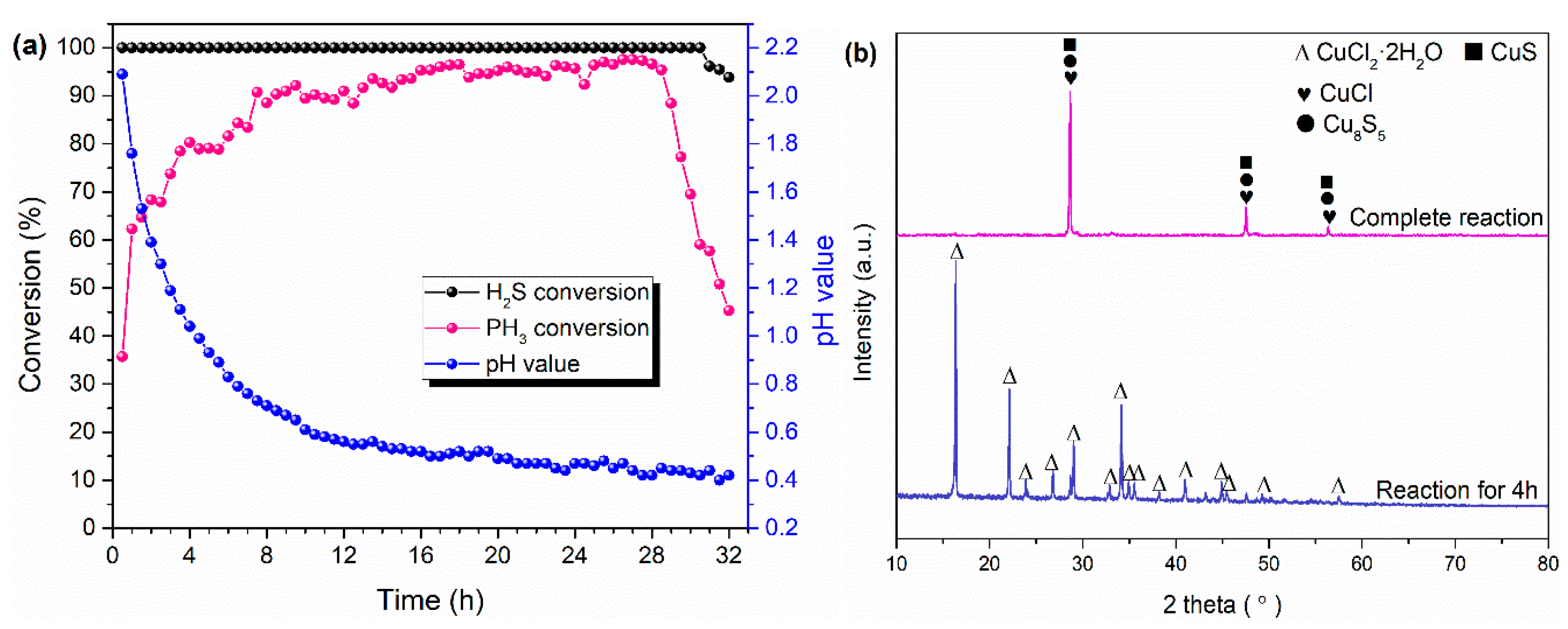
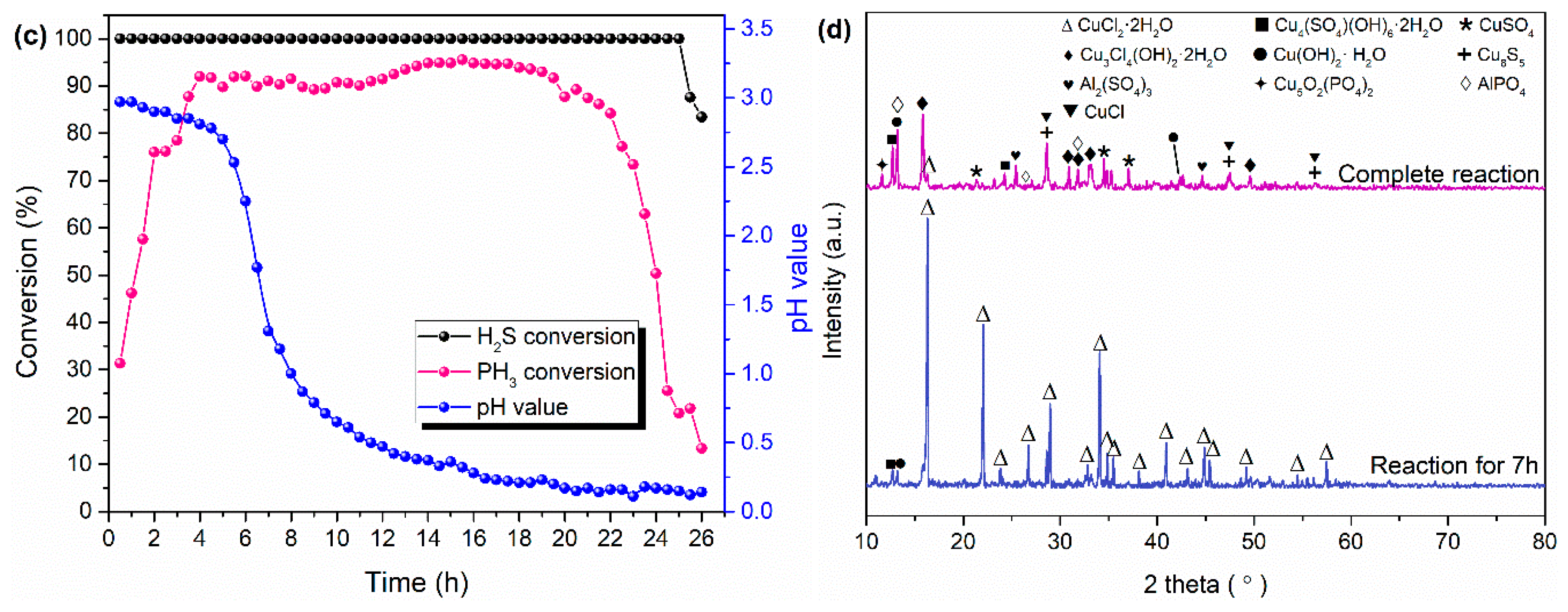
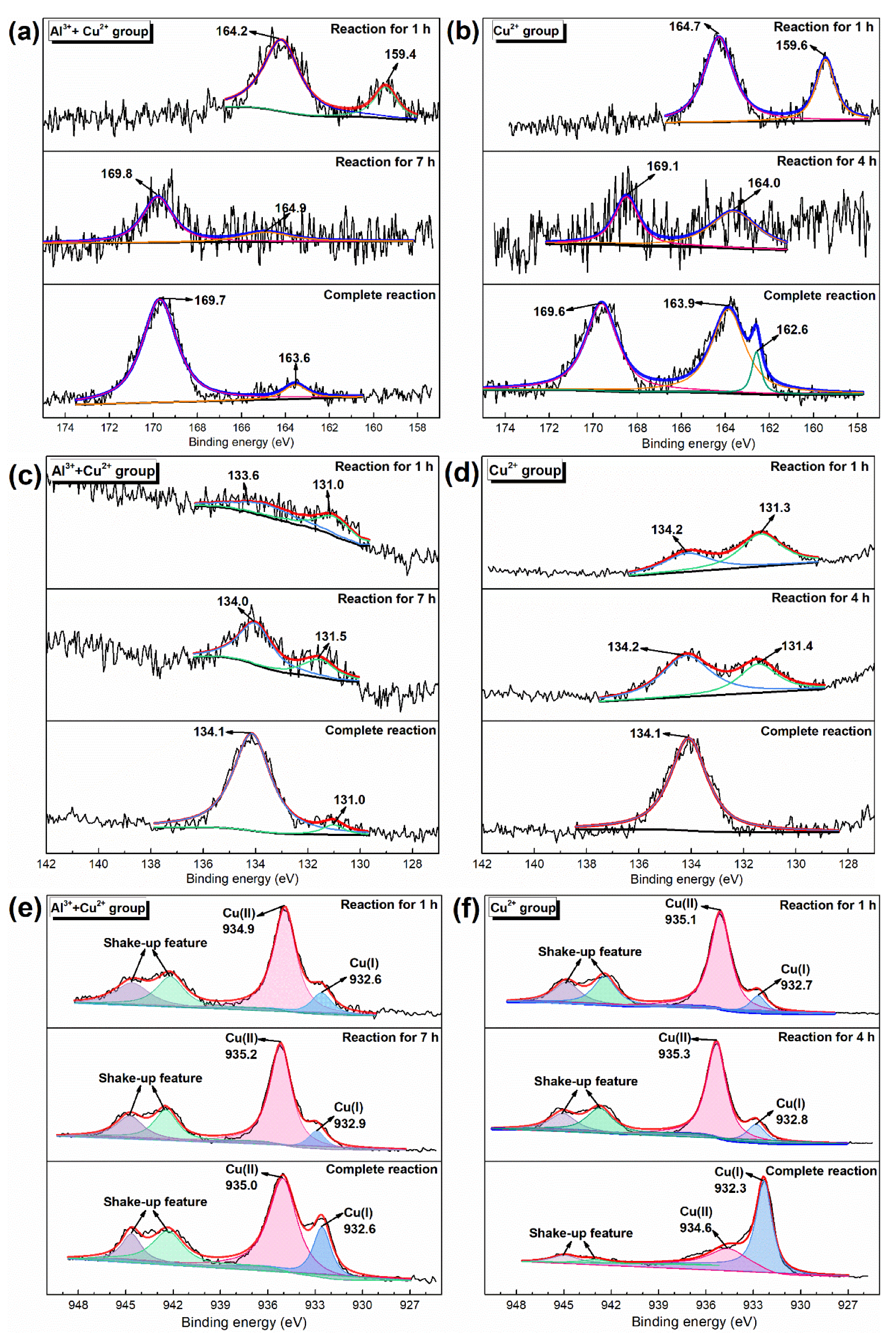
2.4. Reaction Mechanism of Metal Ions to Simultaneous Removal of H2S and PH3
3. Materials and Methods
3.1. Materials
3.2. Acid Leaching Procedure and Preparation of Modified MS Slurry
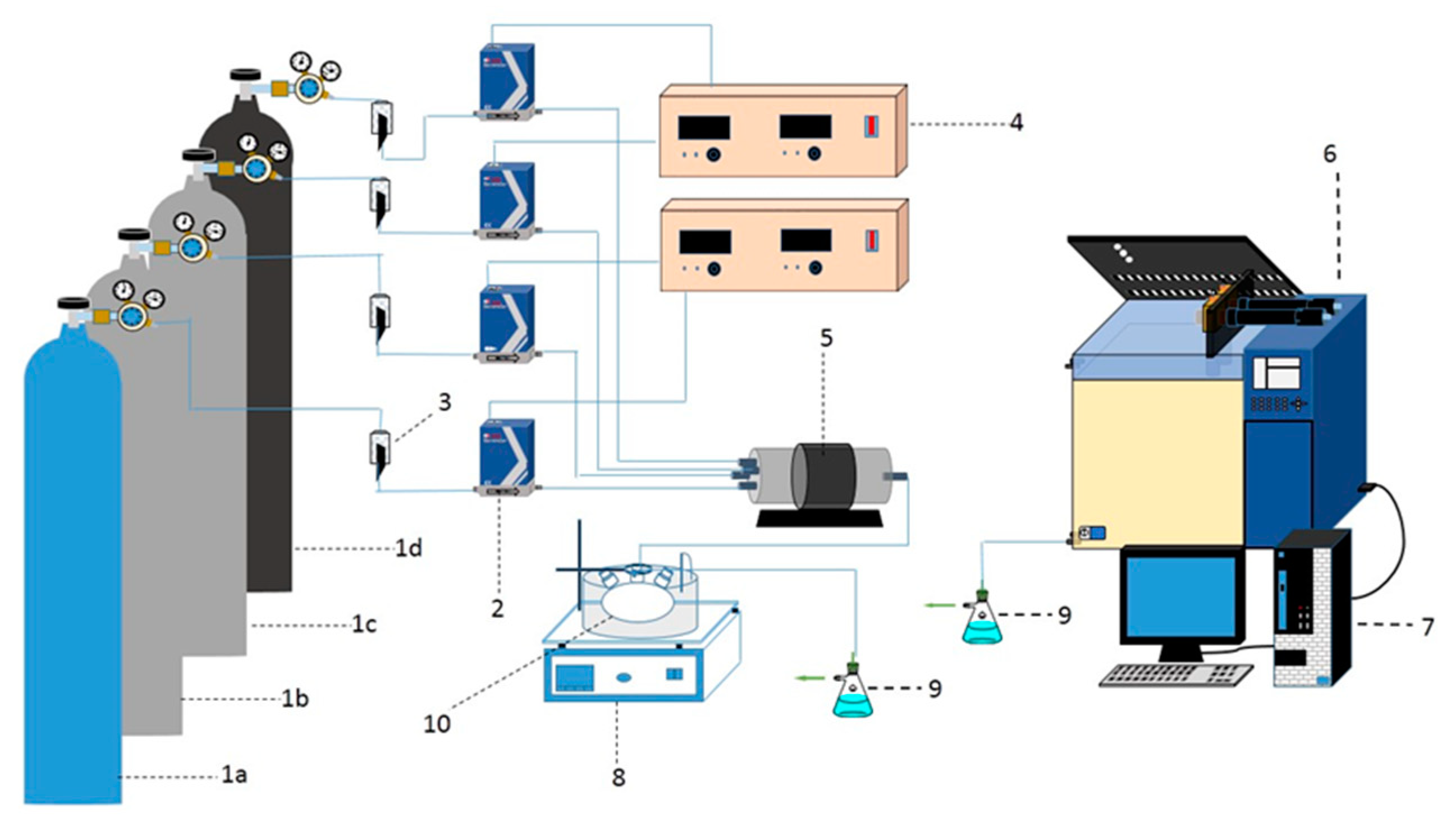
3.3. Analytical Method
3.4. Catalytic Activity Test
4. Conclusions
- (1)
- Through acid leaching experiments, the liquid-phase part after filtration has a leading role in removing H2S and PH3. The highest PH3 conversion efficiency of leaching residue slurry + CuSO4 group can only obtain 15.14%, which indicated that the main active components were consumed by the acid leaching method.
- (2)
- By simulation of the modified MS slurry with metal ions based on real chemical composition, the catalytic activity for H2S and PH3 is relative to the types of metal salts, with the order being metal chlorides > metal nitrates > metal sulfates. All of the metal salts can obtain 100% H2S removal efficiency. In addition, the metal chlorides can maintain above 70% PH3 conversion efficiency for 10.5 h and the highest PH3 conversion efficiency was 86.85%; whereas the highest PH3 removal efficiency of the metal nitrates and metal sulfates can only obtain 47.36% and 27.24%, respectively. Furthermore, Al3+ and Cu2+ has a synergistic effect on removing H2S and PH3 compared to Ca2+, Mg2+, and Mn2+ combined with Cu2+ groups.
- (3)
- H2S was oxidized to element S and sulfate due to the reaction between Cu2+ and H2S and part of the H2S oxidation by O2, while the PH3 was oxidized to PO43− by liquid-phase catalytic oxidation of metal ions with the conversion of Cu2+ to Cu+.
- (4)
- The best PH3 and H2S conversion efficiency was obtained by the modified MS slurry (MS + CuSO4), and the maximum removal efficiency of H2S and PH3 were 100% and ~78%, respectively. The simple modification process for raw MS through adding Cu2+ can effectively improve the H2S and PH3 conversion relative to fresh MS, which can be attributed to the synergistic effect of different metal ions. However, the added Cu2+ in the modified MS slurry would be consumed by conversion of Cu2+ to CuS/Cu2S, thereby leading to the deactivation of modified MS slurry.
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Y.; Wang, X.; Ning, P.; Cheng, C.; Wang, F.; Wang, L.; Lin, Y.; Yu, Y. Simultaneous Removal of PH3, H2S, and Dust by Corona Discharge. Energ. Fuel. 2016, 30, 9580–9588. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, R.H.; Li, S.G.; Liu, C.; Chang, J.; Liu, H. Controls on Hydrogen Sulfide Formation and Techniques for Its Treatment in the Binchang Xiaozhuang Coal Mine, China. Energy Fuels 2019, 33, 266–275. [Google Scholar] [CrossRef]
- Chang, S.-M.; Hsu, Y.-Y.; Chan, T.-S. Chemical Capture of Phosphine by a Sol-Gel-Derived Cu/TiO2 Adsorbent—Interaction Mechanisms. J. Phys. Chem. C 2011, 115, 2005–2013. [Google Scholar] [CrossRef]
- Qu, G.F.; Zhao, Q.; Jian, R.L.; Wang, J.Y.; Liu, D.X.; Ning, P. Mechanism of PH3 absorption by CutILs/H2O two-liquid phase system. Sep. Purif. Technol. 2017, 187, 255–263. [Google Scholar] [CrossRef]
- Govindan, M.; Chung, S.J.; Jang, J.W.; Moon, I.S. Removal of hydrogen sulfide through an electrochemically assisted scrubbing process using an active Co (III) catalyst at low temperatures. Chem. Eng. J. 2012, 209, 601–606. [Google Scholar] [CrossRef]
- Fois, E.; Lallai, A.; Mura, G. Sulfur dioxide absorption in a bubbling reactor with suspensions of Bayer red mud. Ind. Eng. Chem. Res. 2007, 46, 6770–6776. [Google Scholar] [CrossRef]
- Montes-Moran, M.A.; Concheso, A.; Canals-Batlle, C.; Aguirre, N.V.; Ania, C.O.; Martin, M.J.; Masaguer, V. Linz-Donawitz Steel Slag for the Removal of Hydrogen Sulfide at Room Temperature. Environ. Sci. Technol. 2012, 46, 8992–8997. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Asaoka, S.; Yamamoto, T.; Hayakawa, S.; Takeda, K.; Katayama, M.; Onoue, T. Mechanisms of Hydrogen Sulfide Removal with Steel Making Slag. Environ. Sci. Technol. 2012, 46, 10169–10174. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Jung, K.D.; Joo, O.S.; Shul, Y.G. Liquid-phase oxidation of hydrogen sulfide to sulfur over CuO/MgO catalyst. React. Kinet. Catal. Lett. 2005, 87, 115–120. [Google Scholar] [CrossRef]
- Monakhov, K.Y.; Gourlaouen, C. On the Insertion of ML2 (M = Ni, Pd, Pt; L = PH3) into the E-Bi Bond (E = C, Si, Ge, Sn, Pb) of a Bicyclo [1.1.1] Pentane Motif: A Case for a Carbenoid-Stabilized Bi(0) Species? Organometallics 2012, 31, 4415–4428. [Google Scholar] [CrossRef]
- Goncharova, L.V.; Clowes, S.K.; Fogg, R.R.; Ermakov, A.V.; Hinch, B.J. Phosphine adsorption and the production of phosphide phases on Cu(001). Surf. Sci. 2002, 515, 553–566. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Suo, N.; Long, Y.; Li, X.; Shi, Y.; Yu, Y. Preparation of particle electrodes from manganese slag and its degradation performance for salicylic acid in the three-dimensional electrode reactor (TDE). Chemosphere 2019, 216, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Song, X.; Li, K.; Wang, C.; Sun, X.; Ning, P.; Huang, H.B. Preparation of modified manganese slag slurry for removal of hydrogen sulphide and phosphine. Can. J. Chem. Eng. 2020, 98, 1534–1542. [Google Scholar] [CrossRef]
- Fleet, M.E.; Harmer, S.L.; Liu, X.; Nesbitt, H.W. Polarized X-ray absorption spectroscopy and XPS of TiS3: S K-and Ti L-edge XANES and S and Ti 2p XPS. Surf. Sci. 2005, 584, 133–145. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Likhatski, M.; Tomashevich, Y.; Romanchenko, A.; Erenburg, S.; Trubina, S. XAS and XPS examination of the Au-S nanostructures produced via the reduction of aqueous gold(III) by sulfide ions. J. Electron Spectrosc. Relat. Phenom. 2010, 177, 24–29. [Google Scholar] [CrossRef]
- Beattie, D.A.; Arcifa, A.; Delcheva, I.; Le Cerf, B.A.; MacWilliams, S.V.; Rossi, A.; Krasowska, M. Adsorption of ionic liquids onto silver studied by XPS. Colloid. Surface A 2018, 544, 78–85. [Google Scholar] [CrossRef]
- Wu, H.B.; Or, V.W.; Gonzalez-Calzada, S.; Grassian, V.H. CuS nanoparticles in humid environments: adsorbed water enhances the transformation of CuS to CuSO4. Nanoscale 2020, 12, 19350–19358. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Xie, J.W.; Liu, P.; Dai, B. Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts 2016, 6, 120. [Google Scholar] [CrossRef]
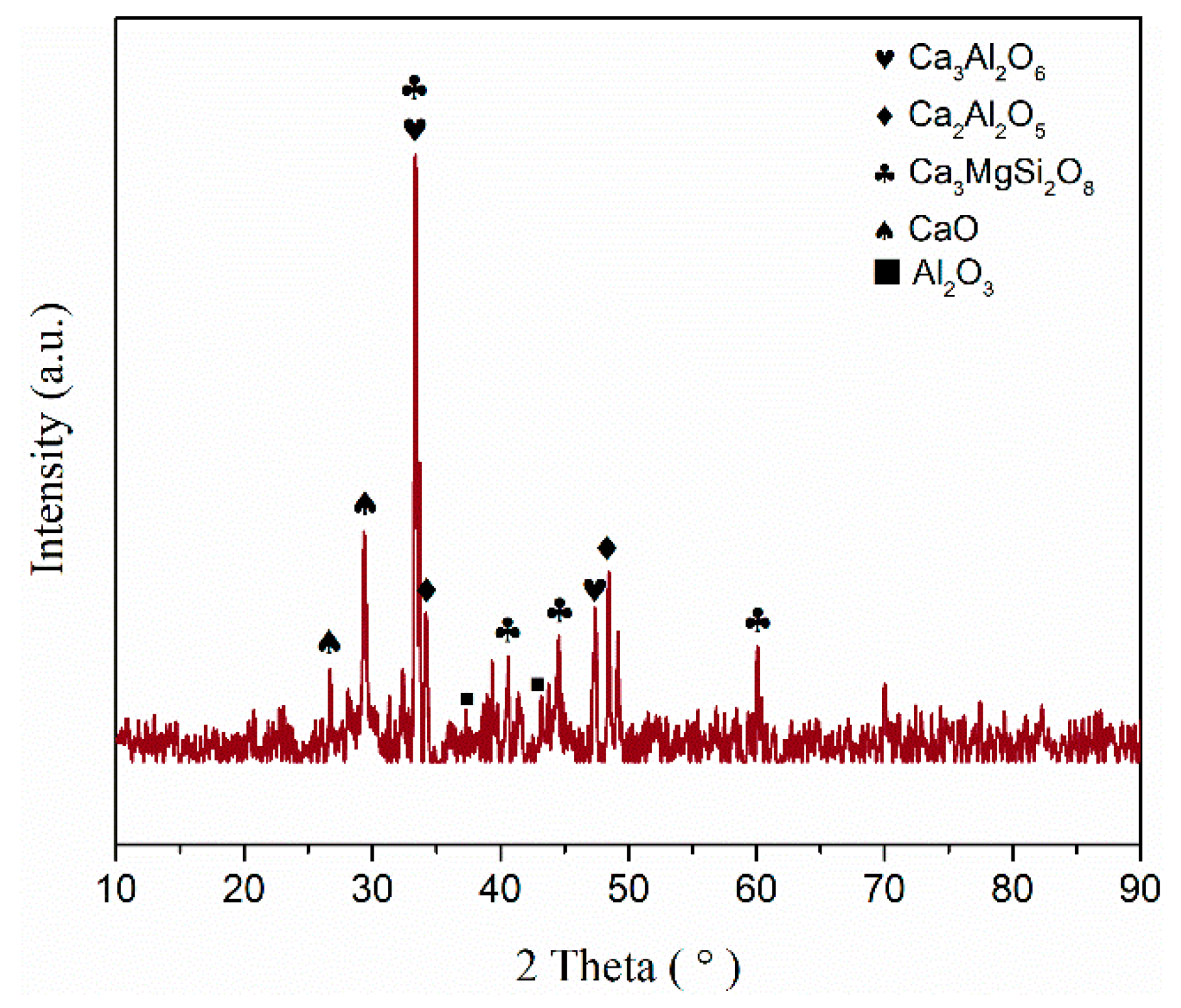


| Element | Ca | Si | Mn | Al | Mg | S | O |
|---|---|---|---|---|---|---|---|
| Raw electrolytic manganese slag | 25.54 | 13.09 | 11.33 | 4.80 | 3.54 | 1.87 | 37.48 |
| Samples | Composition (wt.%), Total Mass = 3 g, Balanced in SiO2 | ||||
|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Mn2+ | Al3+ | Extra Added Cu2+ | |
| Group 1 (metal nitrates) | 25.54 | 3.54 | 11.33 | 4.80 | 0.01 mol |
| Group 2 (metal chlorides) | 25.54 | 3.54 | 11.33 | 4.80 | 0.01 mol |
| Group 3 (metal sulfates) | 25.54 | 3.54 | 11.33 | 4.80 | 0.01 mol |
| Sample | Element | Parameter | |||||||
| 1 h | 4 h | Complete Reaction | |||||||
| Cu2+ Group | S | Position (eV) | 164.7 | 159.6 | 169.1 | 164.0 | 169.6 | 163.9 | 162.6 |
| Atomic ratio (%) | 68.2 | 31.9 | 40.4 | 59.6 | 46.8 | 46.5 | 6.7 | ||
| Substance | Sn1 | S2− | SO42− | S | SO42− | Sn | Sn | ||
| P | Position (eV) | 134.2 | 131.3 | 134.2 | 131.4 | 134.1 | |||
| Atomic ratio (%) | 39.9 | 60.1 | 64.7 | 35.3 | 100.0 | ||||
| Substance | PO43− | P3− | PO43− | P3− | PO43− | ||||
| Cu | Position (eV) | 935.1 | 932.7 | 932.8 | 935.3 | 932.3 | 934.6 | ||
| Atomic ratio (%) | 87.9 | 12.1 | 15.2 | 84.8 | 70.2 | 29.8 | |||
| Substance | CuSO4 | CuS | CuS | CuSO4 | CuS | CuSO4 | |||
| Sample | Element | Parameter | |||||||
| 1 h | 4 h | Complete Reaction | |||||||
| Al3+ + Cu2+ Group | S | Position (eV) | 164.2 | 159.4 | 169.8 | 164.9 | 169.7 | 163.6 | |
| Atomic ratio (%) | 78.4 | 21.6 | 74.2 | 25.8 | 91.4 | 8.6 | |||
| Substance | Sn | S2− | SO42− | S | Sn | Sn | |||
| P | Position (eV) | 133.6 | 131.0 | 134.0 | 131.5 | 134.1 | 131.0 | ||
| Atomic ratio (%) | 51.7 | 48.3 | 69.8 | 30.2 | 94.4 | 5.6 | |||
| Substance | PO43− | P3− | PO43− | P3− | PO43− | P3− | |||
| Cu | Position (eV) | 934.9 | 932.6 | 935.2 | 932.9 | 935.0 | 932.6 | ||
| Atomic ratio (%) | 83.5 | 16.5 | 86.5 | 13.5 | 73.6 | 26.4 | |||
| Substance | Cu(II) | Cu(I) | Cu(II) | Cu(I) | Cu(II) | Cu(I) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, J.; Wang, X.; Li, K.; Wang, F.; Wang, C.; Song, X.; Sun, X.; Ning, P. Reaction Mechanism of Simultaneous Removal of H2S and PH3 Using Modified Manganese Slag Slurry. Catalysts 2020, 10, 1384. https://doi.org/10.3390/catal10121384
Bao J, Wang X, Li K, Wang F, Wang C, Song X, Sun X, Ning P. Reaction Mechanism of Simultaneous Removal of H2S and PH3 Using Modified Manganese Slag Slurry. Catalysts. 2020; 10(12):1384. https://doi.org/10.3390/catal10121384
Chicago/Turabian StyleBao, Jiacheng, Xialing Wang, Kai Li, Fei Wang, Chi Wang, Xin Song, Xin Sun, and Ping Ning. 2020. "Reaction Mechanism of Simultaneous Removal of H2S and PH3 Using Modified Manganese Slag Slurry" Catalysts 10, no. 12: 1384. https://doi.org/10.3390/catal10121384
APA StyleBao, J., Wang, X., Li, K., Wang, F., Wang, C., Song, X., Sun, X., & Ning, P. (2020). Reaction Mechanism of Simultaneous Removal of H2S and PH3 Using Modified Manganese Slag Slurry. Catalysts, 10(12), 1384. https://doi.org/10.3390/catal10121384




