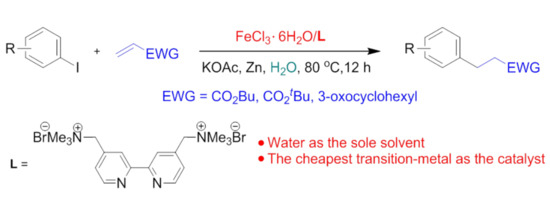
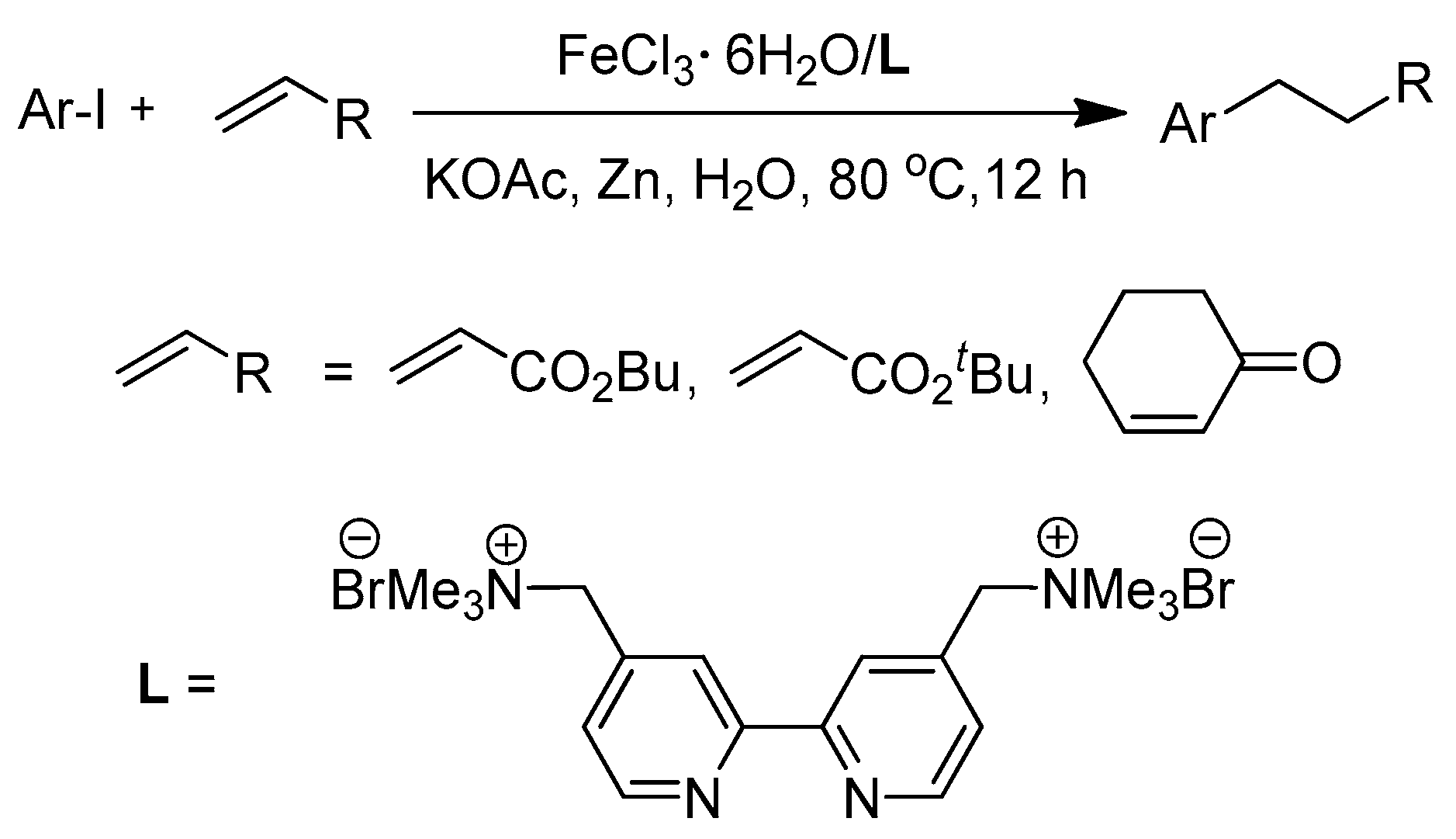
Iron-Catalyzed Conjugate Addition of Aryl Iodides onto Activated Alkenes under Air in Water
Abstract
1. Introduction
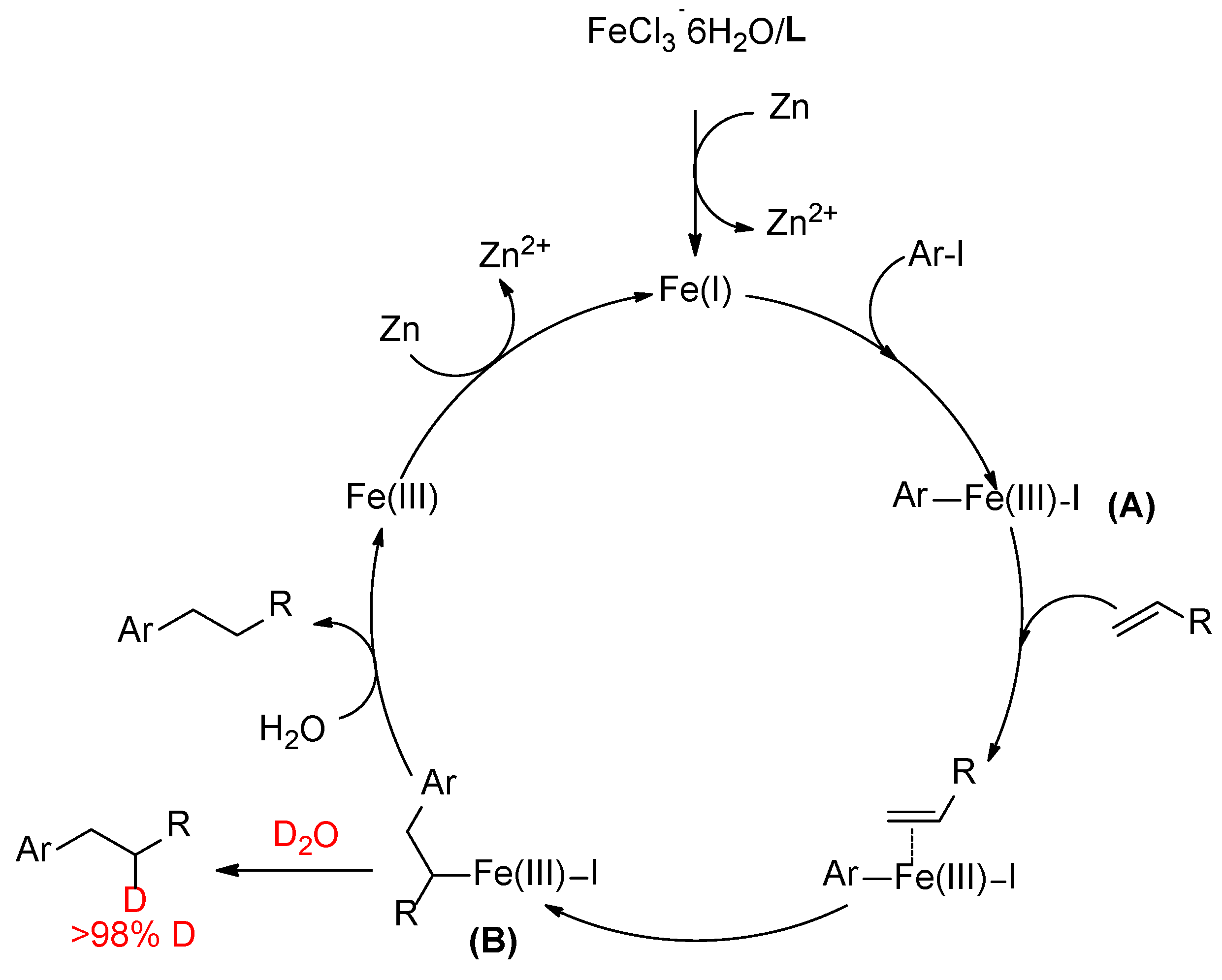
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Procedure for the Conjugate Addition of Aryl Iodides onto Activated Olefins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis; Pergamon Press: Oxford, UK, 1992. [Google Scholar]
- Chen, M.-H.; Mannathan, S.; Lin, P.-S.; Cheng, C.-H. Cobalt(II)-catalyzed 1,4-addition of organoboronic acids to activated alkenes: An application to highly cis-stereoselective synthesis of aminoindane carboxylic acid derivatives. Chem. Eur. J. 2012, 18, 14918–14922. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, E.; Yashuhara, Y.; Hayashi, T. Nickel-catalyzed conjugate addition of arylboron reagents to α,β-unsaturated carbonyl compounds with the aid of a catalytic amount of an alkyne. Chem. Lett. 2006, 35, 768–769. [Google Scholar] [CrossRef]
- Sieber, J.D.; Liu, S.; Morken, J.P. Catalytic conjugate addition of allyl groups to styryl-activated enones. J. Am. Chem. Soc. 2007, 129, 2214–2215. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Yorimitsu, H.; Oshima, K. Nickel-catalyzed 1,4-addition of trialkylboranes to α,β-unsaturated esters: Dramatic enhancement by addition of methanol. Org. Lett. 2007, 9, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.-J.; Gao, M.; Dong, M.; Wei, Y.-P.; Zhang, W.-Q. Catalyzation of 1,4-additions of arylboronic acids to α,β-unsaturated substrates using nickel(I) complexes. Tetrahedron Lett. 2014, 55, 2107–2109. [Google Scholar] [CrossRef]
- Chen, W.; Sun, L.; Huang, X.; Wang, J.; Peng, Y.; Song, G. Ligand-free nickel-catalysed 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds. Adv. Synth. Catal. 2015, 357, 1474–1482. [Google Scholar] [CrossRef]
- Takatsu, K.; Shintani, R.; Hayashi, T. Copper-catalyzed 1,4-addition of organoboronates to alkylidene cyanoacetates: Mechanistic insight and application to asymmetric catalysis. Angew. Chem. Int. Ed. 2011, 50, 5548–5552. [Google Scholar] [CrossRef]
- Yoshida, M.; Ohmiya, H.; Sawamura, M. Enantioselective conjugate addition of alkylboranes catalyzed by a copper−N-heterocyclic carbene complex. J. Am. Chem. Soc. 2012, 134, 11896–11899. [Google Scholar] [CrossRef]
- Liao, Y.-X.; Hu, Q.-S. CuCl/bipyridine-catalyzed addition reactions of arylboroxines with aldehydes, α,β-unsaturated ketones, and N-tosyl aldimines. J. Org. Chem. 2011, 76, 7602–7607. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, X.; Peng, Z.; Fu, L.; Zhang, Z. Ru-catalyzed 1,4-addition of arylboronic acids to acrylic acid derivatives in the presence of phenols. Chem. Commun. 2013, 49, 8797–8799. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 2003, 103, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-Z.; Foster, D.; Sipos, G.; Gao, P.; Skelton, B.W.; Sobolev, A.N.; Dorta, R. Electronic and steric tuning of an atropisomeric disulfoxide ligand motif and its use in the Rh(I)-catalyzed addition reactions of boronic acids to a wide range of acceptors. J. Org. Chem. 2018, 83, 9741–9755. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, K.; Tseng, Y.-Y.; Jian, J.-H.; Takahashi, T.; Ogasawara, M. Planar-chiral phosphine-olefin ligands exploiting a (cyclopentadienyl)manganese(I) scaffold to achieve high robustness and high enantioselectivity. J. Am. Chem. Soc. 2017, 139, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kim, H. Chiral bicyclic bridgehead phosphoramidite (briphos) ligands for asymmetric rhodium-catalyzed 1,2- and 1,4-addition. J. Org. Chem. 2016, 81, 3520–3527. [Google Scholar] [CrossRef]
- Ogasawara, M.; Tseng, Y.-Y.; Arae, S.; Morita, T.; Nakaya, T.; Wu, W.-Y.; Takahashi, T.; Kamikawa, K. Phosphine−olefin ligands based on a planar-chiral (π-arene)chromium scaffold: Design, synthesis, and application in asymmetric catalysis. J. Am. Chem. Soc. 2014, 136, 9377–9384. [Google Scholar] [CrossRef]
- Korenaga, T.; Ko, A.; Shimada, K. Low-temperature Rh-catalyzed asymmetric 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds. J. Org. Chem. 2013, 78, 9975–9980. [Google Scholar] [CrossRef]
- Liu, C.-C.; Janmanchi, D.; Chen, C.-C.; Wu, H.-L. Expanding the C1-symmetric bicyclo[2.2.1]heptadiene ligand family: Highly enantioselective synthesis of cyclic β-aryl-substituted carbonyl compounds. Eur. J. Org. Chem. 2012, 2012, 2503–2507. [Google Scholar] [CrossRef]
- Thaler, T.; Guo, L.-N.; Steib, A.K.; Raducan, M.; Karaghiosoff, K.; Mayer, P.; Knochel, P. Sulfoxide-alkene hybrids: A new class of chiral ligands for the Hayashi-Miyaura reaction. Org. Lett. 2011, 13, 3182–3185. [Google Scholar] [CrossRef]
- Xue, F.; Li, X.; Wan, B. A class of benzene backbone-based olefin-sulfoxide ligands for Rh-catalyzed enantioselective addition of arylboronic acids to enones. J. Org. Chem. 2011, 76, 7256–7262. [Google Scholar] [CrossRef]
- Berhal, F.; Wu, Z.; Genet, J.-P.; Ayad, T.; Ratovelomanana-Vidal, V. Rh-catalyzed asymmetric 1,4-addition of arylboronic acids to α,β-unsaturated ketones with DIFLUORPHOS and SYNPHOS analogues. J. Org. Chem. 2011, 76, 6320–6326. [Google Scholar] [CrossRef]
- Le Boucher d’Herouville, F.; Millet, A.; Scalone, M.; Michelet, V. Room-temperature Rh-catalyzed asymmetric 1,4-addition of arylboronic acids to maleimides and enones in the presence of CF3-substituted MeOBIPHEP analogues. J. Org. Chem. 2011, 76, 6925–6930. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-L.; Iwamura, H.; Shintani, R.; Hayashi, T. Chiral phosphine-olefin ligands in the rhodium-catalyzed asymmetric 1,4-addition reactions. J. Am. Chem. Soc. 2007, 129, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Yamagami, T.; Kimura, T.; Hayashi, T. Asymmetric synthesis of 2-aryl-2,3-dihydro-4-quinolones by rhodium-catalyzed 1,4-addition of arylzinc reagents in the presence of chlorotrimethylsilane. Org. Lett. 2005, 7, 5317–5319. [Google Scholar] [CrossRef]
- Chen, G.; Tokunaga, N.; Hayashi, T. Rhodium-catalyzed asymmetric 1,4-addition of arylboronic acids to coumarins: Asymmetric synthesis of (R)-tolterodine. Org. Lett. 2005, 7, 2285–2288. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Tokunaga, N.; Doi, H.; Hayashi, T. A new entry of nucleophiles in rhodium-catalyzed asymmetric 1,4-addition reactions: Addition of organozinc reagents for the synthesis of 2-aryl-4-piperidones. J. Am. Chem. Soc. 2004, 126, 6240–6241. [Google Scholar] [CrossRef] [PubMed]
- Oi, S.; Moro, M.; Ito, H.; Honma, Y.; Miyano, S. Rhodium-catalyzed conjugate addition of aryl- and alkenyl-stannanes to α,β-unsaturated carbonyl compounds. Tetrahedron 2002, 58, 91–97. [Google Scholar] [CrossRef]
- Oi, S.; Honma, Y.; Inoue, Y. Conjugate addition of organosiloxanes to α,β-unsaturated carbonyl compounds catalyzed by a cationic rhodium complex. Org. Lett. 2002, 4, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Danda, Y.; Fujii, T.; Hirabayashi, K.; Osakada, K. Hydroxorhodium complex-catalyzed carbon-carbon bond-forming reactions of silanediols with α,β-unsaturated carbonyl compounds. Mizoroki-Heck-type reaction vs conjugate addition. J. Am. Chem. Soc. 2001, 123, 10774–10775. [Google Scholar] [CrossRef]
- Takaya, Y.; Ogasawara, M.; Hayashi, T.; Sakai, M.; Miyaura, N. Rhodium-catalyzed asymmetric 1,4-addition of aryl- and alkenylboronic acids to enones. J. Am. Chem. Soc. 1998, 120, 5579–5580. [Google Scholar] [CrossRef]
- Ruiz-Botella, S.; Peris, E. Immobilization of pyrene-adorned N-heterocyclic carbene complexes of rhodium (I) on reduced graphene oxide and study of catalytic activity. ChemCatChem 2018, 10, 1874–1881. [Google Scholar] [CrossRef]
- Mühlhäuser, T.; Savin, A.; Frey, W.; Baro, A.; Schneider, A.J.; Döteberg, H.-G.; Bauer, F.; Köhn, A.; Laschat, S. Role of regioisomeric bicyclo[3.3.0]octa-2,5-diene ligands in Rh catalysis: Synthesis, structural analysis, theoretical study, and application in asymmetric 1,2- and 1,4-additions. J. Org. Chem. 2017, 82, 13468–13480. [Google Scholar] [CrossRef] [PubMed]
- Melcher, M.-C.; da Silva, B.R.A.; Ivšić, T.; Strand, D. Chiral discrimination in rhodium(I) catalysis by 2,5-disubstituted 1,3a,4,6a-tetrahydropenatalene ligands–more than just a twist of the Olefins? ACS Omega 2018, 3, 3622–3630. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, B.; Prakasham, A.P.; Gangwar, M.K.; Ghosh, P. 1,4-Conjugate addition of aryl boronic acids on cyclohexenone as catalyzed by rhodium(I) complexes of C2-symmetric bioxazoline fused N-heterocyclic carbenes. ChemistrySelect 2019, 4, 8526–8533. [Google Scholar] [CrossRef]
- Motokura, K.; Hashiguchi, K.; Maeda, K.; Nambo, M.; Manaka, Y.; Chun, W.-J. Rh-catalyzed 1,4-addition reactions of arylboronic acids accelerated by co-immobilized tertiary amine in silica mesopores. Mol. Catal. 2019, 472, 1–9. [Google Scholar] [CrossRef]
- Borrego, L.G.; Recio, R.; Álvarez, E.; Sánchez-Coronilla, A.; Khiar, N.; Fernández, I. Steric tuning of sulfinamide/sulfoxides as chiral ligands with C1, pseudo-meso, and pseudo-C2 symmetries: Application in rhodium(I)-mediated arylation. Org. Lett. 2019, 21, 6513–6518. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Q.; Zhu, C.; Wu, X.; Li, Y.; Luo, Y.; He, J.-B. Enantioselective conjugate addition of aryl halides and triflates to electron-deficient olefins via nickel- and rhodium-catalyzed sequential relay reactions. Org. Lett. 2019, 21, 8888–8892. [Google Scholar] [CrossRef]
- Pecchioli, T.; Christmann, M. Synthesis of highly enantioenriched propelladienes and their application as ligands in asymmetric Rh-catalyzed 1,4-additions. Org. Lett. 2018, 20, 5256–5259. [Google Scholar] [CrossRef]
- Nikol, A.; Zhang, Z.; Chelouan, A.; Falivene, L.; Cavallo, L.; Herrera, A.; Heinemann, F.W.; Escalona, A.; Frieß, S.; Grasruck, A.; et al. Tricyclic sulfoxide-alkene hybrid ligands for chiral Rh(I) complexes: The “matched” diastereomer catalyzes asymmetric C–C bond formations. Organometallics 2020, 39, 1348–1359. [Google Scholar] [CrossRef]
- Kirchhof, M.; Gugeler, K.; Fischer, F.R.; Nowakowski, M.; Bauer, A.; Alvarez-Barcia, S.; Abitaev, K.; Schnierle, M.; Qawasmi, Y.; Frey, W.; et al. Experimental and theoretical Sstudy on the role of monomeric vs dimeric rhodium oxazolidinone norbornadiene complexes in catalytic asymmetric 1,2- and 1,4-additions. Organometallics 2020, 39, 3131–3145. [Google Scholar] [CrossRef]
- Hamasaka, G.; Muto, T.; Andoh, Y.; Fujimoto, K.; Kato, K.; Takata, M.; Okazaki, S.; Uozumi, Y. Detailed structural analysis of a self-assembled vesicular amphiphilic NCN-pincer palladium complex by using wide-angle X-ray scattering and molecular dynamics calculations. Chem. Eur. J. 2017, 23, 1291–1298. [Google Scholar] [CrossRef]
- Tamura, M.; Ogata, H.; Ishida, Y.; Takahashi, Y. Design and synthesis of chiral 1,10-phenanthroline ligand, and application in palladium catalyzed asymmetric 1,4-addition reactions. Tetrahedron Lett. 2017, 58, 3808–3813. [Google Scholar] [CrossRef]
- de Gracia Retamosa, M.; Álvarez-Casao, Y.; Matador, E.; Gómez, Á.; Monge, D.; Fernández, R.; Lassaletta, J.M. Pyridine-hydrazone ligands in asymmetric palladium-catalyzed 1,4- and 1,6-additions of arylboronic acids to cyclic (di)enones. Adv. Synth. Catal. 2019, 361, 176–184. [Google Scholar] [CrossRef]
- Shimizu, M.; Yamamoto, T. 9-(Diphenylphosphino)anthracene-based phosphapalladacycle catalyzed conjugate addition of arylboronic acids to electron-deficient alkenes. Tetrahedron Lett. 2020, 61, 152257. [Google Scholar] [CrossRef]
- Gerten, A.L.; Stanley, L.M. Palladium-catalyzed conjugate addition of arylboronic acids to 2-substituted chromones in aqueous media. Tetrahedron Lett. 2016, 57, 5460–5463. [Google Scholar] [CrossRef]
- Shockley, S.E.; Holder, J.C.; Stoltz, B.M. Palladium-catalyzed asymmetric conjugate addition of arylboronic acids to α,β-unsaturated cyclic electrophiles. Org. Process Res. Dev. 2015, 19, 974–981. [Google Scholar] [CrossRef]
- Lan, Y.; Houk, K.N. Mechanism of the palladium-catalyzed addition of arylboronic acids to enones: A computational study. J. Org. Chem. 2011, 76, 4905–4909. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Díez, J.; Cadierno, V. Conjugate addition of arylboronic acids to α,β-unsaturated carbonyl compounds in aqueous medium using Pd(II) complexes with dihydroxy-2,2’-bipyridine ligands: Homogeneous or heterogeneous nano-catalysis? Catal. Sci. Technol. 2011, 1, 1605–1615. [Google Scholar] [CrossRef]
- Huang, S.-H.; Wu, T.-M.; Tsai, F.-Y. pH-dependent conjugate addition of arylboronic acids to α,β-unsaturated enones catalyzed by a reusable palladium(II)/cationic 2,2’-bipyridyl system in water under air. Appl. Organometal. Chem. 2010, 24, 619–624. [Google Scholar] [CrossRef]
- Nishikata, T.; Kiyomura, S.; Yamamoto, Y.; Miyaura, N. Asymmetric 1,4-addition of arylboronic acid to α,β-unsaturated esters catalyzed by dicationic palladium(II)-chiraphos complex for short-step synthesis of SmithKline Beecham’s endothelin receptor antagonist. Synlett 2008, 2008, 2487–2488. [Google Scholar] [CrossRef]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. Palladium(II)-catalyzed 1,4-addition of arylboronic acids to β-arylenals for enantioselective syntheses of 3,3-diarylalkanals: A short synthesis of (+)-(R)-CDP 840. Tetrahedron Lett. 2007, 48, 4007–4010. [Google Scholar] [CrossRef]
- He, P.; Lu, Y.; Dong, C.-G.; Hu, Q.-S. Anionic four-electron donor-based palladacycles as catalysts for addition reactions of arylboronic acids with α,β-unsaturated ketones, aldehydes, and α-ketoesters. Org. Lett. 2007, 9, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Gini, F.; Hessen, B.; Feringa, B.L.; Minnaard, A.J. Enantioselective palladium-catalysed conjugate addition of arylsiloxanes. Chem. Commun. 2007, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. Palladium(II)-catalyzed 1,4-addition of arylboronic acids to β-arylenones enantioselective synthesis of 4-aryl-4H-chromenes. Adv. Synth. Catal. 2007, 349, 1759–1764. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iizuka, M.; Ohta, T.; Ito, Y. Palladium catalyzed conjugate 1,4-addition of organoboronic acids to α,β-unsaturated ketones. Chem. Lett. 2006, 35, 198–199. [Google Scholar] [CrossRef]
- Gini, F.; Hessen, B.; Minnaard, A.J. Palladium-catalyzed enantioselective conjugate addition of arylboronic acids. Org. Lett. 2005, 7, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lin, S. Pd(II)-bipyridine catalyzed conjugate addition of arylboronic acid to a,β-unsaturated carbonyl compounds. J. Org. Chem. 2005, 70, 9651–9653. [Google Scholar] [CrossRef] [PubMed]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. Asymmetric 1,4-addition of potassium aryltrifluoroborates [ArBF3]K to enones catalyzed by dicationic palladium(II) complexes. Chem. Lett. 2005, 34, 720–721. [Google Scholar] [CrossRef]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. 1,4-Addition of arylboronic acids and arylsiloxanes to α,β-unsaturated carbonyl compounds via transmetalation to dicationic palladium(II) complexes. Organometallics 2004, 23, 4317–4324. [Google Scholar] [CrossRef]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. Conjugate addition of aryl boronic acids to enones catalyzed by cationic palladium(II)–phosphane complexes. Angew. Chem. Int. Ed. 2003, 42, 2768–2770. [Google Scholar] [CrossRef]
- Ohe, T.; Wakita, T.; Motofusa, S.-I.; Cho, C.-S.; Ohe, K.; Uemura, S. Palladium(II)-catalyzed Michael-type addition reactions using aryltin compounds. Bull. Chem. Soc. Jpn. 2000, 73, 2149–2155. [Google Scholar] [CrossRef]
- Denmark, S.E.; Amishiro, N. Palladium-catalyzed conjugate addition of organosiloxanes to α,β-unsaturated carbonyl compounds and nitroalkenes. J. Org. Chem. 2003, 68, 6997–7003. [Google Scholar] [CrossRef] [PubMed]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. 1,4-Addition of arylsiloxanes to enones catalyzed by dicationic palladium(II) complexes in aqueous media. Chem. Lett. 2003, 32, 752–753. [Google Scholar] [CrossRef]
- Koike, T.; Du, X.; Sanada, T.; Danda, Y.; Mori, A. Iridium-catalyzed Mizoroki-Heck-type reaction of organosilicon reagents. Angew. Chem. Int. Ed. 2003, 42, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Lu, T.; Yang, C.-F.; Chen, X.-T.; Xue, Z.-L. Synthesis, structures, and catalytic properties of dinuclear iridium(I) complexes with a hexadentate macrocyclic diamine tetracarbene ligand. Eur. J. Inorg. Chem. 2018, 2018, 1595–1602. [Google Scholar] [CrossRef]
- Gottumukkala, A.L.; de Vries, J.G.; Minnaard, A.J. Pd–NHC catalyzed conjugate addition versus the Mizoroki–Heck reaction. Chem. Eur. J. 2011, 17, 3091–3095. [Google Scholar] [CrossRef]
- Mannathan, S.; Raoufmoghaddam, S.; Reek, J.N.H.; de Vries, J.G.; Minnaard, A.J. Palladium(II) acetate catalyzed reductive Heck reaction of enones; a practical approach. ChemCatChem 2015, 7, 3923–3927. [Google Scholar] [CrossRef]
- Parveen, N.; Saha, R.; Sekar, G. Stable and reusable palladium nanoparticles-catalyzed conjugate addition of aryl iodides to enones: Route to reductive Heck products. Adv. Synth. Catal. 2017, 359, 3741–3751. [Google Scholar] [CrossRef]
- Yang, W.; Ling, B.; Hu, B.; Yin, H.; Mao, J.; Walsh, P.J. Synergistic N-heterocyclic carbene/palladium-catalyzed umpolung 1,4-addition of aryl iodides to enals. Angew. Chem. Int. Ed. 2020, 59, 161–166. [Google Scholar] [CrossRef]
- Gomes, P.; Gosmini, C.; Nédélec, J.-Y.; Périchon, J. Cobalt bromide as catalyst in electrochemical addition of aryl halides onto activated olefins. Tetrahedron Lett. 2000, 41, 3385–3388. [Google Scholar] [CrossRef]
- Shukla, P.; Hsu, Y.-C.; Cheng, C.-H. Cobalt-catalyzed reductive coupling of saturated alkyl halides with activated alkenes. J. Org. Chem. 2006, 71, 655–658. [Google Scholar] [CrossRef]
- Amatore, M.; Gosmini, C.; Périchon, J. CoBr2(Bpy): An efficient catalyst for the direct conjugate addition of aryl halides or triflates onto activated olefins. J. Org. Chem. 2006, 71, 6130–6134. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-C.; Chu, Y.-H.; Muralirajan, K.; Cheng, C.-H. A simple route to 1,4-addition reactions by Co-catalyzed reductive coupling of organic tosylates and triflates with activated alkenes. Chem. Commun. 2017, 53, 11584–11587. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.-I.; Fukumoto, K.; Kawamoto, T. Effect of phosphine and phosphine sulfide ligands on the cobalt-catalyzed reductive coupling of 2-iodobutane with n-butyl acrylate. Polyhedron 2013, 62, 37–41. [Google Scholar] [CrossRef]
- Lu, S.; Jin, T.; Bao, M.; Yamamoto, Y. Cobalt-catalyzed hydroalkylation of [60] fullerene with active alkyl bromides: Selective synthesis of monoalkylated fullerenes. J. Am. Chem. Soc. 2011, 133, 12842–12848. [Google Scholar] [CrossRef]
- Amatore, M.; Gosmini, C. Direct cobalt-catalyzed conjugate addition of functionalized aryl halides and triflates: A new strategy for the conjugate addition onto methyl vinyl ketone. Synlett 2009, 2009, 1073–1076. [Google Scholar] [CrossRef]
- Qian, Q.; Zang, Z.; Chen, Y.; Tong, W.; Gong, H. Nickel and cobalt-catalyzed coupling of alkyl halides with alkenes via Heck reactions and radical conjugate addition. Mini-Rev. Med. Chem. 2013, 13, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Sharma, A.; Pallavi, B.; Cheng, C.-H. Nickel-catalyzed reductive Heck type coupling of saturated alkyl halides with acrylates and oxabenzonorbornadiene. Tetrahedron 2015, 71, 2260–2266. [Google Scholar] [CrossRef]
- Shrestha, R.; Weix, D.J. Reductive conjugate addition of haloalkanes to enones to form silyl Enol ethers. Org. Lett. 2011, 13, 2766–2769. [Google Scholar] [CrossRef]
- Shrestha, R.; Dorn, S.C.M.; Weix, D.J. Nickel-catalyzed reductive conjugate addition to enones via allylnickel intermediates. J. Am. Chem. Soc. 2013, 135, 751–762. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Cheong, H.-L.; Loh, T.-P. Indium/copper-mediated conjugate addition of unactivated alkyl iodides to α,β-unsaturated carbonyl compounds in water. Tetrahedron Lett. 2009, 50, 1051–1054. [Google Scholar] [CrossRef]
- Fleming, F.F.; Gudipati, S. Alkenenitriles: Zn−Cu promoted conjugate additions of alkyl iodides in water. Org. Lett. 2006, 8, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hu, X.; Zhang, W.; Li, C.-J. Direct conjugate additions using aryl and alkyl organic halides in air and water. Org. Chem. Front. 2018, 5, 3579–3584. [Google Scholar] [CrossRef]
- Zhou, F.; Hu, X.; Zhang, W.; Li, C.-J. Copper-catalyzed radical reductive arylation of styrenes with aryl iodides mediated by zinc in water. J. Org. Chem. 2018, 83, 7416–7422. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Huang, S.; Leong, W.W.Y.; Zhong, G.; Isley, N.A. C−C bond formation via copper-catalyzed conjugate addition reactions to enones in water at room temperature. J. Am. Chem. Soc. 2012, 134, 19985–19988. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.F.; Gudipati, S.; Aitken, J.A. Alkenenitriles: Conjugate additions of alkyl iodides with a silica-supported zinc-copper matrix in water. J. Org. Chem. 2007, 72, 6961–6969. [Google Scholar] [CrossRef]
- Streuff, J.; Gansäuer, A. Metal-catalyzed β-functionalization of Michael acceptors through reductive radical addition reactions. Angew. Chem. Int. Ed. 2015, 54, 14232–14242. [Google Scholar] [CrossRef]
- Pang, H.; Wang, Y.; Gallou, F.; Lipshutz, B.H. Fe-catalyzed reductive couplings of terminal (hetero)aryl alkenes and alkyl halides under aqueous micellar conditions. J. Am. Chem. Soc. 2019, 141, 17117–17124. [Google Scholar] [CrossRef]
- Hung, T.-T.; Huang, C.-M.; Tsai, F.-Y. Sonogashira–Hagihara coupling towards diaryl alkynes catalyzed by FeCl3·6H2O/cationic 2,2’-bipyridyl. ChemCatChem 2012, 4, 540–545. [Google Scholar] [CrossRef]
- Buchwald, S.L.; Bolm, C. On the role of metal contaminants in catalyses with FeCl3. Angew. Chem. Int. Ed. 2009, 48, 5586–5587. [Google Scholar] [CrossRef]
- Zhou, F.; Li, C.-J. The Barbier-Grignard-type arylation of aldehydes using unactivated aryl iodides in water. Nat. Commun. 2014, 5, 4254. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chen, S.-N.; Tsai, F.-Y. Recyclable and highly active cationic 2,2’-bipyridyl palladium(II) catalyst for Suzuki cross-coupling reaction in water. Tetrahedron Lett. 2006, 47, 9267–9270. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Hiyama reaction of aryl bromides with arylsiloxanes catalyzed by a reusable palladium(II)/cationic bipyridyl system in water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]
| Entry | 2a (mmol) | Zn (mmol) | Base (mmol) | pH | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 4 | 3 | -- | 1.8 | 22 |
| 2 | 4 | 3 | KOAc (1) | 5.0 | 74 |
| 3 | 4 | 3 | KOAc (2) | 5.5 | 85 |
| 4 | 4 | 3 | KOAc (3) | 5.8 | 80 |
| 5 | 4 | 3 | K2CO3 (2) | 11.5 | 40 |
| 6 | 4 | 3 | K3PO4 (2) | 13.5 | 35 |
| 7 | 4 | 3 | KOH (2) | 15.4 | 23 |
| 8 | 4 | 3 | Bu3N (2) | 6.8 | 47 |
| 9 | 3 | 3 | KOAc (2) | 77 | |
| 10 | 2 | 3 | KOAc (2) | 52 | |
| 11 | 4 | 2.5 | KOAc (2) | 74 | |
| 12 | 4 | 2 | KOAc (2) | 61 | |
| 13 | 4 | 0 | KOAc (2) | 0 | |
| 14 c | 4 | 3 | KOAc (2) | 86 | |
| 15 d | 4 | 3 | KOAc (2) | 0 | |
| 16 e | 4 | 3 | KOAc (2) | 12 | |
| 17 f | 4 | 3 | KOAc (2) | 0 | |
| 18 g | 20 | 15 | KOAc (10) | 75 | |
| 19 h | 4 | 3 | KOAc (2) | 0 |
| Entry | Aryl Iodide | Product | Yield (%) b |
|---|---|---|---|
| 1 | 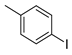 1b 1b | 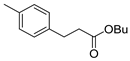 3b 3b | 74 |
| 2 | 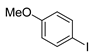 1c 1c | 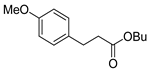 3c 3c | 44 |
| 3 | 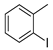 1d 1d | 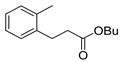 3d 3d | 70 |
| 4 | 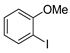 1e 1e | 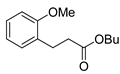 3e 3e | 72 |
| 5 | 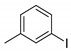 1f 1f | 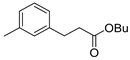 3f 3f | 75 |
| 6 | 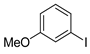 1g 1g | 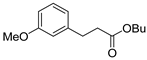 3g 3g | 79 |
| 7 | 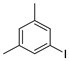 1h 1h | 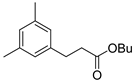 3h 3h | 78 |
| 8 | 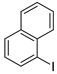 1i 1i | 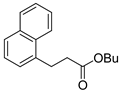 3i 3i | 75 |
| 9 | 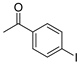 1j 1j | 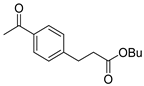 3j 3j | 40 |
| 10 | 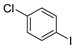 1k 1k | 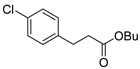 3k 3k | 52 |
| 11 | 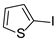 1l 1l | 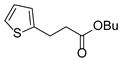 3l 3l | 0 |
| Entry | Aryl Iodide | Alkene | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | 1a | 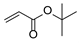 2b 2b | 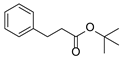 4a 4a | 82 |
| 2 | 1b | 2b | 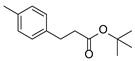 4b 4b | 81 |
| 3 | 1c | 2b | 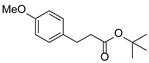 4c 4c | 43 |
| 4 | 1d | 2b | 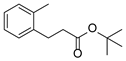 4d 4d | 84 |
| 5 | 1e | 2b | 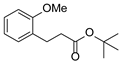 4e 4e | 74 |
| 6 | 1f | 2b | 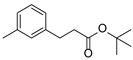 4f 4f | 77 |
| 7 | 1g | 2b | 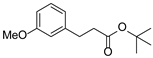 4g 4g | 73 |
| 8 | 1h | 2b | 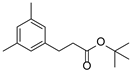 4h 4h | 77 |
| 9 | 1i | 2b | 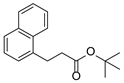 4i 4i | 80 |
| 10 | 1a | 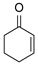 2c 2c | 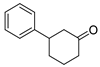 5a 5a | 77 |
| 11 | 1b | 2c | 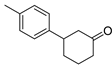 5b 5b | 75 |
| 12 | 1c | 2c | 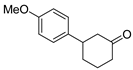 5c 5c | 55 |
| 13 | 1d | 2c | 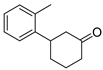 5d 5d | 73 |
| 14 | 1e | 2c | 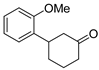 5e 5e | 74 |
| 15 | 1f | 2c | 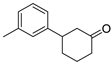 5f 5f | 70 |
| 16 | 1g | 2c | 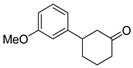 5g 5g | 72 |
| 17 | 1h | 2c | 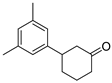 5h 5h | 81 |
| 18 | 1i | 2c | 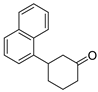 5i 5i | 78 |
| 19 | 1a | 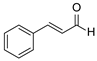 2d 2d | 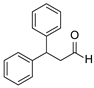 6a 6a | 0 |
| 20 | 1a | 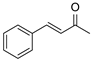 2e 2e | 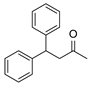 7a 7a | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-M.; Peng, W.-S.; Liu, L.-J.; Wu, C.-C.; Tsai, F.-Y. Iron-Catalyzed Conjugate Addition of Aryl Iodides onto Activated Alkenes under Air in Water. Catalysts 2020, 10, 1320. https://doi.org/10.3390/catal10111320
Huang C-M, Peng W-S, Liu L-J, Wu C-C, Tsai F-Y. Iron-Catalyzed Conjugate Addition of Aryl Iodides onto Activated Alkenes under Air in Water. Catalysts. 2020; 10(11):1320. https://doi.org/10.3390/catal10111320
Chicago/Turabian StyleHuang, Chung-Min, Wen-Sheng Peng, Ling-Jun Liu, Chien-Chi Wu, and Fu-Yu Tsai. 2020. "Iron-Catalyzed Conjugate Addition of Aryl Iodides onto Activated Alkenes under Air in Water" Catalysts 10, no. 11: 1320. https://doi.org/10.3390/catal10111320
APA StyleHuang, C.-M., Peng, W.-S., Liu, L.-J., Wu, C.-C., & Tsai, F.-Y. (2020). Iron-Catalyzed Conjugate Addition of Aryl Iodides onto Activated Alkenes under Air in Water. Catalysts, 10(11), 1320. https://doi.org/10.3390/catal10111320